Abstract
Study Objectives
Provide actigraphic reference values for motor activity during sleep for children and adolescents ages 8–17 years.
Methods
Participants were 671 healthy community-dwelling children and adolescents (52% female, mean age 13.5 + 2.4 years) from the United States (64%) and Australia (36%). All participants wore an Ambulatory-Monitoring Inc. (AMI, Ardsley, NY) actigraph on their nondominant wrist for ≥5 nights and completed daily sleep diaries. Actigraphy data were scored with standard methods and a validated algorithm. Reference values were calculated for three outcome variables: percent sleep (sleep minutes/sleep period), mean activity count (average activity count over the sleep period), and restlessness measured by the activity index (% of epochs in sleep period > 0). Between-group differences were examined for sex and age group. In addition, changes to activity level across the sleep period were explored.
Results
All participants had a minimum of three scorable nights of data, with 95% having at least five scorable nights. Reference values are presented by age group and sex, and reference percentiles are provided. Boys were found to have more activity in sleep across the three outcome variables. Age differences were also found for the three outcomes, but a consistent pattern was not detected across variables.
Conclusions
This study is the first to examine motor activity from actigraphy in a large sample of healthy community-dwelling children and adolescents. Reference tables and percentiles, as well as sample actigrams highlighting different outcomes, are provided for clinicians and researchers who utilize actigraphy in pediatric populations.
Keywords: accelerometer, reference, pediatric, sleep, actigraphy
Statement of Significance
Actigraphy is commonly used in pediatric sleep, both by clinicians and researchers. However, there is limited information on motor activity during sleep for nonclinical children and adolescents as captured by actigraphy. This study provides reference values based on 671 community-dwelling youth and over 4300 nights of data that can be used in pediatric sleep clinics or research studies. As motor activity may be a sign of restless sleep, these reference values can help clinicians evaluate sleep quality in addition to sleep quantity. Similarly, reference values provide a comparison for researchers working with specific populations (e.g. chronic illness, developmental disorder).
Introduction
The use of actigraphy for research and clinical services has increased over the past 15 years [1–3]. One strength of actigraphy is the ability to noninvasively capture sleep–wake patterns for multiple nights in a natural sleeping environment (e.g. home), providing an estimate of sleep useful for clinicians and researchers. Studies have demonstrated that actigraphy has good sensitivity (ability to detect sleep), but poor-to-fair specificity (ability to detect wake after sleep onset) [1, 4]. Because actigraphy data are derived from an algorithm that determines sleep and wake through movement (motor activity), it is important to have a better understanding of the range of motor activity during sleep in a broad sample of healthy youth.
In adults, actigraphy has been found to underestimate wakefulness during the sleep period due to motionless wakefulness, which occurs when someone is awake, but lies still (no motor activity) for prolonged periods of time [2, 5]. Young children, on the other hand, are less likely to lie still while awake, resulting in an overestimate of wakefulness in clinical pediatric populations [6]. Less is known about motor activity during sleep for nonclinical samples of children and adolescents measured by actigraphy [7–9]. This gap is important for clinicians, who may find a patient’s sleep patterns (i.e. bedtime, wake time, sleep opportunity [reported bedtime to reported wake time]) are appropriate, but the child is still sleepy during the day. An increase in motor activity during sleep likely results in poorer quality sleep, which in turn can impact daytime functioning. Similarly, reference values from healthy youth are needed for researchers either working with a specific population (e.g. children with a chronic illness or developmental disorder) to understand whether sleep quality is truly atypical in their samples (e.g. do children and adolescents with asthma have increased motor activity compared to children and adolescents without asthma?), or desiring to know how many young people in their sample have motor activity that lies at the extremes (i.e. <5% or >95%).
Thus, the purpose of this analysis is to provide reference values from a large sample of healthy youth for motor activity during sleep as measured by actigraphy, with reference values and percentiles that can be utilized by both clinicians and researchers. Using a large sample of healthy, community-dwelling children and adolescents, we aimed to provide accessible reference values for accessible measures of sleep quality: percent sleep, mean activity during sleep, and the activity index. Because of known age and sex differences in motor activity during sleep [7–9], values are categorized into these demographic groups. Change in motor activity across the sleep period was also descriptively examined.
Methods
Subjects
Participants were drawn from multiple studies in the United States and Australia that focused on community-dwelling populations of children and adolescents, recruited through schools or the community (e.g. flyers, newspaper advertisements, see Table 1 for details) [10–12]. No participants were drawn from sleep clinics or other inpatient/outpatient medical or psychiatric settings.
Table 1.
