Abstract
Study Objectives
Animal studies suggest a pivotal role of the hyoid bone in obstructive sleep apnea (OSA). We aimed to explore the role of the hyoid bone in humans by testing the hypotheses that muscle paralysis and lung volume (LV) changes displace the hyoid bone position particularly in people with obesity and/or OSA.
Methods
Fifty patients undergoing general anesthesia participated in this study (20 participants with nonobese, non-OSA; 8 people with nonobese OSA; and 22 people with obese OSA). Three lateral neck radiographs to assess the hyoid position (primary variable) and craniofacial structures were taken during wakefulness, complete muscle paralysis under general anesthesia, and LV increase under general anesthesia. LV was increased by negative extrathoracic pressure application and LV changes were measured with a spirometer. Analysis of covariance was used to identify statistical significance.
Results
Muscle paralysis under general anesthesia significantly displaced the hyoid bone posteriorly (95% CI: 1.7 to 4.6, 1.5 to 5.2, and 1.1 to 4.0 mm in nonobese non-OSA, nonobese OSA, and obese OSA groups, respectively), and this was more prominent in people with central obesity. LV increase significantly displaced the hyoid bone caudally in all groups (95% CI: 0.2 to 0.7, 0.02 to 0.6, and 0.2 to 0.6 mm/0.1 liter LV increase in nonobese non-OSA, nonobese OSA, and obese OSA groups, respectively). Waist–hip ratio was directly associated with the caudal displacement during LV increase.
Conclusions
The hyoid bone plays an important role in the pathophysiology of pharyngeal airway obstruction due to muscle paralysis and LV reduction, particularly in people with obesity.
Clinical Trial
UMIN Clinical Trial Registry, https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=cR000022635&language=E, UMIN000019578
Keywords: craniofacial aspects of OSA, obesity, OSA – pathogenesis, respiratory physiology, upper airway, lung volume
Statement of Significance
Among the many characteristic craniofacial features of people with obstructive sleep apnea (OSA), caudal displacement of the hyoid bone has been repeatedly and consistently documented; however, the mechanisms are still yet unknown. This study is the first to evidence that the hyoid bone is displaced as a marker for changes of muscle activation and lung volume, as well as the balance of soft tissue volume for the craniofacial size. Based on the pathophysiological understanding of hyoid bone position, future studies should test the hyoid bone displacement as a possible diagnostic/prognostic indicator for OSA.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive pharyngeal narrowing and closure during sleep, with both obesity and craniofacial abnormalities being important features associated with the presence and development of OSA [1, 2]. Pharyngeal airway size is determined by an interaction between neural regulation of upper airway muscle activity and structural properties of the pharyngeal airway [3, 4]. Among the many characteristic craniofacial features of people with OSA, the caudal displacement of the hyoid bone has been repeatedly and consistently documented, suggesting the importance of the hyoid bone in the OSA pathogenesis [5–7].
The hyoid bone is a horseshoe-shaped bone located within the soft tissue surrounding the pharyngeal airway between the mandible and the thyroid cartilage. Unlike most other vertebrates, the human hyoid bone does not articulate with any other bones. The position of the hyoid bone is suggested to be neurally determined by the muscle force vector between supra- and infra-hyoid muscles that attach to the hyoid bone [8–10]. These muscles are frequently admitted to inspiratory activity possibly producing anterior and caudal traction force on the hyoid bone and leading to its movement in synchrony with anterior movement of the tongue and caudal movement of the trachea and thyroid cartilage during inspiration [11, 12]. Active traction of the hyoid bone by vector forces produced by the infra- and supra-hyoid muscles appears to be one of the potential mechanisms of recovery from pharyngeal obstruction [11, 13]. This neural mechanism is presumably more active in people with OSA, where greater genioglossus muscle activity is evident during wakefulness [14]. In other words, the hyoid bone position could be a marker of the neurally mediated traction on the hyoid bone. This hypothesis can be tested by comparing the hyoid bone position before and during muscle paralysis under general anesthesia.
In addition to the neurally mediated traction of the hyoid bone discussed above, floating hyoid bone within soft tissue could also be displaced by nonmuscular passive forces derived from surrounding soft tissue [15, 16]. Relatively excessive soft tissue within the maxilla–mandible bony structure could shift the hyoid bone caudally, as well as narrow the pharyngeal airway in people with OSA [6, 17]. Furthermore, lung volume (LV) increase could displace the hyoid bone, as well as the trachea, caudally, which consequently could increase the longitudinal tension of the pharyngeal airway wall [18–22] and decrease the extra-luminal tissue pressure [15]. In fact, our research group confirmed stiffening of the passive pharyngeal airway by LV increase under general anesthesia and paralysis [23]. Notably, there was a body mass index (BMI) dependence of the LV influence on the pharyngeal collapsibility [23]. Lung inflation during sleep in people with morbidly obese OSA significantly improved the OSA severity [24]; however, the result was contradicted in another lung inflation study in people with less obese OSA [25]. At lower LV in people with obesity, contraction of the diaphragm with increased curvature is capable of producing a greater longitudinal tracheal traction force and displaces the hyoid bone more caudally. These factors suggest differences in the LV dependence of pharyngeal collapsibility and the hyoid bone position between obese and nonobese people with OSA. This nonmuscular passive mechanism could be tested by comparing the hyoid bone position before and during LV increase under general anesthesia and muscle paralysis in people with obese and nonobese OSA.
Despite the possible roles of the hyoid bone position in OSA pathophysiology, no study has yet to explore the mechanical linkages between the hyoid bone position and the pharyngeal airway maintenance mechanisms, such as pharyngeal muscle activation and LV change. We hypothesized that muscle paralysis displaces the hyoid bone cranially and posteriorly, particularly in people with OSA (primary hypothesis-1); moreover, the LV increase displaces it caudally, particularly in people with obese OSA (primary hypothesis-2). We tested the hypotheses by measuring the hyoid bone position during wakefulness, general anesthesia with muscle paralysis, and LV increase under general anesthesia in male patients undergoing surgeries under general anesthesia.
Methods
Study population
The institutional review board of Chiba University Hospital (Chiba, Japan) approved this prospective study (G27029) and the study was registered in UMIN Clinical Trial Registry (UMIN000019578, October 30, 2015: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=cR000022635&language=E). Written informed consent was obtained from each participant after the aim and potential risks of the study were fully explained. Subject enrollment in this prospective hypothesis testing study was terminated on March 2017. Inclusion criteria were adult male patients undergoing scheduled surgeries under general anesthesia at Chiba University Hospital and exclusion criteria were people with severe co-morbidities, upper airway abnormalities, severe COPD, and uncontrolled asthma. People with respiratory disturbance index (RDI) between 10 and 15 hr−1 were excluded after the sleep study in order to clarify the differences between OSA and non-OSA groups.
