Abstract.
Self-medication with antimalarial drugs is a major factor in the development of drug resistance, exerting subtherapeutic drug pressure on circulating parasite populations. Data on self-medication with antimalarials from the Southern Pacific coast region of Colombia, where 4-aminoquinolines resistance and political instability prevail, are vital to elimination strategies. We present results of an exploratory study of 254 individuals having malaria symptoms who sought malaria diagnosis in two hospitals in Tumaco, Department of Nariño, Colombia. Thirty-two percent (82/254) of participants had positive Saker–Solomons urine tests, indicating self-medication with chloroquine (CQ) before consultation for diagnosis. Notably, among 30 pregnant women participating in the study, 43% were Saker-–Solomons positive. Molecular analysis of the K76T position encoded by the pfcrt gene revealed the mutant allele in all four samples that were both positive for Plasmodium falciparum and positive for the Saker–Solomons test, suggesting persistent CQ pressure. The high frequency of self-medication, particularly among pregnant women merits attention by public health authorities and comprehensive investigation.
INTRODUCTION
The emergence and spread of drug-resistant Plasmodium parasites in Colombia has influenced control measures for malaria. The widespread distribution of chloroquine (CQ)-resistant Plasmodium falciparum motivated the Colombian Ministry of Health to replace CQ with amodiaquine plus sulfadoxine/pyrimethamine as first-line treatment for uncomplicated malaria in 1999.1 Furthermore, variable levels of resistance to these antimalarials led to the implementation of artemisinin-based combination therapies by 2006.2,3 Similarly, Plasmodium vivax resistant to CQ has become an increasing threat in South America4; three clinical studies in Colombia revealed up to 11% clinical failure in patients having vivax malaria treated with CQ.2,4–6
The misuse of antimalarial drugs is a major factor in the emergence of drug resistance.7 Self-medication has been associated with subtherapeutic concentrations leading to selection of resistant parasites.8,9 It is well known that patients living in endemic areas can recognize malaria symptoms and often initiate treatment before diagnosis.8 In addition, because of the antipyretic effect of CQ,10 its use before diagnosis may delay consultation and impair diagnosis.
Self-medication is common in malaria-endemic regions.11 There are several reports from Africa, India, and Latin America, where self-treatment with antimalarials ranged from 13% up to 52%. Community knowledge and experience with malaria symptoms, limited access to health facilities, and relatively easy access to antimalarials without prescription are among the main reasons favoring self-treatment. Efforts to implement home/community-based fever management, education and supervision of pharmacists, and placement of diagnostic kiosks in areas where mining and agricultural activities are being developed should be considered to prevent self-treatment.12–16
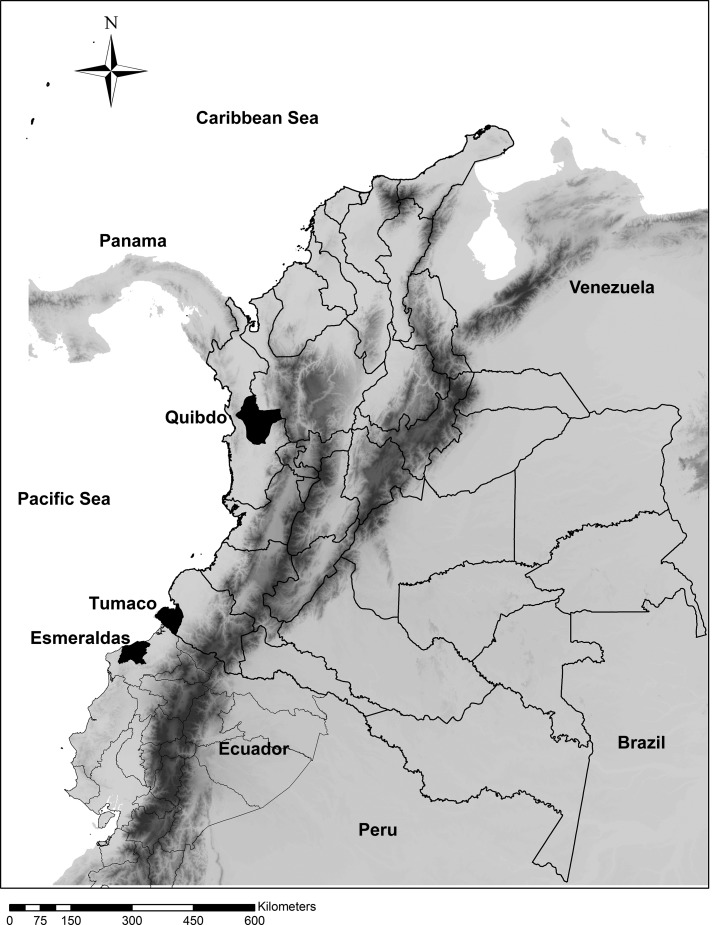
