Abstract
Background:
Most patients risk gaining weight in the years after knee replacement, adding further concern to a population that is mostly overweight/obese prior to surgery.
Objective:
Via a randomised pilot study, we assessed changes in weight during a Patient Centered Weight Loss Program (PACE) initiated either before or after knee replacement, while simultaneously examining the feasibility of recruiting and retaining participants over 26 weeks.
Methods:
Recruitment outreach was made to 133 patients scheduled for knee replacement. Sixteen participants were randomised to a 14-session weight loss program that started either ≤6 weeks before surgery (PACE) or at 12 weeks post-op (Delayed PACE). Repeated measures ANOVAs were used to examine preliminary changes in weight, function, patient-reported outcomes, and physical activity across time (baseline/pre-op, 12 and 26 weeks after surgery) and group.
Results:
Retention was 75% and 69% at 12 and 26 weeks after surgery, respectively. Weight significantly decreased across the 26 weeks (P < 0.001). A group by time interaction (P = 0.03) demonstrated Delayed PACE [−7.6 ± 5.9 kg (−7.9 ± 5.9%)] lost significantly more weight than PACE [−2.5 ± 2.7 kg (−2.6 ± 2.6%)] participants at 26 weeks. Significant improvements across time were seen for all function and patient reported outcomes, however activity did not change.
Conclusion:
Conducting a behavioural intervention was challenging but feasible in a knee replacement population, with preliminary evidence suggesting that initiating a program 12 weeks after surgery produces greater weight losses at 26 weeks compared to a program starting before knee replacement.
Keywords: Weight loss, Surgery, Intervention
Introduction
Osteoarthritis is the leading cause of disability in the United States (US) [1]. Estimates indicate that half of patients diagnosed with knee osteoarthritis will need a total knee replacement during their lifetime [2]. Of patients who need a knee replacement, 80–95% are overweight or obese [3,4]. Physicians often recommend weight loss, as each unit increase of body mass index (BMI) results in an 8% increased risk of surgically-related adverse events, including joint infection and deep-vein thrombosis [5]. Obesity also is linked with poorer outcomes following knee replacement and increased risk of undergoing joint replacement revision [6,7].
Prior to surgery, many patients are encouraged by healthcare providers to lose weight [8], yet few attempts result in a clinically significant weight loss (≥5%) [9]. Immediately after surgery, patients may experience some weight loss, yet by 2 years, patients demonstrate a net weight gain [10]. Several studies have suggested that more than 50% of patients will gain weight after surgery [11,12]. Reducing body weight both before and/or after surgery would lower the risk of surgical and medical complications and improve pain and functional outcomes, yet the optimal time to initiate a weight loss program remains unknown. Immediately before the elective surgery may be an opportune teachable moment to initiate behaviour changes [13]. Conversely, waiting to start a pro-gram after the intensive rehabilitation is completed may be less overwhelming to patients.
Similar to the lack of weight loss observed following surgery, physical activity levels often remain unchanged. Prior to knee replacement, pain is one of the primary barriers to physical activity [14]. Following knee replacement, patients typically report significant reductions in pain and improved function [15] but corresponding increases in physical activity are uncommon [16–18]. Losina et al. [19] recently demonstrated that telephone health coaching and financial incentives could increase knee replacement patients’ steps and physical activity at 6 months, but it is unclear if patients focusing on weight loss would also demonstrate comparable increases in activity.
Guided by knee replacement patients (pre- and post-operative) and healthcare professionals (i.e., orthopedic surgeon, physical therapist) input [8], we developed a 14-week Patient-Centered Weight Loss Program (PACE) specifically for knee replacement patients. During a pilot randomised study comparing changes in weight between patients who started the program up to 6 weeks before knee replacement (PACE) and patients who started 12 weeks after surgery (Delayed PACE), we examined the feasibility of recruiting and retaining patients during a 26-week behavioural intervention. The secondary aims of the study were to examine changes in patient-reported outcomes, physical function, and physical activity. Gaining a better understanding of when to offer a weight loss program to knee replacement patients will help to inform clinician recommendations and the development of future weight loss trials to maximise the long-term effectiveness of the knee replacement.
Materials and methods
Study design and subjects
Patients from nearby orthopedic clinics who had recently scheduled a knee replacement were recruited to participate. Recruitment strategies included placing recruitment postcards in pre-operative packets, referrals from physicians and staff, and direct mailings and emails to potential patients. Interested patients were instructed to visit the study website or contact staff to complete the screening procedures.
