Abstract.
The local signs and symptoms following snakebites are similar to those of cellulitis caused by bacterial infections. This leads to empirical treatment with antibiotics, which however is not supported by evidence. Procalcitonin (PCT) is a biomarker with good diagnostic accuracy for bacterial infection. We studied serum PCT concentration in 100 patients aged 13 years or more, presenting to the hospital with significant local manifestations (crossing the joint proximal to the bitten wound) within 24 hours after snakebite. The extent and progression of local manifestations were monitored 12 hourly. Baseline PCT measurement was carried out for all patients and measurement was repeated 12 hourly only in those patients with progressive local manifestations. The median interqartile range PCT concentration did not differ significantly by the severity of local manifestation at presentation (Grade 2 = 0.28 [0.26–0.30]; Grade 3 = 0.28 [0.26–0.32]; Grade 4 = 0.27 [0.26–0.32] ng/mL; P = 0.15). Furthermore, we did not observe an increase in PCT concentration on serial estimation in those with progressive local manifestation (0.28, 0.29, and 0.29 ng/mL) over 36 hours. These observations suggest that the local manifestations following snakebites were not caused by bacterial infection.
Snakebites are a major health problem in developing countries all over the world, particularly in the Southeast Asian region.1 In India, it is estimated that about 45,900 people die because of snakebites annually, mostly in rural areas.2 Most of the snakebites in India are attributed to the “big four” species, namely Naja naja (Indian cobra), Bungarus caeruleus (Indian krait), Daboia russelii (Russell’s viper), and Echis carinatus (saw-scaled viper).3 Local manifestations such as erythema, warmth, and tender swelling are the early and important signs of envenomation seen in all of the aforementioned snakebites except krait. The proteolytic properties of snake venom cause extensive tissue destruction and devitalization locally, predisposing the wound to bacterial infection from a broad range of aerobic and anaerobic microorganisms commensal on the patient’s skin or present in the snake’s oral cavity.4 Skin and soft tissue infections are a major complication of snakebites. The incidence of secondary skin and soft tissue infection after snakebites varies based on the geographic location and species of snakes, and it is reported to be between 3% and 77%.5–8 Gram-negative microorganisms were isolated in more than 60% patients, whereas in a previous study from our institute Gram-positive microorganisms were more frequently isolated.9
After snakebites, local manifestations usually include redness, warmth, swelling, pain, tenderness, lymphadenopathy, and fever, mimicking those due to bacterial infection. It is hard to tell whether the local manifestations are due to venom or due to bacterial infection. Prophylactic antibiotics are routinely used in patients with local manifestations after snakebites, despite no clear benefits from such therapy reported in previous clinical studies.6,10–13 It is important to know whether local manifestations after snakebites are due to venom or bacteria to avoid inappropriate antibiotic usage.
Inflammatory markers, such as white blood cell count and C-reactive protein, are less specific for bacterial infections.14 Procalcitonin (PCT) has higher diagnostic accuracy for bacterial infections14 and also correlates with severity of bacterial invasion.15 Therefore, this study was carried out to measure the serum PCT concentration in patients with significant local manifestations after snakebite, to correlate its concentration with varying degrees of local manifestations and to document the rise in PCT with increasing severity of local manifestations.
This was a prospective, observational, hospital-based study conducted between August 2014 and July 2016 in a tertiary care teaching hospital in South India. The study protocol was approved by the institutional ethics committee. Written informed consent was obtained from the patients or their legally acceptable representatives before entry into the study. A convenient sample of 100 patients with local manifestations after snakebite was included in the study as there were no previous studies to calculate the sample size. Patients aged 13 years or more, presenting to the hospital with significant local manifestations (crossing the joint proximal to the bitten wound) within 24 hours after snakebites, were included in the study. Patients were excluded if they had been given antibiotics; undergone manipulation of the wound, such as cutting and suturing; or had a focus of bacterial infection identified elsewhere.
