Abstract.
Zika virus (ZIKV) infection is an emerging public health problem, associated with increased risk for Guillain–Barré syndrome and adverse fetal outcomes, including congenital microcephaly. Zika virus sexual transmission is known, but detection of the virus in different parts of the female reproductive tract is not well established. In this case report, we describe prolonged detection of ZIKV RNA in the vaginal secretion and endocervical mucosa from a Brazilian woman convalescent to ZIKV infection. A viral load of 2 × 102 copies/mL was detected up to 31 days after symptom onset in both samples. Other biological fluids, including whole blood, plasma, serum, urine, and saliva samples, were negative for ZIKV RNA. These findings advance the understanding of ZIKV infection and provide data for additional testing strategies.
Introduction
Zika virus (ZIKV) is an emerging Flavivirus that generally causes mild infection in humans but is associated with increased risk for Guillain–Barré syndrome and adverse fetal outcomes, including congenital microcephaly.1 Zika virus is transmitted to humans primarily by Aedes mosquitoes; however, there is growing evidence pointing to ZIKV sexual transmission.2–5 Understanding the mechanisms that underlie ZIKV infections in women and men is critical to develop public guidelines, effective vaccines, and therapies.6 We report the case of ZIKV infection in a Brazilian woman and results from serial samples collected for 54 days. We observed prolonged detection of viral RNA in the vaginal and endocervical mucosa but not in other biological fluids, including blood. These findings might contribute as a potential guide for public health policies in countries with and without autochthonous ZIKV transmission.
Case report
A previously healthy, nonpregnant 39-year-old brown Brazilian woman who did not travel in the previous 3 months presented signs and symptoms consistent with ZIKV infection on February 26 (day 0 after symptoms onset). The first symptoms were fever (39–40°C), fatigue, and anorexia, which persisted until day 16. On day 4, she presented severe nausea which persisted until day 19. On day 6, she was hospitalized in the Municipal Hospital of Maringá city, Paraná state, Brazil, because of the intensity of symptoms. She remained hospitalized until day 28. During the hospitalization, she presented intense headache in the back of the head. On day 28, she developed pruritic rash on the face, chest, back, and arms, which persisted until day 37. On day 54, all symptoms had resolved (Table 1). Several other pathologies were investigated during the hospitalization period, but no other diseases were detected. Therefore, the patient’s symptoms were attributed to ZIKV infection. Her husband did not present symptoms evocative of ZIKV infection. The patient reported not having sexual intercourse during the illness. This study was reviewed and approved by the Committee for Ethics in Research Involving Humans at the State University of Maringá (UEM), Paraná, Brazil (CAAE 56724716.4.0000.0104; permission 2.326.874/2017).
Table 1.
Timeline of acute signs and symptoms and clinical progression/resolution of a Brazilian woman infected with Zika virus
| Day after symptoms onset | Signs, symptoms, and clinical progression |
|---|---|
| 0 | Fever (39–40°C), fatigue, and anorexia |
| 4 | Symptoms continued and severe nausea began |
| 6 | Patient was hospitalized because of the intensity of the symptoms |
| 16 | Fever fatigue and anorexia resolved |
| 19 | Severe nausea had resolved |
| Hospitalization days (6–28) | Patient presented intense headache in the back of the head |
| 28 | She left the hospital; pruritic rash developed on her face, chest, back, and arms |
| 37 | Pruritic rash had resolved |
| 54 | All symptoms had resolved |
On day 6, serum was collected for arbovirus research and differentiation of Zika, dengue, and chikungunya viruses using a qualitative reverse transcription–polymerase chain reaction (RT-PCR). Eluted RNA from the serum was tested using the BIO GENE ZDC MULTIPLEX PCR kit (BioClin, Minas Gerais, Brazil). Previously, to remove any PCR inhibitors, serum was incubated for 15 minutes with proteinase K in phosphate-buffered saline and then centrifuged. RNA was extracted using the AxyPrep™ BodyFluid Miniprep kit (Axygen, San Francisco, CA), according to the manufacturer’s instructions. The results were negative for dengue and chikungunya viruses and positive for ZIKV RNA by qualitative RT-PCR. We detected ZIKV RNA by a qualitative method and quantified using a quantitative reverse transcription–PCR (qRT-PCR) (Bio Gene Zika Vírus PCR kit; BioClin), presenting a viral load of 2 × 103 copies/mL. In addition, we tested the serum sample using enzyme-linked immunosorbent assay (ELISA) to assess serologic immunoglobulin (Ig) M and IgG for ZIKV and chikungunya virus. This assay is composed by microplates coated with ZIKV nonstructural protein 1 (NS1) synthetic antigen and with immunodominant synthetic antigen derived from the chikungunya envelope protein (ENV) region (Biometrix, Paraná, Brazil). Negative results were obtained for both viruses.
