Abstract.
Central nervous system (CNS) strongyloidiasis is a known but rare form of disseminated infection. The diagnosis is often made postmortem, with only five published cases of an antemortem diagnosis. We report two fatal cases of CNS strongyloidiasis diagnosed antemortem, with Strongyloides stercoralis larvae visualized in the CNS sample in one case. Risk factors for disseminated strongyloidiasis common to both cases included origination from the Caribbean, underlying human T-lymphotropic virus-1 infection, and recent prednisone use. Both cases occurred in Canada, where the occurrence of Strongyloides is uncommon, and serve as a reminder to maintain a high index of suspicion in patients with epidemiologic or clinical risk factors for dissemination.
INTRODUCTION
Strongyloides stercoralis, a nematode prevalent in tropical and subtropical regions, is thought to infect 30–100 million people worldwide.1 Although most people with strongyloidiasis remain asymptomatic, severe and fatal clinical presentations such as hyperinfection or disseminated disease can occur.2 Strongyloides stercoralis possesses the unique ability to autoinfect and multiply within the host by penetrating the intestinal mucosa or perianal skin to regain entry into the circulatory system, leading to an enormous worm burden and lifelong persistence, if untreated.1,2 Hyperinfection refers to accelerated autoinfection with increased gastrointestinal and pulmonary symptoms.2,3 Disseminated infection refers to migration of larvae outside of the gastrointestinal-pulmonary autoinfective cycle.2,3 Impaired cellular immunity is a major risk factor for dissemination, especially due to immunosuppressive therapy (such as corticosteroids or posttransplant medications), hematologic malignancies, or human T-lymphotropic virus-1 (HTLV-1) infection.2,4,5
Central nervous system (CNS) strongyloidiasis is a known but rare form of disseminated infection, first described in 1973 as a postmortem diagnosis.6 Since then, five published cases of antemortem diagnosis of CNS strongyloidiasis have been reported,5,7–10 with other cases diagnosed postmortem. We report two cases of CNS strongyloidiasis diagnosed antemortem, with S. stercoralis larvae visualized in the CNS sample in one case. Both cases occurred in Canada, where the occurrence of Strongyloides is uncommon, and serve as a reminder to maintain a high index of suspicion in patients with epidemiologic or clinical risk factors for dissemination.
CASE 1
A 67-year-old Trinidadian male presented to the hospital in August 2014 with a 1-day history of headache, confusion, drowsiness, and seizure, following a period of anorexia, nausea, vomiting, abdominal pain, diarrhea, and weight loss. His background health history included rheumatoid arthritis, systemic lupus erythematosus, benign prostatic hyperplasia, previous prostate cancer, and bilateral knee replacement. For his rheumatologic conditions, he had been recently treated with methotrexate and various doses of prednisone, and had previously also been treated with leflunamide and hydroxychloroquine. On initial presentation to the emergency department, he had an elevated temperature and was suspected to have pneumonia.
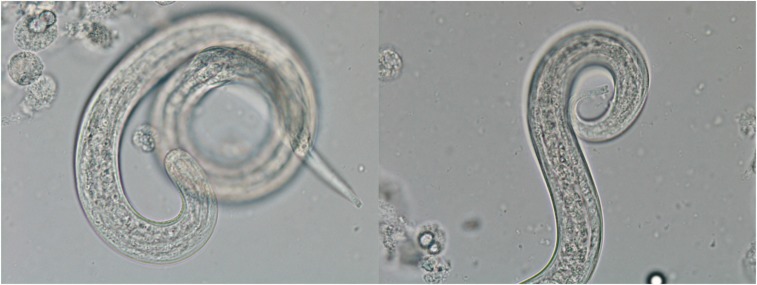
After a series of nondiagnostic investigations during his early hospital stay, he developed worsening confusion and decreasing level of consciousness. Computed tomography (CT) of the abdomen revealed a nonspecific colitis. A lumbar puncture revealed the cerebrospinal fluid (CSF) to have a white blood cell (WBC) count of 22 × 106/L (91% lymphocytes, 2% neutrophils, and 7% monocytes), protein 0.46 g/L, and glucose 8.6 mmol/L. The cerebrospinal fluid culture was positive for Escherichia coli (E. coli), which prompted specific testing for S. stercoralis. Strongyloides stercoralis filariform larvae were found in this initial CSF sample (Figure 1) and also in stool and sputum examinations. Strongyloides serology was positive. Subsequent serology was positive for HTLV-1 and negative for human immunodeficiency virus. Magnetic resonance imaging (MRI) revealed scattered nonspecific white matter changes consistent with microangiopathy.
