Abstract.
The burden of Plasmodium falciparum (Pf) malaria in Kenya is decreasing; however, it is still one of the top 10 causes of morbidity, particularly in regions of western Kenya. Between April 2015 and June 2016, we enrolled 965 apparently healthy children aged 0–15 years in former Nyanza and Western Provinces in Kenya to characterize the demographic, geographic, and household risk factors of asymptomatic malaria as part of an epidemiologic study to investigate the risk factors for endemic Burkitt lymphoma. The children were sampled using a stratified, multistage cluster sampling survey design. Malaria was assessed by rapid diagnostic test (RDT) and thick-film microscopy (TFM). Primary analyses of Pf malaria prevalence (pfPR) are based on RDT. Associations between weighted pfPR and potential risk factors were evaluated using logistic regression, accounting for the survey design. Plasmodium falciparum malaria prevalence was 36.0% (27.5%, 44.5%) by RDT and 22.3% (16.0%, 28.6%) by TFM. Plasmodium falciparum malaria prevalence was positively associated with living in the lake-endemic area (adjusted odds ratio [aOR] 3.46; 95% confidence interval [95% CI] 1.63, 7.37), paternal occupation as peasant farmer (aOR 1.87; 1.08, 3.26) or manual laborer (aOR 1.83; 1.00, 3.37), and keeping dogs (aOR 1.62; 0.98–2.69) or cows (aOR 1.52; 0.96–2.40) inside or near the household. Plasmodium falciparum malaria prevalence was inversely associated with indoor residual insecticide spraying (IRS) (aOR 0.44; 0.19, 1.01), having a household connected to electricity (aOR 0.47; 0.22, 0.98), and a household with two (aOR 0.45; 0.22, 0.93) or ≥ three rooms (aOR 0.41; 0.18, 0.93). We report high but geographically heterogeneous pfPR in children in western Kenya and significant associations with IRS and household-level socioeconomic factors.
INTRODUCTION
Plasmodium falciparum (Pf) malaria exerts substantial morbidity and mortality worldwide and accounted for an estimated 216 million cases and 445,000 deaths in 2016, mostly among children living in sub-Saharan Africa, including Kenya.1,2 Although the burden of malaria in Kenya has been decreasing, mirroring global trends,3 malaria still ranks among the top 10 causes of morbidity and was estimated to cause between 3.5 and 9.6 million clinical cases in 2016.4 The malaria burden in Kenya varies by geography, with areas neighboring Lake Victoria having the highest reported prevalence (∼42.4% by rapid diagnostic test [RDT]) and designated the lake-endemic malaria zone.5,6 Malaria transmission in the adjacent western Kenya highlands is lower (prevalence ∼4.9%), tends to be seasonal, and is characterized by epidemics, whose magnitude vary from year to year; hence, this zone is designated as highland-epidemic malaria zone.6 The well-demarcated malaria-epidemiologic zones in Kenya lend themselves to targeted malaria control,7 but there are challenges about how to deal with > 75% of malaria infections, which are usually asymptomatic.8–10 People who are asymptomatically infected constitute a major source of new infections11,12 because they are more likely to be bitten by mosquitoes than parasite-free individuals,13 they are usually not detected or treated, and are often mobile,10,14 which increases their potential to establish foci of transmission in distant areas.15
A better understanding of the dynamics of asymptomatic malaria in western Kenya may help design interventions to efficiently target high malaria burden regions.16 Our interest in the dynamics of asymptomatic malaria in Kenya had an additional motivation. Plasmodium falciparum malaria is suspected to be causally related to endemic Burkitt lymphoma (eBL),17 but the evidence remains incomplete. Previous research on malaria in Kenya has been conducted in a few relatively small geographical areas,15,18 in children of a restricted age range,19 and in clinical cases20 or children attending school21,22; therefore, those data are imperfect for eBL studies. This study aimed to generate baseline data to enable precise evaluation of malaria patterns in healthy eBL-age children in the regions of western Kenya where eBL incidence is high. Specifically, we were interested in measuring household- and individual-level risk factors of P. falciparum malaria in children enrolled from representative villages in western Kenya.
MATERIALS AND METHODS
Setting of the study.
Between April 2015 and June 2016, we conducted a stratified, multistage cluster design cross-sectional survey to enroll healthy children aged 0–15 years in 100 randomly selected villages (Figure 1) in two administrative regions of Kenya, formerly known as Nyanza and Western Province (Figure 2A). Children enrolled in the cross-sectional survey will serve as controls in a case–control study of eBL called EpideMiology of Burkitt Lymphoma in East African Children and Minors (EMBLEM).23 The two regions wrap around Lake Victoria, which is about 3,700 ft. above sea level and extend inland into the Great Rift Valley that rises to about 4,600 ft. above sea level. The study area was divided into the lake-endemic and highland-epidemic areas, based on definitions used by the Kenya National Malaria Control Program.6 The lake-endemic area was further subdivided into lakeshore districts, defined as districts with a lake shoreline, except for Kuria West and East, which are at a higher altitude, and inland districts, defined as districts separated from the shoreline by one or more districts (as shown in Figure 2B). The climate is warm (18–31°C), wet (48–250 mm of rainfall per month, with two rainfall seasons), and humid and, therefore, conducive to perennial malaria transmission in the lakeshore area, but drier (27–250 mm per month) and cooler (10–25°C), and thus not conducive to malaria transmission in the adjacent highlands.6,24
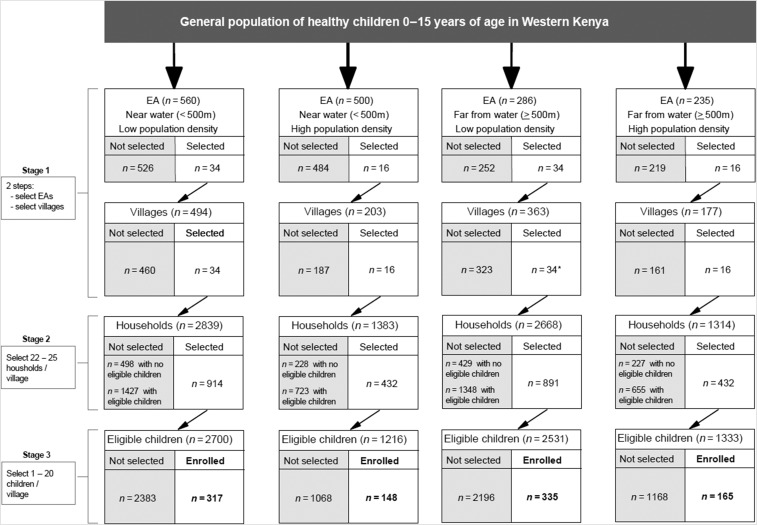
Figure 1.
Diagram of sampling scheme used in the recruitment of apparently healthy children aged 0–15 years between April 2015 and June 2016 from the Western and Nyanza provinces of Kenya. *No child was enrolled from one of the 34 selected villages in the EA far from water and low population density.