In malaria-endemic areas of Colombia, several factors favor the easy access to CQ: 1) CQ is the first-line treatment for P. vivax infection, 2) it is prescribed for rheumatoid arthritis and lupus, and 3) can be obtained without prescription.17 In addition, socioeconomic factors could also be favoring self-medication in Tumaco (a port city and hub of maritime-related activities located in Southwestern Colombia-Figure 1), where approximately 50% of the population lack access to potable water, electricity, education, and basic health services (Department of the National Administration of Statistics of Colombia).18 Under these circumstances, community members who commonly associate fever with malaria will seek to obtain available antimalarial products for self-treatment, including herbal preparations, CQ beverages such as “Tonico Arquin,”19 and “leftover” antimalarial pills from other community members as a “sharing and saving” practice. A study of knowledge, attitudes, and practices of malaria in Tumaco showed that inhabitants not only recognize fever as a symptom of malaria, but also use antimalarials without prescription.8
Figure 1.
Map of Colombia showing the study site (Tumaco) in the Southern Pacific coast area. The map also depicts Quibdo-Choco (Northern Pacific region) and Esmeraldas in the border country of Ecuador, both municipalities have similar social and political characteristics compared withTumaco.
To our knowledge, there are no published data regarding CQ usage by individuals with symptoms of malaria before diagnosis in Tumaco, a high-prevalence area for malaria, and information from other malaria-endemic regions of Colombia is limited. Our study provides evidence that a high proportion of individuals exhibiting malaria symptoms, including pregnant women, self-medicate with CQ before consulting a malaria diagnosis facility.
METHODS
This study was approved by the institutional ethics committee of Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) in compliance with Colombian legislation for research involving human subjects and the Helsinki declaration amended in 2008. Written consent and assent for minors were obtained for all participants. Samples were collected in two public health facilities: Hospital San Andres and Divino Niño, located in Tumaco, a seaport city on the Pacific Coast of the Department of Nariño (Figure 1). Participants were enrolled from April to June 2011 and November to December of 2012; 257 subjects aged ≥ 6 years seeking malaria diagnosis were enrolled. Demographic data (Table 1) and 3–5 mL of urine were obtained from 254 participants. All participants were evaluated for malaria by routine microscopic evaluation of peripheral blood samples. Positive samples for Plasmodium spp., were confirmed by two expert microscopists.
Table 1.
Absence of relationship between sociodemographic variables and chloroquine uptake as determined by the SS test
| Variable | SS− | SS+ | P-value |
|---|---|---|---|
| (n) | (n) | ||
| Gender* | |||
| Male | 32 | 15 | 0.95 |
| Female | 140 | 67 | |
| Age (years)† | |||
| < 18 | 15 | 2 | 0.058 |
| 18–25 | 77 | 49 | |
| 26–35 | 46 | 23 | |
| 36–45 | 23 | 6 | |
| > 45 | 11 | 2 | |
| Falciparum malaria† | |||
| Positive | 16 | 4 | 0.589 |
| Negative | 154 | 78 | |
| Vivax malaria† | |||
| Positive | 6 | 0 | 0.181 |
| Negative | 154 | 78 | |
| Localization† | |||
| Commune 1 | 13 | 9 | 0.799 |
| Commune 2 | 8 | 6 | |
| Commune 3 | 2 | 1 | |
| Commune 4 | 12 | 8 | |
| Commune 5 | 69 | 30 | |
| Commune 6 | 62 | 25 | |
| Health plan† | |||
| Contributory | 9 | 5 | 0.879 |
| Subsidized | 159 | 75 | |
| None | 1 | 1 | |
| Others | 3 | 1 | |
| Occupation† | |||
| Agriculture-related job | 9 | 2 | 0.579 |
| Housewife | 107 | 49 | |
| Student | 33 | 21 | |
| Other | 23 | 10 | |
| Parasitemia (mean [standard deviation])‡ | 7,839 (11,223) | 4,160 (4,348) | Z = 0.37 |
| Days from symptoms to diagnosis (mean [standard deviation])‡ | 2.4 (2.3) | 2.19 (1.63) | Z = 0.341 |
SS = Saker–Solomons.