Figure 1.
Strongyloides stercoralis filariform larvae in the cerebrospinal fluid of the patient in Case 1. Photos courtesy of the Public Health Ontario Laboratory. This figure appears in color at www.ajtmh.org.
He was treated with albendazole (GlaxoSmithKline) 400 mg orally twice daily and ivermectin (Merck & Co., Inc.) 200 mcg/kg subcutaneously daily for disseminated Strongyloides infection, as well as antibiotics for E. coli meningitis. Initially, he did not show any signs of neurologic improvement. Repeat CT and MRI brain imaging showed leptomeningeal enhancement of both cerebral convexities consistent with ongoing meningitis, as well as new subdural collections along the right cerebral convexity and cortical infarcts.
He suffered severe neurological impairment resulting in over 1.5 years in hospital. His course was complicated by thrombosis of his cephalic veins bilaterally, upper gastrointestinal bleed with stress ulcer, Clostridium difficile infection, and recurrent bacteremia with several different organisms. He received intermittent treatment courses with oral ivermectin with no recurrence. Ultimately, the patient passed away in April 2016 with no autopsy performed.
CASE 2
A 55-year-old previously healthy Jamaican man presented to the hospital in August 2016 with 1 month of abdominal pain with intermittent fever. He had multiple emergency department visits with no clear diagnosis despite investigations including abdominal X-ray, abdominal ultrasound, CT abdomen, and chest X-ray. He did not have any skin findings. He had immigrated to Canada in 1981 with no other relevant travel history.
His white blood cell count 1 month before admission was 16.9 × 109/L with 0.46 × 109/L eosinophils (normal range 0–0.8 × 109/L). During the present admission, eosinophils were 0, but WBC had increased to 42.6 × 109/L (37.49 × 109/L neutrophils). A repeat chest X-ray was normal. On August 4, a colonoscopy suggested possible Crohn’s disease with focal crypt architectural distortion and inflamed granulation tissue; he was started on prednisone, ciprofloxacin, and metronidazole on August 10. On August 13, he suddenly developed decreased level of consciousness and hypoxia. Repeat chest X-ray showed bilateral pulmonary infiltrates. Blood cultures were positive for Streptococcus oralis and coagulase-negative Staphylococcus. Sputum and stool parasite examinations showed many larvae suspicious for S. stercoralis. Bronchoalveolar lavage samples were positive for Strongyloides filariform larvae, whereas stool samples were positive for Strongyloides rhabditiform larvae. Cerebrospinal fluid showed WBC 180 × 106/L (50% polymorphonuclear leukocytes and 0% eosinophils), red blood cell (RBC) 10,220 × 106/L, protein 0.32 g/L, and glucose 7.6 mmol/L. Bacterial cultures were negative and no parasites were seen on CSF examination. Computed tomography head suggested hypoxic ischemic encephalopathy, but MRI was normal. Strongyloides serology was reactive, with an optical density of 0.5 (reactive: > 0.3). He was also found to be positive for HTLV-1.
Given that CSF cultures were negative for bacteria and that both the protein and glucose were within normal ranges, the neurological state was best explained by either deep delirium secondary to medical illness or direct CNS involvement from strongyloidiasis in the setting of proven disseminated Strongyloides infection. The CSF findings were not supportive of a bacterial CNS infection and there were no other likely explanations for the neurological state.
Parenteral ivermectin, 12 mg (200 mcg/kg) subcutaneously, was given daily for 2 weeks, then increased to 15 mg because of the lack of response and persistent Strongyloides filariform larvae in sputum. Ivermectin drug levels at this dose were 154 ng/mL. Also, he received albendazole 400 mg twice daily for 1 month.
Although his stool and sputum eventually cleared of Strongyloides larvae (by August 31 and September 6, respectively), he did not have any neurological improvement and care was withdrawn. He died at the end of September 2016, 7 weeks after admission. No autopsy was performed.
