Abstract
Study Objectives
Daytime naps benefit long-term memory relative to taking a break and remaining awake. However, the use of naps as a practical way to improve learning has not been examined, in particular, how memory following a nap compares with spending the equivalent amount of time cramming.
Methods
Young adults learned detailed factual knowledge in sessions that flanked 1 hr spent napping (n = 27), taking a break (n = 27), or cramming that information (n = 30). Recall was examined 30 min and 1 week after learning.
Results
When tested 30 min after learning, cramming and napping led to significantly better memory than taking a break. After a week, napping maintained this significant advantage, but cramming did not.
Conclusions
These findings demonstrate the longer-term benefits of napping for retention of memoranda akin to what students encounter daily and encourage more widespread adoption of napping in education.
Keywords: nap, memory, long-term memory, learning
Statement of Significance
Much research espouses the benefits of short daytime naps for long-term memory, but evidence to support the real-world advantages of napping is limited. A nap may be better than staying awake and taking a break, but is it better than simply continuing to cram information instead of sleeping? We found that taking a 1 hr afternoon nap between learning of educationally realistic material enhanced memory to the same extent as when that hour was spent engaging in further learning. This demonstrates the power of napping in a naturalistic learning environment and has implications for the use of naps as a practical way to improve learning.
Introduction
The cognitive benefits of daytime naps have been studied extensively, particularly in relation to sleep-dependent consolidation of declarative memories. Naps of 6–120 min can reduce forgetting of episodic memories [1–5] and lead to qualitative changes to memories, such as the abstraction of underlying structure in learned material [6]. This transformation of memories is mediated by active processes such as offline memory reactivation [7]. A nap may also refresh memory networks to facilitate the encoding of new episodic memories [8, 9].
These memory improvements have led to the suggestion that naps be adopted as a pedagogical tool [10], but several unanswered questions remain in determining whether naps are a practical way to assist learning. Our current understanding stems from studies that contrast a nap with a period of restful wakefulness, but when studying for an exam a student has the option to remain awake to cram instead. Since the rehearsal of learned material also enhances memory, it is not clear which represents a more productive use of time: napping or cramming.
Furthermore, nap effects are rarely tested beyond the day of learning [3, 4], and several studies have found the behavioral effects of sleep on memory to dissipate when the delay between learning and testing is longer than a few days [11–14]; therefore, it remains an open question whether nap enhancements can be sustained 1 week after learning.
Finally, the short episodic memory tasks used in laboratory nap studies involve simplistic stimuli such as word lists [2] and paired associates [1, 3], whereas educational material more often consists of detailed interrelated facts acquired across several long study sessions. It is not known how napping affects the retention of this type of memoranda.
To answer these questions, participants learned detailed facts about arthropods across a 5 hr period with breaks. Midway through learning, participants either took a 1 hr nap (nap), remained awake and watched a movie (wake), or crammed previously learned information (cram). Participants were tested 30 min after the conclusion of learning and 1 week later via two-alternative forced choice questions that were followed by a confidence rating (certain, somewhat certain, and guess).
We predicted significantly better memory in the nap group relative to wake group 30 min after learning due to the enhanced consolidation and/or encoding associated with sleep. It is well established that increased repetition during encoding will improve long-term memory; therefore, the additional hour of learning for the cram group was expected to yield better memory than the more modest gains associated with napping [1–4], which tend to be expressed as reduced forgetting rather than improvement per se. Forgetting across the 1 week delay was expected to be similar across groups, maintaining differences observed at the 30 min test. These effects were expected for confident declarative memories (certain responses), which are least prone to noise introduced by guessing. Somewhat certain and guess responses were expected to increase across the 1 week delay due to forgetting, but no group differences were predicted for these measures.
Methods
Participants
Ninety healthy students were recruited from a sample of undergraduates at the National University of Singapore, via an advertisement placed on a university website. Students taking biology and ecology majors were excluded. Standardized questionnaires ensured that participants had no history of neurological, psychiatric, or sleep disorders and were taking no medication during the experiment. Participants did not consume more than two caffeinated drinks a day, were fluent English speakers, and had normal or corrected to normal vision.
