Abstract
Hepatitis C virus infection is associated with increased morbidity and mortality. It remains a major challenge for management and treatment, especially in patients with renal transplant. The new direct-acting antiviral agents gave big hopes to both clinicians and patients that they can overcome this challenge without major side effects. Studies recently have supported this claim; however, they are still few, limited, and may give false hopes. In the following case report, we present a case, supported by histological evidence about a possible acute rejection of kidney transplant after treatment with these new medications. This case is limited by the absence of donor-specific antibodies. This report is aimed to increase awareness about the urgent need for further studies.
Key words: Acute rejection, anti-hepatitis C virus medication, daclatasvir, direct-acting antiviral agents, kidney transplant, sofosbuvir
INTRODUCTION
Hepatitis C virus (HCV) infection is prevalent in renal allograft recipient and associated with increased morbidity and mortality. The new direct-acting antiviral agents (DAAs) are highly effective in clearing the virus and considered safe for use in kidney transplant (KT) patients; the early studies did not report acute graft rejection after their use. Here, we are reporting a case of biopsy-proven acute graft rejection after the use of DAAs.
CASE REPORT
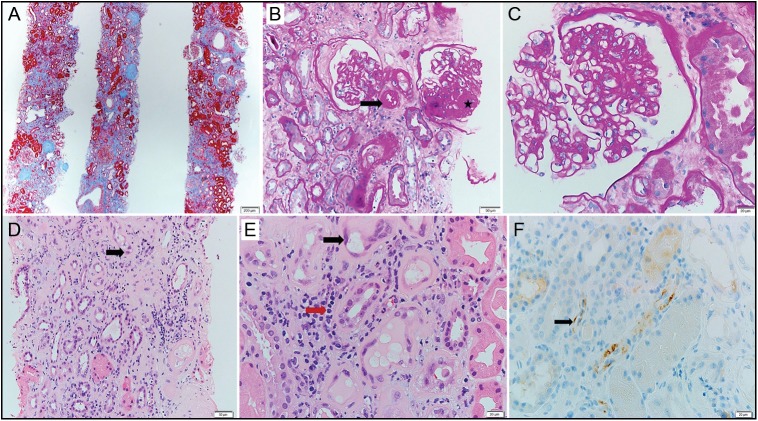
It describes the patient: Male, 47 years old, had a living unrelated kidney transplant outside UAE. Before his transplant, he acquired HCV infection. In 2009, he was found to have 12 million copies/ml of HCV (genotype one) with a deranged liver function test. In 2015, the patient showed F3 fibrosis on FibroScan. However, due to the risk of graft rejection, he was not started on intraneural facilitation therapy and kept on low immunosuppressive treatment consisting of cyclosporine (CYC) 75/50 mg daily, mycophenolic acid 180 mg twice daily, and prednisolone 5 mg daily. Serum creatinine (Cr) level was maintained mostly around 1.4–1.5 mg/dL. Recently, DAAs became available, and he was started on daclatasvir (DAC) 60 mg daily with sofosbuvir (SOF) 400 mg daily. About 3 months after initiating anti-HCV treatment, his Cr raised to 2.4 mg/dL and kept rising steadily reaching 4.78 mg/dL. During the course of DAA treatment, CYC trough level was kept always therapeutic (140–180 ng/mL), and a kidney biopsy was done almost 3 month after starting DAAs (10 years and 6 months posttransplant) and revealed features in keeping with acute/active antibody-mediated rejection (AMR). The biopsy showed diffuse glomerulosclerosis consistent with advanced diabetic neohropathy, acute tubual necrosis, severe interstitial fibrosis and tubular atrophy, and borderline changes suspicious for acute T-cell-mediated rejection [Figure 1] - Banff scores (t1, i2, g1, ci3, ct3, v0, cv2, ah3, ptc2, mm3, and C4d1), there was no evidence of chronic AMR, and CMV and adenovirus tests were negative. As mentioned above, the Findings were suggestive of an acute AMR; however,we could not confirm it by doing donor-specific antibodies (DSAs) in serum due to the unavailability of the tissue typing of the donor, hence, the possibility of advanced diabetic nephropathy and severe interstitial fibrosis couldn’t be completely eliminated Banff scores (t1, i2, g1, ci3, ct3, v0, cv2, ah3, ptc2, mm3, and C4d1). There is no evidence of chronic AMR, CMV, and adenovirus negative. Findings are suggestive of acute AMR; however, we could not confirm it by doing donor-specific antibodies (DSAs) in serum as we do not have the tissue typing of the donor. AMR as well as features of advanced diabetic nephropathy and severe interstitial fibrosis and tubular atrophy. He received methylprednisolone intravenous pulses, and CYC was replaced by tacrolimus together with increasing the dose of mycophenolic acid. The AMR responded partially with the gradual improvement of his Cr [Figure 2].