Detailed information about parent studies from which participants were drawn
| Study name and location | Study aims | Recruitment | Participant ages (years) | Schedule | Season |
|---|---|---|---|---|---|
| Children’s Report of Sleep Patterns (CRSP) Validation (Philadelphia, PA and Denver, CO) | To determine the reliability and validity of the CRSP | Previous research participants, flyers, newspaper ads, cascading recruitment | 8 to 12a | Self | School |
| Intrinsic Period (Providence, RI area) | To measure intrinsic circadian period in normal adolescents | Flyers, newspaper ads | 9.6 to 17.8 | Fixed | Summer |
| Sleep Patterns (Pawucket, RI) | To assess the range of sleep patterns of middle school students | School | 11.0 to 13.0 | Self | School |
| Sleep and Development (Providence, RI area) | To examine the sleep patterns in children with and without a parental history of alcohol use | Flyers, newspaper ads, brochure mailings | 9.2 to 17.8 | Fixed | School |
| Food Choices (Providence, RI area) | To investigate circadian biology and eating patterns in normal weight and overweight adolescents | Flyers, newspaper ads, brochure mailings | 12.3 to 15.9 | Fixed | Summer |
| NSF Light Study (Providence, RI area) | To test the effect of targeted lighting systems on performance, mood, and circadian phase in healthy middle school students | Flyers, newspaper ads, list from Providence Schools | 10.5 to 14.7 | Self | Summer |
| Young Adolescent Sleep-Smart Pacesetter Program (YASS) (Worcester, MA) | To examine the efficacy of a preventative intervention for early adolescents: Sleep Smart Program | Letters to parents of 7th graders in fall health class and evening parent meetings at the schools | 11.0 to 15.0 | Self | School |
| The Prevalence and Cross- Cultural Comparison of Daytime Sleepiness in Adolescents (Adelaide, South Australia) | To estimate sleep and daytime functioning in Australian adolescents and compare them to adolescents in the United States. | Random sampling of students in eight schools in South Australia | 13.8 to 17.4 | Self | School |
aAge data were collected in whole numbers.
Each study was approved by the institutional review board or human research ethics committee at the institution where the research was conducted. Informed consent and assent were obtained for all participants. Table 1 provides detailed information about each study, including study aims, recruitment methods, study sleep schedule (self-selected vs. fixed), and season of study (school year vs. summer).
Actigraphy
All participants wore a Micro-Mini Motionlogger (4th generation, Ambulatory-Monitoring Inc. [AMI], Ardsley, NY) or Micro Sleep Watch (5th generation, AMI) device on their nondominant wrist for at least five nights and completed a daily sleep diary that asked about sleep patterns. For studies that included more than seven nights of data, only the first seven nights were included in analyses.
Actigraphy data were collected in 1-minute epochs using the Zero-Crossing Mode, and the Sadeh algorithm was used for scoring in Action-W 2 software (version 2.7.3045). The different generation of actigraphs are expected to have 100% agreement when using the Zero-Crossing Mode, as this is a measure of movement frequency (T. Kazlausky, personal communication, September 5, 2017). For this study, we focused on motor activity during actigraphically-estimated sleep rather than sleep schedules and sleep continuity. Thus, motor activity during sleep onset latency (the transition from wakefulness to sleep) was not examined.
Sleep diary data were used for data cleaning (e.g. artifact, device removal, atypical night, etc.) and for scoring actigraph records as follows: sleep onset was identified as the first of three consecutive minutes scored as sleep after diary reported bedtime; sleep offset was the last of five consecutive minutes of sleep before diary reported wake time [13]. The sleep period was defined as the interval from sleep onset to sleep offset.
Sleep variables derived from actigraphy data include: (1) percent sleep, the percent of minutes scored as sleep in the sleep period interval; (2) mean activity count, the average activity counts across the sleep period, including waking and sleeping; and (3) the activity index, a measure of “restlessness” calculated as the percent of sleep or wake epochs in the sleep period with >0 activity value.
Data analysis
Analyses were performed using SPSS 20.0 (Statistical Package for the Social Sciences [SPSS], Inc., Chicago, IL). Descriptive analyses (means, frequencies) were used to provide the reference data tables. To examine difference by age groups, participants were divided into the following groups: pre-adolescent = 8–10 years; early-adolescent = 11–13 years; mid-adolescent = 14–15 years; late-adolescent = 16–17 years [14, 15].
Mixed-effects analysis of variance (ANOVA) models were used to test differences in actigraphy parameters between males and females and across age categories, appropriately accounting for both within and between participant variance [16]. All models specified a random effect of participant ID. Models specified mean percent sleep, mean activity count, and the activity index as dependent variables, with fully saturated models (all main and interaction effects) for sex (male/female) and age category (pre-adolescent, early-adolescent, mid-adolescent, late-adolescent). Schedule (self-selected vs. fixed), school timing (school terms vs. school holidays) and sleep period were entered as covariates. Post hoc analyses with least significant differences were conducted to further investigate significant main and interaction effects.
For the exploratory description of motor activity across the sleep period, epoch-by-epoch data were extracted from Action-W Version 2 (AMI, Ardsley, NY), and recoded to provide an average activity count per each hour across the sleep period (i.e. first hour, second hour, etc.). This approach was selected over examining activity by clock hour (i.e. 08:00 pm, 09:00 pm) because sleep timing and duration varied across participants. Recoded data were then averaged across nights for individual participants (e.g. first hour average across seven nights, second hour average across seven nights). Activity during wake was an average of activity counts during all wake periods within the study period (e.g. 7 days).