The proximal extent of local manifestations were marked and measured with a measuring tape at the time of presentation. Local manifestations were monitored 12 hourly, and their progression was noted by measuring the length. Local manifestations were graded based on their extent, complications, and systemic manifestations as follows: Grade 0 – fang marks and minimal local pain; Grade 1 – fang marks, local pain, and 2.5–15 cm of edema and erythema; Grade 2 – fang marks, pain, 15–30 cm of edema and erythema, and systemic symptoms; Grade 3 – fang marks, pain, edema > 30 cm, and systemic symptoms; Grade 4 – Grade 3 + edema of the ipsilateral trunk, and local complications such as necrosis, blebs, blisters, or compartment syndrome.
Blood samples for serum PCT were obtained between 6 and 24 hours after snakebite for all patients and repeated 12 hourly in patients with progressive local manifestations. If an antibiotic was prescribed, further samples were not taken. Samples were centrifuged and stored at −80°C. Serum PCT concentration was measured with the quantitative Ray Bio® Human PCT ELISA kit. The minimum detectable concentration of human PCT was 30 pg/mL (0.03 ng/mL). All patients were adequately treated with snake antivenom and all treatment decisions were based on the treating physician’s discretion.
Comparison between baseline serum PCT concentrations of different grades of local manifestations was carried out using the Kruskal–Wallis test. Comparison between the serum PCT concentrations in patients with progressive local manifestations was carried out using the Wilcoxon signed rank test and analysis of variance (ANOVA) with repeated measures. All statistical analyses were carried out at a 5% level of significance, and a P value < 0.05 was considered significant.
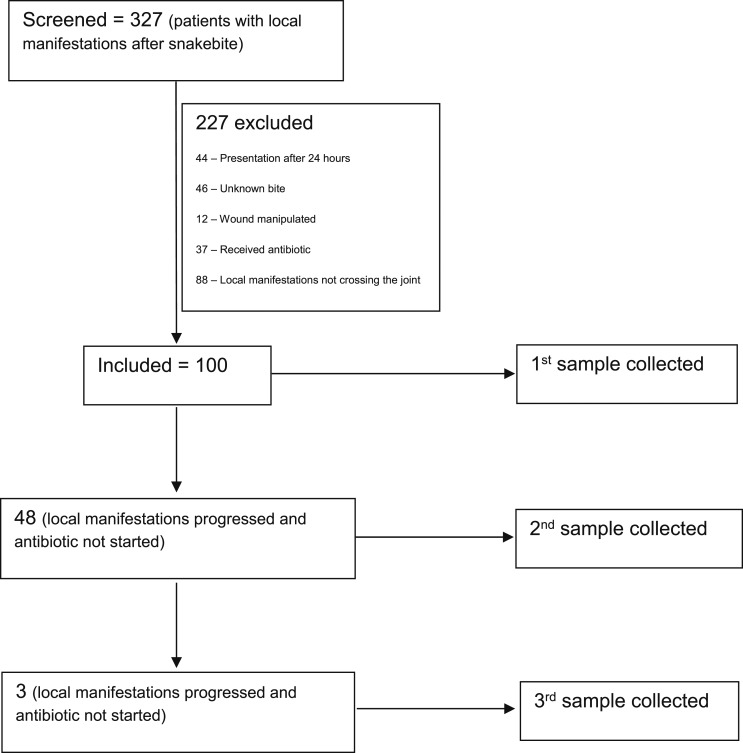
During the study period, 327 patients with snakebites were screened and 100 were included. In 48 patients, local manifestations progressed, so a second sample was taken. Of these 48 patients, three had further progression of local manifestations, so a third sample was taken. The disposition chart of patients is summarized in Figure 1. The median (interqartile range [IQR]) duration from the bite to presentation of patients to our hospital was 7 (4.6–10) hours. The 1st, 2nd, and 3rd samples were taken at a mean (standard deviation [SD]) duration of 13.57 (5.3), 26.6 (5.8), and 39.3 (6.4) hours, respectively, from the time of bite.
Figure 1.
Disposition chart of patients in the study.
