Abstract.
Tick-borne Crimean-Congo hemorrhagic fever virus (CCHFV) is endemic in numerous countries, but the epidemiology and epizoology of Crimean-Congo hemorrhagic fever (CCHF) remain to be defined for most regions of the world. Using a broad database search approach, we reviewed the literature on CCHF and CCHFV in Southern and Western Asia to better define the disease burden in these areas. We used a One Health approach, moving beyond a focus solely on human disease burden to more comprehensively define this burden by reviewing CCHF case reports, human and animal CCHFV seroprevalence studies, and human and animal CCHFV isolations. In addition, we used published literature to estimate the distribution of Hyalomma ticks and infection of these ticks by CCHFV. Using these data, we propose a new classification scheme for organizing the evaluated countries into five categories by level of evidence for CCHF endemicity. Twelve countries have reported CCHF cases, five from Southern Asia and seven from Western Asia. These were assigned to level 1 or 2. Eleven countries that have evidence of vector circulation but did not report confirmed CCHF cases were assigned to level 3 or 4. This classification scheme was developed to inform policy toward strengthening CCHF disease surveillance in the Southern and Western Asia regions. In particular, the goal of this review was to inform international organizations, local governments, and health-care professionals about current shortcomings in CCHFV surveillance in these two high-prevalence regions.
Introduction
Crimean-Congo hemorrhagic fever (CCHF) was originally described in Crimea in 1944–1945 after a disease outbreak was noted by Soviet military personnel.2 Since that time, CCHF is now recognized as the only “viral hemorrhagic fever” that is broadly endemic in both Africa and Eurasia, with more than 30 countries reporting cases.3 During the past two decades, 11 countries have reported their first confirmed CCHF cases (Table 1). Crimean-Congo hemorrhagic fever is caused by Crimean-Congo hemorrhagic fever virus (CCHFV; order Bunyavirales: family Nairoviridae).4,5 Depending on the available health-care system infrastructure, the case fatality rate of CCHF can range from 5% up to 80% during limited outbreaks.6,7 Most cases of CCHF are reported from the Western and Southern Asia, with incidences increasing during the past two decades.8
Table 1.
For the past 20 years, since 1998, 11 countries reported their first autochthonous Crimean-Congo hemorrhagic fever cases
Crimean-Congo hemorrhagic fever virus is a vector-borne virus that is primarily transmitted via Hyalomma tick bites.9 The strength of evidence supporting the role of various Hyalomma ticks as CCHFV vectors varies.10,11 Thus, only ticks with the highest level of evidence, Hyalomma rufipes and Hyalomma marginatum, were included in our search. Various ungulates serve as mammalian CCHF reservoirs.12 Therefore, agrarian countries are more vulnerable to CCHFV endemicity. Indeed, livestock and ticks are the primary source of sustained environmental CCHFV circulation. Humans are infected most frequently by tick bites and primarily serve as dead end hosts.12 The frequency of transmission from animals to humans via direct contact is not well-defined. Studies indicate high CCHFV seroprevalence in abattoir workers.13,14 These data indicate that CCHFV transmission occurs via direct contact with animals or animal products, although tick exposure cannot be ruled out. Furthermore, human-to-human infections have been well-described but are less likely to occur than infection via tick bites. Thus, although several mechanisms are postulated for transmission occur, Hyalomma ticks are required for sustained CCHFV circulation in a given region. Therefore, the future incidence of CCHF is multifactorial and is dependent on changes in human, animal, and tick populations.
Most reports on first autochthonous CCHF cases in individual countries were preceded by epidemiologic surveys that provided evidence of local CCHFV circulation. For example, serologic evidence of CCHFV in animals and humans in Iran was described in 1975 and evidence of CCHFV antigen was found in ticks in 1978.15,16 Yet, it was not until 1999 that the first human case was described.17 In addition, serologic evidence of CCHFV in Turkey existed already in 1975,18 but the first confirmed CCHF case in Turkey was not reported until 2002. This country has since reported the highest number of CCHF cases in the world, totaling more than 10,000 cases.7 Yet, published epidemiologic data are especially sparse for most countries in Southern and Western Asia and have not been updated in great detail since 1979.19 This absence of updated human data is largely because only few countries in these regions have established active surveillance systems.20
This article focuses on Southern and Western Asia to describe regions that are highly affected by CCHFV with an emphasis on countries that may not have been considered CCHFV hotspots in the past. These regions were selected for multiple reasons. First, CCHF case numbers have increased in Afghanistan and Pakistan during the past few years.21,22 To look into entire regions, we therefore turned to the United Nations Geoscheme to determine the official region that Afghanistan and Pakistan belong to (“Southern Asia”) and which countries of that region should also be considered to achieve consistency of our analysis (Bangladesh, Bhutan, India, Iran, Maldives, Nepal, and Sri Lanka).23 Second, the arguably largest expansion of CCHF cases in recent years occurred in Turkey (United Nations Geoscheme: “Western Asia”). Hence, we added the countries of Western Asia to our analysis. Both regions are well-defined areas for political action. Because the vast majority of CCHFV literature is not indexed in open-access medical databases, such as PubMed, and because significant proportions of the CCHFV literature are written in languages other than English (in particular Persian and Russian) in journals that are not easily accessible, this concise review was limited to these two UN Geoscheme regions. Prior reviews on these regions have not fully incorporated governmental human CCHF case detection available through internet search engines or through electronic communication.8,20
To accurately use the epidemiologic results available, we incorporated a One Health approach to our strategy in describing CCHF endemicity. The One Health concept promotes the integration of interdisciplinary ecologic data to guide predictions of pathogen emergence.24 Data on human cases are only one factor in understanding the burden of disease and potential for emergence. We reviewed human, animal, virus, and tick data to assess CCHFV endemicity or potential for emergence to provide more accurate epidemiologic information. Because of the unpredictable effects of environmental changes, we used country-level vector data rather than a strict latitude cut-off that had been used previously.8,25 Our objective was to integrate ecologic research to define the current status of CCHFV circulation. Therefore, we sought to perform a comprehensive search strategy to provide the most up-to-date and accurate overview of CCHF in two regions with the aim of highlighting urgent surveillance needs to inform global health security policy.