Results
Actigraphy data from 671 children and adolescents were included in this study. Participants were from the United States (64%) and Australia (36%), were 52% female, and the mean age was 13.5 years (SD = 2.4, range 8.0 to 17.8 years). Racial data were not available for the Australia participants, but for the US participants 64% of the sample was white, 10% black/African American, 15% Hispanic/Latino, 3% Asian, 4% Multiracial, and 4% Other. Table 2 provides detailed information about the study sample for each of the parent studies. A total of 4373 nights were included for analysis. All participants had a minimum of three scorable nights of actigraphy, with 95% having at least five scorable nights. Mean sleep period for the full sample was 518.5 minutes (SD = 45.1). Controlling for age (which differed by study), study participants on fixed sleep schedules (n = 156) had a longer sleep period than those on self-selected sleep schedules (n = 515) (545.4 vs. 510.3 minutes), F (1,668) = 93.3, p < .001. Similarly, youth whose study was completed during school holidays (n = 99) had a longer sleep period than those studied during school terms (n = 572) (562.8 vs. 510.8 minutes), F (1,668) = 156.1, p < .001. Thus, these variables were controlled in subsequent analyses.
Table 2.
Study participants by parent study
| Study name | N | Mean age (years) (SD, range) | % Female | % White | % Black | % Hispanic/Latino | % Asian | % Multiracial | % Other |
|---|---|---|---|---|---|---|---|---|---|
| Children’s Report of Sleep Patterns (CRSP) Validation | 82 | 9.9 (1.4, 8–12)a | 52.4 | 79.3 | 7.3 | 9.8 | 2.4 | 1.2 | — |
| Intrinsic Period | 39 | 14.7 (2.4, 9.6–17.8) | 51.3 | 89.7 | — | — | — | 7.7 | 2.6 |
| Sleep Patterns | 45 | 12.1 (0.5, 11.0–13.0) | 66.7 | 31.1 | 20.0 | 22.2 | — | 2.2 | 24.4 |
| Sleep and Development | 74 | 13.1 (3.1, 9.2–17.8) | 45.9 | 71.6 | 10.8 | 6.8 | — | 9.5 | 1.4 |
| Food Choices | 43 | 13.7 (0.9, 12.3–15.9) | 44.2 | 69.8 | 9.3 | 14.0 | — | — | 7.0 |
| NSF Light Study | 17 | 12.4 (1.3, 10.5–14.7) | 64.7 | 47.1 | 35.3 | 17.6 | — | — | — |
| Young Adolescent Sleep- Smart Pacesetter Program (YASS) | 130 | 12.5 (0.6, 11.0–15.0) | 60.8 | 53.7 | 8.1 | 24.4 | 7.3 | 2.4 | 4.1 |
| The Prevalence and Cross- Cultural Comparison of Daytime Sleepiness in Adolescentsb | 241 | 15.6 (0.9, 13.8–17.4) | 46.9 | — | — | — | — | — | — |
aAge data were collected in whole numbers.
bRace data were not collected for the Australian study.
Mixed-effects ANOVA models found no interactions between sex and age group, but a significant main effect for all three outcome variables for sex and age group (Table 3). Post hoc analyses showed that boys had more activity during sleep and poorer quality sleep (with both lower percent sleep and a higher activity index) than girls. For age groups, post hoc analyses show that the older age groups had more activity during sleep, with higher average activity counts, compared to the younger age groups. Sleep quality showed a less consistent pattern, with the youngest and oldest age groups having better sleep quality with higher percent sleep than the middle two age groups. In terms of restlessness, early adolescents had a lower activity index than each of the other three age groups, suggesting less restlessness across the sleep period.
Table 3.
Mixed effects ANOVA models
| F-value | df | p-value | Post hoc | |
|---|---|---|---|---|
| Percent sleep | ||||
| Age | 8.14 | 3, 661.49 | <.001 | P>E,M; L>E |
| Sex | 20.75 | 1, 660.49 | <.001 | F>M |
| Age×Sex | .44 | 3, 661.49 | .72 | |
| Mean activity | ||||
| Age | 4.85 | 3, 662.01 | <.001 | P<M,L; E<M |
| Sex | 44.68 | 1, 660.92 | .002 | M>F |
| Age×Sex | 1.68 | 3, 662.01 | .17 | |
| Activity index | ||||
| Age | 12.10 | 3, 661.60 | <.001 | E<P,M,L |
| Sex | 37.86 | 1, 661.03 | <.001 | M>F |
| Age×Sex | 1.11 | 3, 661.60 | .35 |
P = Pre-adolescent (8–10 years); E = Early adolescent (11–13 years); M = Mid-adolescent (14–15 years); L = Late adolescent (16–17 years).