Patients were required to: (1) be 40–79 years of age, (2) have a body mass index (BMI) between 25–45 kg/m2, (3) have a scheduled knee replacement (including primary, staged or independent bilateral, or revision) ≥1 week away from baseline assessment (modified from 6 weeks before surgery due to challenges with recruitment), (4) obtain physician approval to participate, (5) be English speaking, and (6) willing to attend 3 assessments. Patients were excluded if they (1) had any contraindications to diet or weight loss, (2) were undergoing simultaneous bilateral knee replacement or had a scheduled or anticipated knee replacement for the contralateral knee within the next 26 weeks, (3) had a mobility limiting comorbidity besides the knee replacement (e.g., spinal stenosis),(4) were taking anti-obesity medications, (5) were enrolled in a formal weight loss program, (6) had or were planning to have bariatric/gastric/lapband surgery, (7) were planning to relocate out of the area in the next year.
Eligible patients were invited to an in-person session to complete the informed consent process. Following baseline, participants were randomised to a weight loss program that either started prior to surgery (PACE) or 12 weeks after surgery (Delayed PACE). Randomisation was stratified by age (<65 years and ≥65 years) and sex. All procedures were approved by the Northwestern University Institutional Review Board and participants provided written informed consent prior to completing any procedures.
PACE intervention
The PACE intervention is a 14-week Patient-Centered Weight Loss Program that was modelled off the Diabetes Prevention Pro-gram [20] and Look AHEAD [21] and refined based on input from knee replacement patients, orthopedic surgeons, and physical therapists [8,22]. Participants randomised to PACE started the weight loss program 1–6 weeks before their knee replacement and continued for 12 weeks after surgery. During the first session, participants were provided with program materials and were given a 5% weight loss goal based on their baseline body weight. Participants received a calorie goal between 1200–2000 kcal/day to facilitate a 1–2 lb weight loss each week. Patients were also given personalised physical activity (e.g., steps/day, minutes of moderate-to-vigorous activity) goals, which were tailored according to patient preference, comfort, and pre-/post-rehabilitation progress.
Participants were encouraged to monitor their dietary intake and physical activity with their preferred modality (e.g., paper, website, smartphone application, activity tracker). They were pro-vided with paper diaries and a calorie counter reference book, access to an online program or smartphone application, and/or a Fitbit. Study coaches had real-time access to participants’ web or Fitbit physical activity and dietary data; those using paper diaries were asked to mail in diaries regularly. Participants could also opt to receive three text messages a week. Text messages targeted topics relating to diet, activity, recovery from surgery, and behaviour change.
Participants had up to 14 sessions with their coach either inperson or over the telephone on a weekly or bi-weekly basis, based on preference. To standardise the number of sessions between PACE and Delayed PACE and ensure both could receive up to 14 sessions, regardless of when PACE patients were randomised (between 1–6 weeks pre-surgery), participants only received 2 coaching sessions before surgery. Coaches were either bachelors or doctoral level in fields relating to exercise science or psychology and had experience coaching using motivational interviewing. During each coaching session, coaches reviewed participants’ progress with recovery from surgery, discussed behavioural lessons, and provided feed-back on participants’ self-monitoring. PACE participants entered a maintenance period between 12 and 26 weeks after surgery and did not have any contact with coaches.
Delayed PACE
Participants randomised to Delayed PACE received the same intervention as PACE, however, participants started the program 12 weeks after surgery and continued until 26 weeks. Delayed PACE participants did not have any contact with study staff and coaches between baseline and 12 weeks.
Outcomes
Outcomes were assessed at baseline (between 1–6 weeks before knee replacement) and at 12 and 26 weeks after surgery. Participants received $20 for completing the 12 and 26-week assessments.
Body weight
Body weight was assessed with a calibrated balance beam scale with participants wearing lightweight clothing without shoes.
Physical function
Physical function was assessed with the 30-s Chair Stand, the Timed Up and Go, and Six Minute Walk Tests. All physical function tests were completed following Osteoarthritis Research Society International recommended procedures [23]. During the 30-s Chair Stand, patients were asked to complete as many chair stand repetitions as possible during a 30 s period. During the Timed Up and Go Test, the time in seconds taken to rise from a chair, walk 3m, turn, walk back to the chair, and sit down was measured. The fastest time between two Timed Up and Go tests was taken. The Six Minute Walk Test assessed the maximal distance in feet a participant could cover during a six-minute period.
Patient-reported outcomes
Pain and stiffness were obtained from the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [24]. Participants responded on a 5-point Likert scale to five items assessing their level of pain over the last 48 h and two items evaluating stiffness. Also, using the Patient-Reported Outcomes Measurement Information System (PROMIS) in the REDCap shared library [25], pain intensity, pain interference, physical function-mobility, and sleep disturbance were assessed. PROMIS computer adaptive tests were used and the scoring of each factor assessed results in a t-score [26].
Physical activity
Physical activity was objectively measured using Actigraph GT3X monitors over a 7-day period. Participants were instructed to wear the monitor on their waist during waking hours. Only participants with ≥4 valid days of ≥10 h/day were included. Non-wear was defined as ≥90 min with 0 counts/min, allowing up to 2 consecutive min of <100 counts/min [27]. Physical activity was categorised as sedentary (<100 counts/min), light (100–2019 counts/min), and moderate-to-vigorous (MVPA) (≥2020 counts/min) [28]. Total MVPA and bouts of ≥10 MVPA min were examined.