Figure 1.
(a) Low-power view of renal biopsy reveals advanced chronic injury (Masson’s trichrome stain, ×4). (b) One glomerulus displays ischemic changes in the form of thickening and wrinkling of glomerular capillary basement membranes and another (star) displays focal segmental glomerular sclerosis. Severe arteriolar hyaline (arrow) is evident (periodic acid–Schiff stain, ×20). (c) Mild-to-moderate mesangial matrix expansion without significant hypercellularity (periodic acid–Schiff stain, ×40). (d) Acute tubular injury and multifocal nuclear regenerative atypia (arrow) are identified, and polyomavirus, cytomegalovirus, and adenovirus immunostains were negative (H and E, ×20). (e) Prominent peritubular capillary margination by inflammatory cells (red arrow, H and E, ×40). (f) Peritubular capillary stain positive using C4d immunostain (black arrow, ×40)
Figure 2.
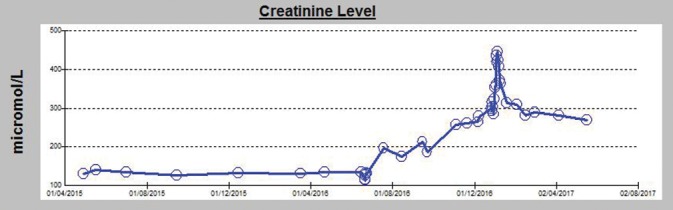
Creatinine level changes
DISCUSSION
It is well known that HCV infection in renal transplant patients significantly affects the overall survival and graft survival.[1] Interferon therapy, which used to be the backbone of HCV infection treatment, is relatively contraindicated in renal transplant patients due to the high risk of acute transplant rejection.[2,3] Recently, the introduction of DAAs made it possible to treat HCV infection in solid organ recipients effectively and safely. The early published data did not report acute transplant rejection in patients with DAAs. DAC and SOF, which were used in our patient, are approved for genotypes one to three. Many studies and reports showed that they are effective and safe when they are used in solid organ recipients. DAC is a NS5A inhibitor that does not require dose adjustments with CYC and tacrolimus coadministration.[4] Schrezenmeier etal.[5] studied the pharmacokinetics of DAC and SOF in a prospective cohort of 16 HCV-positive KT recipients; all had estimated glomerular filtration rate (GFR) >30 ml/min. They found that the administration of DAC/SOF is safe and highly efficient in KTR. There was no dose accumulation of DAC and SOF with GFR between 30 and 60 ml/min).[5]
Kamar et al.[6] reported that a significant decrease in calcineurin inhibitor levels was observed after HCV clearance from 25 KT recipients who received SOF-based therapy. Xue etal.[7] published the results of using DAC/SOF to treat six consecutive Chinese KT patients. They found them highly efficient and safe.[7] Another abstract suggested that the calcineurin levels should be carefully monitored for patients on tacrolimus as it increases or decreases significantly after the initiation of SOF requiring dose adjustments.[8] A recently published meta-analysis evaluating the efficacy and safety of SOF-based therapies for hepatitis C in liver transplant (LT) recipients (22 studies/1730 recipients) found that SOF-based therapy is an effective and well-tolerated treatment strategy for patients with HCV infection recurrence after liver transplantation (Qu Y., 2017). The safety and efficacy of this regimen were reported initially in one case report of a LT patient.[9] Saxena etal.[10] found that in HCV-TARGET study, using DAAs (mainly SOF-based regimens) to treat HCV in transplant recipients (347 LT recipients, 60 KT recipients, and 36 dual liver-kidney transplant recipients) is safe and effective. However, they reported two cases of kidney rejection during treatment (one on SOF + LDV and the other on LDV + SOF + ribavirin [RBV]).[10] Another case published in 2015 reported HCV eradication in a KT patient using SOF and simeprevir with an excellent safety profile.[11] Another French study published recently looked at 333 patients with HCV genotype 3. It lasted for 24 weeks and involved patients with recurrence of HCV infection post-LT and those with an indication for renal or LT. The study concluded that the combination of SOF/DAC with or without RBV has an excellent safety profile in regard to kidney function test.[12] However, taking in consideration, the lack of trials, and previous reports of well-tolerated response to the new anti-HCV meds in transplant patients, our case would be the first of kind to report acute rejection after their use. Another case that we came across in our hospital reacted in the same way to the combination of SOF and DAC, but unfortunately, there was no biopsy taken, and hence, we reported only our case that is supported by histological evidence. In 2015, an abstract presented in the journal of the American Society of Nephrology described the occurrence of antineutrophil cytoplasmic antibody-associated rapid progressive glomerulonephritis, after initiation of SOF with RBV.[13]