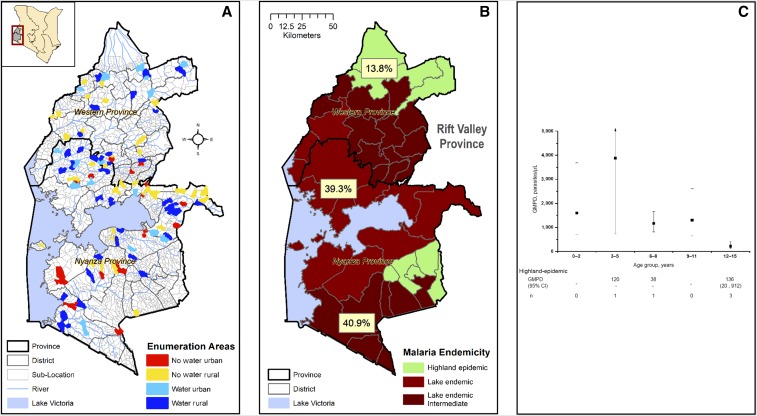
Figure 2.
(A) Map showing the study area in Kenya. The location of the study area is marked by a rectangular box, and the details of study area are shown in the zoom out including regions named Western Province and Nyanza Province, based on the old administrative names present before the Constitutional change of 2013. Details include the provincial, district, and sublocation, which is also the census enumeration area (EA), boundaries. The selected EAs are color coded to indicate their distribution and stratification: rural or urban status and proximity to surface water (see Methods and Materials). (B) This figure shows Plasmodium falciparum prevalence (pfPR) in the study region divided into malaria-epidemiological zones (see Introduction). The lake-endemic area is divided into a lakeshore area (lighter shade of red, labeled lake-endemic) and a more inland area (darker shade of red, lake-endemic intermediate), and the highland-epidemic (lime green). (C) Age-specific patterns of geometric mean parasite density (GMPD), parasites/μL. Data for the lake-endemic area are plotted. The solid square represents the GMPD and short horizontal bars connected by a vertical line represent 95% confidence bands of the GMPD. Because of sparse data, GMPD results for the highland-epidemic area are not graphed but tabulated below the graph. The numbers shown in the graph are for children who were positive on TFM with quantified parasites. The total number of children tested by thick film microscopy (TFM) is similar to those shown in Figure 3B, except for the 6- to 8-year age group in which 18 children were tested by TFM. This figure appears in color at www.ajtmh.org.
Ethics approval and consent to participate.
The Moi University Institutional Research and Ethics Committee (000536) and the National Cancer Institute Special Studies Institutional Review Board reviewed and approved the study (10-C-N133). Written informed consent was obtained from the parents or guardians of the children and written informed assent was obtained from children aged seven years or older prior to enrollment.
Sampling design.
A stratified multistage cluster design (Figure 1) was implemented to enroll a representative sample of children.25 In the first stage, 100 census enumeration areas (EAs) were selected from a national census list,26 grouped into four strata, namely, “low population density,” “high population density,” “near water,” and “far from water.” These were the areas we expected to be associated with malaria transmission.24,25 Enumeration areas were defined as low-density stratum (rural) when they had fewer than 2,235 children aged 0–15 years (i.e., below the average EA childhood population in western Kenya); otherwise the EAs were classified as high-density EA stratum (urban). Based on distances estimated from geographical information system maps of Kenya, EAs with a boundary next to or within 500 m of an all-season surface water body (swamp, river, or lake) were defined as near-water stratum; otherwise they were classified as far-from-water EA stratum. Thus, 50 EAs were sampled from the “far-from-water” stratum and 50 from the “near-water” stratum, both with probability proportional to size, in such a way that 34 of the EAs were from the low-density stratum EAs and 16 of the EAs were from the high-density stratum. In the second stage, one village per EA was randomly selected. In the third stage, all households in a village were counted, listed, and 22–25 households randomly selected per village. Finally, 1–20 children in the selected households were enrolled at their homes, based on a predetermined age and gender distribution of typical eBL cases in western Kenya and invited to take part. Enrollment was implemented such that about half of the villages were enrolled during the dry season and the other half during the wet season. Children aged 0–15 years old who were usual residents of the household and did not report any symptoms of malaria or another illness requiring medical treatment on the day of interview were eligible.
Participant enrollment and malaria testing.
Study activities involved a one-time cross-sectional contact with participants. Following written consent of a parent/guardian and assent of children older than 7 years, experienced field teams administered structured questionnaires to record demographic and household information (Tables 1, 3, and 4). Venous blood specimens for research (10 mL) and clinical tests (4 mL) were collected from each child in ethylenediaminetetraacetic acid (EDTA) tubes. Research blood specimens were transported in cold boxes to local EMBLEM field laboratories within 2 hours of collection; centrifuged for 15 minutes at 1,300 g to separate plasma, buffy coat, and red cell fractions; and all samples were stored in barcoded cryovials at −80°C. Clinical specimens were immediately tested for human immunodeficiency virus (HIV) using commercial RDTs and for malaria parasitemia using thick-film microscopy (TFM) and RDT. As per guidelines, three approved commercial HIV test kits were used, namely, Determine HIV1/2, Stat Pack, and Unigold. Determine HIV1/2 was used as the screening test, Stat Pack was used as the confirmatory test for a Determine positive test, and Unigold was used as the tie breaker, in case Determine and Stat Pack results disagreed. A positive HIV test result was based on at least two positive test results. No child aged < 1 year was found to be HIV-positive, so the issue of positivity from maternal antibodies did not arise. Rapid diagnostic test was based on the P. falciparum malaria histidine-rich protein 2 (HRP2) antigen (CareStart™ MALARIA HRP2 (Pf); ACCESSBIO, Somerset, NJ), which has a false-positive rate of 1.5% for P. falciparum.27 Thick-film microscopy was performed on thick-film slides stained with 10% Giemsa for 10 minutes. Visualized asexual malaria parasite forms were counted against 200 white blood cells (WBCs) and standardized to the WBC count/μL. Thin-film smears were examined to identify Plasmodia species. Plasmodium falciparum was seen in ∼98% of the samples; therefore, this species is assumed in this report. Rapid diagnostic tests have high sensitivity and specificity in different evaluations when compared with conventional light microscopy,28 the gold standard for confirming malaria parasitemia29 and are gaining popularity for malaria surveillance in endemic areas.30,31 The RDTs proved easy to perform, interpret, and robust under field conditions. Furthermore, because malaria antigens appear in blood before asexual parasites and remain in blood for several weeks after asexual parasites have been cleared from blood,32 individuals who are RDT positive but TFM negative can be considered as malaria positive, although without visualized parasites and not as false positives. Only eight (0.6%) children were deemed to have clinical malaria, based on reporting a fever that was verified (≥ 37.5°C) and having > 2,500 parasites/μL at the time of interview.33 These children were not excluded from the study but referred for evaluation and treatment as per National Treatment Guidelines.
Table 1.