Process measures
The option to receive text messages as an additional intervention strategy as well as participants’ self-monitoring (paper, website, app, Fitbit) preference were tracked. All in-person and coaching sessions times were monitored to determine the session duration and number of sessions completed.
PACE evaluation
After completing the intervention (PACE at 12 weeks or Delayed PACE at 26 weeks), participants were asked “Now that you’ve com-pleted the study, if you had the choice to start a weight loss program 6 weeks prior to surgery (PACE) or 12 weeks after surgery (Delayed PACE), which would you choose?”
Statistical analysis
All statistical analyses were conducted using SPSS version 24. Descriptive statistics were computed for baseline characteristics. Differences between completion status and groups were examined using chi-square or independent samples t-tests. The changes in weight, physical function, physical activity, and patient-reported outcomes across time (12 and 26 weeks) by group were examined with repeated measure ANOVAs. Analyses were completed using intent-to-treat (last value carried forward). Shapiro–Wilk tests were used to examine normality of outcomes. Despite the small sample size, the distributions of outcome variables were fairly normal and strong deviations from normality were not seen and therefore the results should be robust. Statistical significance was considered with P < 0.05.
Results
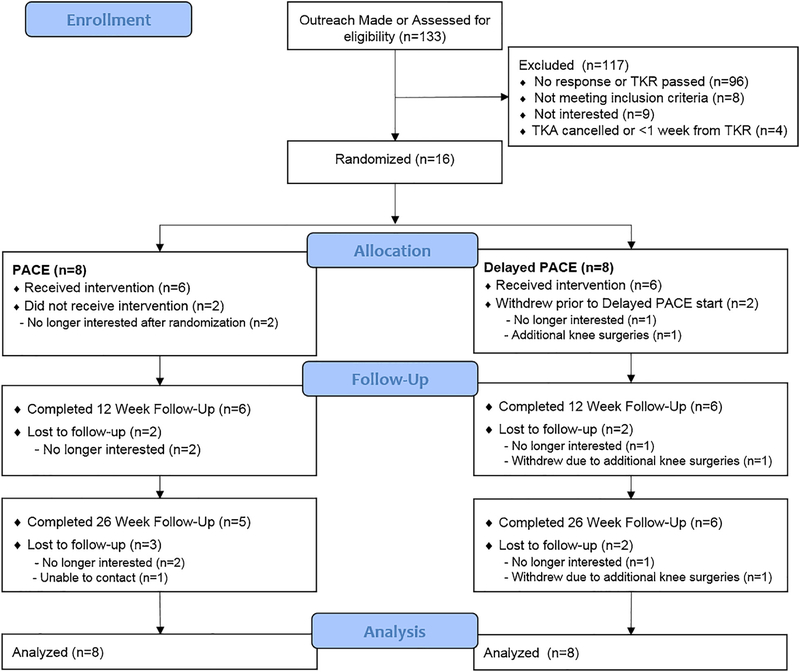
Of the 133 patients who were contacted to participate, 37 responded to our inquiries, and 16 were randomised (Fig. 1). The primary reason for exclusion (n = 96) was due to no response or the date of the knee replacement passing. Retention was 75% and 69% at 12 and 26 weeks after surgery, respectively. Reasons for attrition included no longer interested, withdrawal due to additional knee surgeries, and unable to contact. Three participants had techno-logical issues or chose not to wear the Actigraph accelerometer at baseline. One participant did not complete the PROMIS or WOMAC measures at baseline. There was no difference in retention between PACE and Delayed PACE.
Fig. 1.
Consort diagram of participant flow and analysis of primary outcome (body weight). Abbreviation: PACE: Patient-Centered Weight Loss Program.
Baseline characteristics by randomised condition are presented in Table 1. Participants were primarily female (69%), White (69%), with a mean age of 63.3 ± 7.5 years and BMI 36.5 ± 5.1 kg/m2. Most participants were employed full time (47%) or retired (47%). On average, participants enrolled in the study approximately 20 ± 12.3 days before surgery. There were no significant differences in baseline characteristics between PACE and Delayed PACE.
Table 1.