Methods
We searched PubMed, Google Scholar, Scopus, GenBank, GIDEON, ProMED, and Web of Science records indexed from the original description of CCHF in 19452 until December 31, 2017, to identify and review the scientific literature on reported CCHF cases from each country of Southern and Western Asia as defined by the UN Geoscheme (Southern Asia: Afghanistan, Bangladesh, Bhutan, India, Iran, Maldives, Nepal, Pakistan, and Sri Lanka; Western Asia: Armenia, Azerbaijan, Bahrain, Cyprus, Georgia, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, and Yemen).23 We also reviewed conference proceedings and relevant articles that were cited. Our review included English, French, Georgian, Persian, and Russian language articles. We used Internet search engines for government reports and e-mailed government officials for unpublished data. In addition, we reviewed published human and animal serology data suggestive of CCHFV exposure or infection, data on CCHFV detection or isolation from ticks or vertebrates, and data indicative of the existence of CCHFV surveillance systems. We also reviewed the U.S. National Tick Collection database for data on the presence of CCHFV vectors. Our search was limited to the hard (ixodid) ticks H. marginatum and H. rufipes based on their capacity to transmit CCHFV transstadially, transovarially, and to animals.9,26 For database queries, we used a combination of search terms, including “Crimean,” “Crimean-Congo,” “Congo-Crimean,” “CCHF,” “CCHFV,” “Hyalomma,” and the name of each country of interest.
One health country-level classification scheme.
Based on the results of our search, we developed a classification scheme that integrated vector, animal, and human data to define CCHFV circulation. A similar classification scheme with integration of virus circulation data had been developed by the World Health Organization (WHO) for Zika virus.27 We placed countries in categories defined as follows: level 1: CCHF cases reported annually through established surveillance; level 2: CCHF cases reported intermittently in absence of robust surveillance; level 3: no CCHF cases reported; no robust surveillance established, but available data point toward the possibility of undetected/unreported CCHF cases (animal/human serology, CCHFV detected in Hyalomma ticks); level 4: no CCHF cases reported; no robust surveillance or epidemiologic/epizoologic studies, but Hyalomma ticks are present; and level 5: no available data.
Results
During the past few decades, cases have been reported annually from certain CCHFV-endemic countries (i.e., Georgia, Iran, and Turkey). For example, during the past 10 years, Iran reported 65 confirmed cases each year on average (range 44–150 per year).28–30 Cases have been decreasing in Turkey during the past 3 years, but the incidence of CCHF remains the highest worldwide with 371 confirmed cases reported in 2016.31 Turkey has reported more than 10,000 cases since 2002 (Table 2).7,31 Although cases have been declining in Turkey, Afghanistan and Pakistan have reported increasing numbers of cases during the past few years. In Pakistan, 55 and 103 cases were confirmed in 2016 and 2017, respectively.22,32 In Afghanistan, 242 suspected and 57 confirmed CCHF cases occurred in 2017, a steady increase from 42 suspected and six confirmed cases in 2014.21,33
Table 2.
Total confirmed cases in Southern and Western Asia by country from 1974 to 2017 based on peer-reviewed literature or reports from government organizations
| Country | Total confirmed cases | Total deaths | Cases per year (range) | Years cases reported | References |
|---|---|---|---|---|---|
| Turkey | 10,333 | 469 | 150–1,318 | 2002–2017 | 7,31 |
| Iran | 1,256 | 177 | 18–150 | 1999–2017 | 28–30 |
| Pakistan | 429 | 94 | 3–83 | 1976–2017 | 32,38,39,54–61 |
| Iraq | 377 | 39 | 0–55 | 1979–1980, 1990–2010, 2013, 2015 | 62,63 |
| Afghanistan | 334 | 88 | 1–237 | 1998–2017 | 21,33,64–67 |
| Georgia | 56 | 7 | 0–25 | 2009, 2012–2017 | 42,68,69 |
| India | 47 | 19 | 6–18 | 2011–2015 | 43,70–75 |
| Oman | 34 | 14 | 0–33 | 1995–2014 | 76–79 |
| United Arab Emirates | 24 | 14 | 0–11 | 1979, 1994–1995, 2010 | 6,80–82 |
| Saudi Arabia | 8 | 0 | 0–7 | 1989–1990 | 83 |
| Kuwait | 2 | 0 | 0–2 | 1980, 1982 | 46 |
| Armenia | 1 | 0 | 0–1 | 1974 | 45 |
Total deaths are those among confirmed cases only. Therefore, the case fatality rates were not calculated, as cases were more frequently reported confirmed or suspected than deaths. A conservative approach was used by limiting data to peer-reviewed literature, but this approach likely underestimates the true burden of Crimean-Congo hemorrhagic fever (CCHF). For instance, Pakistan reported 1,339 suspected CCHF cases from 2011 to March 2017, but only 429 cases were confirmed.
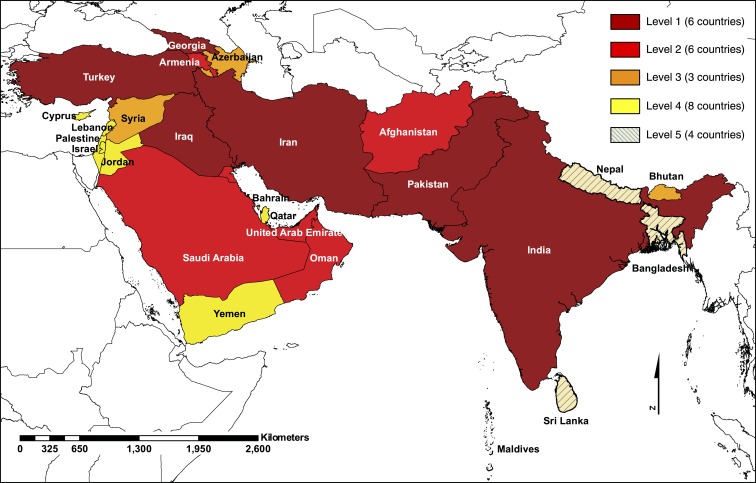
We categorized countries from Southern and Western Asia into five levels that reflect our current CCHFV endemicity assessment (Tables 3 and 4, Figure 1). We assigned 12 countries, five from Southern Asia and seven from Western Asia, to level 1 or 2 because these countries have reported confirmed CCHF cases. Eleven countries, one from Southern Asia and 10 from Western Asia, were assigned to level 3 or 4 because these countries have not reported confirmed CCHF cases, but CCHFV vectors have been found in these areas. Four countries from Southern Asia were assigned to level 5 because no evidence was found for CCHFV endemicity within those countries (Table 3, Figure 1).
Table 3.