Characteristics of 965 asymptomatic children aged 0–15 years surveyed between April 2015 and June 2016 in western Kenya
| Characteristic | N = 965* | Weighted%† (95% confidence interval) |
|---|---|---|
| Demographics | ||
| Age, years | ||
| 0–5 | 334 | 31.1 (27.2, 35.1) |
| 6–10 | 401 | 41.0 (36.8, 45.2) |
| ≥ 11 | 230 | 27.9 (23.6, 32.2) |
| Gender | ||
| Female | 448 | 47.8 (43.7, 52.0) |
| Male | 517 | 52.2 (48.0, 56.3) |
| Stratification variables | ||
| Proximity to water | ||
| Far (> 500 m) | 500 | 34.1 (25.4, 42.7) |
| Near (≤ 500 m) | 465 | 65.9 (57.3, 74.6) |
| Population density of children 0–15 years | ||
| Low (< 2,235) | 652 | 47.7 (38.9, 56.6) |
| High (≥ 2,235) | 313 | 52.3 (43.4, 61.1) |
| Geographical variables | ||
| Region | ||
| Nyanza Province | 648 | 52.6 (38.1, 67.1) |
| Western Province | 317 | 47.4 (32.9, 61.9) |
| Malaria endemicity | ||
| Highland epidemic | 60 | 17.1 (4.5, 29.7) |
| Lake endemic | 905 | 82.9 (70.3, 95.5) |
| Season of enrollment‡ | ||
| Dry | 267 | 26.7 (15.4, 37.9) |
| Wet | 698 | 73.3 (62.1, 84.6) |
| Parental characteristics | ||
| Mother’s education | ||
| Up to standard 4 | 182 | 20.4 (14.5, 26.3) |
| Standard 5–7 | 456 | 45.4 (39.9, 50.9) |
| ≥ Senior secondary school | 316 | 32.8 (26.8, 38.8) |
| Father’s education | ||
| Up to standard 4 | 151 | 17.7 (12.6, 22.8) |
| Standard 5–7 | 371 | 36.4 (30.7, 42.1) |
| ≥ Senior secondary school | 414 | 43.1 (36.3, 49.8) |
| Mother’s occupation | ||
| Trader/sales | 254 | 27.3 (21.3, 33.3) |
| Peasant farmer | 570 | 60.0 (52.6, 67.4) |
| Manual laborer | 133 | 11.6 (8.3, 15.0) |
| Father’s occupation | ||
| Trader/sales | 243 | 24.2 (18.2, 30.3) |
| Peasant farmer | 422 | 45.8 (38.2, 53.5) |
| Manual laborer | 291 | 28.8 (23.2, 34.4) |
| Mother’s income§, Kenyan shillings | ||
| ≤ 5,703 | 856 | 85.5 (81.0, 89.9) |
| > 5,703 | 109 | 14.5 (10.1, 19.0) |
| Malaria prevention | ||
| Slept under mosquito net the night before | ||
| No | 363 | 34.4 (27.8, 41.1) |
| Yes | 591 | 63.5 (57.1, 69.9) |
| Indoor residual insecticide sprayed in house | ||
| No | 880 | 90.8 (86.3, 95.3) |
| Yes | 71 | 7.2 (2.7, 11.7) |
| Don’t know | 4 | 0.1 (0.0, 0.3) |
| History of malaria treatment | ||
| Inpatient | ||
| Yes, past 12 months | 95 | 8.6 (6.1, 11.1) |
| Yes, > 12 months | 117 | 14.5 (10.7, 18.2) |
| Never | 743 | 75.0 (70.6, 79.5) |
| Outpatient | ||
| Yes, past 12 months | 539 | 55.3 (49.4, 61.3) |
| Yes, > 12 months | 64 | 6.7 (4.3, 9.1) |
| Never | 352 | 36.1 (30.0, 42.2) |
The column shows the unweighted frequencies for each category of the characteristics examined.
The column shows the weighted percent for each variable. The weighted percentages may not add up to 100% for some variables because of missing data. The weighted population was estimated to be 1,663,200 with a coefficient of variation of 1.2.
January to March and July to August were classified as dry season months, whereas April to June and September to December were classified as wet season months.
Income categorized based on the international poverty line of $1.90 per a day, which is approximately equal to 5,703 Kenyan shillings for the average 30-day monthly income. Total household income or father’s income was not analyzed because the results for these were considered unreliable.
Table 3.
Unadjusted ORs of factors associated with Plasmodium falciparum parasite infection among children aged 0–15 years surveyed between April 2015 and June 2016 in western Kenya
| Unadjusted | ||||
|---|---|---|---|---|
| Characteristics | N = 949* | Weighted % | OR (95% confidence interval) | P† |
| All subjects | 949 | 36.0 | ||
| Demographics | ||||
| Age, years | 0.14 | |||
| 0–5 | 325 | 36.3 | Ref | |
| 6–10 | 398 | 39.9 | 1.17 (0.77, 1.75) | |
| ≥ 11 | 226 | 29.9 | 0.75 (0.39, 1.43) | |
| Gender | 0.09 | |||
| Female | 441 | 39.6 | Ref | |
| Male | 508 | 32.8 | 0.74 (0.53, 1.05) | |
| Design variables | ||||
| Proximity to water | 0.74 | |||
| Far (> 500 m) | 494 | 34.0 | Ref | |
| Near (≤ 500 m) | 455 | 37.1 | 1.15 (0.51, 2.57) | |
| Population density of children 0–15 years | 0.90 | |||
| Low (< 2,235) | 637 | 36.6 | Ref | |
| High (≥ 2,235) | 312 | 35.5 | 0.96 (0.46, 1.98) | |
| Geographical | ||||
| Region | 0.60 | |||
| Nyanza Province | 637 | 38.2 | Ref | |
| Western Province | 312 | 33.6 | 0.82 (0.39, 1.72) | |
| Malaria endemicity | < 0.001 | |||
| Highland epidemic | 60 | 13.8 | Ref | |
| Lake endemic | 889 | 40.7 | 4.29 (2.06, 8.95) | |
| Season of enrollment‡ | 0.47 | |||
| Dry | 265 | 40.5 | Ref | |
| Wet | 684 | 34.4 | 0.77 (0.38, 1.58) | |
| Parental characteristics | ||||
| Mother’s education | 0.21 | |||
| Up to standard 4 | 180 | 40.7 | Ref | |
| Standard 5–7 | 450 | 38.9 | 0.93 (0.47, 1.83) | |
| ≥ Senior secondary school | 308 | 29.0 | 0.64 (0.29, 1.41) | |
| Father’s education | ||||
| Up to standard 4 | 150 | 41.5 | Ref | 0.32 |
| Standard 5–7 | 363 | 38.2 | 0.87 (0.37, 2.01) | |
| ≥ Senior secondary school | 408 | 31.1 | 0.64 (0.29, 1.41) | |
| Mother’s occupation | 0.28 | |||
| Trader/sales | 251 | 28.8 | Ref | |
| Peasant farmer | 560 | 39.7 | 1.63 (0.88, 2.99) | |
| Manual laborer | 131 | 33.2 | 1.23 (0.62, 2.45) | |
| Father’s occupation | < 0.01 | |||
| Trader/sales | 237 | 22.7 | Ref | |
| Peasant farmer | 418 | 42.6 | 2.53 (1.31, 4.87) | |
| Manual laborer | 285 | 36.3 | 1.94 (1.15, 3.29) | |
| Mother’s income§, Kenyan shillings | 0.63 | |||
| ≤ 5,703 | 841 | 36.7 | Ref | |
| > 5,703 | 108 | 32.2 | 0.82 (0.36, 1.87) | |
| Malaria prevention | ||||
| Slept under mosquito net the night before | 0.45 | |||
| No | 358 | 39.3 | Ref | |
| Yes | 580 | 34.6 | 0.82 (0.48, 1.38) | |
| Indoor residual insecticide sprayed in house | 0.08 | |||
| No | 865 | 37.2 | Ref | |
| Yes | 70 | 22.4 | 0.49 (0.22, 1.08) | |
| History of malaria treatment | ||||
| Inpatient | 0.59 | |||
| Yes, past 12 months | 95 | 41.4 | Ref | |
| Yes, > 12 months | 117 | 38.6 | 0.89 (0.35, 2.24) | |
| Never | 727 | 35.1 | 0.77 (0.41, 1.42) | |
| Outpatient | 0.13 | |||
| Yes, past 12 months | 531 | 39.9 | Ref | |
| Yes, > 12 months | 64 | 23.4 | 0.46 (0.22, 0.98) | |
| Never | 344 | 33.0 | 0.74 (0.43, 1.29) | |
OR = odds ratio.