Baseline characteristics of knee replacement participants.
| Overall (n = 16) | PACE (n = 8) | Delayed PACE (n = 8) | Mean or % difference | |
|---|---|---|---|---|
| between groups (95% CI) | ||||
| Age, years, mean ± SD | 63.3 ± 7.5 | 66.0 ± 6.3 | 60.6 ± 8.0 | 5.4 (−2.3, 13.1) |
| Weight, kg, mean ± SD | 101.2 ± 10.0 | 102.7 ± 9.4 | 99.8 ± 11.1 | 2.9 (−8.1, 13.9) |
| BMI, kg/m2, mean ± SD | 36.5 ± 5.1 | 36.6 ± 5.7 | 36.3 ± 4.8 | 0.3 (−5.4, 6.0) |
| Female, no. (%) | 11 (68.8%) | 6 (75%) | 5 (62.5%) | 0.13 (−0.58, 0.33) |
| Race, no. (%) White | 11 (68.8%) | 6 (75%) | 5 (62.5%) | 0.13 (−0.58, 0.33) |
| Not Hispanic, no. (%) | 15 (93.8%) | 8 (100%) | 7 (87.5%) | 0.13 (−0.10, 0.35) |
BMI = Body mass index, PACE: Patient-Centered Weight Loss Program.
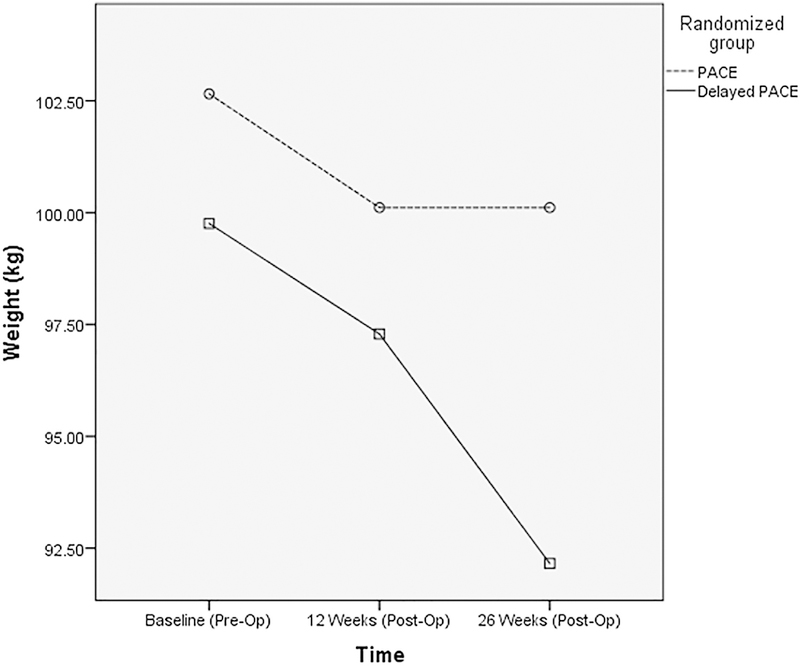
The changes in body weight across all randomised partic-ipants are presented in Fig. 2. Baseline weight did not differ between PACE and Delayed PACE (P = 0.58). Weight signifi-cantly decreased across the 26 weeks (P < 0.001); Delayed PACE (−7.6 ± 5.9 kg, −7.9 ± 5.9%) lost significantly more weight than PACE (−2.5 ± 2.7 kg, −2.6 ± 2.6%) at 26 weeks (P = 0.03 for group by time interaction). Between the preoperative baseline and 12 weeks, both groups lost a similar amount of weight (Delayed PACE: −2.5 ± 4.4 kg; PACE: −2.5 ± 2.9 kg); however, between 12 and 26 weeks, Delayed PACE lost significantly more weight than PACE (−5.1 ± 5.0 kg vs. 0.0 ± 1.6 kg, P < 0.05).
Fig. 2.
PACE and Delayed PACE weight changes from baseline to 26 weeks after surgery. Abbreviation: PACE: Patient-Centered Weight Loss Program.
Changes in physical function and physical activity are shown in Table 2. Significant improvements were observed from before surgery to 26 weeks after knee replacement for the Timed Up and Go (P = 0.01), Six Minute Walk (P < 0.001), and number of chair stands completed (P < 0.001). There were no differences in change of physical function outcomes between PACE and Delayed PACE. Also, there were no significant changes in objectively measured steps per day, bouted or unbouted MVPA, or sedentary time across time or randomised group.
Table 2.
Baseline and changes in physical function (n = 16) and objectively measured physical activity (n = 13) from baseline before surgery to 26 weeks after knee replacement.