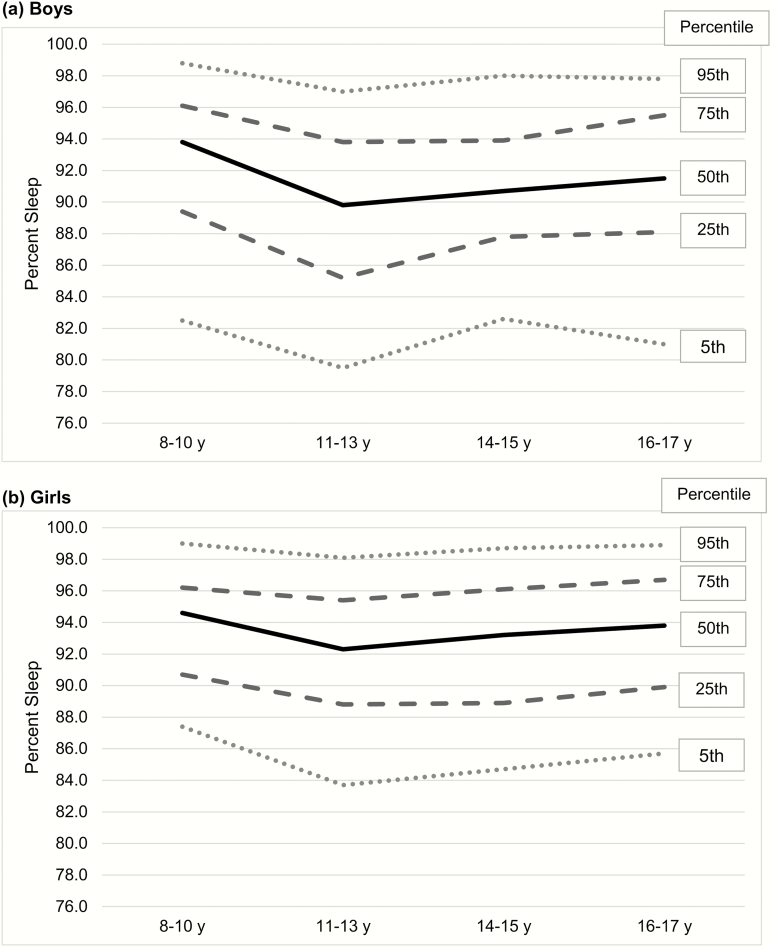
Although there was no interaction between age and sex, Table 4 provides reference values by age group and sex for the three main outcome variables (percent sleep, mean activity count, activity index). Figure 1 provides percentiles for percent sleep displayed by age group and sex.
Table 4.
Reference actigraphic values by age group and sex (mean ± SD [bold font] and range [minimum to maximum values])
| Pre-adolescent (8–10 years) | Early adolescent (11–13 years) | Mid-adolescent (14–15 years) | Late adolescent (16–17 years) | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| n | 50 | 50 | 100 | 145 | 113 | 93 | 59 | 61 |
| Age (years) | 9.7 ± 0.8 | 9.5 ± 1.0 | 12.5 ± 0.7 | 12.3 ± 0.7 | 15.0 ± 0.6 | 15.1 ± 0.5 | 16.7 ± 0.5 | 16.6 ± 0.5 |
| Percent sleep | 92.8 ± 4.4 | 93.8 ± 3.5 | 89.7 ± 5.2 | 91.9 ± 4.4 | 90.6 ± 4.6 | 92.5 ± 4.7 | 91.5 ± 4.9 | 93.2 ± 4.1 |
| Range | 81.3–98.9 | 84.8–99.7 | 76.5–98.4 | 78.7–99.5 | 77.4–98.9 | 76.5–99.5 | 78.6–98.5 | 83.0–99.5 |
| Mean activity count | 12.0 ± 3.3 | 11.0 ± 3.0 | 12.7 ± 3.2 | 11.0 ± 3.0 | 14.3 ± 4.0 | 11.5 ± 3.7 | 13.5 ± 4.3 | 11.4 ± 3.1 |
| Range | 4.3–18.3 | 4.3–16.6 | 6.0–20.1 | 4.4–20.2 | 5.9–26.3 | 5.6–22.5 | 6.1–23.7 | 5.7–19.6 |
| Activity index | 41.0 ± 12.0 | 36.7 ± 11.2 | 37.0 ± 9.6 | 31.9 ± 8.4 | 42.7 ± 11.4 | 35.3 ± 10.1 | 42.0 ± 9.9 | 38.4 ± 10.0 |
| Range | 14.8–70.4 | 14.5–62.6 | 17.8–60.7 | 14.4–55.4 | 19.2–73.6 | 14.5–62.7 | 21.1–60.8 | 17.1–59.4 |
Figure 1.
Percentiles for actigraphic percent sleep by age group and sex.