Results are based on children with complete rapid diagnostic results. Children with missing results (N = 16) are excluded.
The P value is for heterogeneity.
January to March and July to August were classified as dry season months, whereas April to June and September to December were classified as wet season months.
Income categorized based on the international poverty line of $1.90 per a day, which is approximately equal to 5,703 Kenyan shillings for the average 30-day monthly income. Father’s income was not estimated because of concerns about reliability of data.
Table 4.
Unadjusted ORs of additional factors associated with Plasmodium falciparum parasite infection among children aged 0–15 years surveyed between April 2015 and June 2016 in western Kenya
| Unadjusted | ||||
|---|---|---|---|---|
| Characteristics | N = 949* | Weighted % | OR (95% confidence interval) | P† |
| Home characteristics | ||||
| Distance of home from main road | 0.17 | |||
| Far from the main road | 508 | 33.2 | Ref | |
| Near the main road | 330 | 46.0 | 1.71 (0.85, 3.45) | |
| In town or city | 104 | 24.6 | 0.66 (0.27, 1.62) | |
| Distance to water source | 0.73 | |||
| Near (≤ 500 m) | 351 | 34.7 | Ref | |
| Far (>500 m) | 591 | 36.7 | 1.09 (0.67, 1.79) | |
| Source of drinking water | 0.20 | |||
| Unprotected spring/well | 562 | 38.9 | Ref | |
| Protected spring/well | 170 | 38.7 | 0.99 (0.58, 1.70) | |
| Public tap/piped household | 207 | 26.8 | 0.57 (0.30, 1.11) | |
| Connected to electricity grid | 0.01 | |||
| No | 822 | 38.9 | Ref | |
| Yes | 115 | 19.6 | 0.38 (0.18, 0.80) | |
| Number of rooms in house | 0.04 | |||
| 1 room | 138 | 55.9 | Ref | |
| 2 rooms | 389 | 34.7 | 0.42 (0.21, 0.83) | |
| ≥ 3 rooms | 415 | 31.1 | 0.36 (0.15, 0.85) | |
| Number of people sleeping in the same room as child | 0.04 | |||
| 1–2 people | 383 | 32.2 | Ref | |
| 3 people | 255 | 28.8 | 0.85 (0.49, 1.47) | |
| ≥ 4 people | 304 | 45.3 | 1.74 (0.84, 3.61) | |
| Number of usual children residents | 0.95 | |||
| 1–2 people | 346 | 37.0 | Ref | |
| 3 people | 228 | 35.1 | 0.92 (0.50, 1.69) | |
| ≥ 4 people | 368 | 35.2 | 0.93 (0.55, 1.56) | |
| Number of usual adult residents | 0.63 | |||
| 1–2 people | 565 | 35.1 | Ref | |
| 3 people | 181 | 39.7 | 1.22 (0.79, 1.89) | |
| ≥ 4 people | 196 | 34.5 | 0.98 (0.56, 1.71) | |
| Other malaria prevention | ||||
| Owns treated mosquito bed net | 0.20 | |||
| No | 315 | 41.3 | Ref | |
| Yes | 623 | 34.2 | 0.74 (0.46, 1.18) | |
| Regularly uses mosquito insecticide sprays | 0.25 | |||
| No | 908 | 36.8 | Ref | |
| Yes | 24 | 21.8 | 0.48 (0.14, 1.70) | |
| History of fevers and hospital admission | ||||
| Has fever at enrollment | 0.40 | |||
| No | 820 | 36.6 | Ref | |
| Yes | 120 | 30.6 | 0.76 (0.40, 1.44) | |
| Reported ≥ 1 fever in the last 12 months | 0.90 | |||
| No | 312 | 37.2 | Ref | |
| Yes | 509 | 36.5 | 0.97 (0.64, 1.48) | |
| Reported ≥ 1 fever due to malaria in the past 6 months | 0.34 | |||
| No | 281 | 31.9 | Ref | |
| Yes | 659 | 37.6 | 1.29 (0.76, 2.17) | |
| Reported ≥ 1 fever not due to malaria in the last 6 months | 0.59 | |||
| No | 761 | 36.6 | Ref | |
| Yes | 176 | 32.9 | 0.85 (0.46, 1.55) | |
| Reported ≥ 1 hospital admission | 0.71 | |||
| No | 682 | 35.7 | Ref | |
| Yes | 254 | 37.6 | 1.08 (0.71, 1.66) | |
| Animals kept near or inside house | ||||
| Chicken | 0.31 | |||
| No | 111 | 28.2 | Ref | |
| Yes | 831 | 37.1 | 1.50 (0.68, 3.34) | |
| Pigs | 0.04 | |||
| No | 883 | 34.4 | Ref | |
| Yes | 59 | 58.8 | 2.73 (1.07, 6.92) | |
| Goat | 0.46 | |||
| No | 648 | 34.8 | Ref | |
| Yes | 294 | 38.8 | 1.19 (0.75, 1.89) | |
| Sheep | 0.36 | |||
| No | 748 | 35.2 | Ref | |
| Yes | 194 | 39.9 | 1.23 (0.79, 1.90) | |
| Cows | 0.07 | |||
| No | 365 | 29.9 | Ref | |
| Yes | 577 | 39.3 | 1.52 (0.96, 2.40) | |
| Birds | 0.39 | |||
| No | 881 | 36.6 | Ref | |
| Yes | 61 | 26.5 | 0.63 (0.21, 1.84) | |
| Dogs | 0.03 | |||
| No | 298 | 29.1 | Ref | |
| Yes | 642 | 40.5 | 1.66 (1.05, 2.63) | |
OR = odds ratio.
Results shown do not include children with missing rapid diagnostic test results (N = 16).
The P value is for heterogeneity.
Data management.
Questionnaires and laboratory forms were reviewed for completeness, accuracy, and clarity and then computerized by DataFax. Consistency checks were performed on the electronic data and corrections applied, in consultation with field staff.
Statistical analysis.