| PACE | Delayed PACE | |
|---|---|---|
| Physical function tests (n = 16) | ||
| Mean or mean change (95% confidence intervals) | ||
| Timed Up & Go (s) | ||
| Baseline | 10.0 (6.6, 12.3) | 9.5 (7.1, 11.4) |
| 12 week change | −1.2 (−2.9, 0.5) | −0.5 (−1.3, 0.3) |
| 26 week change | −1.5 (−3.3, 0.2) | −0.7 (−1.8, 0.4) |
| Six Minute Walk (ft) | ||
| Baseline | 1113.0 (831.6, 1394.3) | 1228.7 (937.8, 1519.6) |
| 12 week change | 158.5 (35.1, 281.9) | 116.8 (39.3, 194.3) |
| 26 week change | 218.1 (38.6, 397.5) | 224.7 (78.5, 370.8) |
| Chair stands | ||
| Baseline | 10.3 (8.3, 12.2) | 9.8 (7.2, 12.4) |
| 12 week change | 0.5 (−1.4, 2.4) | 2.6 (0.8, 4.4) |
| 26 week change | 1.9 (0.7, 3.1) | 3.8 (1.3, 6.2) |
| Physical activity (n = 13) | ||
| Mean or mean change (95% confidence intervals) | ||
| Steps/day | ||
| Baseline | 5715.7 (2464.5, 8966.9) | 6062.4 (3456.5, 8668.2) |
| 12 week change | −1460.1 (−3837.3, 917.1) | −1118.7 (−3605.3, 1367.8) |
| 26 week change | −723.9 (−2604.5, 1156.7) | 262.0 (−2706.6, 3230.7) |
| Bouted MVPA (min/week) | ||
| Baseline | 81.2 (−67.6, 229.9) | 37.6 (−41.7, 116.8) |
| 12 week change | −108.5 (−291.2, 74.2) | 5.9 (−67.6, 79.3) |
| 26 week change | −44.7 (−123.5, 34.2) | 30.3 (−28.1, 88.7) |
| Unbouted MVPA (min/week) | ||
| Baseline | 156.5 (−42.5, 355.5) | 131.6 (56.0, 207.2) |
| 12 week change | −77.7 (−221.3, 65.9) | 24.6 (−22.0, 71.1) |
| 26 week change | −47.8 (−130.4, 34.7) | 40.6 (−11.6, 92.8) |
| Sedentary time (%/day) | ||
| Baseline | 70.1 (65.5, 74.8) | 67.8 (63.5, 72.1) |
| 12 week change | 1.3 (−3.0, 5.6) | 2.2 (−1.5, 6.0) |
| 26 week change | −0.1 (−10.0, 9.9) | 0.4 (−3.7, 4.4) |
MVPA: Moderate-to-vigorous intensity physical activity, PACE: Patient-Centered Weight Loss Program.
Table 3 shows the changes in patient reported outcomes assessed by WOMAC and PROMIS. Among all participants, significant improvements across time were seen for PROMIS pain intensity, pain interference, physical function/mobility, and sleep disturbance (P < 0.05). WOMAC pain and stiffness also improved significantly from before surgery to 26 weeks after knee replacement (P < 0.001). There were no differences over time between PACE and Delayed PACE for any of the patient reported outcomes.
Table 3.
Baseline and changes in patient-reported outcomes from baseline before surgery to 26 weeks after knee replacement (n = 15).
| PACE | Delayed PACE | |
|---|---|---|
| Patient-reported outcomes (n = 15) | ||
| Mean or mean change (95% confidence intervals) | ||
| WOMAC pain | ||
| Baseline | 9.4 (6.6, 12.3) | 9.3 (7.1, 11.4) |
| 12 week change | −5.1 (−8.0, −2.3) | −2.8 (−4.7, −0.8) |
| 26 week change | −6.1 (−9.2, −3.1) | −4.6 (−8.2, −1.1) |
| WOMAC stiffness | ||
| Baseline | 4.3 (3.4, 5.2) | 5.3 (3.4, 7.0) |
| 12 week change | −1.6 (−3.1, −0.1) | −1.8 (−2.9, −0.6) |
| 26 week change | −2.0 (−3.4, −0.6) | −2.1 (−3.3, −0.9) |
| PROMIS pain intensity (t score) | ||
| Baseline | 52.6 (47.8, 57.4) | 51.4 (48.0, 54.8) |
| 12 week change | −8.3 (−16.1, −0.4) | −7.3 (−13.0, −1.6) |
| 26 week change | −12.6 (−21.0, −4.2) | −10.3 (−17.4, −3.1) |
| PROMIS pain interference (t score) | ||
| Baseline | 60.0 (54.5, 65.6) | 60.9 (57.3, 64.5) |
| 12 week change | −5.0 (−10.7, 0.7) | −6.7 (−12.6, −0.8) |
| 26 week change | −11.3 | |
| −8.7 (−16.9, −0.4) | (−19.6, −2.9) | |
| PROMIS mobility (t score) | ||
| Baseline | 36.1 (31.8, 40.4) | 36.5 (33.3, 39.6) |
| 12 week change | 3.5 (−0.7, 7.7) | 4.9 (1.1, 8.6) |
| 26 week change | 4.6 (1.0, 8.2) | 7.6 (1.7, 13.5) |
| PROMIS sleep disturbance (t score) | ||
| Baseline | 52.1 (44.5, 59.8) | 52.8 (48.5, 57.0) |
| 12 week change | −1.2 (−9.1, 6.7) | −1.5 (−4.9, 1.8) |
| 26 week change | −6.2 (−11.5, −0.8) | −3.7 (−10.1, 2.8) |
PACE: Patient-Centered Weight Loss Program, PROMIS: Patient-Reported Outcomes Measurement Information System, WOMAC: Western Ontario and McMaster Uni-versities Arthritis Index.