Six participants were excluded due to absence for the day-8 test (n = 3), computer error during retrieval (n = 1), and scoring >2 SD above the mean during the 30 min test (n = 2). The remaining 84 participants (19–27 years, age M = 21.51, and SD = 1.81 years) were assigned to three experimental groups: wake (n = 27, 13 females, age M = 21.89, SD = 2.08 years), nap (n = 27, 17 females, age M = 21.11, SD = 1.5 years), and cram (n = 30, 16 females, age M = 21.53, SD = 1.83 years).
There were no significant group differences in age, Pittsburg Sleep Quality Index (PSQI) global score, Morningness–Eveningness Questionnaire (MEQ) score, or habitual napping (p > .05; Table 1). All participants gave written informed consent in compliance with the protocol approved by the National University of Singapore Institutional Review Board.
Table 1.
Comparison of group demographics, sleep habits, and actigraphically assessed sleep during the experiment
| Wake | Nap | Cram | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F | P | |
| Age (y) | 21.89 | 2.08 | 21.11 | 1.5 | 21.53 | 1.83 | 1.25 | .29 |
| Pittsburgh Sleep Quality Index global score | 3.35 | 1.57 | 3.26 | 1.63 | 3.55 | 1.70 | .24 | .79 |
| Morningness–Eveningness Questionnaire score | 50.58 | 7.65 | 49.93 | 8.83 | 51.59 | 8.33 | .29 | .75 |
| Habitual napping (proportion) | 0.59 | 0.50 | 0.59 | 0.50 | 0.63 | 0.49 | .065 | .94 |
| Actigraphy | ||||||||
| TIB—mean of 3 days prior to experiment (h) | 7.81 | 0.63 | 7.73 | 0.76 | 7.56 | 0.63 | .89 | .42 |
| TIB—mean of retention interval (h) | 7.88 | 0.77 | 7.90 | 0.67 | 7.64 | 0.57 | 1.19 | .31 |
y = year; SD = standard deviation; h = hour; actigraphy threshold = medium.
Habitual napping represents the proportion of participants that reported napping once or more per week.
Materials
Cognitive tests
Several cognitive tests were performed during a briefing session held 3–14 days prior to the experiment to establish that groups did not differ in general measures of cognition. Tests evaluated processing speed (Symbol Search), short-term memory (Digit Span; from Weschler Adult Intelligence Scale, WAIS), fluid intelligence (12-item shortened version of RAVENS advanced progressive matrices), and verbal memory (Rey-Auditory Verbal Learning Test, RAVLT).
Factual knowledge task—learning
Participants learned about six species of crab, split into Spider (Japo, Oatii, and Cornis) and Hermit Crabs (Amatus, Latro, and Peatus), and six species of ant split into Symbiotic (Texana, Styga, and Myrmex) and Hunter Ant (Pontu, Gulosa, and Dorylus), adapted from Hennies et al. [15].
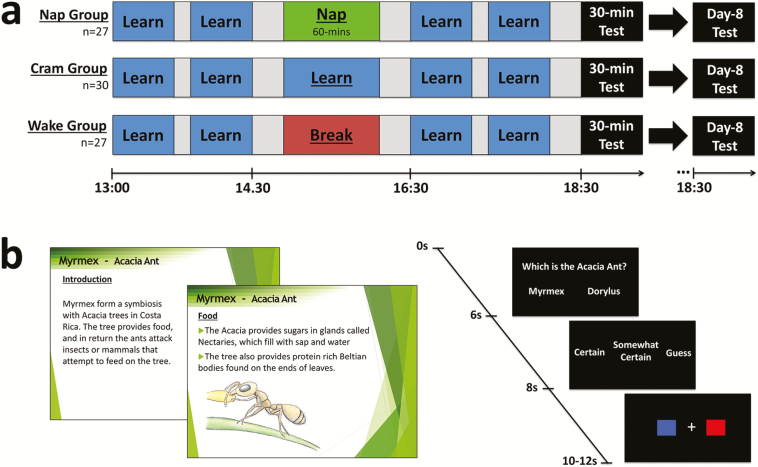
Participants learned in four blocks across the day separated by breaks (2 hr 20 min learning in total). This followed the pattern of two pairs of blocks—with each pair containing all information about both ants and crabs—that were separated by a 2 hr interval containing the nap/wake/cram manipulation (Figure 1b). The manipulation period was placed between learning periods, rather than after all of them, to ensure the time between learning and test was matched across groups. Had the manipulation been placed after learning, the cramming group would have finished learning 30 min before the test, whereas the other groups would finish 1 hr 30 min before the test and therefore would have more time to forget. The order of ants and crabs within each pair of blocks was counterbalanced. Participants were instructed to not look up information about arthropods during breaks.