Current evidence for CCHFV circulation in Southern Asia
| Country | CCHF cases reported | Human serology | Animal serology | Hyalomma ticks | Virus detected in Hyalomma ticks |
|---|---|---|---|---|---|
| Afghanistan | 1998–201721,53 | 199884 | 197453,64,84 | Yes* | NA19,38,64 |
| Bangladesh | No | NA | NA | NA | NA |
| Bhutan | No | NA | NA | NA | NA |
| India | 2011–201743,70 | 197385 | 1973, 2010–201172,85 | NA | 1973, 2010–201172,85 |
| Iran | 1999–201728,52 | 1975†, 2004–2005, 201713,15,86 | 1975†, 2004–2005, 2010–201115,86–89 | Yes10 | 2004–2016†‡10,89–91 |
| Maldives | No | NA | NA | NA | NA |
| Nepal | No | NA | NA | NA | NA |
| Pakistan | 1976–201760,92 | 2007–201356 | 201638,93 | Yes* | 1970†94 |
| Sri Lanka | No | NA | NA | NA | NA |
CCHF = Crimean-Congo hemorrhagic fever; CCHFV = Crimean-Congo hemorrhagic fever virus; NA = no information available. Years are listed if there is peer-reviewed evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
* Information from the United States National Tick Collection.
† Year represents time of publication rather than time of sample collection.
‡ CCHFV antigen was isolated from an Ornithodoros (Alveonasus) lahorensis soft tick in Iran in 1978.
Table 4.
Current evidence for CCHFV circulation in Western Asia
| Country | CCHF cases reported | Human serology | Animal serology | Hyalomma ticks | Virus detected in Hyalomma ticks |
|---|---|---|---|---|---|
| Armenia | 197445,95 | 197296 | 197296 | Yes97 | 1972–197497,98 |
| Azerbaijan | No | 200799 | 1967–1970100 | Yes97 | 1972–197497,101 |
| Bahrain | No | NA | NA | NA | NA |
| Cyprus | No | NA | NA | Yes* | NA |
| Georgia | 2009–201742,68,102 | 201468,102–105 | NA | Yes19 | NA |
| Iraq | 1979–1980, 1990–2010, 2013, 201562,63,106 | 1979–198063 | 1980107 | Yes* | NA |
| Israel | No | NA | NA | Yes* | NA |
| Jordan | No | NA | NA | Yes* | NA |
| Kuwait | 1980, 198246 | 1979–1982108 | NA | Yes* | NA |
| Lebanon | No | NA | NA | Yes* | NA |
| Oman | 1995–201779,109,110 | 2000†14 | 2000†14 | Yes* | 2000†14 |
| Palestine | No | NA | NA | Yes* | NA |
| Qatar | No | NA | NA | NA | NA |
| Saudi Arabia | 1990, 200983 | 200983,111,112 | 1995111 | Yes* | 1995113 |
| Syria | No | NA | 199614 | Yes114 | 2014†11 |
| Turkey | 2002–20177,31 | 1974, 2012, 2016†115–117 | 2011118 | Yes* | 2013–2015119 |
| United Arab Emirates | 1979, 1980, 1994–1995, 20106,80,82 | 1997†81 | 1997†81 | Yes* | 1997†81 |
| Yemen | No | NA | NA | Yes120 | NA |
CCHF = Crimean-Congo hemorrhagic fever; CCHFV = Crimean-Congo hemorrhagic fever virus; NA = no information available. Years are listed if there is peer-reviewed evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
* United States National Tick Collection.
† Year represents time of publication rather than time of sample collection.Years are listed if there is peer-reviewed evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
Figure 1.
Burden of CCHF in Southern and Western Asia using a One Health approach. Data support CCHFV circulation in lower Level (1 and 2) countries, whereas further study and surveillance of CCHFV circulation is recommended in countries of higher Levels (3, 4, and 5). Classification at the country level was performed for policy implications. Country boundaries do not necessarily reflect the geographic area at risk. Map was created using ArcGIS Release 10.61. Source: Database of Global Administrative Areas (GADM). This figure appears in color at www.ajtmh.org.1
Discussion
Crimean-Congo hemorrhagic fever virus presents a significant threat to human health within these high-prevalence regions and throughout the world. The WHO included CCHF in its blueprint of priority diseases, which lists emerging diseases that are understudied.34 We assessed the burden of CCHF in Western and Southern Asia because these regions report the most cases of CCHF worldwide. However, there is significant variability in surveillance activities and capabilities between countries.
The increasing incidence of CCHF cases in Afghanistan is unlikely to have been noticed if it were not for a Disease Early Warning System (DEWS) established in 2006.21,35 This system was developed with the technical assistance of WHO and financial assistance from the United States Agency for International Development. Given the recent increasing incidence of CCHF in Pakistan and Afghanistan, strengthening of human surveillance systems in this region is critically important. Only with intergovernmental collaborations and support similar to the development of the Afghanistan DEWS can we strive toward global health preparedness in level 2–5 countries, where the true burden of CCHFV remains largely unknown.
Certain general trends in the epidemiology of CCHF cases have been observed. First, CCHF cases occur most frequently during the summer months because tick populations are lower during the colder winter months.36 CCHF cases may increase during more temperate winters.36 It is therefore speculated that climate change has contributed to the increasing incidence of CCHF cases worldwide.37 With milder winters projected, CCHF emergence may continue to be observed. Further research should evaluate historical climate change data with trends in CCHF incidence. Second, it has been speculated that increasing trends in CCHF could be related to livestock migration and sacrifice for the Eid al-Adha festival in Pakistan.38 However, this migration has occurred in late summer during the past few years and would be difficult to differentiate from the effects of temporal variation.39 Yet, given livestock and population migration, increased surveillance and infection precautions are warranted.36 By contrast, Saudi Arabia, a country with intermittent CCHF cases, has developed surveillance systems for Eid al-Adha and has not encountered increased cases.40 Third, CCHF cases have been increasingly recognized in areas that were not historically reporting cases. Although outside the scope of this review, Egypt, Georgia, India, and Spain have reported their first autochthonous cases within the past decade.41–44 This review was limited to UN Geoscheme regions with the highest CCHF endemicity, but because of international travel, increasing human population density, and climate change, CCHF is silently expanding its boundaries.
The WHO map for demonstrating CCHF endemicity25 is limited to numbers of reports of human disease, positive human serology, and presence or absence of ticks. Our country classification more comprehensively integrated other factors as part of a One Health approach, including animal serology and inclusion of more granular data on CCHFV vector presence. Our broad search strategy updated and uniquely integrated epidemiologic information to make the following recommendations:
1. Countries of Southern and Western Asia in general would benefit from intergovernmental support and multi-institutional collaborations to develop or to strengthen human and ecologic CCHF surveillance networks.