Among participants that completed either the PACE (n = 6) or Delayed PACE (n = 6) intervention, the average duration of the first session was 34.0 ± 12.1 min. After the first session, all sessions were completed over the telephone. On average, participants completed 8.6 ± 3.2 sessions and each call lasted 12.2 ± 3.4 min. No differences were observed in the number of calls or call durations between groups. For dietary self-monitoring and activity tracking, the major-ity of participants used a Fitbit provided by the study (79%); the remaining participants used their own Fitbits (14%) or used LoseIt! (7%). The majority (69%) of participants opted to receive text messages during the intervention. When asked their preference regarding the timing of starting a program, either pre-surgery (PACE) or post-surgery (Delayed PACE), of the twelve participants who completed an evaluation after the intervention, 7 participants (five of whom were randomised to PACE) indicated that they would have preferred to be in PACE.
Discussion
As knee replacement utilisation continues to rise [29], it is important to identify both relevant methods as well as the optimal timing to assist patients with weight management before and/or after surgery. The current study examined the feasibility of con-ducting a randomised pilot behavioural intervention examining changes in weight, physical function, physical activity, and patient-reported outcomes between patients who started a weight loss program either before or after knee replacement. The results suggest that initiating a weight loss program 12 weeks after surgery produces greater weight losses at 26 weeks compared to a program starting before knee replacement. Significant improvements were also observed with physical function, pain, and stiffness, but there were no differences between PACE or Delayed PACE. Objectively measured physical activity did not change between before surgery and 26 weeks after knee replacement.
This study provides preliminary insight on the best time to approach weight management in knee replacement patients. From before surgery to 12 weeks after knee replacement, regardless of randomised group, participants lost approximately 2.5 kg. This suggests that even outside of a weight management program, natural weight losses may be occurring. Although previous studies have examined weight changes longer than 4 months after surgery [30,31], these results are consistent with some of literature indicating that small weight losses may occur around the time of the surgery [10,32]. The difference in weight change between the groups emerged at 12 weeks after surgery, when PACE entered a maintenance phase and Delayed PACE started the weight loss pro-gram. Delayed PACE participants on average lost 5.4% during this time and exceeded the 5% program weight loss goal, whereas PACE participants’ weight remained stable. The overall weight losses in PACE (2.6%) were comparable to a dietician led weight loss program pre- and post-knee replacement by Gandler et al. [33], which saw a 3.2% weight loss; however these are both below the recommended 5–10% goal [34]. Initiating a weight loss program post-knee replacment may be a more opportune time because it allows patients to benefit from the natural post-operative weight loss, and may provide additional motivation to reach the recommended weight losses for adults with overweight/obesity.
Interestingly, among participants who completed the evaluation after the intervention, >50% of participants indicated that if given the choice, they would have chosen to start the program before surgery. Similarly, during the development of PACE, most patients interviewed indicated that they would want to start a weight loss program before surgery — only 5% of patients reported a preference to wait until after surgery [8]. One of the challenges faced in the current study was recruiting patients who scheduled the knee replacement 6 weeks (42 days) in advance. The initial goal was to ensure that patients had ample time for weight loss to occur in the event they were randomised to PACE; however, due to challenges with recruitment, the inclusion criteria were modified to begin enrolling patients at least 1 week before surgery instead of a minimum of 6 weeks before. The current study made outreach to a total of 133 patients who were scheduled for knee replacement, yet only 16 patients were enrolled into the study. On average, participants enrolled 20 days before surgery (range 7–39 days). Initiating weight loss prior to the knee replacement could help to reduce the risk of surgical complications [35], but identifying candidates for knee replacement early may be difficult. Patients will often explore several treatment options prior to deciding to undergo knee replacement, making it challenging to determine exactly when surgery may occur [36]. Additionally, there may be limited options and flexibility with scheduling the surgery depending on the orthopedic clinic and surgeon.
Significant improvements were seen across time for WOMAC pain and stiffness for both groups of participants. The changes were similar to what is typically observed following knee replacement [37]. Compared to WOMAC, fewer studies of patients undergoing knee replacement have utilised PROMIS measures; however, more information is emerging as the computer adaptive tests are beginning to be integrated into orthopedic clinics [38]. Similar to the changes in the patient-reported outcomes, the changes observed in physical function in the current study are comparable to previous studies [39]. While there was not a detectable benefit from the weight loss in the pain and functional outcomes at 26 weeks after surgery, future studies should examine long-term benefits of weight loss and the knee replacement.