After ZIKV RNA detection on day 6, serial specimens were longitudinally collected for 54 days, more specifically on days 31 and 54. Samples were collected in these time points because the patient had a medical appointment scheduled for clinical follow-up. At day 6, the samples were obtained in the hospital. After day 54, the patient did not return to the appointments and, therefore, no new clinical samples were collected.
Specimens included whole blood, plasma, serum, urine, saliva, vaginal, and endocervical samples. Vaginal samples were collected using an Ayre spatula and endocervical samples using cytobrush, which was inserted and rotated 360 over the cervix. The cytological smears were sent to the Clinical Cytology Laboratory/UEM and graded according to the Bethesda System.7 The cytological findings were negative for intraepithelial lesion or malignancy. Saliva swabs were collected by using the Culture Swab Collection and Transport System (Becton Dickinson, Franklin Lakes, NJ). Vaginal secretion and endocervical mucosa samples were separately and immediately suspended in ThinPrep (Cytyc, Marlborough, MA) solution after collection for ZIKV RNA detection. For molecular analysis, all samples were processed and the RNA was extracted as described previously for the initial serum sample. Eluted RNAs from all samples were tested by qRT-PCR (Bio Gene Zika Vírus PCR kit; BioClin) according to the manufacturer’s instructions.
Between days 6 and 31, the virus was cleared from blood. Serum, plasma, and whole blood were negative for ZIKV RNA at day 31. Saliva and urine samples also presented negative results. Interestingly, we detected ZIKV RNA in the vaginal and endocervical samples up to day 31, with a viral load of 2 × 102 copies/mL in both samples. Between days 31 and 54, the virus was cleared from the vaginal and endocervical samples (Table 2) and remained negative in all other tested samples.
Table 2.
Quantitative reverse transcription–polymerase chain reaction with viral load values of days after the onset of signs and symptoms for different samples obtained from a 39-year-old brown Brazilian woman infected with Zika virus
| Samples | Day 6 | Day 31 | Day 54 |
|---|---|---|---|
| Whole blood | – | Negative | Negative |
| Plasma | – | Negative | Negative |
| Serum | 2 × 103 copies/mL | Negative | Negative |
| Urine | – | Negative | Negative |
| Vaginal | – | 2 × 102 copies/mL | Negative |
| Endocervical | – | 2 × 102 copies/mL | Negative |
– = not performed.
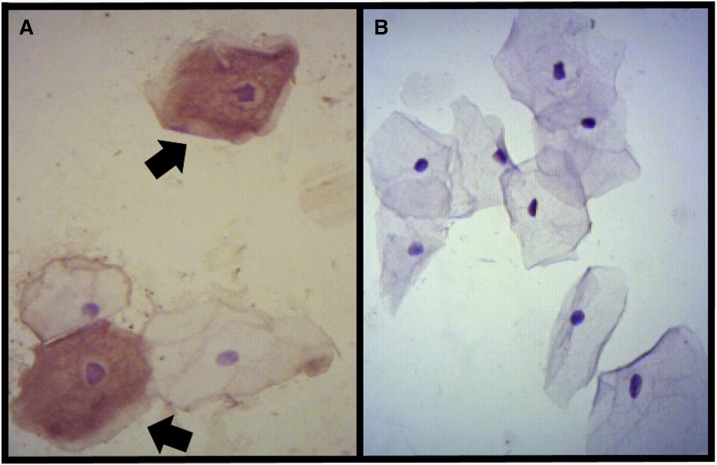
In addition, we performed immunocytochemistry staining of the vaginal–endocervical smears collected on days 31 and 54. The biotin–streptavidin–peroxidase method was performed for immunostaining of the vaginal–endocervical smears to detect the viral antigen using a polyclonal anti-ZIKV antibody.8,9 The sample of day 31 presented a brown color in the squamous cells, which was defined as a positive immunostaining for ZIKV infection (Figure 1A). The sample of day 54 presented a negative immunostaining for ZIKV (Figure 1B).