Hour-by-hour exploration of activity during sleep
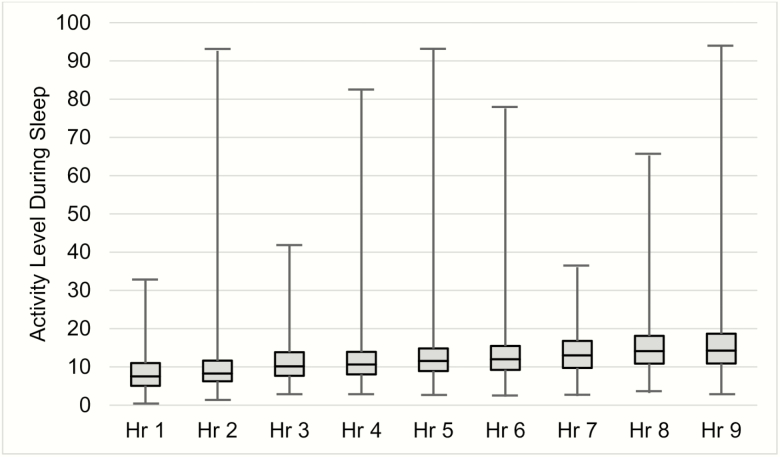
Mean activity during wake was 215.6 counts per minute (SD = 23.0, range 129.7 to 268.0). As shown in Figure 2, hourly mean activity count during the sleep period increased over the night, from an average activity count of 8.5 in hour 1 to 15.6 in hour 9. The range of hourly mean activity counts during the sleep period ranged from 0.4 counts per hour to 94 counts per hour. Although a repeated measures ANOVA found the increase of mean activity counts from hour to hour was statistically significant for the full sample, F(1,308) = 49.6, p < .001, the average increase between hours ranged from 0.1 to 1.9. This increase was consistent for all four age groups and both sexes (data not shown).
Figure 2.
Range of average activity levels for each hour of sleep.
Discussion
With 671 participants and over 4300 nights of data, this study provides reference values for motor activity during sleep as measured by actigraphy for children and adolescents, with separate reference values provided for both boys and girls, as well as youth in different age groups. These data can be used by pediatric sleep clinicians and researchers, allowing for the comparison of an individual patient’s activity during sleep (that may be a factor contributing to daytime sleepiness) or in clinical studies when it is important to determine if the measured activity levels may be due to a study sample’s characteristics (e.g. increased movement in youth with attention-deficit/hyperactivity disorder, increased movement in youth with atopic dermatitis due to scratching). There will also be inherent benefits for future studies comparing their samples against the reference values provided here, for instance, determining how many young people lie at the extremes (<5% and >95%), as has been done for body mass index (BMI) and IQ [17, 18].
Overall, this study found significantly lower activity in girls than boys, resulting in better sleep quality, as measured by both percent sleep and the activity index. Findings from this study are consistent with previous work examining sex-differences in sleep motor activity for both school-aged children and adolescents [7–9]. More research is needed to determine why school-aged boys experience more motor activity during sleep than girls, and whether these differences are linked to physiological or psychosocial changes during puberty [7–9].
By directly comparing sleep motor activity and sleep quality in the sleep period across age groups, this study provides a novel contribution to the literature. Previous studies have limited the ability to compare actigraphically-measured sleep efficiency across age groups due to differences in calculations (dividing time asleep by either time in bed or time in sleep period) [3]. One study found lower sleep efficiency in adolescents (13–18 years) compared to school-aged children (8–12 years) [6]; however that study had a smaller sample size and results were collapsed across age groups, making it difficult to compare the current results. In adolescents, a higher percent sleep may suggest more motionless wakefulness. With both school-aged children and late adolescents having the highest percent sleep in the current study, it is possible that this capacity to lie still while awake develops during the school-age period. Notably, age group difference between the average highest sleep percent (93.3%, pre-adolescents) and the lowest sleep percent (90.8%, early-adolescents) is only 2.5%. Thus, although a statistical difference was found, it is unlikely this difference is clinically meaningful.
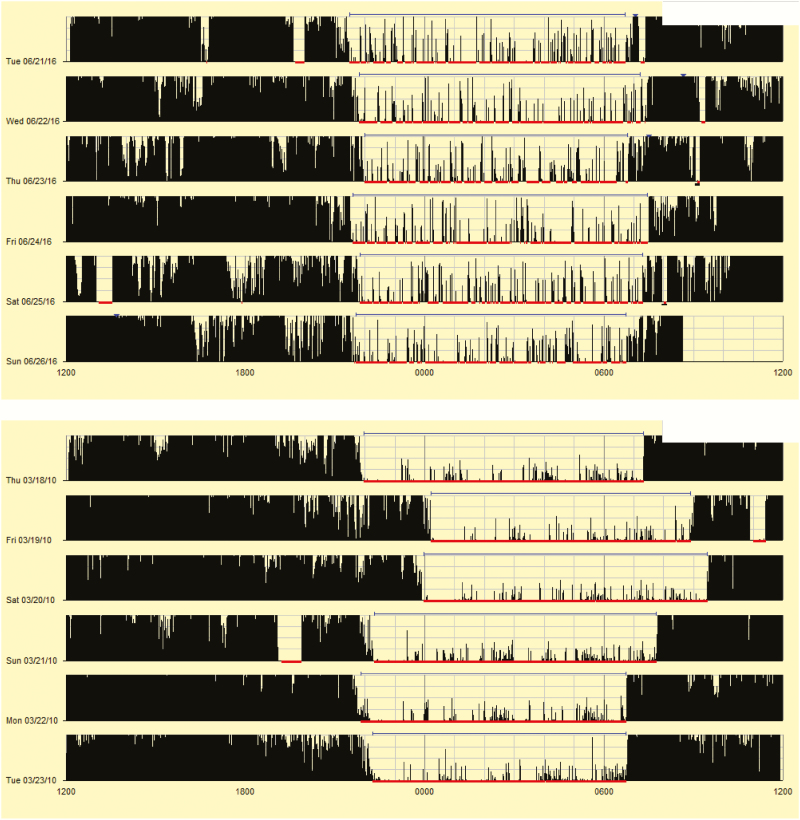
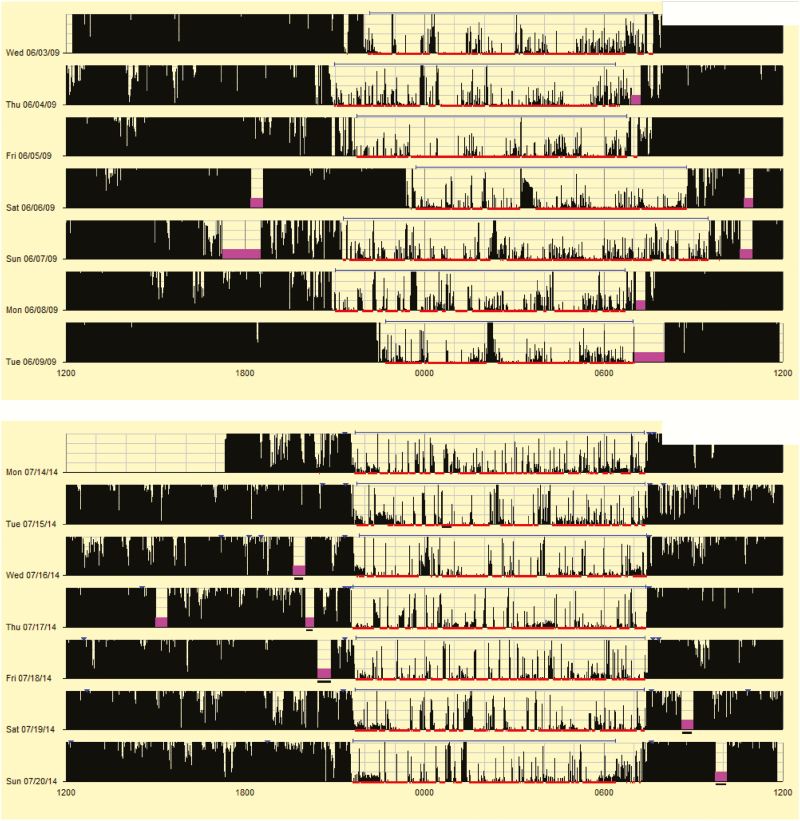