2. Hyalomma ticks are present in countries clustered by the Eastern Mediterranean basin, including Cyprus, Israel, Jordan, Lebanon, and Syria. However, information about CCHFV circulation is limited. These countries may benefit from testing of Hyalomma ticks for CCHFV and from performing seroprevalence studies in humans and animals.
3. Countries with civil strife, particularly Syria and Yemen, have weakened health-care infrastructures and the presence of the Hyalomma tick vector. These countries may be at high risk for propagated CCHF outbreaks and would benefit from mobile diagnostic support and personal protective equipment (PPE).
4. Armenia, Azerbaijan, and Kuwait are associated with strong evidence of CCHFV circulation in the past, but no data have been published over the past three decades. Reported CCHF cases in Armenia (in 1974) and Kuwait (in 1980 and 1982) have been largely ignored.45,46 Cases may be occurring undetected and, therefore, these countries may benefit from active surveillance for CCHF cases.
5. Multi-institutional collaborations should focus on establishing improved health-care infrastructures to prevent community and nosocomial CCHFV transmission from index cases with provision of resources for PPE.
6. Regional reference laboratories should be established or strengthened to provide rapid diagnostic support during CCHF outbreaks.
There are limitations with our approach. Our data are only as good as the surveillance systems in the countries for assessing endemicity. In addition, in countries with widely varying topography and risk for disease spread, it would be more accurate to categorize the risk by regions within a country rather than a country as a whole. In addition, borders are artificially created and have little to no effect on the transmission of disease by arthropods. We maintained the unit of country political borders to emphasize the need for policy to improve regional and country-level surveillance strengthening. Future research should integrate human, animal, and vector data to define or predict hot spots for precise geographic estimates on CCHFV distribution.
In summary, we call on public health organizations, including WHO or the World Organization for Animal Health, to consider creating or expanding partnerships with local governments to provide support for human, tick, and animal CCHFV surveillance in high priority areas in Southern and Western Asia.
Acknowledgments:
We thank Laura Bollinger (Integrated Research Facility at Fort Detrick, Frederick, MD) for critically editing the manuscript.
REFERENCES
- 1.ESRI , 2017. ArcGIS: Release 10.6. Redlands, CA: Environmental Systems Research Institute.
- 2.Čumakov MP, 1945. Krymskaâ Gemorragičeskaâ Lihoradka (Ostryj Infekcionnyj Kapillârotoksikoz). Simferopol, USSR: Izdanie Otdel’noj Primorskoj Armii. [Google Scholar]
- 3.Papa A, Weber F, Hewson R, Weidmann M, Koksal I, Korukluoglu G, Mirazimi A, 2015. Meeting report: first international conference on Crimean-Congo hemorrhagic fever. Antiviral Res 120: 57–65. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization , 2016. ICD-10 Version: 2016. Available at: http://apps.who.int/classifications/icd10/browse/2016/en. Accessed February 3, 2018.
- 5.Maes P, et al. 2018. Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018. Arch Virol 163: 2295–2310. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz TF, Nsanze H, Ameen AM, 1997. Clinical features of Crimean-Congo haemorrhagic fever in the United Arab Emirates. Infection 25: 364–367. [DOI] [PubMed] [Google Scholar]
- 7.Leblebicioglu H, Ozaras R, Irmak H, Sencan I, 2016. Crimean-Congo hemorrhagic fever in Turkey: current status and future challenges. Antiviral Res 126: 21–34. [DOI] [PubMed] [Google Scholar]
- 8.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M, 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 100: 159–189. [DOI] [PubMed] [Google Scholar]
- 9.Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA, 2017. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antiviral Res 144: 93–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telmadarraiy Z, Chinikar S, Vatandoost H, Faghihi F, Hosseini-Chegeni A, 2015. Vectors of Crimean Congo hemorrhagic fever virus in Iran. J Arthropod Borne Dis 9: 137–147. [PMC free article] [PubMed] [Google Scholar]
- 11.Široký P, Bělohlávek T, Papoušek I, Jandzik D, Mikulíček P, Kubelová M, Zdražilová-Dubská L, 2014. Hidden threat of tortoise ticks: high prevalence of Crimean-Congo haemorrhagic fever virus in ticks Hyalomma aegyptium in the Middle East. Parasit Vectors 7: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron E, Bente DA, 2016. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res 135: 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostafavi E, Pourhossein B, Esmaeili S, Bagheri Amiri F, Khakifirouz S, Shah-Hosseini N, Tabatabaei SM, 2017. Seroepidemiology and risk factors of Crimean-Congo hemorrhagic fever among butchers and slaughterhouse workers in southeastern Iran. Int J Infect Dis 64: 85–89. [DOI] [PubMed] [Google Scholar]
- 14.Williams RJ, Al-Busaidy S, Mehta FR, Maupin GO, Wagoner KD, Al-Awaidy S, Suleiman AJM, Khan AS, Peters CJ, Ksiazek TG, 2000. Crimean-Congo haemorrhagic fever: a seroepidemiological and tick survey in the sultanate of Oman. Trop Med Int Health 5: 99–106. [DOI] [PubMed] [Google Scholar]
- 15.Saidi S, Casals J, Faghih MA, 1975. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am J Trop Med Hyg 24: 353–357. [DOI] [PubMed] [Google Scholar]
- 16.Sureau P, Klein J-M, 1980. Arbovirus en Iran. Méd Trop (Mars) 40: 549–554. [PubMed] [Google Scholar]
- 17.Mardani M, 2000. An outbreak of Crimean-Congo haemorrhagic fever in the Islamic Republic of Iran. 1999. Clin Infect Dis 31: 315. [Google Scholar]
- 18.Casals J, 1978. Crimean-Congo hemorrhagic fever. Pattyn SR, ed. Ebola Virus Haemorrhagic Fever. Proceedings of an International Colloquium on Ebola Virus Infection and Other Haemorrhagic Fevers held in Antwerp, Belgium, 6–8 December, 1977. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press, 301–318. [Google Scholar]
- 19.Hoogstraal H, 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol 15: 307–417. [DOI] [PubMed] [Google Scholar]
- 20.Al-Abri SS, et al. 2017. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization eastern Mediterranean region: issues, challenges, and future directions. Int J Infect Dis 58: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afghanistan National Public Health Institute , 2017. Disease Early Warning System Weekly Report. Available at: http://moph.gov.af/en/documents. Accessed February 3, 2018.
- 22.Haider S, Hassali MA, Iqbal Q, Anwer M, Saleem F, 2016. Crimean-Congo haemorrhagic fever in Pakistan. Lancet Infect Dis 16: 1333. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Statistics Division , 2018. Standard Country or Area Codes for Statistical Use (M49). Available at: https://unstats.un.org/unsd/methodology/m49/. Accessed February 3, 2018.