The mean age of patients included in this study was 40.15 ± 12.84 years. Snakebites were more common among males (59%). Most of the patients with snakebites were farmers (78%). More than half of the patients had bites to the lower extremities (66%). There were no patients with Grade 0 or 1 local manifestations, 23 had Grade 2 local manifestations, 62 had Grade 3 local manifestations, and 15 had Grade 4 local manifestations. The mean (SD) extent of local manifestations while taking the first sample for 100 patients was 40 (11.28) cm. The local manifestations of the 48 patients for whom the second sample was taken had progressed to 83.32 (20.02) cm. Three patients had further progression of local manifestations to the extent of 111.67 (24.54) cm while taking the 3rd sample.
The median (IQR) PCT concentrations at baseline (n = 100) for patients with Grade 2, 3, and 4 local envenomation were 0.28 (0.26–0.30), 0.28 (0.26–0.32), and 0.27 (0.26–0.32) ng/mL, respectively (Table 1). The difference between the serum PCT concentrations of different grades of local manifestations was not statistically significant (P = 0.15). In patients with progressive local manifestations, the 1st, 2nd, and 3rd median PCT concentrations were 0.28, 0.29, and 0.29 ng/mL, respectively (Table 2). Differences between the 1st, 2nd, and 3rd PCT concentrations were not found to be statistically significant (F = 1.30, P = 0.37).
Table 1.
Procalcitonin concentrations at baseline in various grades of local manifestations after snakebite
| Procalcitonin (ng/mL) | Grade 1 (n = 0) | Grade 2 (n = 23) | Grade 3 (n = 62) | Grade 4 (n = 15) | P-value |
|---|---|---|---|---|---|
| Mean + SD | – | 0.40 ± 0.50 | 0.40 ± 0.41 | 0.40 ± 0.32 | – |
| Median (IQR) | – | 0.28 (0.26–0.30) | 0.28 (0.26–0.32) | 0.27 (0.26–0.32) | 0.15* |
| Minimum | – | 0.08 | 0.05 | 0.17 | – |
| Maximum | – | 2.62 | 3.04 | 1.34 | – |
* IQR = interqartile range; SD = standard deviation; Kruskal–Wallis test.
Table 2.
Serial PCT concentrations in patients with progressive local manifestations after snakebite
| Procalcitonin (ng/mL) | 1st sample (n = 100) | 2nd sample (n = 48) | 3rd sample (n = 3) | P-value |
|---|---|---|---|---|
| Mean ± SD | 0.36 ± 0.36 | 0.39 ± 0.36 | 0.30 + 0.02 | – |
| Median (IQR) | 0.28 (0.27–0.30) | 0.29 (0.27–0.35) | 0.29 | 0.42* |
| Minimum | 0.05 | 0.07 | 0.27 | – |
| Maximum | 2.62 | 2.43 | 0.33 | – |
IQR = interqartile range; PCT = procalcitonin; SD = standard deviation.
* P-value (calculated by Wilcoxon signed rank test) is for the difference between the 1st and 2nd values.
The PCT concentration in normal individuals is less than 0.05 ng/mL,16 and in patients with local bacterial infection, it is between 0.05 and 2.0 ng/mL.17–19 In our study, the median PCT concentration at baseline was 0.28 ng/mL. This value was elevated when compared with normal. At baseline, patients with higher grades of local manifestations did not have higher PCT concentrations than patients with lower grades of local manifestations. Subsequently, there was no increase in PCT concentrations with increasing grades of local manifestations, suggesting that bacterial infection is unlikely to be the cause of local manifestations in patients with snakebites. It is possible that noninfectious inflammatory response produced by the snake venom is responsible for the elevation of PCT values in patients with snakebites as compared with normal individuals.
To our knowledge, this is the first study to measure serum PCT concentration in patients with local manifestations after snakebites. We included patients with significant local manifestations irrespective of systemic symptoms. Therefore, our study results are applicable to patients with snakebites in our geographic area. However, we did not measure the PCT concentration after initiation of antibiotics. Similarly, measuring PCT in a control population such as those with diabetic foot infections matched for the extent of local manifestations may help to clarify the role of bacterial infection in the local manifestations in patients with snakebites.