Sleep study and study grouping before radiographic assessments
A sleep study was performed by a nocturnal portable monitor which measured respiratory airflow and SaO2 (SAS2100, Nihon Kohden, Tokyo, Japan) in all patients with consent. An oximetry finger probe and nasal cannula were attached before sleep and removed on awakening. After checking quality of the recordings by A.K., respiratory and oximetry variables were calculated by a computer software (QP-021W, Nihon Kohden, Tokyo, Japan). Severity of OSA was quantified by RDI, frequency of desaturation 3% or more from the baseline, the percent of time spent at SaO2 less than 90%, and lowest SaO2. RDI was calculated by the frequencies of apneas and hypopneas with 3% or more desaturation per hour of the monitoring time. Apnea and hypopnea were determined by absence of airflow for 10 s or more and more than 50% decrease of the nasal pressure signal for 10 s or more, respectively. The participants were divided into three groups based on BMI and the results of the portable sleep study: participants with BMI less than 25 kg/m2 and RDI equal to or less than 10 hr−1 were denoted as the nOB-nOSA group; participants with BMI less than 25 kg/m2 and RDI greater than 15 hr−1 as the nOB-OSA group; and participants with BMI equal to or greater than 25 kg/m2 and RDI greater than 15 hr−1 as the OB-OSA group.
Lateral head and neck radiographs during wakefulness, general anesthesia with muscle paralysis, and LV increase under general anesthesia and paralysis
On the day of the surgery, lateral head and neck radiographs were obtained at three different conditions in the operating room in the supine position while maintaining the neutral head and neck position (no head elevation, with face straight up). An inverter-type mobile X-ray system (Sirius Star Mobile 130H, Hitachi Ltd, Tokyo, Japan) with a wireless digital radiography system (AeroDR1417, Konica Minorta Inc., Tokyo, Japan) was used to obtain high-quality digitized radiograph images with a constant source to image-receptor distance (150 cm) and a constant radiation setting (80 kV, 5 mAs, no removing scatter grid). A scale attached on the center of the sternum was used to obtain absolute values of the radiograph measurements. The mouth was kept closed with a chin strap. The head posture was maintained constant by adjusting the head angle between the operation table and a line connecting two small markers stuck on the side-face skin near the tragus and the corner of the eye immediately before each radiograph. First, a radiograph was taken at the end of quiet expiration during wakefulness (AW) and the second and third radiographs were taken during cessation of mechanical ventilation under general anesthesia and paralysis either with (AN-LV(+)) or without (AN) lung inflation which were randomly determined before the measurements. The quality of the images was confirmed for each radiograph by a diagnostic imaging workstation (CS-7, Konica Minolta Inc., Tokyo, Japan).
General anesthesia and lung inflation procedures
Under standard cardiorespiratory monitoring, general anesthesia was induced by intravenous infusion of fentanyl 2 μg kg−1 and propofol 1–2 mg kg−1 and maintained by either continuous infusion of propofol or inhalation of desuflurane. Rocuronium 1 mg kg−1 produced complete paralysis confirmed by a muscle relaxation monitor (TOF watch SX, Nihon Kohden Inc., Tokyo, Japan) throughout the experiment while the participant was ventilated through an anesthesia full-face mask with positive pressure through an anesthetic machine. The mechanical ventilation was ceased for 1 min to obtain lateral head and neck radiographs for the AN-LV(+) and AN conditions.
LV was increased by application of negative extrathoracic pressure up to −50 cmH2O through a rigid plastic shell covering the anterior part of the thorax between axilla and ilium. An AN-LV(+) radiograph was taken after measuring the LV change by a spirometer connected to a tightly fitted full face mask at atmospheric pressure. To counteract the possibility of a closed airway during anesthesia and paralysis, the triple airway maneuver (mandible advancement, neck extension, and mouth opening) with anesthesiologist’s two hands was performed to open the airway and allow oxygen to flow in order to increase LV during negative extra-thoracic pressure application. Airway opening and absence of mask leak were confirmed by progressive increase of spirometer tracing in response to the negative pressure application. The triple airway maneuver was applied during LV change, but was released for the radiographs.
Analyses of lateral head and neck radiographs
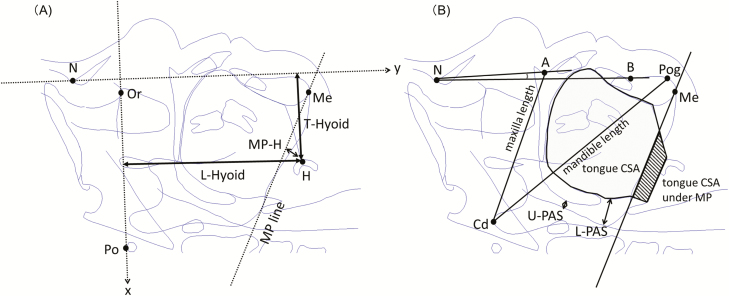
Using an image enhancement processing software (CS-7, Konica Minolta Inc., Tokyo, Japan), digitized radiographic images were modified to obtain clear boundaries of targeted structures and craniofacial dimensions were measured by the investigator (A.K.) using a cephalometric analysis program (WinCeph version 10, Rise Corporation, Miyagi, Japan), without prior knowledge of the anthropometric and sleep characteristics (Figure 1). To systematically assess the direction and amount of the hyoid bone movement, we defined a coordinate on the lateral radiograph with anterior–posterior (x-axis) and cranial–caudal (y-axis) axes. Frankfort plane was set as the x-axis, and a line perpendicular to the Frankfort plane through the nasion (N) was set as the y-axis (Figure 1). Using the coordinate superimposed on the radiographic images, longitudinal and transverse distances of the hyoid bone to the origin (L-Hyoid and T-Hyoid, respectively) were defined and measured. Due to variable LV increase among the participants, these variables were normalized by a unit LV change (0.1 liter), L-Hyoid/LV and T-Hyoid/LV, respectively, in order to assess the effects of LV increase on the hyoid bone position. As commonly measured in scientific studies and for clinical purposes, the distance between the hyoid and the mandibular plane line (MP-H) was also measured. In addition to the hyoid bone position analyses, other cephalometric variables such as pharyngeal airway spaces, craniofacial bony structures, and soft tissue sizes were also assessed. Narrowest pharyngeal airway spaces above and below the tip of the uvula were measured as the upper pharyngeal airway space (U-PAS) and lower pharyngeal airway space (L-PAS), respectively. Relative position of the mandible to the maxilla was assessed by the angle between the lines subspinale to nasion and supramentale to nasion. Maxillary length and mandibular length were defined as distances between the medial condylar point of the mandible and subspinale, and medial condylar point of the mandible and porin, respectively. Total tongue cross-sectional area was outlined by the dorsum of the tongue surface and lines that connect tongue tip, retrognathion, and base of epiglottis. Tongue cross-sectional area below the mandibular plane line was also measured. To assess constancy of head and neck position during the radiography for each participant, a head and neck angle formed by the x-axis and a line connecting the posterior edge of the odontoid process and posterior inferior tip of the third cervical vertebra (head/neck angle) was measured.
Figure 1.