DISCUSSION
The two cases of CNS strongyloidiasis highlight the clinical presentation of an uncommon but devastating form of disseminated strongyloidiasis. The mechanism of CNS strongyloidiasis has been hypothesized to occur by 1) direct entry of migrating larvae through the arterial circulation, 2) a reaction to substances produced by dead or dying larvae, or 3) immune complex deposition in the meninges.8 The latter two mechanisms may explain why it is rare to find larvae in the CSF.
In both cases, epidemiological and clinical risk factors for disseminated strongyloidiasis were present. Regions with the highest risk for Strongyloides exposure include Southeast Asia, Oceania, sub-Saharan Africa, South America, and the Caribbean.2 Both our patients were originally from the Caribbean (Trinidad and Jamaica). Although both had already spent decades in Canada, the unique ability of Strongyloides spp. to autoinfect means that strongyloidiasis can persist lifelong if not treated.1,2
Key clinical risk factors for dissemination include corticosteroid therapy (equivalent to 20 mg/day of prednisone for ≥ 2 weeks), other immunomodulatory agents (including agents used in the management of solid-organ transplants and other agents affecting cellular immunity), hematologic malignancies, and HTLV-1 infection.2,4,5 It has been shown that impairment in cell-mediated immunity leads to a lack of granulomatous immune reaction to Strongyloides larvae in tissues.11 Both our patients had HTLV-1 infection and both received prednisone in immunosuppressant doses. Case 1 received a prolonged course of prednisone, whereas case 2 received prednisone only 4 days before presenting with disseminated disease.
The relationship between HTLV-1 and Strongyloides infection appears to be bidirectionally influential. That is, strongyloidiasis appears to accelerate the disease course of HTLV-1 to become T-cell leukemia, possibly due to increased levels of CD4+ CD25+ T cells in coinfected patients yielding increased circulating proviral DNA. Concurrently, HTLV-1 infection appears to render patients more tolerant to Strongyloides infection by biasing the immune system toward the Th1 system, thereby producing more gamma interferon and less polyclonal and parasite-specific immunoglobulin E.3,12
Corticosteroids are implicated in most cases of hyperinfection and dissemination of Strongyloides infection, with reports of low-dose steroids, high-dose steroids, locally injected steroids, and adrenocorticotropin being associated with hyperinfection.3 This may be due to the acute suppression of eosinophilia and lymphocyte activation by corticosteroids, and/or possibly direct effects of corticosteroids on the parasite by either accelerating the rhabditiform to infective filariform larvae transformation or increasing the reproduction of adult females.3
Five previously published cases of antemortem diagnosed CNS strongyloidiasis with confirmed larvae found in the CSF are outlined in Table 1, along with Case 1 of this paper. All previous reports were from the United States. Of note, similar to our patients, most cases did not have peripheral eosinophilia during disseminated Strongyloides, although there was often eosinophilia before the dissemination stage. The most common presenting symptoms were meningeal signs, usually with a neutrophilic rather than eosinophilic CSF profile; this may be because of the fact that disseminated strongyloidiasis is often complicated by concomitant spread of enteric organisms during the autoinfective cycle when filariform larvae penetrate the intestinal mucosa.4,8 Only one patient survived. All previous cases in the literature were predisposed to disseminated strongyloidiasis by epidemiological and clinical risk factors.
Table 1.