Figure 1.
Protocol and stimuli. (a) Participants learned in four 40 min blocks, interspersed with breaks. Groups diverged for a 60 min period at 15:00: the nap group slept in the lab, the cram group revised the same learning materials, and the wake group watched a movie. Testing took place 30 min after the final learning block, and again 1 week later (day-8). (b) Learning was self-paced and consisted of detailed information about 12 species of arthropods presented on slides. Each test included 120 two alternative forced-choice questions of varying difficulty. Each question was followed by a confidence rating and a baseline task where participants indicated the color of a central cross.
Each block included all crab or ant species and contained the same information each time so that participants were exposed to the same material before and after the manipulation period. Blocks contained approximately 80 slides detailing factual information in the form of sentences, numbered points, and images (Figure 1b). To assist learning, participants were asked to write down on paper what they could remember about specific species for some slides during learning blocks, after which the paper was turned over. Movement through slides was self-paced, although participants were asked to observe a minimum speed to allow them to see all the slides within each block of learning. To this end, a timer was placed on the desk and markers in the slides informed the minimum amount of time that should have passed at 5 min intervals. The last slide advised participants to go back and recap the information if any time was remaining.
Factual knowledge task—tests
A total of 360 questions were split into three test sets of 120 questions (60 ants and 60 crabs), matched for difficulty, and pseudorandomly assigned to 30 min and day-8 tests. Different test sets were used to minimize test-enhanced learning. Participants answered questions in separate blocks for ants and crabs, separated by a 2 min break, with order counterbalanced (i.e. crabs or ants first). The order of questions was randomized and arranged so that no questions provided the answer to another question in that test set. Questions had two alternative forced-choice answers where the foil was most often the answer to the same question for a different species, or incorrect information that was similar to the correct answer. An additional 60 questions of varying difficulty were utilized in a pretest to establish prior knowledge.
Trials began with a fixation cross (500 ms) followed by the question displayed for 6000 ms (Figure 1b). Participants selected answers via a response box held in the right hand, upon which a confirmation fixation-cross appeared below the question. Failure to respond triggered on-screen instructions to respond faster. A confidence rating scale (“Certain,” “Somewhat Certain,” and “Guess”) was displayed for 2000 ms and participants responded with one of three buttons. This was followed by the baseline task involving a white fixation cross-flanked by a red box (left) and blue box (right). Following a pseudorandom delay, the fixation cross turned red or blue and participants pressed the corresponding button as quickly and accurately as possible. Baseline task duration varied randomly between 2000 and 4000 ms but averaged across each block to be 3000 ms. Target onset was randomly generated between 500 ms from onset to 2000 ms, always allowing at least 1000 ms to respond.
Psychomotor vigilance and subjective alertness
Vigilance and subjective alertness were assessed via 3 min psychomotor vigilance tests (PVT) and Karolinska Sleepiness scales (KSS) prior to learning (12:55, 16:25) and retrieval sessions (18:25). Trials of the PVT required participants to respond as quickly as possible with the space bar when a counter appeared on screen at random intervals (2000–10 000 ms). Response speed (1/RT) was measured.
Procedure
Participants attended a briefing 3–14 days prior to the experiment where cognitive tests and questionnaires were administered. Participants were instructed to keep to a sleep schedule (6.5–9 hr sleep per night, sleep before 12.30 am, and wake before 9 am) 3 days prior to the experiment and during the 1 week retention period, verified by sleep diary and actigraphy.
Participants from the wake and cram groups arrived for the first experimental session at 12:15, with nap participants arriving 45 min earlier to allow application of polysomnography (PSG) electrodes. The factual knowledge pretest began at 12:30. Participants were instructed to remember the names and characteristics of each species as they would be tested later. Example test questions using the name of a species not featured in the learning were shown.