* Pearson χ2.
† Fisher’s exact.
‡ Mann–Whitney test.
Presence of CQ and its principal metabolite desethyl-CQ in urine samples was determined using the Saker–Solomons test as an indicator of CQ intake.20 In brief, a stock solution of tetrabromophenolphthalein ethyl ester (TBPEE) was prepared by diluting the TBPEE reagent in chloroform (0.5 mg/mL) and mixing with 10 mL of HCl 2N. The aqueous phase was removed and 10 mL of buffer phosphate pH = 8.0 added to the organic phase. This stock solution was stored at 2–6°C protected from light. The Saker–Solomons working solution was prepared adding 0.2 mL of TBPEE stock solution to 1.0 mL of phosphate buffer. The limit of detection of the Saker–Solomons reagent was evaluated in triplicate against a CQ solution at 0.5 ppm. In addition, cross reactivity with the antimalarials amodiaquine, desethylamodiaquine, mefloquine, lumefantrine, and dihydroartemisinin was evaluated over a concentration range of 1–5 ppm. Two milliliters of urine from each participant were added to 1 mL of Saker–Solomons working solution, agitated for 30 seconds, and left resting vertically for 10 minutes. The formation of a violet–red precipitate indicated the presence of CQ; yellow–green precipitate indicated the absence of CQ. The limit of detection of the Saker–Solomons reagent was determined to be 0.5 ppm of CQ, which is lower than the previously established limit of detection (1.0 ppm).20 No cross-reactivity of the Saker–Solomons reagent with antimalarial drugs (other than CQ) was detected, consistent with previous findings.20
To explore the frequency of the CQ resistance molecular marker K76T encoded by the pfcrt gene, the subset of samples from positive P. falciparum subjects with a positive Saker–Solomons test were screened for this mutation by nested polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP), using primers at 1 µM, deoxynucleotides (dNTPs) at 200 nM each, and MgCl2 at 2.5 mM as described elsewhere with modifications.21 The sequences of the primers for the primary amplification were forward: 5′ CCGTTAATAATAAATACACGCAG 3′ and reverse: 5′ CGGATGTTACAAAACTATAGTTACC 3′, to obtain a 538-bp DNA fragment. For the second amplification, the forward primer was 5′ TGTGCTCATGTCTTTAAACTT 3′ and the reverse primer was 5′ CAAAACTATAGTTACCAATTTTG 3′, to obtain a 145-bp DNA fragment. The Platinum® Taq DNA Polymerase (Invitrogen; Fisher Scientific, Waltham, MA) was used for PCRs and the restriction was performed with the enzyme Apol (New England Biolabs, Beverly, MA). The relationships among sociodemographic variables and Saker–Solomons test were evaluated using STATA/SE12 (StataCorp. 2011. Release 12; StataCorp LP, College Station, TX).
RESULTS
Frequency of self-medication with CQ.
The present study substantiated a high frequency of self-medication (CQ intake before consultation/diagnosis) evidenced by 82/254 (32%) symptomatic individuals consulting for malaria diagnosis having a positive Saker–Solomons test (Table 1). False-positive results due to the uptake of amodiaquine, mefloquine, lumefantrine, and dihydroartemisinin-containing drugs are unlikely in this study because cross-reactivity with these compounds did not occur under the conditions of evaluation. However, previous reports showed that quinine and proguanil exhibit a positive reaction with the Saker–Solomons test.20 Nevertheless, such cross-reactions would still indicate self-medication before consultation. Of concern, 43% (13/30) of pregnant women included in this study had a positive Saker–Solomons test, indicating self-medication.