The results were weighted by the inverse probability of children in the two regions (approximately 1,663,200 children aged 0–15 years) being sampled into the study.25 Weights were trimmed by replacing the value of the weights in the highest 5th percentile of the weight distribution with the value of the weight at the 95th percentile to minimize the impact of outlier weights. The variance estimation took sampling weights and the clustering of children in the village into account. The performance of RDT to detect asexual parasites was validated by calculating sensitivity and specificity against TFM performed by experienced local microscopists.28,30 Because malaria antigens appear in blood before asexual parasites and remain in blood for several weeks after asexual parasites have been cleared from blood,32 our primary analyses of Pf prevalence (pfPR) are based on RDT, which captures both current and recent infection. The geometric mean parasite density (GMPD) was calculated in participants with asexual parasites visualized by TFM. Associations between pfPR and study covariates were assessed by odds ratios (ORs) and associated Wald-type 95% confidence intervals (95% CIs) using Proc Survey logistic (SAS version 9.4, Cary, NC). The independent association between pfPR and 10 uncorrelated variables with a P < 0.10 in the unadjusted analysis or those selected a priori as risk factors for malaria or eBL was assessed using multivariate models. As the goal of this study was hypothesis generation, these results were not corrected for multiple comparisons, such as with a Bonferroni method, and a two-sided P ≤ 0.05 was considered statistically significant.
RESULTS
Characteristics of study population.
Overall, 965 (98.6%) of 979 children invited were enrolled, representing 7,851 eligible children living in 2,696 selected households in 99 of 100 selected villages. One village from which one child was selected to be enrolled was dropped because one parent insisted that the other parent be present to give permission, but it was not possible to arrange a second visit to this village because this happened toward the end of the fieldwork, which ended shortly thereafter.
The weighted population distribution is shown in Table 1 and Supplementary Table 1: 41.0% of children were aged 6–10 years, 52.2% were males, 52.3% lived in high-density villages, 65.9% lived in villages near (within 500 m) surface water, and 52.6% were from Nyanza Province. Most (82.9%) children were from the lake-endemic malaria area, with 44.2% living in subareas abutting Lake Victoria and 38.7% in more inland areas, and 17.1% lived in the highland-epidemic malaria area (as shown in Figure 2B). Most mothers and fathers had attained standard 5 or higher education (78.2% and 79.5%, respectively); occupation was peasant or subsistence farmer for 60.0% of the mothers and 45.8% of the fathers; and 85.5% of mothers had a monthly income ≤ 5,703 Kenyan shillings (equivalent to US$ 1.90 per day, based on the poverty index by the World Bank). Ownership of long-lasting treated mosquito bed nets was 69.7%, and 63.5% of the children were reported to have slept under a treated net the night before the interview. By contrast, only 7.2% of households were reported to have received indoor residual insecticide spraying (IRS). History of inpatient malaria treatment was reported for 23.1% of the children, including 8.6% with inpatient treatment in the past 12 months and 14.5% more than 12 months before interview. Outpatient malaria treatment was reported for 62.0% of the children, including 55.3% in the past 12 months and another 6.7% in the period more than 12 months before the interview. HIV infection was positive in 1.1% (n = 15; youngest HIV‐positive child was age 2 years) of the children.
Weighted P. falciparum malaria prevalence and density.
The weighted cross-sectional pfPR was 36.0% (95% CI: 27.5%, 44.5%) by RDT and 22.3% (16.0%, 28.5%) by TFM performed by experienced local technicians (Table 2). The sensitivity of RDT to detect parasitemia was 82.0% (75.1%, 89.0%) and the specificity was 76.8% (69.4%, 84.1%), as compared with TFM performed by experienced local technicians. Following the rationale described in the Materials and Methods, the 157 individuals who were RDT positive but TFM negative (18.1%; Table 2) were considered as having malaria antigenemia but not visualized parasites rather than as false positives.
Table 2.
Weighted results showing the validation of RDT to detect parasitemia using thick-film microscopy by experienced local microscopists, among children 0–15 years surveyed between April 2015 and June 2016 in western Kenya
| TFM by experienced local technicians | |||
|---|---|---|---|
| Negative weighted % (n) | Positive weighted % (n) | Total weighted % (n) | |
| Rapid diagnostic test | |||
| Negative | 59.6 (532) | 4.0 (39) | 63.6 (571) |
| Positive | 18.1 (157) | 18.3 (206) | 36.4 (363) |
| Total | 77.7 (689) | 22.3 (245) | 100.0 (N = 934*, N weighted = 1,612,821) |
RDT = rapid diagnostic test; TFM = thick film microscopy. The sensitivity of detecting parasitemia by RDT was 82.0% (75.1%, 89.0%) and the specificity was 76.8% (69.4%, 84.1%) as compared with TFM by experienced local technicians.
Results do not include 31 children not successfully tested: two missing RDT results, 15 missing TFM results, and 14 missing both.
The weighted GMPD, among those with visualized parasites on TFM, was 901 parasites/μL (95% CI: 460, 1,767). Geometric mean parasite density was higher in the TFM-positive/RDT-positive group than the TFM-positive/RDT-negative group (1,122 versus 179 parasites/μL; P < 0.001). Geometric mean parasite density was higher among children in the lake-endemic area than those in the highland-epidemic area (1,046 versus 132 parasites/μL; P = 0.04) and peaked in children aged < 6 years compared with older children (2,917 versus 554 parasites/μL; P < 0.001). These GMPD patterns were driven by results in the lake-endemic but could not be assessed in the highland-epidemic area because only five children were TFM positive (Figure 2C). No differences in GMPD by gender were seen (P = 0.36).
Geographical, demographical, and seasonal pfPR patterns.
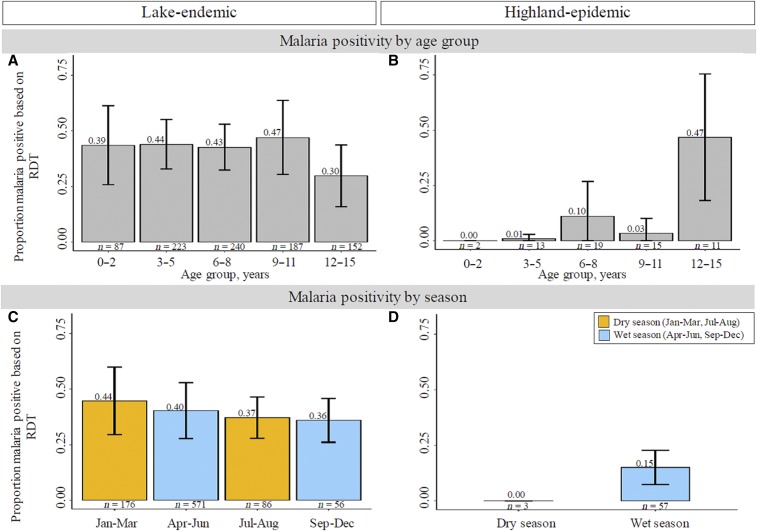
The pfPR was higher in the lake-endemic area than the highland-epidemic area (40.7% versus 13.8%, P < 0.001). However, within the lake-endemic area, pfPR was not different in children living in areas abutting the lakeshore versus those living more inland but sharing similar climate (39.9% versus 41.7%; Figure 2B). Age-specific pfPR in the lake-endemic area was 39.0% in children aged 0–2 years old, slightly higher (43.0–44.0%) in children aged 3–8 years old and peaked (47.0%) in children aged 9–11 years, then moderately lower (30.0%) in those aged 12–15 years (Figure 3A). The age-specific pfPR patterns in the highland-epidemic area children were low (0.0–10.0%) in children aged 0–11 years and spiked in those aged 12–15 years (46.8%), although these results are based on small numbers (Figure 3B). Thus, the age-specific pfPR patterns differed from the age-specific GMPD patterns described previously.