Definitions of the cephalometric variables. (A) Coordinate on the cephalogram with transverse and longitudinal axes was defined with using the Frankfort plane (a line connecting Po and Or) as the transverse axis, and a line perpendicular to Frankfort plane through the nasion (N) as the longitudinal axis. Using the coordinate, the longitudinal and transverse distances of the hyoid bone to the origin were measured as the L-Hyoid and T-Hyoid, respectively. (B) Narrowest pharyngeal airway spaces above and below the tip of the uvula were measured as the U-PAS and L-PAS, respectively. Relative position of the mandible to the maxilla was assessed by ANB angle (angle between the lines A to N and B to N). Maxillary length (Cd-A) and mandibular length (Cd-Po) were defined as distances between the Cd and A, and Cd and Po, respectively. Total tongue cross-sectional area was outlined by the dorsum of the tongue surface and lines that connect tongue tip, retrognathion, and base of epiglottis. Shaded area indicates tongue cross-sectional area below the MP line. A = subspinale; B = supramentale; Cd = medial condylar point of the mandible; H = hyoid bone; Me = menton; MP = mandibular plane; N = nasion; Or = orbitale; Po = porin; Pog = pogonion.
Statistical methods
The primary outcome was change of the L-Hyoid after interventions such as induction of general anesthesia with muscle paralysis (ΔL-Hyoid) and LV increase (ΔL-Hyoid/LV). Since no previous study measured L-Hyoid, we calculated a sample size by using the reported values of distance between the hyoid and the mandible plane (MP-H: 23.4 ± 5.8 mm) although the MP-H was obtained by a different radiographic technique and in a different posture from this study [5]. In our preliminary study of 26 people with awake OSA, changes of the LV from residual capacity to total lung capacity resulted in an MP-H change of 8.8 mm on average. We expected a much smaller LV change in this study because our previous experiment revealed only a 0.72 liter LV increase by the same technique [23]. In sample size calculation, a pathophysiologically meaningful change was considered to be 50% of MP-H change (4.4mm) possibly produced by maximum voluntary LV change in the preliminary experiment. The minimum sample size was calculated as 20 participants for each group assuming α = 0.05(two-tailed), β = 0.8 (SigmaPlot 12.0, Systat Software Inc., Point Richmond, CA). Accordingly, we set the sample size to be 22 participants for each group.
To assess displacements of the hyoid bone in response to induction of general anesthesia with muscle paralysis and LV increase (primary variables: ΔL-Hyoid and ΔL-Hyoid/LV), intergroup and intragroup comparisons were performed through analysis of a covariance (ANCOVA) model, taking into account the variation caused by using the waist/hip ratio and neck circumference as covariates. As a secondary analysis, we specifically focused on possible changes of the upper and lower pharyngeal airway spaces (ΔU-PAS and ΔL-PAS, respectively) and tongue cross-sectional area (Δtongue-CSA) in response to induction of general anesthesia with muscle paralysis and LV increase. Statistical differences of patient characteristics and radiographic variables were assessed by analysis of variance (ANOVA) and group comparisons were performed by Turkey’s test for multiple comparisons. A value of p < 0.05 was considered statistically significant, and all p values were two-sided. Statistical analyses were performed using SAS Ver. 9.4 (SAS Institute, Cary, NC).
Results
The study was successfully performed in 50 patients, but terminated before completing the predetermined sample size particularly in nOB-OSA group due to difficulty in recruiting nOB-OSA participants during the scheduled study period. However, we considered this analysis of the 50 participants as meaningful, as a result of this prospective hypotheses-testing pathophysiological study. Table 1 presents significant differences of background participant characteristics and results of the portable sleep study between the groups in accordance with our study protocol. As shown in Table 2, LV increase was successfully performed in all participants but varied among the participants (794 ± 312 ml). The maximum level of the negative pressure application was limited particularly in participants with obesity because the shell was filled with the thorax of the participant.
Table 1.
Background participant characteristics and results of the portable sleep study for each of the groups
| All participants | nOB-nOSA | nOB-OSA | OB-OSA | P | |
|---|---|---|---|---|---|
| Number of participants | 50 | 20 | 8 | 22 | |
| Age (year) | 59.3 ± 12.8 | 56.9 ± 15.4 | 67.9 ± 5.4 | 58.5 ± 11.1 | 0.102 |
| Height (cm) | 168.5 ± 6.2 | 170.3 ± 7.1 | 168.0 ± 5.1 | 166.9 ± 5.5 | 0.146 |
| Weight, kg | 71.8 ± 13.5 | 63.3 ± 7.1 | 64.0 ± 6.2 | 82.3 ± 12.7 | <0.0001 |
| BMI, kg/m2 | 25.3 ± 4.5 | 21.8 ± 2.0 | 22.6 ± 1.4 | 29.4 ± 3.4 | <0.0001 |
| Neck circumference (cm) | 38.4 ± 3.1 | 36.1 ± 2.2 | 37.1 ± 1.2 | 40.9 ± 2.5 | <0.0001 |
| Waist circumference (cm) | 90.1 ± 12.1 | 80.5 ± 6.8 | 86.9 ± 6.8 | 99.9 ± 9.5 | <0.0001 |
| Hip circumference (cm) | 95.9 ± 8.9 | 89.7 ± 5.5 | 93.9 ± 4.7 | 102.4 ± 8.2 | <.0001 |
| Waist/hip ratio | 0.936 ± 0.059 | 0.897 ± 0.049 | 0.925 ± 0.044 | 0.976 ± 0.047 | <.0001 |
| Mallampati (I/II/III/IV) | 6, 4, 9, 31 | 6, 3, 5, 6 | 0, 0, 0, 8 | 0, 1, 4, 17 | 0.002 |
| RDI, events/hr | 21.8 ± 18.2 | 4.7 ± 2.6 | 20.8 ± 5.5 | 37.6 ± 14.7 | <0.0001 |
| 3% ODI, events/hr | 17.2 ± 15.0 | 4.2 ± 2.6 | 15.1 ± 4.0 | 29.8 ± 13.7 | <0.0001 |
| CT90, % | 3.0 ± 5.7 | 0.1 ± 0.2 | 0.9 ± 0.9 | 6.4 ± 7.2 | <0.0001 |
| Nadir SaO2, % | 92.7 ± 2.6 | 94.6 ± 1.2 | 93.4 ± 0.9 | 90.7 ± 2.4 | <0.0001 |
| Lowest SaO2, % | 84.4 ± 7.8 | 90.5 ± 3.5 | 82.8 ± 7.8 | 79.5 ± 7.1 | <0.0001 |
Values are means ± SD. Group differences were assessed by ANOVA.
nOB-nOSA = people with non-obese non-OSA; nOB-OSA = people with non-obese OSA; OB-OSA = people with obese OSA; ODI = oxygen desaturation index; CT90 = percent of time spent SaO2 < 90%; SaO2 = arterial oxygen saturation; 3%ODI = 3% oxygen desaturation index.
Table 2.