Low frequency of malaria diagnosis among individuals exhibiting malaria-like symptoms.
Even though all patients included in this study consulted the hospital while experiencing clinical manifestations compatible with malaria, only 8.7% (22/254) had a positive diagnosis for either P. falciparum (16/254) or P. vivax (6/254). In this study, more women (81.5%) than men (18.5%) consulted malaria diagnosis facilities; however, positive malaria results were significantly higher (P < 0.001, χ2-test) in males (12/47, 25%) than in females (10/207, 4.8%). Interestingly, the proportion of males having malaria and positive Saker–Solomons test was higher (2/12, 16.7%) than the proportion of females (1/10, 10%). Overall, none of the sociodemographic variables examined showed significant association with the results of the Saker–Solomons test (Table 1).
Pfcrt mutation conferring resistance to CQ.
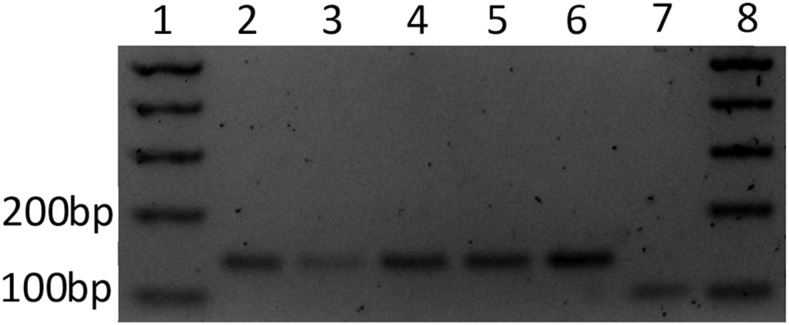
Among 16 patients infected with P. falciparum, four had a positive Saker–Solomons test. The K76T mutant allele was detected in all four corresponding samples (Figure 2).
Figure 2.
Agarose gel of restriction fragments from four subjects infected with Plasmodium falciparum and positive Saker–Solomons test (lanes 2–5). DNA from a positive sample for 76T (Mutant control) (lane 6). DNA from the P. falciparum reference strain 3D7 wild-type for Pfcrt 76 (Lane 7). Wild-type amplicons are cleaved with ApoI into 98-bp and 47-bp fragments (lane 7, showing the 98-bp fragment), whereas mutants exhibiting 76T are not cleaved showing a 145-bp fragment (lanes 2–6). One kilobase DNA ladder (lanes 1 and 8).
DISCUSSION
Most health-care providers recognize that self-medication with CQ is common in Tumaco and other malaria-endemic areas of Colombia. However, there are limited reports/studies of the magnitude of this practice in Colombia. In a previous study in Tumaco and Turbo (a malaria-endemic area in northwest Colombia), interviews regarding self-medication with antimalarials, suggested that up to 13% of the participants self-medicated.22 In this report, we confirmed that self-medication with CQ is a relevant problem in Tumaco. Self-medication among 43% of 30 pregnant women was particularly concerning. This practice should be taken into consideration and discouraged by prenatal control programs. Self-medication with CQ in a patient with falciparum malaria could control fever without necessarily controlling parasitemia, thereby increasing the risk for complicated and/or placental malaria in pregnant women. A comprehensive assessment of and attention to self-medication with antimalarial drugs among pregnant women in this region having limited access to diagnosis is warranted.