For readers who do not regularly use actigraphy, Figure 3 provides actigrams from two participants, one participant with low percent sleep (77.1%) and a different participant with high percent sleep (99.5%). While it is always important to extract the data for more precise reporting (see Appendix for more details), these pictures provide a “quick glance” at what clinicians and researchers may see when reviewing actigrams (the visual output from actigraphy studies), highlighting differences in motor activity during sleep. When comparing the percent sleep of each participant to the reference Figure 1, it quickly becomes apparent that the first participant (77.1% sleep) falls below the 5th percentile, suggesting very poor quality sleep, while the other participant (99.5% sleep) falls at the 95th percentile, suggesting very good quality sleep.
Figure 3.
Examples of actigrams with low and high percent sleep.
One surprising finding was that although early adolescents had the lowest percent sleep, this group also had the lowest activity index, suggesting the least restless sleep. This contrary result would suggest possible differences measures of “sleep quality.” For example, a youth with a low percent sleep could have more frequent night wakings, but when he/she is asleep, there is less restlessness as seen by a lower activity index. More research is needed to better understand sleep quality as measured by motor activity, as well as age-related changes in actigraphically-measured movement during sleep.
Finally, this study explored changes in sleep motor activity across the course of the sleep period, with small increases in activity occurring during the latter part of the sleep period. This may be a result of increased wakefulness and longer wake episodes that are likely to occur towards the end of the sleep period, resulting in higher activity counts. In addition, these results are consistent with previous studies that have shown PSG measured sleep motor activity varies according to sleep stage, with lighter stages of sleep having more activity than deeper sleep [19, 20].
The strengths of this study include the use of the largest sample of nonclinical children and adolescents reported thus far in the literature, and the examination of important outcomes variables for clinicians and researchers. One limitation of this study is the inclusion of only one type of actigraph device. Previous work has shown that sleep outcomes (i.e. total sleep time, wake after sleep onset, sleep efficiency) differ across different brands of actigraphs [21, 22].
It is also important to emphasize that the goal of this study was to examine motor activity during sleep, thus we did not focus on participants’ sleep timing or sleep schedules, thus no conclusions can be drawn about sleep opportunity (bedtime to wake time or time in bed) or sleep onset latency (time from reported bedtime to sleep onset). Further, without a measure of sleep opportunity, it is not possible to calculate sleep efficiency, which takes into account both sleep onset latency and wake after sleep onset. In general, however, sleep onset latency as measured by actigraphy is considered less reliable than other variables [1–3, 23], as it requires accurate input data from patients/research participants in the form of event markers or sleep diary data. As it is common, especially in clinical settings, for actigraphy studies to be completed without this complementary data, the findings from the current study have practical use for clinicians and researchers. Additionally, identifying when sleep onset begins for youth can be difficult as media use in bed may blur the line between going to bed and trying to fall asleep, especially for youth who use media to facilitate sleep onset [24, 25].