Participants performed the first learning block (13:00–13:40) and then had a 10 min break before the second learning block (13:50–14.30). The following 2 hr period differed for each group (14:30–16:30). The nap group was prepared for bed, with lights out by 14:40 to ensure that they could fall asleep by approximately 15:00. The experimenter allowed a 60 min nap from the time of stage 1 onset. Participants were only awoken from stage 1 or stage 2 sleep, and no later than 16:00 to reduce the influence of sleep inertia in the following learning block at 16:30. Electrodes were removed after participants awoke. During this same period, the cram group had a 30 min break, followed by a 60 min learning block containing all the material about ants and crabs, and another 30 min break. The wake group had the same breaks, but watched a movie for 60 min instead of learning. All participants then performed the penultimate (16:30–17:10) and final learning blocks (17:20–18:00). During the day, participants subjective and objective alertness was also measured via PVT and KSS prior to block 1 (12:55), block 3 (16:25), and the 30 min test (18:25).
The 30 min test took place in the MRI scanner (results not presented here). Participants viewed the screen through a mirror on the head coil and responded via a button box. Five practice questions that were unrelated to the learning stimuli allowed participants to familiarize themselves with the buttons and speed of presentation. Memory was tested in separate blocks of 60 questions for ants and crabs, with the order counterbalanced and a 2 min break between. In the following week, participants were instructed not to revise what they had learned or actively seek out information about arthropods. The day-8 test took place at the same time of day and followed an identical procedure. It was preceded by a PVT and KSS.
Statistical analysis
Group differences were assessed with one-way ANOVA (nap/cram/wake), to examine cognitive tests, actigraphy, and pretest performance. For memory, correct trials for certain, somewhat certain, guess, and all responses combined (overall memory) were analyzed separately via a 3 × 2 mixed ANOVA with group as the between-participants factor (nap/cram/wake) and delay as the within-participants factor (30 min/day-8). Response bias correction (correct—incorrect) was performed separately within each level of confidence (certain, somewhat certain, and guess). Questions answered correctly where no confidence response was recorded in time were included in the overall memory measure. Similar ANOVA was used to analyze response times (RTs) and performance accuracy at the baseline task, KSS and PVT. Planned group comparisons were tested with independent-samples t-tests. Spearman’s ρ correlations explored the relationship between memory, alertness, and sleep characteristics. All statistical tests were two-tailed, significance level, p < .05.
Polysomnography
Recordings were acquired with a 16-channel MR amplifier (BrainAmp, Brain Products GmbH, Gilching, Germany) from seven scalp derivations (F3, F4, C3, C4, Cz, O1, and O2) referenced to linked mastoids (M1 and M2), according to the 10–20 system. Electrooculogram (EOG), electromygram (EMG), and forehead ground electrodes were also attached. Impedance of <5 kΩ was verified for electroencephalography (EEG) and <10 kΩ for EOG and EMG. Signals were collected at a digital sampling rate of 500 Hz (bandpass filtered 0.1–250 Hz). Sleep data were assessed online and scored offline according to standardized criteria via an in-house automated algorithm [16] in Matlab 2012 (MathWorks, Inc., Natick, MA) and verified by experimenters. Analysis of spindles and slow-wave activity (SWA) utilized C3 referenced to A2. Slow (12–13.5 Hz) and fast (13.5–15 Hz) spindle density (spindles per minute) was assessed using an adapted automated detection algorithm [17]. EEG spectral analysis was performed on nonoverlapping 5 s epochs. Power spectral density focused on SWA (0.6–4 Hz) using Welch’s method (Hamming window; 0.2 Hz bin resolution). Total and mean SWA was summed across all nonrapid eye movement (NREM) epochs.
Results
Actigraphy
One-way ANOVA confirmed that groups did not differ in actigraphically assessed time in bed (TIB) for the mean of 3 days prior to the experiment or during the 1 week retention interval (p > .05; Table 1). This suggests that participants were equally well rested when beginning the learning session and had a similar opportunity for sleep-dependent consolidation during the retention interval.
Cognitive tests
No significant group differences were observed for processing speed, F(2,81) = 1.64, p = .201, ηp2 = .04, forward short-term memory span, F(2,81) = 0.33, p = .720, ηp2 = .01, or backward span, F(2,81) = 0.13, p = .876, ηp2 = .00, verbal learning for RAVLT trial 7, F(2,81) = 1.0, p = .374, ηp2 = .02, and trial 8, F(2,81) = 1.44, p = .244, ηp2 = .03, and fluid intelligence, F(2,81) = 0.77, p = .467, ηp2 = .02 (Supplementary Table S1). Thus, participants were comparable in cognitive abilities.
Factual knowledge task
There were no group differences in prior knowledge of the learning materials in the pretest performed before the main experiment, F(2,81) = 0.21, p = .815, ηp2 = .01.