One of the most negative effects of self-medication is the selection of drug-resistant microorganisms.11 It has previously been shown that the availability and use of CQ is linked to high prevalence of pfcrt mutant alleles,23 and that withdrawal of CQ has been associated with the reduction of CQ-resistant parasites.24 The frequencies of CQ-susceptible parasites as high as 91% were recently reported in samples from countries of Africa where CQ use was discontinued.25 Chloroquine resistance in P. falciparum is conferred by single nucleotide polymorphisms (SNPs) on the pfcrt gene, including the well-characterized K76T mutation. Consistent with maintenance of selective pressure, we found that the infecting parasites of the four patients that were P. falciparum and Saker–Solomons positive exhibited the mutant allele. Several studies in Colombia have reported a high frequency of parasites exhibiting the pfcrt-K76T mutation even though national health authorities stopped the use of CQ for treatment of falciparum malaria since 1999.26–28 A recent study that evaluated 111 P. falciparum isolates from three different malaria-endemic regions in Colombia (including Tumaco) found the K76T mutation in all tested samples.28 The persistence of P. falciparum CQ–resistant phenotypes despite the discontinuance of CQ for treatment of falciparum malaria almost 20 years ago could be explained by the routine use of CQ to treat P. vivax infection or by extensive self-medication as shown in this exploratory study.
Our results indicate that CQ is widely used even before patients are diagnosed with malaria, preserving CQ-resistant phenotypes and potentially increasing the risk for complicated malaria associated with the apparent relief (antipyretic effect) of symptoms without eliminating parasitemia. Unpublished results from previous studies carried out in Tumaco by CIDEIM investigators discerned patients with falciparum malaria and positive Saker–Solomons test during the years 2006 (4/23), 2007 (1/31), 2009 (2/28), and 2010 (2/39), further supporting the evidence of use of CQ before diagnosis, among malaria patients in this Pacific coast municipality.
In Tumaco, malaria could be considered an occupational disease with males accounting for most of the cases (as corroborated by our results).29 However, our data suggested that men seek medical attention less frequently. Both circumstances, higher frequency of malaria and fewer men consulting diagnostic facilities, could be partially explained by occupational exposure in areas of difficult access involving legal and illegal activities. During the last several years the incidence of malaria in Tumaco has almost quadrupled from an Annual Parasite Index (API) (confirmed number of positive slides for parasites during 1 year for the population under surveillance × 1,000) of 6.9 in 2011 to 25.2 in 2017 (Sistema de Vigilancia en Salud Publica de Colombia - SIVIGILA).30 A contrasting situation has been experienced in Esmeraldas-Ecuador (near the border with Colombia-Figure 1), where the reduction of malaria cases during the last decade was close to 99%.31 These contrasting situations underscore the influence of effective public health policy and intervention (in Esmeraldas), as well as armed conflict, illegal gold mining, and cultivation of illicit crops in promoting the high incidence of malaria and self-treatment in Tumaco. A similar situation occurred on the northern Pacific coast of Colombia in Quibdo-Choco (Figure 1), where political instability and poverty are comparable with that in Tumaco. In Choco, the API increased from 24 in 2011 to 97 in 2016. Similarly, social conflict is affecting malaria control in other areas of the region, a recent study from French Guiana showed that 52.4% of illegal gold miners self-medicated with antimalarials.12 Inaccessible areas, for whatever reason (criminal activity, war, geographic barriers, and poverty) and pharmacy practices of providing medication without prescription could individually and collectively create a path of “least resistance” for febrile individuals to self-medicate for what is perceived to be the most common serious febrile illness in these regions. The dimension and circumstances of self-medication are neither well documented nor understood. As control programs are successful and incidence of malaria declines, other febrile illness prevalent in malaria-endemic regions such as leptospirosis, dengue, chikungunya, and typhoid may be mistreated as malaria, creating yet another problem.
A major limitation of the present study was the sample size, which was determined by convenience. Nevertheless, this exploratory study provides the first direct evidence of CQ usage by individuals in an endemic region of the Colombian Pacific coast with clinical symptoms of malaria, before consulting malaria diagnostic facilities. This evidence provides basis and motivation to address this problem and to design a comprehensive investigation of the magnitude and intervenable circumstances of self-medication with CQ and other antimalarial drugs, and to determine whether this practice could be favoring the selection of resistant P. vivax parasites in the region.
In conclusion, one-third of patients seeking malaria diagnosis had a positive Saker–Solomons test indicating the use of CQ before diagnosis, confounding diagnosis and treatment, and potentially contributing to the loss of drug susceptibility particularly for P. vivax infections.