Baseline radiographic variables during wakefulness for each of the patient groups
| Lateral radiograph during wakefulness | All participants | nOB-nOSA | nOB-OSA | OB-OSA | P |
|---|---|---|---|---|---|
| ANB angle (degree) | 4.4 ± 3.1 | 4.4 ± 2.6 | 5.1 ± 3.3 | 4.3 ± 3.6 | 0.91 |
| maxilla length (mm) | 89.1 ± 6.0 | 90.7 ± 4.8 | 86.6 ± 8.3 | 88.6 ± 6.0 | 0.25 |
| mandible length (mm) | 122 ± 7.9 | 123 ± 7.4 | 118 ± 12.8 | 122 ± 6.0 | 0.27 |
| L-Hyoid (mm) | 99.9 ± 8.6 | 99.3 ± 5.6 | 98.1 ± 15.0 | 101 ± 8.0 | 0.47 |
| T-Hyoid (mm) | 52.3 ± 16.1 | 50.5 ± 16.8 | 52.6 ± 15.2 | 54.0 ± 16.3 | 0.78 |
| MP-H (mm) | 16.6 ± 6.3 | 14.2 ± 4.0 | 18.0 ± 7.7 | 18.3 ± 7.0 | 0.16 |
| U-PAS (mm) | 5.4 ± 3.1 | 5.3 ± 2.5 | 5.1 ± 3.8 | 5.7 ± 3.5 | 0.79 |
| L-PAS (mm) | 11.7 ± 5.1 | 11.5 ± 4.2 | 10.5 ± 4.5 | 12.3 ± 6.0 | 0.53 |
| tongue CSA (cm2) | 3300 ± 387 | 3212 ± 317 | 3170 ± 594 | 3428 ± 331 | 0.052 |
| tongue CSA under MP (cm2) | 603 ± 229 | 538 ± 205 | 630 ± 318 | 653 ± 210 | 0.19 |
Values are means ± SD. Group differences were assessed by ANOVA.
ANB = angle between the lines subspinale to nasion and supramentale to nasion; CSA = cross-sectional area; L-Hyoid = longitudinal distance of the hyoid bone to the origin; L-PAS = lower pharyngeal airway space; MP-H = distance from the mandibular plane to the hyoid bone; nOB-nOSA = people with nonobese non-OSA; nOB-OSA = people with non-obese OSA; OB-OSA = people with obese OSA; T-Hyoid = transverse distance of the hyoid bone to the origin; U-PAS = upper pharyngeal airway space.
See Figure 1 for definitions of the radiographic variables.
Possible confounding factors associated with the primary variables
The head/neck angle did not statistically differ between the three conditions when the radiographs were taken (95% CI: 93.6 to 98.8, 93.3 to 98.4, and 93.5 to 98.6 degree during wakefulness, general anesthesia with muscle paralysis, and LV increase under anesthesia and paralysis, respectively), suggesting constancy of head and neck position during radiography within the participants. Table 3 presents baseline radiographic variables during wakefulness which were not different between the groups. Table 4 presents results of univariate correlation analyses between the hyoid bone displacement and anthropometric and sleep characteristics. Besides the BMI and RDI used for the classifications in this study, waist/hip ratio and neck circumference, which characterize obesity pattern, were significantly associated with the primary variables and these possible confounding factors were used as covariates for the ANCOVA in this study population.
Table 3.
Lung volume change in response to application of negative extra-thoracic pressure or each of the patient groups
| All participants | nOB-nOSA | nOB-OSA | OB-OSA | P | |
|---|---|---|---|---|---|
| Extrathoracic pressure (cmH2O) | 30.5 ± 13.7 | 39.4 ± 13.9 | 32.6 ± 14.8 | 21.7 ± 6.0 | 0.0002 |
| Lung volume change (ml) | 794 ± 312 | 910 ± 311 | 841 ± 95 | 671 ± 327 | 0.16 |
Values are means ± SD. Group differences were assessed by ANOVA.
nOB-nOSA = people with non-obese non-OSA; nOB-OSA = people with non-obese OSA; OB-OSA = people with obese OSA.
Table 4.
Results of univariate correlation analyses between the hyoid bone displacement and background variables
| n = 50 | Wakefulness to general anesthesia with muscle paralysis | Lung volume increase under general anesthesia with muscle paralysis | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔL-Hyoid | ΔT-Hyoid | ΔL-Hyoid/LV | ΔT-Hyoid/LV | |||||
| r | P | r | P | r | P | r | P | |
| Age | −0.032 | 0.826 | 0.028 | 0.85 | −0.098 | 0.5 | −0.021 | 0.886 |
| BMI | −0.451 | 0.001 | −0.301 | 0.034 | 0.161 | 0.263 | 0.063 | 0.665 |
| Neck circumference | −0.352 | 0.012 | −0.235 | 0.1 | 0.026 | 0.858 | 0.065 | 0.656 |
| Waist/hip ratio | −0.375 | 0.007 | −0.281 | 0.048 | 0.312 | 0.027 | 0.218 | 0.128 |
| RDI | −0.404 | 0.004 | −0.23 | 0.107 | −0.029 | 0.839 | −0.065 | 0.656 |
Values are correlation coefficient (p value) obtained by Pearson correlation analyses.
ΔL-Hyoid = the amount of caudal displacement of the hyoid bone by induction of general anesthesia and muscle paralysis; ΔL-Hyoid/LV = change of longitudinal distance of the hyoid bone to the origin per 0.1 liter lung volume increase; ΔT-Hyoid = the amount of posterior displacement of the hyoid bone by induction of general anesthesia and muscle paralysis; ΔT-Hyoid/LV = change of transverse distance of the hyoid bone to the origin per 0.1 liter lung volume increase.
Results related to the primary hypothesis-1: influences of muscle paralysis and general anesthesia on hyoid positions and other radiographic variables
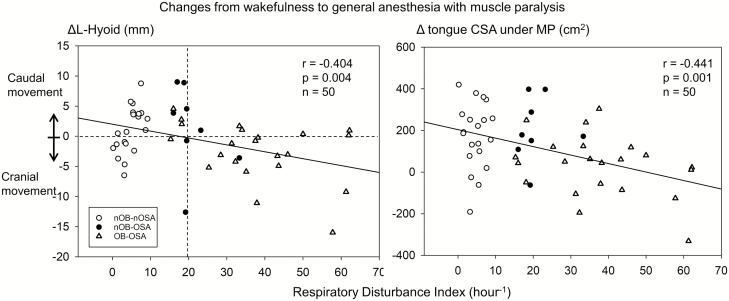
The ΔL-Hyoid (primary variable) ranged widely (−0.5 ± 5.2 mm, −16 to 9 mm) and direction of the longitudinal hyoid bone displacement was significantly associated with severity of RDI (r = −0.404, p = 0.004, n =50) (Figure 2, left). Cranial hyoid bone displacement was more commonly observed in people with OSA with RDI greater than 20 hr−1 (14 out of 20 patients), whereas caudal hyoid bone displacement was more common in people with normal or less severe OSA (19 out of 30 patients) as clearly illustrated in Figures 2 and 3 (Fisher exact test: p = 0.042, n = 50). Similar indirect association was noted between Δtongue CSA under MP and RDI (r = −0.441, p = 0.001, n = 50; Figure 2, right).
Figure 2.
Relationships between respiratory disturbance index and ΔL-Hyoid (left panel), and Δtongue CSA under MP (right panel) from wakefulness to general anesthesia and muscle paralysis. Pearson’s correlation analysis was performed. Values are correlation coefficient, p value, and the number of participants. ΔL-Hyoid = the amount of caudal displacement of the hyoid bone by induction of general anesthesia and muscle paralysis; Δtongue CSA under MP = the amount of increased tongue cross-sectional area under mandibular plane by induction of general anesthesia and muscle paralysis.