Published cases of Strongyloides stercoralis disseminated infection involving the CNS diagnosed antemortem with the presence of Strongyloides larvae in CSF
| Authors and year of publication | Year of case | Age/gender | Risk factors for disseminated strongyloidiasis | Clinical presentation | Relevant investigations | Antiparasitic treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Bradley et al.5 | 1977 | 30/M | Lymphoma, chemotherapy, prednisone | Shortness of breath 1 month after diagnosis of lymphoma, and chemotherapy and prednisone initiation, shortly followed by intestinal obstruction and progressive unresponsiveness and meningismus | White blood cell count: 36.8 × 109/L with 1% eosinophils | Thiabendazole 1.5 g NG | Death |
| Born in West Indies; other travel history unknown | |||||||
| CXR: extensive bilateral infiltrates | |||||||
| Bronchoalveolar lavage: S. stercoralis larval forms | |||||||
| cerebrospinal fluid: S. stercoralis filariform larvae, Gram-negative bacilli | |||||||
| Meltzer et al.7 | 1977 | 56/F | Prednisone (up to 60 mg/day) for ITP for 9 months before presentation | Abdominal distension, fever, headache 1 week after splenectomy for ITP and 9 months of prednisone, followed by worsening mental statusHad eosinophilia with abdominal cramping of unknown etiology for 7 years prior | White blood cell count: 15.8 × 109/L (eosinophilia % on admission not reported, but was 12% previous year) | Thiabendazole 25 mg/kg BID × 8 days + levamisole 150 mg on days 1, 2, 7, 8 and 14 | Death (septic shock from Klebsiella pneumoniae on DOA 56) |
| Born in Southern Italy and lived there until age 6, then visited 6 months before presentation; also lived in Florida for 10 years | |||||||
| CXR: extensive bilateral infiltrates | |||||||
| Blood: Escherichia coli | |||||||
| Urine: E. coli and Pseudomonas aeruginosa | |||||||
| Sputum, stool, CSF: S. stercoralis filariform larvae | |||||||
| Cerebrospinal fluid also grew Pseudomonas aeruginosa, Enterococcus faecium, and Candida albicans at various times during admission | |||||||
| Belani et al.8 | 1985 | 46/F | Prednisone (5 mg BID) for presumed asthma | Headache, stiff neck, nausea, vomiting, fever, vague abdominal pain; eventually became more confused with generalized weakness and bilateral extremity pain, wheezingHad two episodes of neutrophilic meningitis in 1977 and 1978 with negative bacterial and fungal cultures | White blood cell count: 9.5 × 109/L with 3% eosinophilia | Thiabendazole 25 mg/kg/day divided BID × 8 days | Survived (continued with monthly stool examinations and prophylactic 2-day courses of thiabendazole monthly while on steroids) |
| CXR: few granulomatous densities, but no infiltrate | |||||||
| Born in Kentucky and mainly lived in Florida for last 22 years, working as a sharecropper and frequently walking barefootTreated for Strongyloides in 1979 with thiabendazole × 3 days when found in stool during work-up for eosinophilia | |||||||
| Computed tomography head: cerebral atrophy with slightly dilated ventricles | |||||||
| Magnetic resonance imaging brain: periventricular areas of high signal, no abscess | |||||||
| Sputum, stool, CSF: S. stercoralis filariform larvae | |||||||
| Cerebrospinal fluid was 97% neutrophilic | |||||||
| Dutcher et al.9 | 1985 | 45/M | Non-Hogdkin’s Burkitt-like lymphoma with CNS involvement with chemotherapy started 8 months before presentation; human immunodeficiency virus positive | Right 3rd cranial nerve palsy, right Babinski sign positive, urinary retention → thought to be lymphomatous involvement so WBI and IT chemotherapy → developed hyponatremia, seizures, and respiratory distress shortly after | Cerebrospinal fluid: malignant cells, S. stercoralis filariform larvae | Thiabendazole 1.5 g NG BID × 14 days | Death |
| Bronchoalveolar lavage, gastric washings, stool: S. stercoralis filariform and rhabditiform larvae | |||||||
| Born and raised in Puerto Rico, moved to and resides in the United States for the last 22 years | |||||||
| Blood: E. coli | |||||||
| 3 months prior, had been noted to have 20% eosinophilia of unclear etiology | |||||||
| Dokmeci et al.10 | ? | 75/F | High-dose corticosteroids for presumed asthma with intermittent steroids twice in the last 4 months | Respiratory distress refractory to steroids followed by mental status deterioration, progressive lethargy, seizure, and bradycardic arrest | White blood cell count: 19.7 × 109/L (no eosinophilia, but 11% the week prior) | Ivermectin 200 mcg/kg/day via NG × 7 days | Death (DOA 12) |
| CXR: unremarkable | |||||||
| Lived in Angola for 7 years 4 decades ago before coming to US | |||||||
| Cerebrospinal fluid: WBC 2074/mm3 with 100% neutrophils, rare S. stercoralis organisms | |||||||
| Sputum, stool: many Strongyloides organisms | |||||||
| Brain imaging: no gross hemorrhage or structural abnormalities | |||||||
| Had chronic intermittent diarrhea for years without clear etiology despite colonoscopy with biopsy | |||||||
| Case 1 of this paper | 2014 | 67/M | Prednisone, HTLV-1 infectionBorn in Trinidad with multiple subsequent visits | Headache, confusion, decreased level of consciousness, seizures; also history of nausea, vomiting, diarrhea, abdominal pain, anorexia; suspected pneumonia | White blood cell count: 9.7 × 109/L (no eosinophilia) | Ivermectin | Death |
| Albendazole | |||||||
| Computed tomography abdomen: colitis | |||||||
| Computed tomography head/MRI brain: leptomeningeal enhancement with subdural collections | |||||||
| Cerebrospinal fluid: WBC 22 × 106/L with 91% lymphocytes; E. coli, S. stercoralis | |||||||
| Stool, sputum: S. stercoralis larvae |
BID = twice daily; CNS = central nervous system; CSF = cerebrospinal fluid; CXR = chest X-ray; DOA = day of admission; HTLV-1 = human T-lymphotropic virus-1; IT = intrathecal; ITP = idiopathic thrombocytopenic purpura; MRI = magnetic resonance imaging; NG = nasogastric; WBC = white blood cell; WBI = whole brain irradiation.