Despite setting regular activity goals, discussing activity during coaching sessions, and the majority of participants using an activity tracker, there were no changes in MVPA or steps between surgery and 26 weeks after knee replacement. Increasing activity in this population remains challenging. Previous literature indicates that physical activity does not automatically increase after knee replacement, even in the presence of improvements with pain and function [16,18]; however, recent interventions have shown promise with increasing activity in knee replacement patients. Increases in activity above pre-operative levels were seen using supervised exercise sessions [40] and financial incentives and health coaching [19]. Although the current study included a strong physical activity intervention as part of the weight loss program, alternative and more intensive approaches may be necessary to help knee replacement patients increase their activity levels, particularly as physical activity plays a vital role in preventing weight regain after weight loss.
This study is not without limitations. First, this was a pilot study with a small sample size consisting of likely the most motivated patients and short-term follow-up. It is unclear if participants in either condition maintained weight loss, increased physical activity levels, or saw additional improvements in any of the patient-reported outcomes past the 26 weeks after knee replacement. Further, because physical activity did not change, it is likely that most of the weight loss was attributed to dietary changes. Future studies are needed to more closely examine the dietary changes made in this population. Additionally, participants enrolled in the study on average 20 days before surgery. Participants in PACE may have needed more than 3 weeks of time to initiate weight losses before undergoing knee replacement. The current study also has many strengths. The use of accelerometers to objectively mea-sure physical activity provides further evidence of the challenge of increasing physical activity levels in knee replacement patients. Second, this study utilised PROMIS to assess pain intensity, pain interference, physical function, and sleep disturbance. The use of these computer adaptive tests in knee replacement patients is an innovative method to more efficiently collect patient-reported out-comes.
In summary, conducting a behavioural weight loss intervention within a knee replacement population was challenging but feasible. The results suggest that starting a weight loss program 12 weeks after knee replacement produces greater weight losses at 26 weeks compared to a weight loss program starting before surgery. Although differences in physical function and pain did not emerge between groups, future studies are needed to evaluate long-term benefits of weight loss post-knee replacement. Further, the results of this study highlight the need to identify strategies to help this population increase physical activity levels after undergoing knee replacement.
Acknowledgements
This study was supported by K12HS023011 from the AHRQ and P60-AR064464 from the NIAMs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AHRQ, or NIAMS. Study data were collected and managed using REDCap hosted at Feinberg School of Medicine by Northwestern University Clinical and Translational Science (NUCATS) Institute. Research reported in this publication was supported, in part, by the NIH’s NCATS (UL1TR001422).
Footnotes
Declaration of interest
BS reports personal fees from serving on the Scientific Advisory Board of Actigraph, outside the submitted work. The remaining authors declare no conflict of interest.
References
- [1].Felson DT, Lawrence RC, Dieppe PA, Hirsch CG, Jordan JM, Kington RS, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000;133(8):635–46. [DOI] [PubMed] [Google Scholar]
- [2].Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am 2013;95(5):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Changulani M, Kalairajah Y, Peel T, Field RE. The relationship between obesity and the age at which hip and knee replacement is undertaken. J Bone Joint Surg Br 2008;90-B(3):360–3. [DOI] [PubMed] [Google Scholar]
- [4].Fehring TK, Odum SM, Griffin WL, Mason JB, McCoy TH. The obesity epidemic: its effect on total joint arthroplasty. J Arthroplast 2007;22(6, Supplement):71–6. [DOI] [PubMed] [Google Scholar]
- [5].Dowsey MM, Liew D, Stoney JD, Choong PF. The impact of pre-operative obesity on weight change and outcome in total knee replacement: a prospective study of 529 consecutive patients. Bone Joint J 2010;92-B(4):513–20. [DOI] [PubMed] [Google Scholar]
- [6].Foran JRH, Mont MA, Rajadhyaksha AD, Jones LC, Etienne G, Hungerford DS. Total knee arthroplasty in obese patients: a comparison with a matched control group. J Arthroplast 2004;19(7):817–24. [DOI] [PubMed] [Google Scholar]
- [7].Foran JR, Mont MA, Etienne G, Jones LC, Hungerford DS. The outcome of total knee arthroplasty in obese patients. J Bone Joint Surg Am 2004;86-A(8):1609–15. [DOI] [PubMed] [Google Scholar]
- [8].Pellegrini CA, Ledford G, Hoffman SA, Chang RW, Cameron KA. Preferences and motivation for weight loss among knee replacement patients: implications for a patient-centered weight loss intervention. BMC Musculoskelet Disord 2017;18(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Inacio MCS, Kritz-Silverstein D, Raman R, Macera CA, Nichols JF, Shaffer RA, et al. The impact of pre-operative weight loss on incidence of surgical site infection and readmission rates after total joint arthroplasty. J Arthroplast 2014;29(3):458–64, e451. [DOI] [PubMed] [Google Scholar]