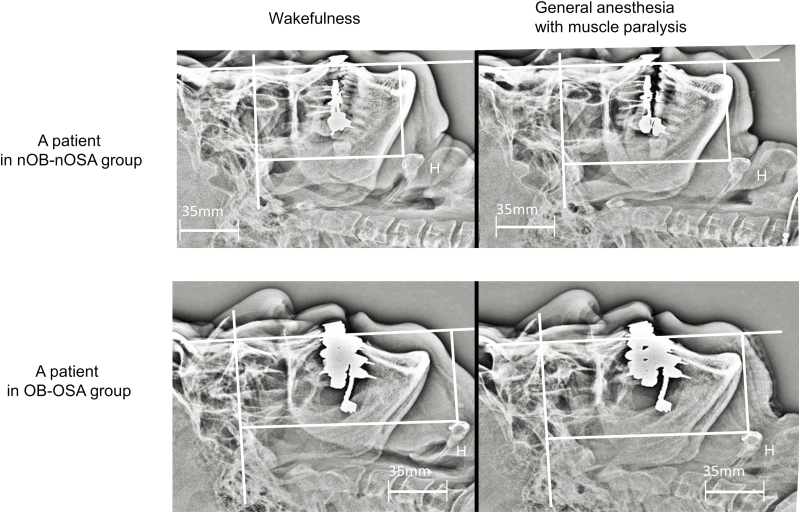
Figure 3.
Lateral head and neck radiographs during wakefulness and during muscle paralysis under general anesthesia in a participant of non-obese and non-OSA (nOB-nOSA) group (upper panel) and a participant of obese and OSA (OB-OSA) group (lower panel). (Upper panel) In this 58-year-old male patient with BMI 23.7 kg/m2, and RDI 7.5 hr−1, the hyoid bone was posteriorly (6.0 mm) and cranially (8.8 mm) displaced during general anesthesia with muscle paralysis. Note the increase of submandible tongue CSA and reduction of posterior pharyngeal airway space. (Lower panel) In this 66-year-old male patient with BMI 26.9 kg/m2 and RDI 37.9 hr−1, the hyoid bone was posteriorly (3.7 mm) and caudally (11.1 mm) displaced during general anesthesia with muscle paralysis. Note the reduction of submandible tongue CSA and reduction of posterior pharyngeal airway space. CSA = cross-sectional area; nOB-nOSA = non-obese and non-OSA; OB-OSA = obese and OSA; OSA = sleep-disordered breathing.
Results of the ANCOVA for the ΔL-Hyoid between AW and AN indicated no significant differences among the groups (p = 0.726) and within the group (95% CI: −3.0 to 2.5, −2.8 to 4.3, and −3.9 to 1.6 mm in nOB-nOSA, nOB-OSA, and OB-OSA groups, respectively; Table 5). In contrast, the ANCOVA for the ΔT-Hyoid between AW and AN revealed significant increase in all groups (95% CI: 1.7 to 4.6, 1.5 to 5.2, and 1.1 to 4.0 mm in nOB-nOSA, nOB-OSA, and OB-OSA groups, respectively), whereas no significant group difference was indicated (p = 0.815).
Table 5.
Results of an analysis of variance (ANCOVA) analyses for assessing craniofacial responses to induction of general anesthesia with muscle paralysis with and without lung volume increase
| Measurement conditions and craniofacial variables | Adjusted mean value (95% confidential interval) | Group difference p value | |||
|---|---|---|---|---|---|
| nOB-nOSA | nOB-OSA | OB-OSA | |||
| AW control position | L-Hyoid (mm) | 100 (94.1 to 104) | 98.1 (91.6 to 105) | 101 (96.3 to 106) | 0.755 |
| T-Hyoid (mm) | 54.8 (45.8 to 63.8) | 55.4 (43.6 to 67.1) | 49.0 (39.9 to 58.0) | 0.673 | |
| changes (Δ) from AW to AN-LV(−) | ΔL-Hyoid (mm) | −0.26 (−3.0 to 2.5) | 0.77 (−2.8 to 4.3) | −1.14 (−3.9 to 1.6) | 0.726 |
| ΔT-Hyoid (mm) | 3.1 (1.7 to 4.6)* | 3.3 (1.5 to 5.2)* | 2.5 (1.1 to 4.0)* | 0.815 | |
| ΔMP-H (mm) | −1.3 (−3.6 to 1.0) | −1.5 (−4.5 to 1.5) | −3.2 (−5.6 to −0.9)* | 0.569 | |
| ΔU-PAS (mm) | −4.2 (−6.0 to −2.4)* | −4.8 (−7.2 to −2.5)* | −5.8 (−7.6 to −4.0)* | 0.58 | |
| ΔL-PAS (mm) | −9.0 (−11.5 to −6.5)* | −9.3 (−12.6 to −6.0)* | −9.6 (−12.1 to −7.1)* | 0.96 | |
| Δtongue CSA (mm2) | 218 (95 to 341)* | 285 (124 to 445)* | 263 (139 to 387)* | 0.768 | |
| Δtongue CSA under MP (mm2) | 155 (68 to 242)* | 194 (81 to 307)* | 50 (−37 to 138) | 0.165 | |
| changes (Δ) from AN-LV(−) to AN-LV(+) (per 0.1 liter LV increase) | ΔL-Hyoid/LV (mm) | 0.4 (0.2 to 0.7)** | 0.3 (0.02 to 0.6)** | 0.4 (0.2 to 0.6)** | 0.776 |
| ΔT-Hyoid/LV (mm) | 0.20 (−0.03 to 0.4) | 0.004 (−0.3 to 0.3) | 0.1 (−0.1 to 0.4) | 0.564 | |
| ΔMP-H/LV (mm) | 0.2 (0.03 to 0.4)** | 0.1 (−0.2 to 0.3) | 0.2 (0.02 to 0.4)** | 0.44 | |
| ΔU-PAS/LV (mm) | 0.1 (−0.06 to 0.2) | 0.03 (−0.1 to 0.2) | −0.1 (−0.24 to 0.01) | 0.232 | |
| ΔL-PAS/LV (mm) | −0.2 (−0.4 to 0.1) | −0.10 (−0.4 to 0.2) | −0.3 (−0.5 to −0.1)** | 0.566 | |
| Δtongue CSA/LV (mm2) | 17 (−3 to 36) | 4 (−22 to 29) | 5 (−14 to 25) | 0.652 | |
| Δtongue CSA under MP/LV (mm2) | 15 (6 to 23)** | 8 (−3 to 19) | 13 (5 to 22)** | 0.596 | |
Between-groups and within-group comparisons were performed by the ANCOVA model with taking into account the variation caused by using the waist/hip ratio and neck circumference as covariates.
*p < 0.05 versus AW; †p < 0.05 versus AN.