Procalcitonin concentrations in patients with local manifestations after snakebites were lower than those expected in patients with cellulitis due to bacterial infection. Procalcitonin concentrations were not different in different grades of local manifestations and did not increase with a significant increase in local manifestations. Therefore, we suggest that local manifestations of snakebites may be due to venom itself and not due to bacterial infection. Randomized controlled trials of routine versus as clinically indicated use of antibiotics are needed in patients with local manifestations after snakebites to investigate the appropriate use of antibiotics.
REFERENCES
- 1.Kasturiratne A, Wickremasinghe AR, Silva ND, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ, 2008. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 5: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, Rodriguez PS, Mishra K, Whitaker R, Jha P; Million Death Study Collaborators , 2011. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis 5: e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO , 2016. Guidelines for the Management of Snake-Bites, 2nd edition New Delhi, India: World Health Organization, Regional Office for South-East Asia; Available at: http://apps.searo.who.int/PDS_DOCS/B5255.pdf?ua=1. [Google Scholar]
- 4.Goldstein EJC, 2016. Bites. Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier, 3510–3515. [Google Scholar]
- 5.Clark RF, Selden BS, Furbee B, 1993. The incidence of wound infection following crotalid envenomation. J Emerg Med 11: 583–586. [DOI] [PubMed] [Google Scholar]
- 6.Kerrigan KR, Mertz BL, Nelson SJ, Dye JD, 1997. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg 21: 369–373. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-M, Wu K-G, Chen C-J, Wang C-M, 2011. Bacterial infection in association with snakebite: a 10-year experience in a northern Taiwan medical center. J Microbiol Immunol Infect 44: 456–460. [DOI] [PubMed] [Google Scholar]
- 8.Mao YC, Liu PY, Hung DZ, Lai WC, Huang ST, Hung YM, Yang CC, 2016. Bacteriology of Naja atra snakebite wound and its implications for antibiotic therapy. Am J Trop Med Hyg 94: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A, Sujatha S, Garg J, Acharya NS, Chandra Parija S, 2009. Wound infections secondary to snakebite. J Infect Dev Ctries 3: 221–223. [DOI] [PubMed] [Google Scholar]
- 10.Palappallil DS, 2015. Pattern of use of antibiotics following snake bite in a tertiary care hospital. J Clin Diagn Res 9: OC05–OC09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorge MT, et al. 2004. Failure of chloramphenicol prophylaxis to reduce the frequency of abscess formation as a complication of envenoming by Bothrops snakes in Brazil: a double-blind randomized controlled trial. Trans R Soc Trop Med Hyg 98:529–534. [DOI] [PubMed] [Google Scholar]
- 12.Kularatne SA, Kumarasiri PV, Pushpakumara SK, Dissanayaka WP, Ariyasena H, Gawarammana IB, Senanayake N, 2005. Routine antibiotic therapy in the management of the local inflammatory swelling in venomous snakebites: results of a placebo-controlled study. Ceylon Med J 50: 151–155. [DOI] [PubMed] [Google Scholar]
- 13.Sachett JAG, et al. 2017. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: a randomized controlled clinical trial. PLoS Negl Trop Dis 11: e0005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, Nusbaumer C, Tamm M, Christ-Crain M, 2007. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C, 1993. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP, 2008. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia 51: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eder J, Hlavin G, Haushofer A, Trubert-Exinger D, Trautinger F, 2012. Correlation of serum procalcitonin with the severity of skin and skin structure infections–a pilot study. J Dtsch Dermatol Ges 10: 564–571. [DOI] [PubMed] [Google Scholar]
- 18.Enguix A, Rey C, Concha A, Medina A, Coto D, Diéguez MA, 2001. Comparison of procalcitonin with C-reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensive Care Med 27: 211–215. [DOI] [PubMed] [Google Scholar]
- 19.Aikawa N, et al. 2005. Multicenter prospective study of procalcitonin as an indicator of sepsis. J Infect Chemother 11: 152–159. [DOI] [PubMed] [Google Scholar]