The use of different inclusion/exclusion criteria across studies (i.e. one about a third of participants were from studies that excluded for sleep disorders) meant that the reference sample may have included youth with genetic predispositions or disorders of movements during sleep (e.g. periodic limb movements of sleep). However, the inclusion of a broad sample of youth should only strengthen concerns about patients or research participants whose motor activity during sleep falls outside the reference values provided in this paper. Similarly, the use of only wrist actigraphy may underestimate discrete movement that occur in the legs or other muscles that may disrupt sleep quantity and quality. However, this limitation should again raise concerns for clinicians and researchers when a youth’s motor activity during sleep falls outside of the reference values.
Summary
In summary, this analysis provides reference values for motor activity during sleep as measured by actigraphy in children and adolescents aged 8 to 17 years.
These values can be used by clinicians to help identify potential sleep disturbances in children and adolescents, while researchers may find these values helpful to compare different populations of research participants. More research is needed to better understand sex and age-related differences in sleep motor activity, as well as factors that may increase or decrease motor activity during sleep.
Funding
This study was supported in part by National Institutes of Health (NIH) grants MH045945, MH076969, MH077662, HD47928, and HL119441; Australian Research Council grant DP0881261.
Conflict of interest statement. None declared.
Acknowledgments
The authors thank the children and adolescents who participated in these studies, Kay Orzech, Caroline Gredvig, Ashley Ernst, Hayley Dohnt, Anna Johnston, and Kassie Flewelling for their assistance with compiling data, and Ashley Peightal for her assistance with scoring.
Appendix—Application of Study Findings
The following information and examples are offered to help clinicians and researchers apply these findings to their own work and to further research questions. While this section is specific for the AMI devices, similar variables could be calculated with other brands of validated wrist-worn actigraphy devices.
Calculation of Variables
Data in this study were scored from sleep onset to sleep offset manually using the Custom interval. We would recommend this approach when the focus is primarily on the sleep period. However, if reported bedtime and reported wake time are set using the Down interval (based on sleep diary and/or event marker data), Action-W will score the sleep period automatically. When extracting percent sleep, activity mean, or activity index as reported in this paper, one should select these variables to appear on the statistics grid for the O-O interval.
Example Actigrams Highlighting Differences in Outcomes
For those not familiar with actigrams, Figures 3–5 present a picture summary of the actigraphy study. Each line represents a unique day (starting and ending at noon). Activity is represented by vertical black lines, with higher amplitude lines indicating a higher activity count for that epoch (minute). The thin red lines mark scored sleep, and gaps in the red line suggest wakefulness. The thin blue lines represent the sleep period (sleep onset to sleep offset).
Figure 3 presents two actigrams highlighting youth with a very low and very high percent sleep. In the top figure frequent high amplitude activity is seen, with multiple night wakings, resulting in a percent sleep of 77.1%. The bottom figure shows low amplitude activity during sleep and few night wakings, resulting in a percent sleep of 99.5%. As previously described, Figure 1 suggests the first participant has very poor quality sleep, while the second participant has very good quality sleep.
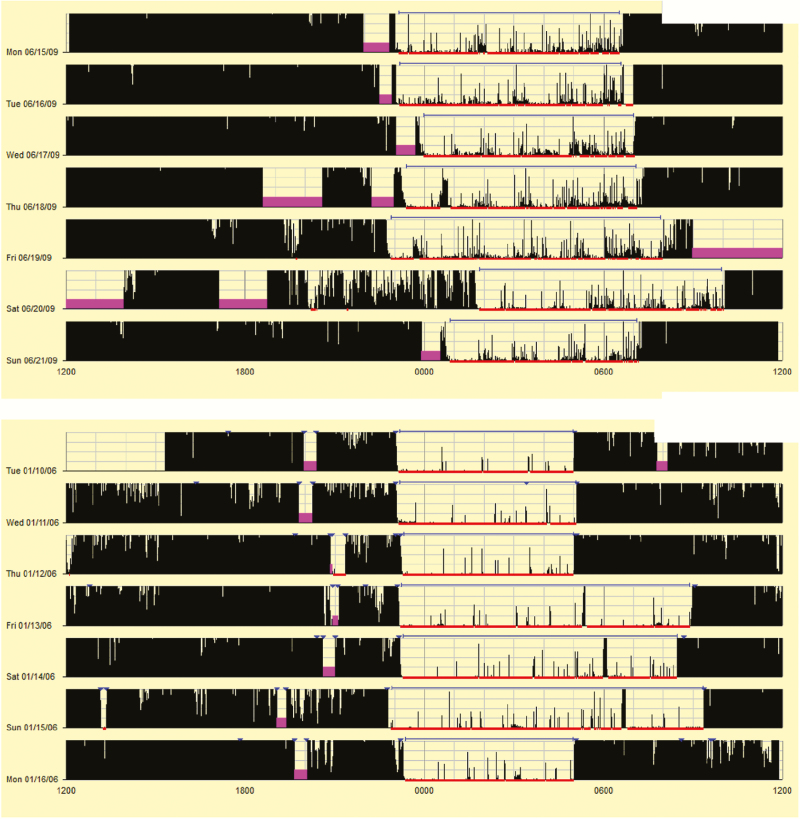
The activity index, a measure of restlessness, is seen in the two actigrams in Figure 4. The top figure highlights activity throughout the night, resulting in an activity index of 67.2. When compared to the reference values in Table 4 it is apparent that this is more than 2 SDs above the mean. It is important to note that much of the activity is low amplitude; however, that does not result in night wakings, resulting in a percent sleep of 89.8%. While frequent low amplitude activity could be a sign of artifact (e.g. child places hand on stomach during sleep providing regular movement), this is also seen in children with self- and parent-described “restless sleep,” thus should be considered in the overall clinical picture. The bottom figure shows a low activity index of 16.7 (more than 2 SDs below the mean according to Table 4), with few epochs that include any level of activity. High amplitude activity scored as a brief waking can be seen on each of the 3 days with oversleep (it was a long holiday weekend).