- 24.World Health Organization , 2017. One Health. Available at: http://www.who.int/features/qa/one-health/en/. Accessed February 3, 2018.
- 25.World Health Organization , 2017. Geographic Distribution of Crimean-Congo Haemorrhagic Fever. Available at: http://www.who.int/emergencies/diseases/crimean-congo-haemorrhagic-fever/Global_CCHFRisk_2017.jpg?ua=1. Accessed December 20, 2018.
- 26.Zeller HG, Cornet J-P, Camicas J-L, 1994. Experimental transmission of Crimean-Congo hemorrhagic fever virus by west African wild ground-feeding birds to Hyalomma marginatum rufipes ticks. Am J Trop Med Hyg 50: 676–681. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization , 2017. Zika Virus Country Classification Scheme. Available at: http://apps.who.int/iris/bitstream/handle/10665/254619/WHO-ZIKV-SUR-17.1-eng.pdf?sequence=1. Accessed December 20, 2018.
- 28. Mardani M, Namazee N, 2017. CCHF in Iran: An Eighteen Years Review. 2nd International Conference on Crimean-Congo Hemorrhagic Fever, Thessaloniki, Greece, September 10–12, 2017.
- 29.Jalali T, 2015. Epidemiology, Phylogenetics, Biosafety and Molecular Detection of CCHF. Available at: http://www.isv.org.ir/attachments/article/87/Jalali.pdf. Accessed February 3, 2018.
- 30.Baghi HB, Aghazadeh M, Oskouee MA, 2017. CCHF in Iran and Future Challenges. 2nd International Congress on Crimean-Congo Hemorrhagic Fever Thessaloniki, Greece, September 10–12, 2017. [Google Scholar]
- 31.Cosgun Y, Menemenlioglu D, Caglayik DY, Bayrakdar F, Giray BG, Seda H, Ozmen E, Korhan A, Korukluoglu G, 2017. Evaluation of the CCHF Results Analyzed in Public Health Laboratories in Turkey Between 2014 and 2017. Papa A, ed. 2nd International Conference on Crimean-Congo Hemorrhagic Fever Thessaloniki, Greece, September 10–12, 2017, 53–54. [Google Scholar]
- 32.World Health Organization , 2017. Health threats reported in 2017. Wkly Epidemiol Monit 10: 1. [Google Scholar]
- 33.World Health Organization , 2017. Crimean-Congo hemorrhagic fever in Afghanistan. Wkly Epidemiol Monit 10: 1. [Google Scholar]
- 34.World Health Organization , 2016. An R&D Blueprint for Action to Prevent Epidemics. Available at: http://www.who.int/blueprint/about/r_d_blueprint_plan_of_action.pdf. Accessed February 3, 2018.
- 35.Wagner AL, Mubarak MY, Johnson LE, Porth JM, Yousif JE, Boulton ML, 2017. Trends of vaccine-preventable diseases in Afghanistan from the disease early warning system, 2009–2015. PLoS One 12: e0178677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atif M, Saqib A, Ikram R, Sarwar MR, Scahill S, 2017. The reasons why Pakistan might be at high risk of Crimean Congo haemorrhagic fever epidemic; a scoping review of the literature. Virol J 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medlock JM, Leach SA, 2015. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect Dis 15: 721–730. [DOI] [PubMed] [Google Scholar]
- 38.Karim AM, Hussain I, Lee JH, Park KS, Lee SH, 2017. Surveillance of Crimean-Congo haemorrhagic fever in Pakistan. Lancet Infect Dis 17: 367–368. [DOI] [PubMed] [Google Scholar]
- 39.Alam MM, Khurshid A, Sharif S, Shaukat S, Rana MS, Angez M, Zaidi SS, 2013. Genetic analysis and epidemiology of Crimean Congo hemorrhagic fever viruses in Baluchistan province of Pakistan. BMC Infect Dis 13: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Tawfiq JA, Memish ZA, 2014. Mass gathering medicine: 2014 Hajj and Umra preparation as a leading example. Int J Infect Dis 27: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negredo A, et al. Crimean Congo Hemorrhagic Fever@Madrid Working G , 2017. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med 377: 154–161. [DOI] [PubMed] [Google Scholar]
- 42.Zakhashvili K, Tsertsvadze N, Chikviladze T, Jghenti E, Bekaia M, Kuchuloria T, Hepburn MJ, Imnadze P, Nanuashvili A, 2010. Crimean-Congo hemorrhagic fever in man, Republic of Georgia, 2009. Emerg Infect Dis 16: 1326–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra AC, Mehta M, Mourya DT, Gandhi S, 2011. Crimean-Congo haemorrhagic fever in India. Lancet 378: 372. [DOI] [PubMed] [Google Scholar]
- 44.El-Bahnasawy MMM, Sabah AAA, Saleh HAA, Morsy TA, 2012. The tick-borne Crimean-Congo hemorrhagic fever in Africa, Asia, Europe, and America: what about Egypt? J Egypt Soc Parasitol 42: 373–384. [DOI] [PubMed] [Google Scholar]
- 45.Semaško IV, Čumakov MP, Karapetân RI, Vorob’ev AG, Zavodova TI, Matevosân KŠ, Nersesân MA, 1974. Pervyj slučaj izolâcii virusa Krymskoj gemorragičeskoj lihoradki v Armenii iz krovi bol’nogo. Med Virusol 22: 25–28. [Google Scholar]
- 46.Alnakib W, Elmekki A, Alkandari S, Majeed HA, 1983. Congo Crimean hemorrhagic fever in Kuwait–1st report of 2 laboratory documented cases. J Kuwait Med Assoc 17: 163–166. [Google Scholar]
- 47.Aradaib IE, Erickson BR, Mustafa ME, Khristova ML, Saeed NS, Elageb RM, Nichol ST, 2010. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg Infect Dis 16: 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papa A, Velo E, Papadimitriou E, Cahani G, Kota M, Bino S, 2009. Ecology of the Crimean-Congo hemorrhagic fever endemic area in Albania. Vector Borne Zoonotic Dis 9: 713–716. [DOI] [PubMed] [Google Scholar]
- 49.Nabeth P, Thior M, Faye O, Simon F, 2004. Human Crimean-Congo hemorrhagic fever, Senegal. Emerg Infect Dis 10: 1881–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karti SS, et al. 2004. Crimean-Congo hemorrhagic fever in Turkey. Emerg Infect Dis 10: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunster L, Dunster M, Ofula V, Beti D, Kazooba-Voskamp F, Burt F, Swanepoel R, DeCock KM, 2002. First documentation of human Crimean-Congo hemorrhagic fever, Kenya. Emerg Infect Dis 8: 1005–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keshtkar-Jahromi M, Sajadi MM, Ansari H, Mardani M, Holakouie-Naieni K, 2013. Crimean-Congo hemorrhagic fever in Iran. Antiviral Res 100: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization , 1998. Crimean-Congo Haemorrhagic Fever in Afghanistan. Geneva, Switzerland: WHO. Available at: http://www.who.int/csr/don/1998_05_08a/en/. Accessed September 22, 2017.