Acknowledgments:
We would like to thank the patients who participated in this study. We thank the microscopists and health-care workers in the Hospitals Divino Niño and San Andres de Tumaco. Special thanks to Nancy Gore Saravia for her thoughtful revision of the manuscript.
REFERENCES
- 1.Castillo CM, Osorio LE, Palma GI, 2002. Assessment of therapeutic response of Plasmodium vivax and Plasmodium falciparum to chloroquine in a malaria transmission free area in Colombia. Mem Inst Oswaldo Cruz 97: 559–562. [DOI] [PubMed] [Google Scholar]
- 2.Osorio L, Pérez Ldel P, González IJ, 2007. Assessment of the efficacy of antimalarial drugs in Tarapacá, in the Colombian Amazon basin [article in Spanish]. Biomedica 27: 133–140. [PubMed] [Google Scholar]
- 3.Corredor V, Murillo C, Echeverry DF, Benavides J, Pearce RJ, Roper C, Guerra AP, Osorio L, 2010. Origin and dissemination across the Colombian Andes mountain range of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum. Antimicrob Agents Chemother 54: 3121–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonçalves LA, Cravo P, Ferreira MU, 2014. Emerging Plasmodium vivax resistance to chloroquine in South America: an overview. Mem Inst Oswaldo Cruz 109: 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeño N, Dunne M, Berman J, 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg 65: 90–93. [DOI] [PubMed] [Google Scholar]
- 6.Arias AE, Corredor A, 1989. Low response of Colombian strains of Plasmodium vivax to classical antimalarial therapy. Trop Med Parasitol 40: 21–23. [PubMed] [Google Scholar]
- 7.White NJ, 2004. Antimalarial drug resistance. J Clin Invest 113: 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forero DA, Chaparro PE, Vallejo AF, Benavides Y, Gutiérrez JB, Arévalo-Herrera M, Herrera S, 2014. Knowledge, attitudes and practices of malaria in Colombia. Malar J 13: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mawili-Mboumba DP, Ndong Ngomo JM, Maboko F, Guiyedi V, Mourou Mbina JR, Kombila M, Bouyou Akotet MK, 2014. Pfcrt 76T and pfmdr1 86Y allele frequency in Plasmodium falciparum isolates and use of self-medication in a rural area of Gabon. Trans R Soc Trop Med Hyg 108: 729–734. [DOI] [PubMed] [Google Scholar]
- 10.Bojang KA, Schneider G, Forck S, Obaro SK, Jaffar S, Pinder M, Rowley J, Greenwood BM, 1998. A trial of Fansidar plus chloroquine or Fansidar alone for the treatment of uncomplicated malaria in Gambian children. Trans R Soc Trop Med Hyg 92: 73–76. [DOI] [PubMed] [Google Scholar]
- 11.Ocan M, Obuku EA, Bwanga F, Akena D, Richard S, Ogwal-Okeng J, Obua C, 2015. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health 15: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douine M, et al. 2018. Predictors of antimalarial self-medication in illegal gold miners in French Guiana: a pathway towards artemisinin resistance. J Antimicrob Chemother 73: 231–239. [DOI] [PubMed] [Google Scholar]
- 13.Leyva-F R, Erviti-E J, Ramsey JM, Gasman N, 1997. Medical drug utilization patterns for febrile patients in rural areas of Mexico. J Clin Epidemiol 50: 329–335. [DOI] [PubMed] [Google Scholar]
- 14.Nsimba SE, Rimoy GH, 2005. Self-medication with chloroquine in a rural district of Tanzania: a therapeutic challenge for any future malaria treatment policy change in the country. J Clin Pharm Ther 30: 515–519. [DOI] [PubMed] [Google Scholar]
- 15.Blenkinsopp A, Bradley C, 1996. Patients, society, and the increase in self medication. BMJ 312: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez AD, Penilla RP, Henry-Rodríguez M, Hemingway J, Francisco Betanzos A, Hernández-Avila JE, 2003. Knowledge and beliefs about malaria transmission and practices for vector control in southern Mexico. Salud Publica Mex 45: 110–116. [PubMed] [Google Scholar]
- 17.Management Sciences for Health/Strengthening Pharmaceutical Systems , 2010. Technical Report: Assessment of the Availability of Antimalarial Medicines in the Public and Private Markets in Countries of the Amazon Basin. Presented to the U.S. Agency for International Development by the Strengthening Pharmaceutical Systems (SPS) Program. Arlington, VA: Management Sciences for Health. [Google Scholar]
- 18.Departamento Administrativo Nacional de Estadística (DANE) , 2012. Indicador de Necesidades Básicas Insatisfechas (NBI) por Municipios. Colombia. Available at: www.dane.gov.co. Accessed September 27, 2018.