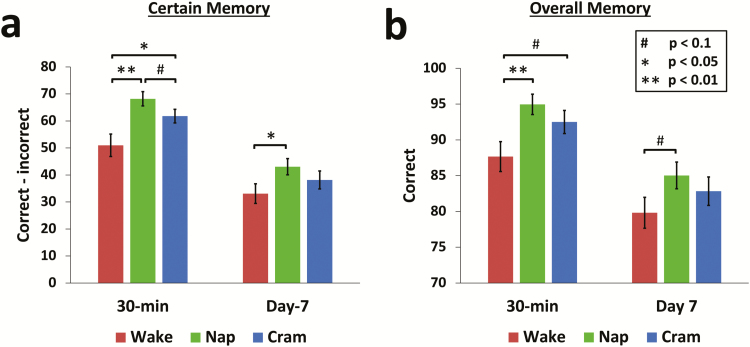
For certain responses (correct—incorrect), we observed a main effect of group, F(2,81) = 4.89, p = .010, ηp2 = .11, and a main effect of delay, F(1,81) = 278.44, p < .001, ηp2 = .78, but there was no significant group*delay interaction, F(2,81) = 2.68, p = .074, ηp2 = .06. As predicted, certain memory was higher for the nap group compared with wake group after 30 min, t(52) = 3.50, p = .001, Cohen’s d = .95, and also on day-8, t(52) = 2.11, p = .039, Cohen’s d = .56, showing that napping enhanced the retention of factual knowledge relative to a period of rested wakefulness (Figure 2).
Figure 2.
Results. (a) For memories rated as certain (correct—incorrect), the nap group remembered significantly more than the wake group at 30 min and day-8 tests. The cram group remembered significantly more than the wake group at 30 min but not the day-8 test. (b) A similar pattern emerged for overall memory, except the only significant difference was between nap and wake groups at the 30 min test. Mean ± SEM.
The cram group also had significantly better certain memory than the wake group after 30 mins, t(55) = 2.28, p = .027, Cohen’s d = .60, but this difference was no longer significant on day-8, t(55) = 1.03, p = .308, Cohen’s d = .27. There was a trend for enhanced memory of the nap group relative to the cram group at 30 min, t(55) = 1.76, p = .084, Cohen’s d = .46, but not day-8, t(55) = 1.09, p = .282, Cohen’s d = .29.
Overall memory (hits) showed a similar pattern to certain memory, with main effects of group, F(2,81) = 3.31, p = .041, ηp2 = .08, delay, F(1,81) = 107.1, p < .001, ηp2 = .57, and no group*delay interaction, F(2,81) = .53, p = .589, ηp2 = .01. The nap group remembered significantly more than the wake group at the 30 min test, t(52) = 2.90, p = .006, Cohen’s d = .79, but other group comparisons were not significant for either delay (p > .05). There were no significant group differences for somewhat certain or guess responses (p > .05; Supplementary Material).
Psychomotor vigilance and sleepiness
Some participants were removed from specific analyses due to corrupted data, for PVT (n = 6), KSS (n = 6), and the baseline task (n = 2). A 3 × 4 mixed ANOVA with group and time (12:55, 16:25, 18:25, and day-8 18:25) for PVT response speed (1/RT) showed a main effect of time, F(3,225) = 3.36, p = .020, ηp2 = .04, reflecting a general slowing of responses across the experiment. There was no significant main effect of group, F(2,75) = 1.34, p = .269, ηp2 = .03, and no group*time interaction, F(6,225) = 0.83, p = .550, ηp2 = .02.
Subjective alertness (KSS) showed significant effects of group, F(1,75) = 6.08, p = .004, ηp2 = .14, time, F(3,225) = 3.38, p = .019, ηp2 = .04, and a group*time interaction, F(6,225) = 4.01, p = .001, ηp2 = .10. Follow-up comparisons found no group differences prior to learning or prior to day-8 retrieval (p > .247). The nap group were significantly more alert than cram and wake groups shortly after the nap period (p < .012) and prior to the 30 min test (p < .031), suggesting the nap improved subjective alertness for up to 2.5 hr postnap. The cram group was also less alert than the wake group after the nap period (p = .024), but not prior to the 30 min test (p = .183). Next we correlated certain memory at 30 min and day-8 tests with each KSS to examine whether memory effects were related to improved feelings of alertness, but found no significant relationships (p > .05).