- [10].Pellegrini CA, Song J, Semanik PA, Chang RW, Lee J, Gilbert AL, et al. Patients less likely to lose weight following a knee replacement: results from the osteoarthritis initiative. J Clin Rheumatol 2017;23(7):355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthr Cartil 2010;18(4):510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwartsmann CR, Borges AM, Schuck de Freitas GL, Migon EZ, Kaempf de Oliveira G, Rodrigues MW. Do patients lose weight after total knee replace-ment? Revista Brasileira de Ortopedia (English Edition) 2017;52(2):159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Warner DO. Surgery as a teachable moment: lost opportunities to improve public health. Arch Surg 2009;144(12):1106–7. [DOI] [PubMed] [Google Scholar]
- [14].Pellegrini CA, Ledford G, Chang RW, Cameron KA. Understanding barriers and facilitators to healthy eating and physical activity from patients either before and after knee arthroplasty. Disabil Rehabil 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86-A(5):963–74. [DOI] [PubMed] [Google Scholar]
- [16].Hammett T, Simonian A, Austin M, Butler R, Allen KD, et al. Changes in physical activity after total hip or knee arthroplasty: a systematic review and meta-analysis of 6 and 12 month outcomes. Arthritis Care Res 2017;70(6):892–901. [DOI] [PubMed] [Google Scholar]
- [17].Kahn TL, Schwarzkopf R. Does total knee arthroplasty affect physi-cal activity levels? Data from the osteoarthritis initiative. J Arthroplasty 2015;30(9):1521–5. [DOI] [PubMed] [Google Scholar]
- [18].Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res 2014;472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Losina E, Collins JE, Deshpande BR, Smith SR, Michl GL, Usiskin IM, et al. Finan-cial incentives and health coaching to improve physical activity following total knee replacement: a randomized controlled trial. Arthritis Care Res 2017;(July) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Diabetes Prevention Program Research Group. The Diabetes Prevention Pro-gram. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clini-cal trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24(5):610–28. [DOI] [PubMed] [Google Scholar]
- [22].Pellegrini CA, Ledford G, Chang RW, Cameron KA. Understanding barriers and facilitators to healthy eating and physical activity from patients either before and after knee arthroplasty. Disabil Rehabil 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance-based tests to assess physical function in peo-ple diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage Cartil 2013;21(8):1042–52. [DOI] [PubMed] [Google Scholar]
- [24].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically impor-tant patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–40. [PubMed] [Google Scholar]
- [25].Obeid JS, McGraw CA, Minor BL, Conde JG, Pawluk R, Lin M, et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 2013;46(2):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl. 1):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerome-ter wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- [29].Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the united states cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am 2012;94(3):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ast MP, Abdel MP, Lee Y-y Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty. J Bone Joint Surg Am 2015;97(11):911–9. [DOI] [PubMed] [Google Scholar]
- [31].Inacio MS, Kritz-Silverstein D, Paxton E, Fithian D. Do patients lose weight after joint arthroplasty surgery? A systematic review. Clin Orthop Relat Res 2013;471(1):291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Teichtahl AJ, Quirk E, Harding P, Holland AE, Delany C, Hinman RS, et al. Weight change following knee and hip joint arthroplasty—a six-month prospective study of adults with osteoarthritis. BMC Musculoskelet Disord 2015;16(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gandler N, Simmance N, Keenan J, Choong PF, Dowsey MM. A pilot study investigating dietetic weight loss interventions and 12 month functional out-comes of patients undergoing total joint replacement. Obes Res Clin Pract 2016;10(2):220–3. [DOI] [PubMed] [Google Scholar]
- [34].Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014;22(S2):S5–39.24961825 [Google Scholar]
- [35].Kerkhoffs GMMJ, Servien E, Dunn W, Dahm D, Bramer JAM, Haverkamp D The influence of obesity on the complication rate and outcome of total knee arthroplasty. J Bone Joint Surg Am 2012;94(20):1839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jacobson AF, Myerscough RP, Delambo K, Fleming E, Huddleston AM, Bright N, et al. Patients’ perspectives on total knee replacement. Am J Nurs 2008;108(5):54–63. [DOI] [PubMed] [Google Scholar]
- [37].Bachmeier CJM, March LM, Cross MJ, Lapsley HM, Tribe KL, Courtenay BG, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage Cartil 2001;9(2):137–46. [DOI] [PubMed] [Google Scholar]
- [38].Haskell A, Kim T. Implementation of patient-reported outcomes measurement information system (PROMIS) data collection in a private orthopedic surgery practice. Foot Ankle 2017;2(2), 2473011416S2473000013. [DOI] [PubMed] [Google Scholar]
- [39].Stevens-Lapsley JE, Schenkman ML, Dayton MR. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. PM R 2011;3(6):541–9. [DOI] [PubMed] [Google Scholar]
- [40].Piva SR, Almeida GJ, Gil AB, DiGioia AM, Helsel DL, Sowa GA. A comprehensive behavioral and exercise intervention improves physical function and activity participation after total knee replacement—a pilot randomized study. Arthritis Care Res (Hoboken) 2017;69(12):1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]