- 19.Valero-Bernal MV, Tanner M, Muñoz-Navarro S, Valero-Bernal JF, 2017. Proportion of fever attributable to malaria in Colombia: potential indicators for monitoring progress towards malaria elimination. Rev Salud Publica 19: 45–51. [DOI] [PubMed] [Google Scholar]
- 20.Mount DL, Nahlen BL, Patchen LC, Churchill FC, 1989. Adaptations of the Saker-Solomons test: simple, reliable colorimetric field assays for chloroquine and its metabolites in urine. Bull World Health Organ 67: 295–300. [PMC free article] [PubMed] [Google Scholar]
- 21.Veiga MI, Ferreira PE, Björkman A, Gil JP, 2006. Multiplex PCR-RFLP methods for pfcrt, pfmdr1 and pfdhfr mutations in Plasmodium falciparum. Mol Cel Probes 20: 100–104. [DOI] [PubMed] [Google Scholar]
- 22.Tobón CA, Giraldo SC, Pineros JJG, Arboleda NM, Blair TS, Carmona-Fonseca J, 2006. Epidemiologia de la malaria falciparum complicada: estudio de casos y controles en Tumaco y Turbo, Colombia, 2003. Revista Brasileira de Epidemiologia 9: 283–296. [Google Scholar]
- 23.Asare KK, Boampong JN, Afoakwah R, Ameyaw EO, Sehgal R, Quashie NB, 2014. Use of proscribed chloroquine is associated with an increased risk of pfcrt T76 mutation in some parts of Ghana. Malar J 13: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frosch AE, Venkatesan M, Laufer MK, 2011. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J 10: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu F, et al. 2017. Return of chloroquine sensitivity to Africa? Surveillance of African Plasmodium falciparum chloroquine resistance through malaria imported to China. Parasit Vectors 10: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restrepo E, Carmona-Fonseca J, Maestre A, 2008. Plasmodium falciparum: high frequency of pfcrt point mutations and emergence of new mutant haplotypes in Colombia. Biomedica 28: 523–530. [PubMed] [Google Scholar]
- 27.Echeverry DF, Holmgren G, Murillo C, Higuita JC, Björkman A, Gil JP, Osorio L, 2007. Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am J Trop Med Hyg 77: 1034–1038. [PubMed] [Google Scholar]
- 28.Aponte S, Guerra Á, Álvarez-Larrotta C, Bernal SD, Restrepo C, González C, Yasnot MF, Knudson-Ospina A, 2017. Baseline in vivo, ex vivo and molecular responses of Plasmodium falciparum to artemether and lumefantrine in three endemic zones for malaria in Colombia. Trans R Soc Trop Med Hyg 111: 71–80. [DOI] [PubMed] [Google Scholar]
- 29.Bonilla E, Rodriguez A, 1993. Determining malaria effects in rural Colombia. Soc Sci Med 37: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 30.Instituto Nacional de Salud de Colombia Sistema de Vigilancia en Salud Publica (SIVIGILA), 2017. Boletines Epidemiologicos de Malaria de 2011 a 2017. Available at: https://www.ins.gov.co/buscador-eventos/Paginas/Info-Evento.aspx. Accessed August 30, 2018.
- 31.Sáenz FE, et al. 2017. Malaria epidemiology in low-endemicity areas of the northern coast of Ecuador: high prevalence of asymptomatic infections. Malar J 16: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]