- 54.Ali N, Chotani RA, Anwar M, Nadeem M, Karamat KA, Tariq WUZ, 2007. A Crimean–Congo haemorrhagic fever outbreak in northern Balochistan. J Coll Physicians Surg Pak 17: 477–481. [PubMed] [Google Scholar]
- 55.Smego RA, Jr., Sarwari AR, Siddiqui AR, 2004. Crimean-Congo hemorrhagic fever: prevention and control limitations in a resource-poor country. Clin Infect Dis 38: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 56.Hasan Z, Atkinson B, Jamil B, Samreen A, Altaf L, Hewson R, 2014. Short report: diagnostic testing for hemorrhagic fevers in Pakistan: 2007–2013. Am J Trop Med Hyg 91: 1243–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Athar MN, Khalid MA, Ahmad AM, Bashir N, Baqai HZ, Ahmad M, Balouch AH, Bashir K, 2005. Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002: contact tracing and risk assessment. Am J Trop Med Hyg 72: 471–473. [PubMed] [Google Scholar]
- 58.Hussain Q, Shaikh BH, Bhutto AR, Sohaib M, 2016. An unusual case of Crimean Congo hemorrhagic fever: prolonged bleeding with successful recovery. J Coll Physicians Surg Pak 26: 151–153. [PubMed] [Google Scholar]
- 59.Sheikh AS, Sheikh AA, Sheikh NS, Rafi US, Asif M, Afridi F, Malik MT, 2005. Bi-annual surge of Crimean-Congo haemorrhagic fever (CCHF): a five-year experience. Int J Infect Dis 9: 37–42. [DOI] [PubMed] [Google Scholar]
- 60.Burney MI, Ghafoor A, Saleen M, Webb PA, Casals J, 1980. Nosocomial outbreak of viral hemorrhagic fever caused by Crimean hemorrhagic fever-Congo virus in Pakistan, January 1976. Am J Trop Med Hyg 29: 941–947. [DOI] [PubMed] [Google Scholar]
- 61.Altaf A, Luby S, Ahmed AJ, Zaidi N, Khan AJ, Mirza S, McCormick J, Fisher-Hoch S, 1998. Outbreak of Crimean-Congo haemorrhagic fever in Quetta, Pakistan: contact tracing and risk assessment. Trop Med Int Health 3: 878–882. [DOI] [PubMed] [Google Scholar]
- 62.Majeed B, Dicker R, Nawar A, Badri S, Noah A, Muslem H, 2012. Morbidity and mortality of Crimean-Congo hemorrhagic fever in Iraq: cases reported to the National Surveillance System, 1990–2010. Trans R Soc Trop Med Hyg 106: 480–483. [DOI] [PubMed] [Google Scholar]
- 63.Al-Tikriti SK, et al. 1981. Congo/Crimean haemorrhagic fever in Iraq. Bull World Health Organ 59: 85–90. [PMC free article] [PubMed] [Google Scholar]
- 64.Mustafa ML, et al. 2011. Crimean-Congo hemorrhagic fever, Afghanistan, 2009. Emerg Infect Dis 17: 1940–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khurshid A, Hassan M, Alam MM, Aamir UB, Rehman L, Sharif S, Shaukat S, Rana MS, Angez M, Zaidi SS, 2015. CCHF virus variants in Pakistan and Afghanistan: emerging diversity and epidemiology. J Clin Virol 67: 25–30. [DOI] [PubMed] [Google Scholar]
- 66.Ölschlager S, Gabriel M, Schmidt-Chanasit J, Meyer M, Osborn E, Conger NG, Allan PF, Günther S, 2011. Complete sequence and phylogenetic characterisation of Crimean-Congo hemorrhagic fever virus from Afghanistan. J Clin Virol 50: 90–92. [DOI] [PubMed] [Google Scholar]
- 67.Mofleh J, Ahmad Z, 2012. Crimean-Congo haemorrhagic fever outbreak investigation in the western region of Afghanistan in 2008. East Mediterr Health J 18: 522–526. [DOI] [PubMed] [Google Scholar]
- 68.Georgia NCDC , 2018. Health Care Statistical Yearbook 2016. Available at: http://www.ncdc.ge/Handlers/GetFile.ashx?ID=31eee2a3-9bf5-4558-959b-a4b92f600555. Accessed October 31, 2018.
- 69.Nana Mamuchishvili KZ, Chakhunashvili G, lmnadze P, Keshtkar-Jahromi M, Pecor D, Rivard R, 2017. CCHF in Georgia. 2nd International Conference on Crimean-Congo Hemorrhagic Fever Thessaloniki, Greece, September 10–12, 2017. [Google Scholar]
- 70.Mourya DT, Viswanathan R, Jadhav SK, Yadav PD, Basu A, Chadha MS, 2017. Retrospective analysis of clinical information in Crimean-Congo haemorrhagic fever patients: 2014–2015, India. Indian J Med Res 145: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav PD, et al. 2014. Emergence of Crimean-Congo hemorrhagic fever in Amreli district of Gujarat state, India, June to July 2013. Int J Infect Dis 18: 97–100. [DOI] [PubMed] [Google Scholar]
- 72.Mourya DT, Yadav PD, Shete AM, Gurav YK, Raut CG, Jadi RS, Pawar SD, Nichol ST, Mishra AC, 2012. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010–2011. PLoS Negl Trop Dis 6: e1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Makwana D, Yadav PD, Kelaiya A, Mourya DT, 2015. First confirmed case of Crimean-Congo haemorrhagic fever from Sirohi district in Rajasthan state, India. Indian J Med Res 142: 489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yadav PD, et al. 2016. Nosocomial infection of CCHF among health care workers in Rajasthan, India. BMC Infect Dis 16: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yadav PD, Raut CG, Mourya DT, 2013. Re-occurrence of Crimean-Congo haemorrhagic fever in Ahmedabad, Gujarat, India (2012): a fatal case report. Indian J Med Res 138: 1027–1028. [PMC free article] [PubMed] [Google Scholar]
- 76.Yadav PD, Thacker S, Patil DY, Jain R, Mourya DT, 2017. Crimean-Congo hemorrhagic fever in migrant worker returning from Oman to India, 2016. Emerg Infect Dis 23: 1005–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Idris Al-Abaidani SSA-A, 2014. Cluster of Crimean-Congo Haemorrhagic Fever Cases in Oman: October 2014. Available at: http://www.promedmail.org/direct.php?id=20141108.2939718. Accessed January 7, 2018.