Figure 3.
Multipanel figure showing bar graphs of weighted age group Plasmodium falciparum prevalence (pfPR) in children aged 0–15 years in western Kenya in the lake-endemic (A) and highland-epidemic (B) areas and seasonal pfPR patterns in the lake-endemic (C) and highland-epidemic (D) areas. The dry season was defined as January to March and July to August; the wet season was defined as April to June and September to December. The results from the early and late dry and wet season months were combined for the highland-epidemic areas because of sparse data. The horizontal bars connected by a vertical line represent 95% confidence bands for the pfPR. This figure appears in color at www.ajtmh.org.
Pf malaria prevalence was perennial (unrelated to season) in the lake-endemic areas (Figure 3C) but it was high only in in the wet season in the highland-epidemic areas (Figure 3D), although this interpretation is based on only three children enrolled in the dry season.
Factors associated with asymptomatic malaria infection.
The unadjusted associations between pfPR and studied covariates are shown in Tables 3 and 4. An increased pfPR was associated with living in the lake-endemic (versus the highland-epidemic area: OR 4.29; 95% CI: 2.06, 8.95) and with having a father whose occupation was reported as peasant farmer (OR 2.53; 95% CI: 1.31, 4.87) or manual laborer (versus trader or sales: OR 1.94; 95% CI: 1.15, 3.29; Table 3). An increased pfPR was associated with keeping pigs (OR 2.73; 95% CI: 1.07, 6.92), dogs (OR 1.66; 95% CI: 1.05, 2.63), and, to a lesser extent, cows inside or near the house (versus not: OR 1.52; 95% CI: 0.96, 2.40; Table 4).
Pf malaria prevalence was inversely associated with male gender (versus female: OR 0.74; 95% CI: 0.53, 1.05) and with reporting IRS application to the household (versus not: OR 0.49; 95% CI: 0.22, 1.08; Table 3). Plasmodium falciparum malaria prevalence tended to be inversely associated with higher educational attainment by parents, but the trend results did not reach statistical significance (Table 3). In addition, pfPR was inversely associated with living in a household connected to electricity (versus not: OR 0.38; 95% CI: 0.18, 0.80) and with living in a house with two rooms (OR 0.42; 95% CI: 0.21, 0.83) or more than two rooms (versus one room: OR 0.36; 95% CI: 0.15, 0.85; Table 4).
Pf malaria prevalence was not associated with sleeping under a mosquito bed net the night before the interview, a history of inpatient or outpatient malaria treatment, proximity of village to surface water, population density, season, fever at the time of enrollment, reported fevers due to malaria or other illnesses, the number of lifetime hospital admissions, or HIV status (Tables 3 and 4).
In the adjusted analysis, pfPR was positively associated with living in the lake-endemic area (adjusted OR [aOR] 3.46; 95% CI: 1.63, 7.37) and with the father’s occupation reported as peasant farmer (aOR 1.87; 95% CI: 1.08, 3.26) or manual laborer (aOR 1.83; 95% CI: 1.00, 3.37; Table 5). Keeping dogs and cows inside or near the house was associated with a higher pfPR, albeit not statistically significant. Plasmodium falciparum malaria prevalence was inversely associated with living in a house connected to electricity (aOR 0.47; 95% CI: 0.22, 0.98), living in a house where IRS was applied (aOR 0.44; 95% CI: 0.19, 1.01), and living in a house with two rooms (aOR 0.45; 95% CI: 0.22, 0.93) or more than two rooms (aOR 0.41; 95% CI: 0.18, 0.93), but the relationship was not monotonic. The inverse association between pfPR and male gender tended to persist in the adjusted analysis (aOR 0.70; 95% CI: 0.48, 1.02; Table 5), albeit not statistically significant.
Table 5.
Adjusted ORs of factors associated with Plasmodium falciparum parasite infection among children aged 0–15 years surveyed between April 2015 and June 2016 in western Kenya
| Characteristics | OR* (95% confidence interval) | P† |
|---|---|---|
| Demographical | ||
| Age, years | 0.22 | |
| 0–5 | Ref | |
| 6–10 | 1.22 (0.77, 1.95) | |
| ≥ 11 | 0.85 (0.40, 1.80) | |
| Gender | 0.06 | |
| Female | Ref | |
| Male | 0.70 (0.48, 1.01) | |
| Geographical | ||
| Malaria endemicity | < 0.01 | |
| Highland epidemic | Ref | |
| Lake endemic | 3.46 (1.63, 7.37) | |
| Parental characteristics | ||
| Father’s occupation | 0.05 | |
| Trader/sales | Ref | |
| Peasant farmer | 1.87 (1.08, 3.26) | |
| Manual laborer | 1.83 (1.00, 3.37) | |
| Malaria prevention | ||
| Indoor residual insecticide sprayed in house | 0.05 | |
| No | Ref | |
| Yes | 0.44 (0.19, 1.01) | |
| Home characteristics | ||
| Connected to electricity grid | 0.04 | |
| No | Ref | |
| Yes | 0.47 (0.22, 0.98) | |
| Number of rooms in house | 0.08 | |
| 1 room | Ref | |
| 2 rooms | 0.45 (0.22, 0.93) | |
| ≥ 3 rooms | 0.41 (0.18, 0.93) | |
| Animals kept near or inside house | ||
| Pigs | 0.36 | |
| No | Ref | |
| Yes | 1.52 (0.62, 3.75) | |
| Cows | 0.09 | |
| No | Ref | |
| Yes | 1.60 (0.92, 2.78) | |
| Dogs | 0.06 | |
| No | Ref | |
| Yes | 1.61 (0.97, 2.68) | |
OR = odds ratio.
All variables in the table are adjusted for one another (see Methods).
The P value is for heterogeneity.