Current Canadian recommendations for treatment of disseminated strongyloidiasis include ivermectin 200 μg/kg/day orally or subcutaneously once daily plus albendazole 400 mg orally twice daily until clinical improvement and cessation of larval shedding.2
The Centers for Disease Control and Prevention in the United States recommends ivermectin 200 μg/kg/day orally until stool and/or sputum tests are negative for 2 weeks.1
Despite advances in treatment over time, mortality remains high with a fatality rate of up to 86%13; timely diagnosis and treatment remain paramount. Disseminated Strongyloides can be prevented by screening those from endemic countries with serology and stool for ova and parasite examination before starting corticosteroids or other immune suppressive medications, especially when eosinophilia is present.
In conclusion, CNS strongyloidiasis is a rare but severe form of disseminated infection with high morbidity and mortality. Central nervous system strongyloidiasis should be considered in patients presenting with neurological, respiratory, and gastrointestinal signs when there are epidemiological and clinical risk factors for disseminated strongyloidiasis.
REFERENCES
- 1.Centers for Disease Control and Prevention , 2014. Parasites—Strongyloides. Available at: https://www.cdc.gov/parasites/strongyloides/. Accessed April 9, 2018.
- 2.Boggild A, Libman M, Greenaway C, McCarthy A; on behalf of the Committee to Advise on Tropical Medicine and Travel (CATMAT) , 2016. CATMAT statement on disseminated strongyloidiasis: prevention, assessment and management guidelines. Can Comm Dis Rep 42: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keiser PB, Nutman TB, 2004. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim S, Katz K, Krajden S, Fuksa M, Keystone JS, Kain KC, 2004. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. Can Med Assoc J 171: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley SL, Dines DE, Brewere NS, 1978. Disseminated Strongyloides stercoralis in an immunosuppressed host. Mayo Clin Proc 53: 332–335. [PubMed] [Google Scholar]
- 6.Neefe LI, Pinilla O, Garagusi VF, Bauer H, 1973. Disseminated strongyloidiasis with cerebral involvement: a complication of corticosteroid therapy. Am J Med 55: 832–838. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer RS, Singer C, Armstrong D, Mayer K, Knapper WH, 1979. Antemortem diagnosis of central nervous system strongyloidiasis. Am J Med Sci 277: 91–98. [DOI] [PubMed] [Google Scholar]
- 8.Belani A, Leptrone D, Shands JW, 1987. Strongyloides meningitis. South Med J 80: 916–918. [DOI] [PubMed] [Google Scholar]
- 9.Dutcher JP, Marcus SL, Tanowitz H, Wittner M, Fuks JZ, Wiernik PH, 1990. Disseminated strongyloidiasis with central nervous system involvement diagnosed antemortem in a patient with acquired immunodeficiency syndrome and Burkitts lymphoma. Cancer 66: 2417–2420. [DOI] [PubMed] [Google Scholar]
- 10.Dokmeci O, Forshay B, Scholand SJ, 2013. Worms on the brain: fatal meningoencephalitis from disseminated strongyloides infection. Conn Med 77: 31–34. [PubMed] [Google Scholar]
- 11.Purtilo DT, Meyers WM, Connor DH, 1974. Fatal strongyloidiasis in immunosuppressed patients. Am J Med 56: 488–493. [DOI] [PubMed] [Google Scholar]
- 12.Montes M, et al. 2009. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis 3: e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB, 1981. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis 3: 397–407. [DOI] [PubMed] [Google Scholar]