- 78.Scrimgeour EM, Mehta FR, Suleiman AJ, 1999. Infectious and tropical diseases in Oman: a review. Am J Trop Med Hyg 61: 920–925. [DOI] [PubMed] [Google Scholar]
- 79.Al-Zadjali M, Al-Hashim H, Al-Ghilani M, Balkhiar A, 2013. A case of Crimean-Congo hemorrhagic fever in Oman. Oman Med J 28: 210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al Dabal LM, Rahimi Shahmirzadi MR, Baderldin S, Abro A, Zaki A, Dessi Z, Al Eassa E, Khan G, Shuri H, Alwan AM, 2016. Crimean-Congo hemorrhagic fever in Dubai, United Arab Emirates, 2010: case report. Iran Red Crescent Med J 18: E38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan AS, et al. 1997. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med Hyg 57: 519–525. [DOI] [PubMed] [Google Scholar]
- 82.Suleiman MNEH, Muscat-Baron JM, Harries JR, Satti AGO, Platt GS, Bowen ETW, Simpson DIH, 1980. Congo/Crimean haemorrhagic fever in Dubai. An outbreak at the Rashid Hospital. Lancet 316: 939–941. [PubMed] [Google Scholar]
- 83.el-Azazy OME, Scrimgeour EM, 1997. Crimean-Congo haemorrhagic fever virus infection in the western province of Saudi Arabia. Trans R Soc Trop Med Hyg 91: 275–278. [DOI] [PubMed] [Google Scholar]
- 84.Todd CS, Mansoor GF, Buhler C, Rahimi H, Zekria R, Fernandez S, Mikhail AFW, Scott PT, Yingst SL, 2016. Prevalence of zoonotic and vector-borne infections among Afghan national army recruits in Afghanistan. Vector Borne Zoonotic Dis 16: 501–506. [DOI] [PubMed] [Google Scholar]
- 85.Shanmugam J, Smirnova SE, Chumakov MP, 1976. Presence of antibody to arboviruses of the Crimean haemorrhagic fever-Congo (CHF-Congo) group in human beings and domestic animals in India. Indian J Med Res 64: 1403–1413. [PubMed] [Google Scholar]
- 86.Chinikar S, et al. 2012. Serological evaluation of Crimean-Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan province of Iran and genetic analysis of circulating strains. Vector Borne Zoonotic Dis 12: 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mostafavi E, Chinikar S, Esmaeili S, Amiri FB, Tabrizi AM, KhakiFirouz S, 2012. Seroepidemiological survey of Crimean-Congo hemorrhagic fever among sheep in Mazandaran Province, northern Iran. Vector Borne Zoonotic Dis 12: 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mostafavi E, Haghdoost A, Khakifirouz S, Chinikar S, 2013. Spatial analysis of Crimean Congo hemorrhagic fever in Iran. Am J Trop Med Hyg 89: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Telmadarraiy Z, Ghiasi SM, Moradi M, Vatandoost H, Eshraghian MR, Faghihi F, Zarei Z, Haeri A, Chinikar S, 2010. A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis 42: 137–141. [DOI] [PubMed] [Google Scholar]
- 90.Tahmasebi F, Ghiasi SM, Mostafavi E, Moradi M, Piazak N, Mozafari A, Haeri A, Fooks AR, Chinikar S, 2010. Molecular epidemiology of Crimean- Congo hemorrhagic fever virus genome isolated from ticks of Hamadan Province of Iran. J Vector Borne Dis 47: 211–216. [PubMed] [Google Scholar]
- 91.Mohammadian M, et al. 2016. Molecular assay on Crimean Congo hemorrhagic fever virus in ticks (Ixodidae) collected from Kermanshah Province, western Iran. J Arthropod Borne Dis 10: 381–391. [PMC free article] [PubMed] [Google Scholar]
- 92.Alam MM, Khurshid A, Rana MS, Aamir UB, Salman M, Ahmad M, 2017. Surveillance of Crimean-Congo haemorrhagic fever in Pakistan. Lancet Infect Dis 17: 806. [DOI] [PubMed] [Google Scholar]
- 93.Darwish MA, Hoogstraal H, Roberts TJ, Ghazi R, Amer T, 1983. A sero-epidemiological survey for Bunyaviridae and certain other arboviruses in Pakistan. Trans R Soc Trop Med Hyg 77: 446–450. [DOI] [PubMed] [Google Scholar]
- 94.Begum F, Wisseman CL, Jr., Casals J, 1970. Tick-borne viruses of west Pakistan. IV. Viruses similar to or identical with, Crimean hemorrhagic fever (Congo-Semunya), Wad Medani and Pak Argas 461 isolated from ticks of the Changa Manga forest, Lahore district, and of Hunza, Gilgit agency, W. Pakistan. Am J Epidemiol 92: 197–202. [DOI] [PubMed] [Google Scholar]
- 95.Karapetân RM, Vorob’ev AG, Semaško IV, Matevosân KŠ, Vopân DS, 1974. Slučaj krymskoj gemorragičeskoj lihoradki v Armânskoj SSR. Med Virusol 22: 260–265. [Google Scholar]
- 96.Matevosân KŠ, Semaško IV, Rubin SG, Čumakov MP, 1974. Antitela k virusu KGL v syvorotkah krovi lûdej i krupnogo rogatogo skota v Armânskoj SSR. Med Virusol 22: 173–175. [Google Scholar]
- 97.Semaško IV, Čumakov MP, Matevosân Kš, Safarov RK, Marutân ÈM, Postoân SR, Karapetân RM, Baškircev VN, Tkačenko EA, Čunihin SP, 1975. Itogi rabot v 1972–1974 gg. po vydeleniû i izučeniû virusov KGL-Kongo, Dhori i Bhandža v Azerbajdžane i Armenii. Čumakov MP, ed. Voprosy medicinkoj virusologii, October 21–23, Moscow, USSR, 354–355. [Google Scholar]
- 98.Matevosân KŠ, Semaško IV, Marutân ÈM, Rubin SG, Čumakov MP, 1974. Obnaruženie v Armânskoj SSR virusa Krymskoj gemorragičeskoj lihoradki v kleŝah Hyalomma plumbeum plumbeum, Hyalomma anatolicum, Rhipicephalus bursa, Boophilus calcaratus. Med Virusol 22: 169–172. [Google Scholar]
- 99.Hepburn MJ, Rivard R, 2018. A seroprevalence study of prior exposure to select arthropod-borne and zoonotic infections among rural communities in three regions of Azerbaijan (unpublished). United States Army Medical Research Institute of Infectious Diseases.