AW = wakefulness; AN = general anesthesia with muscle paralysis without lung volume increase; AN-LV(+) = general anesthesia with muscle paralysis with lung volume increase; Δ = difference between the conditions; ΔL-Hyoid = the amount of caudal displacement of the hyoid bone; ΔMP-H = the amount of hyoid bone displacement relative to the mandibular plane; ΔT-Hyoid = the amount of posterior displacement of the hyoid bone; ΔL-PAS = the amount of increase of lower pharyngeal airway space; ΔU-PAS = the amount of increase of upper pharyngeal airway space; Δtongue-CSA = the amount of increase of tongue cross-sectional area; MP = mandibular plane; nOB-nOSA = people with non-obese non-OSA patients; nOB-OSA = people with non-obese OSA; OB-OSA = people with obese OSA.
See Figure 1 for definitions of radiographic variables.
Muscle paralysis and anesthesia induction significantly narrowed the pharyngeal airway space in all groups and no group difference was indicated (Table 5). ΔU-PAS and ΔL-PAS were not significantly associated with ΔT-Hyoid between AW and AN (p = 0.955, 0.958, respectively, n =50). The tongue CSA significantly increased with muscle paralysis and general anesthesia suggesting tongue musculature volume shift from lateral to anteroposterior direction. The tongue musculature was significantly displaced to the submandible region in nOB-nOSA and nOB-OSA groups, but not in the OB-OSA group. The Δtongue CSA under MP between AW and AN was significantly associated with the ΔL-Hyoid (r = 0.573, p < 0.001, n = 50).
Results related to the primary hypothesis-2: influences of LV increase under general anesthesia on hyoid positions and other radiographic variables
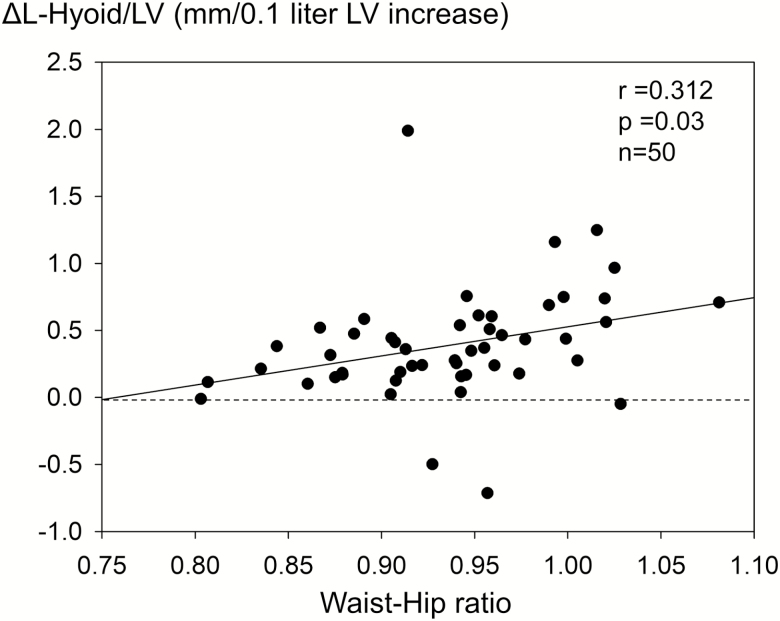
As demonstrated in Figure 4, LV increase under general anesthesia and muscle paralysis displaced the hyoid bone and the tongue musculature caudally, whereas no anteroposterior hyoid bone displacement was observed. The already closed pharyngeal airway remained closed even during LV increase. The ANCOVA analyses revealed caudal hyoid bone displacement (95% CI: 0.2 to 0.7, 0.02 to 0.6, and 0.2 to 0.6 mm/0.1 liter LV increase in nOB-nOSA, nOB-OSA, and OB-OSA groups, respectively), whereas no significant group difference was identified (p = 0.776; Figure 5, Table 5). Notably, the ANCOVA identified the waist/hip ratio to be a significant confounding factor for the ΔT-Hyoid/LV (p = 0.001). The amount of the ΔL-Hyoid/LV was directly associated with the waist/hip ratio (r = 0.312, p = 0.03, n = 50; Figure 5) and the Δtongue CSA under MP/LV (r = 0.679, p < 0.001, n = 50). No significant change of the ΔT-Hyoid/LV was indicated by the ANCOVA.
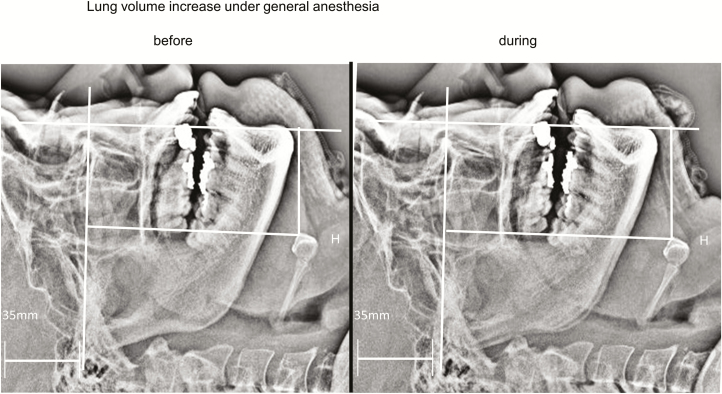
Figure 4.
Lateral head and neck radiographs before and during LV increase under general anesthesia with muscle paralysis in a participant of OB-OSA group (46-year-old male patient with BMI 26.1 kg/m2 and RDI 16 hr−1). Note 1 liter LV increase under general anesthesia and paralysis caudally displaced the hyoid bone by 6.1 mm. OB-OSA = obese and OSA.
Figure 5.
Relationship between the amount of the ΔL-Hyoid/LV and waist/hip ratio. Pearson’s correlation analysis indicates direct association between them. Values are correlation coefficient, p value, and the number of participants. ΔL-Hyoid/LV = the amount of longitudinal displacement of the hyoid bone under general anesthesia with muscle paralysis by increase of 0.1 liter LV.
Discussion
This is the first study which demonstrates hyoid bone displacement with anesthesia and muscle paralysis and subsequent LV increase. Consistent with pharyngeal airway narrowing during muscle paralysis and anesthesia, the hyoid bone was posteriorly displaced, but its longitudinal movements varied and were associated with severity of obesity and OSA. LV increase caudally displaced the hyoid bone regardless of the severity of BMI and OSA, and the waist/hip ratio was directly associated with the amount of caudal hyoid bone displacement. The longitudinal hyoid bone movement was significantly associated with changes of the tongue volume at submandible region. These results strongly suggest the hyoid bone position as a marker for changes of upper airway muscle activation and longitudinal pharyngeal wall tension due to LV change as well as the balance between soft tissue volume and craniofacial size.