DISCUSSION
Our study was conducted to generate population-level data about low-grade, mostly asymptomatic, malaria in the western regions of Kenya that also has a high incidence of eBL.34 This aggressive childhood cancer has been linked to perennial malaria transmission,35,36 it shows a strong ecological correlation with malaria transmission zones in western Kenya,34 and peaks in children aged 6–9 years who are morel likely to be asymptomatic parasite carriers.37 Thus, our comprehensive analysis of household- and individual-level exposures in healthy children of an age typical of eBL cases in western Kenya38 provides baseline data about malaria patterns in this region relevant to malaria public health authorities and eBL researchers.23,25
Our study found high but geographically heterogeneous pfPR in children in the eBL age range in western Kenya. We observed strong inverse associations between pfPR and IRS application in the household and with characteristics indicative of high socioeconomic status (a house with two or more rooms, or connected to electricity), and positive associations with characteristics indicative of low socioeconomic status (having a father employed as a peasant farmer or manual laborer). Age-specific pfPR peaked in older children, particularly in the lake-endemic area, mirrors the patterns observed in eBL cases,37 whereas GMPD peaked in younger children, consistent with the notion that younger children have lower antiparasite immunity 39 and that high GMPD might not be a risk factor for eBL.39 Further research on pfPR/GMPD patterns could shed new insights about the role of malaria in eBL.37,40
We confirm the high ownership and use of insecticide-treated bed nets, as reported by 69.7% of the population.41 However, in contrast to the expected reduction in pfPR associated with bed net use, as was shown in a community trial conducted in two districts of western Kenya in 2005–2007,42 bed net use the night before was not associated with significant reduction in the odds of malaria infection in our study. Our results confirm the strong protective effect of IRS against malaria infection, as reported previously in Kenya43 and elsewhere.25,44 Our findings that pfPR was elevated in children with fathers engaged in peasant or manual laborer jobs and decreased in children living in bigger houses and those connected to electricity reaffirm the important role socioeconomic environment of the child plays in influencing malaria risk.6 These results highlight the potential to enhance malaria control through greater use of IRS and policies that improve income and housing construction.45
Our finding of high pfPR in the lake-endemic area is not surprising because Lake Victoria is the main geographical factor influencing the warm, wet, and humid climate that is conducive for perennial malaria transmission.5 However, our finding that pfPR was similar in the areas abutting the lake than those that were more inland (39.3% versus 40.9%) suggests that breeding sites located closer to people’s homes, such as slow-flowing streams, rain ponds, and vegetation, play an important small-area role in maintaining high malaria transmission patterns in both lakeshore and inland areas.46 This reasoning is consistent with the observation that most mosquito vectors tend to breed near sites of human habitation47 and that most fly relatively short distances (< 3 km) to find blood meals,48 which is considerably shorter than the average distance across districts used to define lakeshore versus more inland areas. Our finding of high GMPD (∼1,000 parasites/μL) among asymptomatic children in the lake-endemic area may have public health relevance if we consider that GMPD > 1,000 parasites/μL may be a surrogate of carrying gametocytes.49 Conversely, a lower GMPD, such as that observed in asymptomatic children in the highland-epidemic area (mean < 200 parasites/μL) may indicate a lower likelihood of carrying gametocytes.49 Given the public health interest in suppressing malaria, public health authorities could stress the potential for high GMPD carriers establishing foci for new infections during short-term visits to low endemicity areas.14
We found that keeping certain animals inside or near the house was associated with higher pfPR. These associations may be relevant because animals may divert mosquitoes either away from human hosts, which is also termed zooprophylaxis, or they may attract mosquitoes and incidentally increase mosquito bites for humans tending or in close proximity with the animals, leading to the so-called zoopotentiation.50 Although these mosquito–animal–human interactions are plausible, their directionality is also influenced by the feeding preference of prevalent mosquito vectors, either for humans, also called anthropophilic, or for animals, also called zoophilic.51 In view of the potential animal-related risk for pfPR to households,52,53 further research is needed to understand the human–animal–vector interactions around households and their impact on malaria risk.54
The strengths of our study include using a general population sample of healthy children in western Kenya to study malaria patterns. Our results complement those from studies that enrolled children in clinics55 or schools.56 The limitations of our study include having only cross-sectional data based on a one-time contact with the participant, which may overestimate asymptomatic malaria, and the use of RDT to estimate asymptomatic malaria, which may miss some cases. We also lacked data on the number, proximity of animals in relation to humans, and any recent changes in livestock,57,58 or housing design.47,59 In addition, we did not collect data about specific vectors prevalent in the different areas or the quality of the bed nets.60 We acknowledge that the sample size was relatively small and many comparisons were performed; thus, the results should be considered exploratory.
CONCLUSION
This study confirmed high but geographically variable asymptomatic malaria prevalence in children with low-grade GMPD in eBL-age children in two provinces in western Kenya. Age-specific pfPR and GMPD patterns were discordant (pfPR high in older children, GMPD high in younger children), supporting the hypothesis that chronic carriage of parasites at older ages occurs in children with malaria immunity, which may be relevant for malaria biology in eBL. Our findings about IRS and measures of high socioeconomic status, including house design, highlight approaches that could be implemented to enhance malaria control in Kenya.
Supplementary Material
Acknowledgments:
We thank the study population and communities for their participation. We thank Janet Lawler-Heavner at Westat Inc., (Rockville, MD) and Erisa Sunday at the African Field Epidemiology Network (Kampala, Uganda) for managing the study. We are grateful to the leadership of Moi University, Webuye District, and Homa Bay District Hospital for hosting local field offices and laboratories and supporting the fieldwork. We thank Laurie Buck, Carol Giffen, Greg Rydzak, and Jeremy Lyman at Information Management Services Inc. (Calverton, MD) for coordinating data and preparing data analysis files, and Jeremy for developing study maps.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM, 2014. Malaria. Lancet 383: 723–735. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization , 2017. World Malaria Report 2017 Geneva, Switzerland: WHO. Available at: http://apps.who.int/iris/bitstream/handle/10665/259492/9789241565523-eng.pdf;jsessionid=78C722919AE7E766074B0161AC904208?sequence=1. Accessed April 20, 2017.
- 3.Snow RW, Sartorius B, Kyalo D, Maina J, Amratia P, Mundia CW, Bejon P, Noor AM, 2017. The prevalence of Plasmodium falciparum in sub-Saharan Africa since 1900. Nature 550: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenya National Bureau of Statistics , 2015. National Top 10 Incidences of Diseases: 2009 to 2015 Available at: https://knbs.or.ke/visualizations/?page_id=3215. Accessed March 5, 2018.
- 5.Noor AM, Gething PW, Alegana VA, Patil AP, Hay SI, Muchiri E, Juma E, Snow RW, 2009. The risks of malaria infection in Kenya in 2009. BMC Infect Dis 9: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Malaria Control Programme , 2015. Kenya Malaria Indicator Survey 2015. Available at: https://dhsprogram.com/pubs/pdf/MIS22/MIS22.pdf. Accessed April 20, 2017.
- 7.Goncalves BP, et al. 2017. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]
- 9.Galatas B, Bassat Q, Mayor A, 2016. Malaria parasites in the asymptomatic: looking for the hay in the Haystack. Trends Parasitol 32: 296–308. [DOI] [PubMed] [Google Scholar]
- 10.Walldorf JA, et al. 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One 10: e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z, et al. 2016. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J 15: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coalson JE, et al. 2016. High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J 15: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson A, et al. 2018. Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci USA 115: E4209–E4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, Buckee CO, 2012. Quantifying the impact of human mobility on malaria. Science 338: 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt A, Obala AA, MacIntyre C, Otsyula B, Meara WPO, 2018. Dynamic malaria hotspots in an open cohort in western Kenya. Sci Rep 8: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission , 2017. malERA: an updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med 14: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legason ID, et al. 2017. Evaluating the causal link between malaria infection and endemic Burkitt lymphoma in northern Uganda: a mendelian randomization study. EBioMedicine 25: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC, 2006. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly CE, et al. 2012. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med 9: e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipanga PN, Omondi D, Mireji PO, Sawa P, Masiga DK, Villinger J, 2014. High-resolution melting analysis reveals low Plasmodium parasitaemia infections among microscopically negative febrile patients in western Kenya. Malar J 13: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kepha S, et al. 2016. Plasmodium falciparum parasitaemia and clinical malaria among school children living in a high transmission setting in western Kenya. Malar J 15: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson JC, et al. 2013. Reliability of school surveys in estimating geographic variation in malaria transmission in the western Kenyan highlands. PLoS One 8: e77641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simbiri KO, et al. 2014. Burkitt lymphoma research in east Africa: highlights from the 9(th) African organization for research and training in cancer conference held in Durban, South Africa in 2013. Infect Agent Cancer 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minakawa N, Sonye G, Mogi M, Githeko A, Yan G, 2002. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol 39: 833–841. [DOI] [PubMed] [Google Scholar]
- 25.Maziarz M, et al. 2017. Age and geographic patterns of Plasmodium falciparum malaria infection in a representative sample of children living in Burkitt lymphoma-endemic areas of northern Uganda. Malar J 16: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenya National Bureau of Statistics , 2013. Overview of Census 2009 Available at: https://www.knbs.or.ke/overview-of-census-2009/. Accessed April 16, 2018.