- 100.Ismailova ST, Rubin SG, Čhumakov MP, Khankišhiev AM, Manafov IN, Berezin VV, Rešhetnikov IA, 1972. Izučenie potencial’nyh očagov Krymskoj gemorragičeskoj lihoraki (KGL) v Azerbajdžane po dannym serologičeskogo obsledovaniâ domašnih životnyh v reakcii diffuzionnoj precipitacii v agare (RDPA). Čumakov MP, ed. Aktual’nye problemy virusologii i profilaktiki virusnyh zabolevanij. October 24–27, Moscow, USSR, 365–366.
- 101.Sokolova EI, Mirzoeva NM, Kulieva NM, 1976. O vydelenii arbovirusov iz kleŝej v Azerbajdžanskoj SSR. Čumakov MP, ed. Tezisy dokladov na III Vsesoûznom soveŝanii po teoretičeskoj i prikladnoj akarologii, October 4–6, Tashkent, USSR, 218.
- 102.Vashakidze E, Mikadze I, 2015. Epidemiology, clinical and laboratory features of Crimean-Congo hemorrhagic fever in Georgia. Georgian Med News 247: 54–58. [PubMed] [Google Scholar]
- 103.Greiner AL, Mamuchishvili N, Salyer SJ, Stauffer K, Geleishvili M, Zakhashvili K, Morgan J; Centers for Disease Control And Prevention , 2015. Notes from the field: increase in reported Crimean-Congo hemorrhagic fever cases—country of Georgia, 2014. MMWR Morb Mortal Wkly Rep 64: 228–229. [PMC free article] [PubMed] [Google Scholar]
- 104.Greiner AL, Mamuchishvili N, Kakutia N, Stauffer K, Geleishvili M, Chitadze N, Chikviladze T, Zakhashvili K, Morgan J, Salyer SJ, 2016. Crimean-Congo hemorrhagic fever knowledge, attitudes, practices, risk factors, and seroprevalence in rural Georgian villages with known transmission in 2014. PLoS One 11: e0158049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuchuloria T, et al. 2014. Viral hemorrhagic fever cases in the country of Georgia: acute febrile illness surveillance study results. Am J Trop Med Hyg 91: 246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khistawi AN, 2017. Electronic communication. Baghdad, Iraq: Iraq CDC Surveillance section.
- 107.Tantawi HH, Shony MO, Al-Tikriti SK, 1981. Antibodies to Crimean-Congo haemorrhagic fever virus in domestic animals in Iraq: a seroepidemiological survey. Int J Zoonoses 8: 115–120. [PubMed] [Google Scholar]
- 108.Al-Nakib W, Lloyd G, El-Mekki A, Platt G, Beeson A, Southee T, 1984. Preliminary report on arbovirus-antibody prevalence among patients in Kuwait: evidence of Congo/Crimean virus infection. Trans R Soc Trop Med Hyg 78: 474–476. [DOI] [PubMed] [Google Scholar]
- 109.Schwarz TF, Nitschko H, Jäger G, Nsanze H, Longson M, Pugh RNH, Abraham AK, 1995. Crimean-Congo haemorrhagic fever in Oman. Lancet 346: 1230. [DOI] [PubMed] [Google Scholar]
- 110.International Society of Infectious Diseases , 2017. Crimean-Congo Hem. Fever–Oman: Case Numbers. Available at: http://www.promedmail.org/. Accessed September 22, 2017.
- 111.Hassanein KM, el-Azazy OME, Yousef HM, 1997. Detection of Crimean-Congo haemorrhagic fever virus antibodies in humans and imported livestock in Saudi Arabia. Trans R Soc Trop Med Hyg 91: 536–537. [DOI] [PubMed] [Google Scholar]
- 112.Memish ZA, et al. 2011. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg Infect Dis 17: 2316–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hassanein KM, El-Azazy OME, 2000. Isolation of Crimean-Congo hemorrhagic fever virus from ticks on imported Sudanese sheep in Saudi Arabia. Ann Saudi Med 20: 153–154. [DOI] [PubMed] [Google Scholar]
- 114.Buczek A, Bartosik K, Buczek S, Zając Z, 2017. Anomalies in Hyalomma marginatum larvae (Acari: Ixodidae) in relation to taxonomic studies. Syst Appl Acarology 22: 423–430. [Google Scholar]
- 115.Cikman A, Aydin M, Gulhan B, Karakecili F, Kesik OA, Ozcicek A, Akin H, Kara M, 2016. Seroprevalence of Crimean-Congo hemorrhagic fever virus in Erzincan Province, Turkey, relationship with geographic features and risk factors. Vector Borne Zoonotic Dis 16: 199–204. [DOI] [PubMed] [Google Scholar]
- 116.Bayram Y, Parlak M, Özkaçmaz A, Çıkman A, Güdücüoǧlu H, Kılıç S, Berktaş M, Andac CA, 2017. Seroprevalence of Crimean-Congo hemorrhagic fever in Turkey’s Van Province. Jpn J Infect Dis 70: 65–68. [DOI] [PubMed] [Google Scholar]
- 117.Serter D, 1980. Present status of arbovirus sero-epidemiology in the Aegean region of Turkey. Zentralbl Bakteriol 9: 155–161. [Google Scholar]
- 118.Mertens M, Schuster I, Sas MA, Vatansever Z, Hubalek Z, Güven E, Deniz A, Georgiev G, Peshev R, Groschup MH, 2016. Crimean-Congo hemorrhagic fever virus in Bulgaria and Turkey. Vector Borne Zoonotic Dis 16: 619–623. [DOI] [PubMed] [Google Scholar]
- 119.Orkun Ö, Karaer Z, Çakmak A, Nalbantoğlu S, 2017. Crimean-Congo hemorrhagic fever virus in ticks in Turkey: a broad range tick surveillance study. Infect Genet Evol 52: 59–66. [DOI] [PubMed] [Google Scholar]
- 120.McCartan BM, Hunter AG, Pegram RG, Bourne AS, 1987. Tick infestations on livestock in the Yemen Arab Republic and their potential as vectors of livestock diseases. Trop Anim Health Prod 19: 21–31. [DOI] [PubMed] [Google Scholar]