Limitations of the study
Clearly, this study has many methodological limitations. First, complete muscle paralysis under general anesthesia differs from the reduction of upper airway muscle activity during sleep [26, 27]. Therefore, the results do not directly reflect the conditions of sleeping humans but should be able to clarify fundamental roles of the upper airway muscle activation in the maintenance of the pharyngeal airway patency. Secondly, distribution of forces experimentally produced by negative extra-thoracic pressure application for increase of the LV in this study would significantly differ from that produced by contraction of the chest wall muscles. The difference would also be significantly influenced by obesity as indicated by smaller LV increase in response to negative extra-thoracic pressure in obese OSA group. Thirdly, the initial LV and the amount of LV reduction by induction of general anesthesia and paralysis were not measured and controlled [28]. In fact, the functional residual capacity is reported to decrease by more than 1 liter in people with obesity immediately after induction of general anesthesia; consequently, this study would have only reversed part of the LV reduction (0.67 liter) [29]. Accordingly, the changes of hyoid bone position from wakefulness to general anesthesia with muscle paralysis are the sum of muscle paralysis and LV reduction and would be more significant in people with obesity. Fourthly, the amount of LV increase was not controlled and significantly differed between participants as evident in Table 2. Since no difference in variation was observed between groups, intergroup comparisons were possible. Potential nonlinearity of LV dependence of the hyoid bone displacement would result in inaccurate quantitative estimation of the effect. Fifthly, the sample size for the nOB-OSA group is insufficient, and therefore comparisons between nOB-OSA and other groups are underpowered. We tried to minimize this limitation by using ANCOVA. Sixthly, we did not study patients with RDI between 10 and 15 hr−1 who would be habitual snorers with less apnea. Although responses to muscle paralysis and LV increase would be intermediate between those in the nOSA and OSA as indicated by Figure 2, future studies need to include this unique population. Lastly, we used neither a three-dimensional imaging technique nor a lateral cephalogram technique both of which are commonly used to characterize the craniofacial structures, and therefore, the accuracy of our craniofacial measurements might be questioned. To improve and assure accuracy of the measurements, the head posture was checked immediately before each radiograph by measuring the head angle relative to the operation table and the mouth opening was prevented by using a chin strap. Furthermore, we used a digitized radiography technique that increases the accuracy of determining boundaries of the soft tissue and bony structures. In fact, we confirmed no difference in the craniofacial variables for the three radiographs taken in this study.
Hyoid bone and muscle activation
Changes of the hyoid bone position after induction of general anesthesia and muscle paralysis mainly reflect activation of the supra- and infra-hyoid muscles (neurally mediated caudal traction of the hyoid bone). In accordance with the previous study [30], the hyoid bone was posteriorly displaced in response to induction of general anesthesia and muscle paralysis. This would be the result of abolition of wakeful genioglossus and suprahyoid muscle activity. Interestingly, changes of the anteroposterior hyoid bone position were not associated with narrowing of the retroglossal or retropalatal airways. This suggests that although the anteroposterior hyoid position is possibly a marker of pharyngeal dilator muscle activity, the pharyngeal airway space is influenced by both neural and mechanical factors.
Dependency of the longitudinal hyoid bone displacement on severity of OSA found in this study has never been documented in the literature. Interestingly, Lim et al., orthopedic surgeons, recently alerted the use of the hyoid bone position as a surgical landmark for the C3 cervical level because of variable and significant hyoid bone displacement by induction of general anesthesia and paralysis [31]. They found no significant association between the degree of the hyoid bone displacement and gender, obesity, and age. We consider that the association between the longitudinal hyoid bone position and the OSA severity does not mean a causal relationship but rather reflects possible increased activity of the infra-hyoid muscles during wakefulness as evidenced in animal studies which demonstrated electrical stimulation of the infra-hyoid muscles to decrease the upper airway closing pressure [32]. However, this speculation needs to be tested by assessing the magnitude of infra- and supra-hyoid muscle activities in people with OSA in future studies.
Hyoid bone and LV change
In people with OSA, we previously reported that a 0.72 liter increase of the LV decreased the velopharyngeal closing pressures by 1.2 cmH2O [23]. Although this suggests mechanical linkage between the lung and the pharynx, no human study has provided its evidence. Recently, in anesthetized rabbits spontaneously breathing through tracheostomy, Amatoury et al. demonstrated that caudal tracheal displacement resulted in smaller caudal hyoid bone displacement (approximately 20% of the tracheal displacement) and proportional reduction of extra-luminal tissue pressure [15]. Although the magnitude of hyoid bone displacement during lung inflation appears to be small in our study, the observed hyoid bone displacement (0.4 mm longitudinal hyoid bone displacement in response to 100 ml LV increase) would correspond to 2 mm tracheal displacement per 100 ml LV increase according to the Amatoury’s findings [15]. The hyoid bone position would reflect the magnitude of mechanical linkage between the lung and the pharyngeal airway. The results of the present study are the first to provide direct evidence suggesting that the mechanical link between lung inflation/tracheal traction and caudal hyoid bone displacement is an important mechanism mediating effects on the human upper airway.
Furthermore, the direct association between the waist/hip ratio and the amount of caudal hyoid bone displacement also agrees with the previous finding that the BMI was directly associated with the magnitude of LV dependence of the pharyngeal collapsibility [23]. Interestingly, the Hoffstein’s pioneer work demonstrated that the pharyngeal cross-sectional area decreased significantly more during slow exhalation from total lung capacity to residual volume in people with obese OSA than in weight-matched control participants without OSA [20]. Notably, the functional residual capacity of participants with obese OSA was significantly lower than that of participants with obese non-OSA [20]. Similarly, lung inflation during sleep was only effective for reducing OSA severity in people with morbidly obese OSA [24], and not in people with less obese OSA [25]. Increased curvature of the diaphragm in a person with obesity at a lower initial LV could mechanically produce greater longitudinal tracheal traction force and displace the hyoid bone more caudally. These observations suggest a BMI dependence of the LV influence on the hyoid bone position. Results of our study clarified that the type of obesity does matter and supports that LV dependence of the pharyngeal collapsibility is augmented particularly in people with central obesity.
Hyoid bone and the balance of the soft tissue volume for the craniofacial structure
The pharyngeal airway is a space inside rigid craniofacial bony enclosure formed by mandible, maxilla, and cervical vertebrae. When the upper airway muscle activation is eliminated, the anatomical balance of the soft tissue volume for the craniofacial structure is considered to determine the airway space and one of the significant mechanisms for pharyngeal airway obstruction in people with OSA [6, 17]. In this context, displacement of the tongue musculature to the outside of the bony enclosure could decrease extra-luminal tissue pressure [33] and serve to compensate for the pharyngeal airway narrowing during muscle paralysis. As evident from Figures 2 and 3, this compensation was less evident, but rather lost during muscle paralysis in people with severe OSA. Accordingly, the results of this study indicate that the upper airway muscles dilate the pharyngeal airway partly by displacing the upper airway soft tissue to submandible region, particularly in people with severe OSA during wakefulness, and the neural mechanism is lost when the muscle activation is suppressed [14]. The hyoid bone locates at the bottom of the tongue, and therefore, the displacement of the tongue is perceived by the movement of the hyoid bone as a marker of the anatomical balance surrounding the pharyngeal airway [6, 17]. The interaction between the upper airway soft tissue and the hyoid bone position is relevant to the effects of neck circumference changes caused by nocturnal fluid shift in people with OSA with cardiac failure [34].