We also examined RTs to questions and accuracy for the baseline color response task as indirect measures of alertness and found no significant group differences (p > .05; Supplementary Material).
To summarize, objective alertness levels did not differ between groups during learning and retrieval sessions; therefore, these measures can be reasonably discounted as a source of between-group variability in encoding or retrieval ability. Subjective alertness on the other hand did suggest an advantage for the nap group and may have contributed to their enhanced learning.
Polysomnography
Participants were only awoken from stage 1 or 2 sleep and were not permitted to sleep beyond 16:00, to reduce the potential influence of sleep inertia on postnap learning blocks. This, combined with spontaneous awakenings in some other participants, led to a range of total sleep lengths (range = 31.5–79.5 min) and a mean that was close to 60 min (M = 60.35 min, SD = 10.78 min; Table 2). One participant was excluded from analyses due to corrupted data.
Table 2.
Nap group sleep parameters
| Nap time (min) | ||
|---|---|---|
| Mean | SD | |
| Stage 1 | 9.02 | 5.35 |
| Stage 2 | 25.88 | 8.56 |
| Slow-wave sleep | 20.23 | 13.15 |
| Rapid eye movement sleep | 5.21 | 6.13 |
| Total sleep time | 60.35 | 10.78 |
| Time in bed | 74.29 | 8.91 |
Next we examined correlations between sleep macroarchitecture and certain memory at 30 min and day-8 tests. We observed no significant correlations (p > .05) between memory measures and duration of sleep stage 1, stage 2, slow-wave sleep, rapid eye movement (REM) sleep, total-sleep-time, NREM fast spindle density (13.5–15 Hz), NREM slow spindle density (12–13.5 Hz), or NREM mean SWA (0.5–4 Hz).
Discussion
We found that a 1 hr nap provided comparable improvement to learning as an equivalent time spent cramming. When tested 30 min after learning, retention of factual knowledge was significantly greater after an hour spent napping or cramming relative to taking a break. The nap benefit remained after 1 week, while cramming no longer provided significantly better retention than taking a break.
The majority of nap experiments compare a short bout of daytime sleep with a comparable period of wakefulness, and this has provided consistent evidence for the role of sleep in memory consolidation [1–4, 6, 7]. We extended this design to probe the practical utility of naps and show that in a naturalistic learning environment, naps provide the equivalent benefits to memory as when that period of time is spent revising the same material. When retention was tested immediately after learning, both napping and cramming produced better retention than taking a break, but only the nap benefit remained significant when tested 1 week later. It is tempting to speculate that napping between learning sessions leads to more enduring memories than cramming. Notably however, direct comparisons between these groups revealed only a trend for a nap benefit at the 30 min test; therefore, we can only conclude that these two learning approaches are likely to produce similar outcomes for memory. These findings extend prior observations on the long-term memory benefits of napping: one week for paired associates [3] and up to 4 years for emotional texts [4].
Although the nap benefited memory, our protocol was not intended to determine whether consolidation, encoding, or some combination of these processes was responsible. Learning materials from prenap blocks may have been preferentially strengthened, stabilized [1–4, 6], and reorganized in order to promote subsequent relearning [18]. Encoding of information in postnap learning blocks may have also benefited from the nap, since naps can restore encoding capacity [8, 9]. Prior work has linked sleep spindles and slow-wave sleep to consolidation [3, 6, 19, 20] and refreshed encoding capacity [9], but we failed to find any relationship between these features and memory. As mentioned, memory performance following the nap may have benefited from a combination of memory processes, making it difficult to identify simple relationships between either one of these processes and characteristics of sleep. Future studies could adapt the factual knowledge task to investigate relative contributions of these memory processes.
In addition to the memory benefits, those who napped felt significantly less sleepy prior to the final two blocks of learning, which is consistent with findings that a nap can restore lowered levels of alertness during the circadian dip [21, 22]. Sleepiness can be a predictor of academic performance [23] and a reduction in sleepiness could potentially have contributed to the nap-related memory benefit observed here, although we observed no significant correlations between memory and subjective alertness.