Figure 1.
Immunocytochemistry staining on the vaginal–cervical cytological smear. (A) Positive immunostaining of Zika virus (ZIKV) antigens in squamous cells (arrow). (B) Negative immunostaining of ZIKV antigens in cells (×400 magnification). This figure appears in color at www.ajtmh.org.
Discussion
The recent ZIKV outbreak evidenced the occurrence of ZIKV vertical transmission and consequently fetal outcomes.10 Male-to-woman, woman-to-male, and male-to-male sexual transmission11 highlighted the presence of infectious virus in almost all body secretions, including those from male and female genital tracts.12–15 We had the unique opportunity to prospectively monitor the clinical course of ZIKV infection in a patient starting on day 0, collecting biological samples on days 6, 31, and 54.
We detected viral shedding in vaginal and endocervical samples on day 31, with a viral load of 2 × 102 copies/mL in both samples. Between days 31 and 54, the virus was cleared from vaginal and endocervical samples. Interestingly, between days 6 and 31, the virus was cleared from serum but remained positive in the vaginal and endocervical samples, although the viral load was lower in vaginal and endocervical samples than in serum of day 6. Moreover, we report for the first time the detection of positive immunostaining for ZIKV infection in the squamous cells of vaginal–endocervical smears, on the same day that the ZIKV RNA was positive in both vaginal and cervical samples (day 31). Interestingly, immunostaining was negative for ZIKV on day 54, in which all samples were also negative for viral RNA, including vaginal and endocervical samples. Taken together, these results could reflect a ZIKV association to cervical and vaginal epithelial cells, despite low viral loads detected in both genital secretions. The absence of viral RNA in urine and saliva on days 31 and 54 reinforces this hypothesis. Although we cannot assert that whether positive results by RT-PCR indicated replicating virus, our findings could give a deeper insight in ZIKV sexual and vertical transmission.
To our knowledge, there are only two human studies reporting ZIKV RNA in vaginal secretion up to days 3 and 1412,16 and only one study reporting ZIKV RNA in cervical mucosa up to day 11.17 The transient presence of ZIKV in the female genital tract in these studies contrasts with an extensive literature about ZIKV persistence in semen, where viral RNA has been detected up to 6 months after the patient’s return from endemic areas.13,18–22 However, in the present case report, vaginal and endocervical samples remained positive for ZIKV RNA for a much longer period than described in these studies (≥ 31 days), even after the serum became negative for ZIKV RNA. This is evidence that as in semen, variations in the rates of positivity and duration of infection in female genital secretions may occur.
We evaluated the serum by performing an ELISA assay to assess IgM and IgG for ZIKV and chikungunya virus; negative results were obtained. These findings reinforced the higher sensitivity of the RT-PCR for the detection of ZIKV in the acute phase.
Finally, there is uncertainty on the recommendation of pregnancy after ZIKV exposure because it is not definite that RNA detection corresponds to active replicating ZIKV. However, our results indicate that a period for safe sexual practices or sexual abstinence is at least 2 months on ZIKV infection.
In conclusion, this case study advances the understanding of ZIKV pathogenesis and provides new findings, including prolonged detection of ZIKV RNA in vaginal and endocervical secretions ≥ 31 days. Moreover, ZIKV RNA was not detected in other biological fluids including whole blood, plasma, serum, urine, and saliva on day 31. Finally, the pruritic rash developed on the patient’s face, chest, back, and arms in the late stage of infection (day 37), which we presume was associated to the infection. To our knowledge, this finding has not been previously described. These findings might contribute as a potential guide for public health policies in countries with and without autochthonous ZIKV transmission. However, further investigations involving larger cohorts in individuals with acute ZIKV infection are needed to understand the implications of viral genital shedding on vertical and sexual transmission.