Clinical implications of the study
The results of this study clearly demonstrated a role of the hyoid bone position as a marker for neural and structural mechanisms for pharyngeal airway maintenance. Therefore, we consider that the caudally located hyoid bone commonly seen in people with OSA is a result of interactions between the infra-hyoid muscle activation [11, 12], compensatory displacement of the excessive soft tissue outside the enclosure [33, 35], and tracheal traction by lung inflation [19, 36]. It should be noted that the hyoid bone is displaced anteriorly or caudally in people with OSA whenever the pharyngeal airway collapsibility is improved either by neural or structural mechanisms mentioned above. It is widely believed that longer pharyngeal airway (caudally located hyoid bone position) increases pharyngeal airway resistance and is a disadvantage for breathing [37], but this does not mean that the intervention to shorten the pharyngeal airway length leads to improvement of the OSA severity [38]. Similarly, the association between the pharyngeal length and upper airway critical closing pressure does not mean that the longer pharyngeal airway increases pharyngeal airway collapsibility [6, 7]. On the contrary, our results strongly suggest that interventions to displace the hyoid bone anteriorly and caudally should decrease the pharyngeal collapsibility and the frequency of obstructive events in people with OSA while interventions such as mandible advancement cranially displace the hyoid bone but decrease the pharyngeal collapsibility [38, 39].
In conclusion, both muscle paralysis and LV influence the hyoid bone position. Anterior and caudal displacement of the hyoid bone are considered to be linked to improvement of the pharyngeal airway patency and collapsibility particularly in people with OSA. Changes of the hyoid bone position should be interpreted in terms of the balances in neural and structural influences involved in pharyngeal airway maintenance.
Acknowledgments
We would like to thank Dr. Takeshi Sugawara, associate professor, Clinical Research Center, Chiba University Hospital, Chiba, for conducting accurate and reliable case registration and data management for this clinical study. Sara Shimizu (Shimizu Orthopedic Plastic Surgery Clinic) greatly helped us to improve this manuscript.
Work Performed: This work was conducted at Chiba University Hospital.
Funding
This study was supported by Japan Society for the Promotion of Science KAKENHI Grant number 15H04967.
References
- 1. Strohl KP, et al. Mechanical properties of the upper airway. Compr Physiol. 2012;2(3):1853–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17(1):32–42. [DOI] [PubMed] [Google Scholar]
- 3. Remmers JE, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931–938. [DOI] [PubMed] [Google Scholar]
- 4. Isono S, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol (1985). 1997;82(4):1319–1326. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson KA, et al. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108(2):375–381. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe T, et al. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(2):260–265. [DOI] [PubMed] [Google Scholar]
- 7. Genta PR, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep. 2014;37(10):1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts JL, et al. Pharyngeal airway-stabilizing function of sternohyoid and sternothyroid muscles in the rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(6):1790–1795. [DOI] [PubMed] [Google Scholar]
- 9. Van de Graaff WB, et al. Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(1):197–204. [DOI] [PubMed] [Google Scholar]
- 10. van Lunteren E, et al. Mechanical function of hyoid muscles during spontaneous breathing in cats. J Appl Physiol (1985). 1987;62(2):582–590. [DOI] [PubMed] [Google Scholar]
- 11. Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985). 1988;65:2124–2131. [DOI] [PubMed] [Google Scholar]
- 12. Hollowell DE, et al. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol (1985). 1991;71(6):2267–2273. [DOI] [PubMed] [Google Scholar]
- 13. Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol (1985). 1991;70(3):1328–1336. [DOI] [PubMed] [Google Scholar]
- 14. Mezzanotte WS, et al. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest. 1992;89(5):1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amatoury J, et al. Peripharyngeal tissue deformation and stress distributions in response to caudal tracheal displacement: pivotal influence of the hyoid bone?J Appl Physiol (1985). 2014;116(7):746–756. [DOI] [PubMed] [Google Scholar]
- 16. Amatoury J, et al. Development and validation of a computational finite element model of the rabbit upper airway: simulations of mandibular advancement and tracheal displacement. J Appl Physiol (1985). 2016;120(7):743–757. [DOI] [PubMed] [Google Scholar]
- 17. Tsuiki S, et al. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108(6):1009–1015. [DOI] [PubMed] [Google Scholar]
- 18. van der Weide L, et al. Analysis of carina position as surrogate marker for delivering phase-gated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71(4):1111–1117. [DOI] [PubMed] [Google Scholar]
- 19. Thut DC, et al. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol (1985). 1993;75(5):2084–2090. [DOI] [PubMed] [Google Scholar]
- 20. Hoffstein V, et al. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130(2):175–178. [DOI] [PubMed] [Google Scholar]
- 21. Stanchina ML, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26(7):851–856. [DOI] [PubMed] [Google Scholar]
- 22. Heinzer RC, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(1):114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tagaito Y, et al. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol (1985). 2007;103(4):1379–1385. [DOI] [PubMed] [Google Scholar]
- 24. Heinzer RC, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sériès F, et al. Influence of lung volume in sleep apnoea. Thorax. 1989;44(1):52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tangel DJ, et al. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol (1985). 1992;73(3):1058–1066. [DOI] [PubMed] [Google Scholar]
- 27. Carberry JC, et al. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep. 2016;39(3):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedenstierna G, et al. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology. 1985;62(3):247–254. [DOI] [PubMed] [Google Scholar]
- 29. Damia G, et al. Perioperative changes in functional residual capacity in morbidly obese patients. Br J Anaesth. 1988;60(5):574–578. [DOI] [PubMed] [Google Scholar]
- 30. Sivarajan M, et al. The position and the state of the larynx during general anesthesia and muscle paralysis. Anesthesiology. 1990;72(3):439–442. [DOI] [PubMed] [Google Scholar]
- 31. Lim JH, et al. Positional change of hyoid bone after anesthesia in anterior surgery of upper cervical spine. Spine J. 2014;14(9):1890–1894. [DOI] [PubMed] [Google Scholar]
- 32. Eisele DW, et al. The effects of selective nerve stimulation on upper airway airflow mechanics. Arch Otolaryngol Head Neck Surg. 1995;121(12):1361–1364. [DOI] [PubMed] [Google Scholar]
- 33. Kairaitis K, et al. Mass loading of the upper airway extraluminal tissue space in rabbits: effects on tissue pressure and pharyngeal airway lumen geometry. J Appl Physiol (1985). 2009;106(3):887–892. [DOI] [PubMed] [Google Scholar]
- 34. Yumino D, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121(14):1598–1605. [DOI] [PubMed] [Google Scholar]
- 35. Kairaitis K, et al. Mandibular advancement decreases pressures in the tissues surrounding the upper airway in rabbits. J Appl Physiol (1985). 2006;100(1):349–356. [DOI] [PubMed] [Google Scholar]
- 36. Kairaitis K, et al. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30(2):179–186. [DOI] [PubMed] [Google Scholar]
- 37. Malhotra A, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166(10):1388–1395. [DOI] [PubMed] [Google Scholar]
- 38. Susarla SM, et al. Upper airway length decreases after maxillomandibular advancement in patients with obstructive sleep apnea. J Oral Maxillofac Surg. 2011;69(11):2872–2878. [DOI] [PubMed] [Google Scholar]
- 39. Tsuiki S, et al. Effects of a titratable oral appliance on supine airway size in awake non-apneic individuals. Sleep. 2001;24(5):554–560. [DOI] [PubMed] [Google Scholar]