Utilizing sleep as a tool for improving educational outcomes has received little attention, despite it being a low cost and effective way to improve memory [10]. Many Chinese schools already facilitate naps as part of a healthy lifestyle, and this has been linked to enhanced daytime function in young adolescents [24]. Naps were also suggested to aid the retention of lecture material in a more applied school setting, where students given a 2 hr nap opportunity retained more than those who attended regular classes instead [5]. It is therefore practically feasible to allow napping in educational institutions, and we provide evidence that this has the potential to improve learning and academic achievement.
The nap length of 60 min is short enough to be practical for use in education, less likely to disrupt nocturnal sleep, contains slow-wave sleep which is important for consolidation of declarative memories [3, 7], and only induces a short period of sleep inertia after awakening [21]. There is evidence that naps as short as 6–10 min can enhance alertness [25] and memory consolidation [2], raising the possibility that a shorter nap might be beneficial while intruding less into existing school schedules.
In sum, we show that an hour long mid-afternoon nap between learning periods may be a viable means to assist long-term retention of educationally relevant factual knowledge. Coupled with findings that naps can alleviate deficits to mood and cognition associated with insufficient sleep [22, 26], there is a growing case for educational institutions to adopt napping for the promotion of learning and wellbeing.
Funding
This work was supported by grants from the National Medical Research Council Singapore (STaR/0015/2013), the National Research Foundation, Singapore (NRF2016-SOL002-001), and the Far East Organization.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We thank Nicholas Chee, Xuan Kai Lee, and Ju Lynn Ong for assistance in data collection and analysis of sleep architecture.
References
- 1. Tucker MA, et al. . A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86(2):241–247. [DOI] [PubMed] [Google Scholar]
- 2. Lahl O, et al. . An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17(1):3–10. [DOI] [PubMed] [Google Scholar]
- 3. Alger SE, et al. . Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012;98(2):188–196. [DOI] [PubMed] [Google Scholar]
- 4. Wagner U, et al. . Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60(7):788–790. [DOI] [PubMed] [Google Scholar]
- 5. Lemos N, et al. . Naps in school can enhance the duration of declarative memories learned by adolescents. Front Syst Neurosci. 2014;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau H, et al. . Daytime napping: effects on human direct associative and relational memory. Neurobiol Learn Mem. 2010;93(4):554–560. [DOI] [PubMed] [Google Scholar]
- 7. Rasch B, et al. . About sleep’s role in memory. Physiol Rev. 2014;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mander BA, et al. . Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonenko D, et al. . Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37(7):1142–1151. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro S, et al. . Sleep and school education. Trends Neurosci Educ. 2014;3(1):18–23. [Google Scholar]
- 11. Gais S, et al. . Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104(47):18778–18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterpenich V, et al. . Sleep promotes the neural reorganization of remote emotional memory. J Neurosci. 2009;29(16):5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schönauer M, et al. . Evidence for two distinct sleep-related long-term memory consolidation processes. Cortex.. 2015;63:68–78. [DOI] [PubMed] [Google Scholar]
- 14. Kuriyama K, et al. . Sleep deprivation facilitates extinction of implicit fear generalization and physiological response to fear. Biol Psychiatry. 2010;68(11):991–998. [DOI] [PubMed] [Google Scholar]
- 15. Hennies N, et al. . Sleep spindle density predicts the effect of prior knowledge on memory consolidation. J Neurosci. 2016;36(13):3799–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patanaik A, et al. . An end-to-end framework for real-time automatic sleep stage classification. Sleep. 2018;41(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrarelli F, et al. . Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3): 483–492. [DOI] [PubMed] [Google Scholar]
- 18. Mazza S, et al. . Relearn faster and retain longer: along with practice, sleep makes perfect. Psychol Sci. 2016:27(10): 1321–1330. [DOI] [PubMed] [Google Scholar]
- 19. Gais S, et al. . Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marshall L, et al. . Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 21. Lovato N, et al. . The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–166. [DOI] [PubMed] [Google Scholar]
- 22. Lo JC, et al. . Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2016;40(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin C, et al. . Sleep habits, excessive daytime sleepiness and school performance in high school students. Psychiatry Clin Neurosci. 2003;57(4):451–453. [DOI] [PubMed] [Google Scholar]
- 24. Ji X, et al. . The relationship between midday napping and neurocognitive function in early adolescents. Behav Sleep Med. 2018;Feb 2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks A, et al. . A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006;29(6):831–840. [DOI] [PubMed] [Google Scholar]
- 26. Faraut B, et al. . Napping: a public health issue. From epidemiological to laboratory studies. Sleep Med Rev. 2017;35:85–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.