Finally, Figure 5 presents two actigrams with similar high mean activity counts (22.8 and 22.5), although different percent sleep. In the top figure, longer night wakings and prolonged periods of low-mid amplitude activity are noted, resulting in a percent sleep of 85.5%. In the bottom figure, epochs with high amplitude activity are more spaced out throughout the night, yet still frequent enough to result in night wakings that reduced percent sleep to 77.9%. Both studies suggest poor quality sleep, although more information would be needed to determine whether this activity is due to motor restlessness during sleep, or frequent night wakings that result in “tossing and turning.”
Figure 4.
Examples of actigrams with low and high values for the activity index.
Figure 5.
Examples of actigrams with similar high activity means for different reasons.
Data collection for this research project took place at Children’s Hospital of Philadelphia, National Jewish Health, Brown University, College of the Holy Cross, Rhode Island College, and Flinders University. Research analyses took place at National Jewish Health.
References
- 1. Meltzer LJ, et al. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. [DOI] [PubMed] [Google Scholar]
- 3. Galland BC, et al. Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep. 2018. [DOI] [PubMed] [Google Scholar]
- 4. Morgenthaler T, et al. ; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. [DOI] [PubMed] [Google Scholar]
- 5. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 6. Meltzer LJ, et al. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35(1):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadeh A, et al. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. [DOI] [PubMed] [Google Scholar]
- 8. Gaina A, et al. Gender and temporal differences in sleep-wake patterns in Japanese schoolchildren. Sleep. 2005;28(3):337–342. [PubMed] [Google Scholar]
- 9. Johnson NL, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep. 2007;30(7):899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Short MA, et al. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13(4):378–384. [DOI] [PubMed] [Google Scholar]
- 11. Marco CA, et al. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 2011;10(1): 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meltzer LJ, et al. The children’s report of sleep patterns (CRSP): a self-report measure of sleep for school-aged children. J Clin Sleep Med. 2013;9(3):235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meltzer LJ, et al. A comparison of actigraphy scoring rules used in pediatric research. Sleep Med. 2011;12(8):793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu T, et al. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 2002;110(4):752–757. [DOI] [PubMed] [Google Scholar]
- 15. Karpati AM, et al. Stature and pubertal stage assessment in American boys: the 1988-1994 Third National Health and Nutrition Examination Survey. J Adolesc Health. 2002;30(3):205–212. [DOI] [PubMed] [Google Scholar]
- 16. Van Dongen HP, et al. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol. 2004;384:139–171. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Use and Interpretation of the WHO and CDC Growth Charts for Children From Birth to 20 Years in the United States. 2015. [Google Scholar]
- 18. Sattler J. Assessment of Children: Cognitive Foundations. 5th ed. La Mesa, CA: Jerome M. Sattler, Publisher, Inc; 2008. [Google Scholar]
- 19. Horne JA, et al. Patterns of spontaneous and evoked body movements during sleep. Sleep. 1995;18(3):209–211. [DOI] [PubMed] [Google Scholar]
- 20. Liefting B, et al. Electromyographic activity and sleep states in infants. Sleep. 1994;17(8):718–722. [PubMed] [Google Scholar]
- 21. Meltzer LJ, et al. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep. 2015;38(8):1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LeBourgeois MK, et al. Comparing estimates of adolescent sleep and wake from two activity monitoring systems. Sleep. 2002;25:A273–A274. [Google Scholar]
- 23. Ancoli-Israel S, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13 (Suppl 1):S4–S38. [DOI] [PubMed] [Google Scholar]
- 24. Exelmans L, et al. Technology and sleep: how electronic media exposure has impacted core concepts of sleep medicine. Behav Sleep Med. 2015;13(6):439–441. [DOI] [PubMed] [Google Scholar]
- 25. Eggermont S, et al. Nodding off or switching off? The use of popular media as a sleep aid in secondary-school children. J Paediatr Child Health. 2006;42(7-8):428–433. [DOI] [PubMed] [Google Scholar]