CONCLUSION
in our patient, the use of DAAs was associated with what seems to be an acute AMR. However, despite the availability of a histological evidence, the possibility of a chronic rejection still stands in the light of absent DSAs which was not available in our report. This report aims at raising the need to be more cautious when using these medications in KT patients. More studies are needed to establish the link between this treatment and graft rejection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18:97–115. doi: 10.1002/rmv.565. [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2008;73:S1–99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 3.Wéclawiack H, Kamar N, Mehrenberger M, Guilbeau-Frugier C, Modesto A, Izopet J, et al. Alpha-interferon therapy for chronic hepatitis C may induce acute allograft rejection in kidney transplant patients with failed allografts. Nephrol Dial Transplant. 2008;23:1043–7. doi: 10.1093/ndt/gfm678. [DOI] [PubMed] [Google Scholar]
- 4.Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idiosyncratic hepatotoxicity in the liver transplant setting. J Hepatol. 2014;60:872–84. doi: 10.1016/j.jhep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrezenmeier E, Galander P, Hoffmann F, Jaeger C, Lisec J, Schrezenmeier J, et al. Pharmakokinetics of daclatasvir, sofosbuvir and GS-331007 in a prospective cohort of HCV positive kidney transplant recipients. Am J Transplant. 2017;17(Suppl 3) doi: 10.1097/FTD.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 6.Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, et al. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant. 2016;16:1474–9. doi: 10.1111/ajt.13518. [DOI] [PubMed] [Google Scholar]
- 7.Xue Y, Zhang LX, Wang L, Li T, Qu YD, Liu F, et al. Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection. World J Gastroenterol. 2017;23:5969–76. doi: 10.3748/wjg.v23.i32.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn KS, Martin MT, Tang IY. Effectiveness of simeprevir and sofosbuvir in the treatment of hepatitis C virus in genotype 1 post-kidney transplant recipients. American Society of Nephrology. 2017 [Google Scholar]
- 9.Qu Y, Guo Y, Li T, Ye Q, Sun C, Wang L, et al. Efficacy and safety of sofosbuvir-based interferon-free therapies for hepatitis C in liver transplant recipients. Journal of gastroenterology and hepatology. 2017;32:740–8. doi: 10.1111/jgh.13614. [DOI] [PubMed] [Google Scholar]
- 10.Saxena V, Koraishy FM, Sise M, Lim JK, Chung RT, Liapakis A, et al. LP08: Safety and efficacy of sofosbuvir-containing regimens in hepatitis C infected patients with reduced renal function: Real-world experience from HCV-target. J Hepatol. 2015;62:S263–864. [Google Scholar]
- 11.Bonacci M, Londoño MC, Esforzado N, Forns X, Sotoca JM, Campistol JM, et al. Antiviral treatment with sofosbuvir and simeprevir in a kidney transplant recipient with HCV-decompensated cirrhosis: Viral eradication and removal from the liver transplant waiting list. Transpl Int. 2015;28:1345–9. doi: 10.1111/tri.12622. [DOI] [PubMed] [Google Scholar]
- 12.Hézode C, Lebray P, De Ledinghen V, Zoulim F, Di Martino V, Boyer N, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a french early access programme. Liver Int. 2017;37:1314–24. doi: 10.1111/liv.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadde S. Antineutrophil cytoplasmic antibody crescentic allograft. World J Nephrol. 2016;5:547–50. doi: 10.5527/wjn.v5.i6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]