- 27.World Health Organization , 2015. WHO Prequalification of in vitro Diagnostics Programmes Public Report Product: CareStart™ Malaria HRP2/pLDH (Pf/PAN) COMBO Number: PQDx 0136-049-00. Geneva, Switzerland: WHO. Available at: http://www.who.int/diagnostics_laboratory/evaluations/150528_final_report_0136_049_00_malaria_hrp2pldh_pfpan.pdf.
- 28.Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR, 2006. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis 6: 582–588. [DOI] [PubMed] [Google Scholar]
- 29.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH, 2007. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77: 119–127. [PubMed] [Google Scholar]
- 30.World Health Organization Informal Consultation on Recent Advances in Diagnostic Techniques and Vaccines for Malaria , 1996. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. WHO informal consultation on recent advances in diagnostic techniques and vaccines for malaria. Available at: http://www.who.int/iris/handle/10665/49976 Bull World Health Organ 74: 47–54. Accessed April 20, 2017. [PMC free article] [PubMed] [Google Scholar]
- 31.Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, Hoffman SL, 1994. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343: 564–568. [DOI] [PubMed] [Google Scholar]
- 32.Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ, 2001. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg 95: 179–182. [DOI] [PubMed] [Google Scholar]
- 33.Schellenberg JR, Smith T, Alonso PL, Hayes RJ, 1994. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today 10: 439–442. [DOI] [PubMed] [Google Scholar]
- 34.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML, 2007. Spatial clustering of endemic Burkitt’s lymphoma in high-risk regions of Kenya. Int J Cancer 120: 121–127. [DOI] [PubMed] [Google Scholar]
- 35.Kafuko GW, Burkitt DP, 1970. Burkitt’s lymphoma and malaria. Int J Cancer 6: 1–9. [DOI] [PubMed] [Google Scholar]
- 36.Morrow RH, Jr., 1985. Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt’s lymphoma. IARC Sci Publ 60: 177–186. [PubMed] [Google Scholar]
- 37.Emmanuel B, et al. 2011. African Burkitt lymphoma: age-specific risk and correlations with malaria biomarkers. Am J Trop Med Hyg 84: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckle G, et al. 2016. Factors influencing survival among Kenyan children diagnosed with endemic Burkitt lymphoma between 2003 and 2011: a historical cohort study. Int J Cancer 139: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Barraquer I, et al. 2016. Quantifying heterogeneous malaria exposure and clinical protection in a cohort of Ugandan children. J Infect Dis 214: 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston WT, et al. 2014. Relationship between Plasmodium falciparum malaria prevalence, genetic diversity and endemic Burkitt lymphoma in Malawi. Sci Rep 4: 3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noor AM, Amin AA, Akhwale WS, Snow RW, 2007. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med 4: e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fillinger U, Ndenga B, Githeko A, Lindsay SW, 2009. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ 87: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gimnig JE, et al. 2016. The effect of indoor residual spraying on the prevalence of malaria parasite infection, clinical malaria and anemia in an area of perennial transmission and moderate coverage of insecticide treated nets in western Kenya. PLoS One 11: e0145282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinhardt LC, et al. 2013. The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda. Am J Trop Med Hyg 88: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rek JC, et al. 2018. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health 2: e83–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, Matano AS, Owuor PO, Ouma C, 2018. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou G, Munga S, Minakawa N, Githeko AK, Yan G, 2007. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg 77: 29–35. [PubMed] [Google Scholar]
- 48.Hamer GL, et al. 2014. Dispersal of adult Culex mosquitoes in an urban west Nile virus hotspot: a mark-capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl Trop Dis 8: e2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins WE, Warren M, Sullivan JS, Galland GG, 2001. Plasmodium coatneyi: observations on periodicity, mosquito infection, and transmission to Macaca mulatta monkeys. Am J Trop Med Hyg 64: 101–110. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly B, Berrang-Ford L, Ross NA, Michel P, 2015. A systematic, realist review of zooprophylaxis for malaria control. Malar J 14: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaburi JC, Githuto JN, Muthami L, Ngure PK, Mueke JM, Mwandawiro CS, 2009. Effects of long-lasting insecticidal nets and zooprophylaxis on mosquito feeding behaviour and density in Mwea, central Kenya. J Vector Borne Dis 46: 184–190. [PubMed] [Google Scholar]
- 52.Sota T, Mogi M, 1989. Effectiveness of zooprophylaxis in malaria control: a theoretical inquiry, with a model for mosquito populations with two bloodmeal hosts. Med Vet Entomol 3: 337–345. [DOI] [PubMed] [Google Scholar]
- 53.Bouma M, Rowland M, 1995. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans R Soc Trop Med Hyg 89: 351–353. [DOI] [PubMed] [Google Scholar]
- 54.Iwashita H, Dida GO, Sonye GO, Sunahara T, Futami K, Njenga SM, Chaves LF, Minakawa N, 2014. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit Vectors 7: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Githinji S, Noor AM, Malinga J, Macharia PM, Kiptui R, Omar A, Njagi K, Waqo E, Snow RW, 2016. A national health facility survey of malaria infection among febrile patients in Kenya, 2014. Malar J 15: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooker S, Kolaczinski JH, Gitonga CW, Noor AM, Snow RW, 2009. The use of schools for malaria surveillance and programme evaluation in Africa. Malar J 8: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mala AO, Irungu LW, Shililu JI, Muturi EJ, Mbogo CM, Njagi JK, Mukabana WR, Githure JI, 2011. Plasmodium falciparum transmission and aridity: a Kenyan experience from the dry lands of Baringo and its implications for Anopheles arabiensis control. Malar J 10: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nah K, Kim Y, Lee JM, 2010. The dilution effect of the domestic animal population on the transmission of P. vivax malaria. J Theor Biol 266: 299–306. [DOI] [PubMed] [Google Scholar]
- 59.Wanzira H, Kakuru A, Arinaitwe E, Bigira V, Muhindo MK, Conrad M, Rosenthal PJ, Kamya MR, Tappero JW, Dorsey G, 2014. Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis 59: 509–516. [DOI] [PubMed] [Google Scholar]
- 60.Githinji S, Herbst S, Kistemann T, Noor AM, 2010. Mosquito nets in a rural area of western Kenya: ownership, use and quality. Malar J 9: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.