Acknowledgments:
We would like to acknowledge Pedro Fernando da Costa Vasconcelos/Instituto Evandro Chagas for providing the polyclonal anti-ZIKV antibody.
REFERENCES
- 1.Yun SI, Lee YM, 2017. Zika virus: an emerging Flavivirus. J Microbiol 55: 204–219. [DOI] [PubMed] [Google Scholar]
- 2.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB, 2011. Probable non–vectorborne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P, 2016. Male-to-male sexual transmission of Zika virus–Texas, January 2016. MMWR Morb Mortal Wkly Rep 65: 372–374. [DOI] [PubMed] [Google Scholar]
- 4.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D, 2016. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep 65: 716–717. [DOI] [PubMed] [Google Scholar]
- 5.Russell K, et al. 2017. Male-to-female sexual transmission of Zika virus-United States, January–April 2016. Clin Infect Dis 64: 211–213. [DOI] [PubMed] [Google Scholar]
- 6.Durbin AP, 2016. Vaccine development for Zika virus-timelines and strategies. Semin Reprod Med 34: 299–304. [DOI] [PubMed] [Google Scholar]
- 7.Nayar R, Wilbur DC, 2017. The Bethesda System for reporting cervical cytology: a historical perspective. Acta Cytolog 61: 359–372. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo RSS, et al. 2016. Zika virus epidemic in Brazil. I. Fatal disease in adults: clinical and laboratorial aspects. J Clin Virol 85: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azevedo RSS, et al. 2018. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci Rep 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez S, et al. 2016. Confirmed case of Zika virus congenital infection, Spain, March 2016. Euro Surveill 21: 30261. [DOI] [PubMed] [Google Scholar]
- 11.Moreira J, Peixoto TM, Siqueira AM, Lamas CC, 2017. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect 23: 296–305. [DOI] [PubMed] [Google Scholar]
- 12.Murray KO, et al. 2017. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis 23: 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G, 2016. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill 21: 30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicastri E, Castilletti C, Di Caro A, Capobianchi MR, Ippolito G, 2016. Diagnosis of Zika virus infection in pregnant women travelling to or residing in endemic areas. Lancet Infect Dis 16: 771–772. [DOI] [PubMed] [Google Scholar]
- 15.Barzon L, Lavezzo E, Palù G, 2017. Zika virus infection in semen: effect on human reproduction. Lancet Infect Dis 17: 1107–1109. [DOI] [PubMed] [Google Scholar]
- 16.Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, Leparc-Goffart I, Molina JM, 2017. Infectious Zika virus in vaginal secretions from HIV-infected woman, France, August 2016. Euro Surveill 22: 30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G, 2016. Zika virus in the female genital tract. Lancet Infect Dis 16: 1000–1001. [DOI] [PubMed] [Google Scholar]
- 18.Harrower J, Kiedrzynski T, Baker S, Upton A, Rahnama F, Sherwood J, Huang QS, Todd A, Pulford D, 2016. Sexual transmission of Zika virus and persistence in semen, New Zealand, 2016. Emerg Infect Dis 22: 1855–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y, 2016. Long-lasting persistence of Zika virus in semen. Clin Infect Dis 63: 1264. [DOI] [PubMed] [Google Scholar]
- 20.Gaskell KM, Houlihan C, Nastouli E, Checkley AM, 2017. Persistent Zika virus detection in semen in a traveler returning to the United Kingdom from Brazil, 2016. Emerg Infect Dis 23: 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, Palù G, 2016. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill 21: 30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson B, Thorburn F, Petridou C, Bailey D, Hewson R, Simpson AJ, Brooks TJ, Aarons EJ, 2017. Presence and persistence of Zika virus RNA in semen, United Kingdom, 2016. Emerg Infect Dis 23: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]