Abstract.
A subset of multibacillary (MB) leprosy patients manifest with clinical “nonresponsiveness” to the fixed-duration, World Health Organization multidrug therapy MB regimen (WHO-MDT-MBR). The aim of this retrospective study was to assess the effectiveness and safety of alternate anti-leprosy therapy (ALT) in such patients. This is an analysis of patients’ records, registered in the leprosy clinic of our institute over a period of 6 years (2010–2015). The criteria for inadequate response/nonresponsiveness to treatment were as follows: 1) persistent/new lesions after completing ≥ 12 months of WHO-MDT-MBR (isolated reactions were ruled out histopathologically) and 2) persistent positive/increasing value of the morphological index (MI) and a 2 log increase in the bacteriological index (BI) after ≥ 12 months of WHO-MDT-MBR. Such cases were treated with ALT consisting of minocycline, clofazimine, and ofloxacin (24 months). Of 556 patients registered during the study period, 40.3% (224) were slit-skin smear (SSS) positive and 59.7% (332) were SSS negative. Of all, 35 patients (6.3%) satisfied the criteria for clinical nonresponsiveness. Of 224 SSS-positive patients, these 35 patients amounted to 15.6%. The mean BI and MI of these patients after completion of ≥ 12 months of WHO-MDT-MBR were 5.3 ± 0.6 and 14 ± 6.8%, respectively. After 6 months of treatment with ALT, MI became negative (0) in all these patients. After completion of ALT, the mean BI and MI became 1.7 ± 0.7 and 0%, respectively (P < 0.0001). There were 16 patients with corticosteroid-dependent recurrent/chronic erythema nodosum leprosum, who had excellent response with significant reduction in the number of reactional episodes and mean dose of prednisolone required (P < 0.0001). No serious adverse effects were noted. We conclude that ALT is safe and effective in the management of MB leprosy patients who are nonresponsive to 12 months of WHO-MDT-MBR.
Introduction
The introduction of World Health Organization (WHO) multidrug therapy (MDT) has played a pivotal role in achieving the epidemiological target of “elimination of leprosy as a public health problem” (global-2000, India-December 2005).1 With more than 16 million treated leprosy cases and a current world prevalence of 0.23, WHO MDT has been instrumental in our fight against leprosy. But, sadly, the annual new case detection rate or the child rate has not decreased significantly in the last decade, suggesting the presence of an ongoing, unabated, and active transmission of the disease.2 A recent survey from western India (unpublished) concluded that the proportion of patients presenting with multibacillary (MB) disease and deformity/nerve function impairments (both of which are indicators of delay in diagnosis and treatment) has almost doubled since December 2005.
Of late, many centers in India are observing a subset of MB patients not responding satisfactorily (clinically and microbiologically) to the current fixed duration (FD) of WHO-MDT-MB regimen (MBR). In the absence of definite guidelines for management of such patients, they are generally continued on the same regimen for a longer duration, with some being additionally offered immunotherapy in the form of vaccines (MIP [Mycobacterium indicus pranii] or BCG [Bacillus Calmette–Guérin]). Although reports of emerging drug resistance in Mycobacterium leprae have emerged from various parts of the world, including India, data on the clinico-epidemiological features and management of this subset of patients are lacking.3–6 We have faced similar state of affairs over the past few years in the leprosy unit of our tertiary care center and, therefore, attempted to perform a retrospective analysis of such “nonresponsive” MB cases. Herein, we attempt to share our experience regarding the effectiveness and safety of alternate anti-leprosy treatment (ALT) comprising minocycline, ofloxacin, and clofazimine in treating WHO-MDT-MBR refractory leprosy patients, in the absence of facilities for resistance studies.
Materials and methods
Patients registered at the leprosy unit of a tertiary care and referral center in north India over a duration of 6 years (2010–2015) were screened. Data were collected with respect to the demographic profile of patients, morphology of lesions, and investigations, including slit-skin smear (SSS) and histopathology. Slit-skin smear and skin histopathology are performed routinely in our clinic at the baseline and completion of treatment. Number and type of leprosy reactions were noted. Type 2 reactions (erythema nodosum leprosum [ENL]) were further classified as recurrent (recurring within 6 weeks of stopping treatment for ENL and > 6 episodes of ENL in a year) or chronic (lasting > 24 weeks).7
The criteria for the diagnosis of “nonresponsiveness” to MDT were as follows: 1) persistent/new lesions after completing ≥ 12 months of WHO-MDT-MBR (reactions were ruled out histopathologically from these lesions) and 2) persistent positive/increasing values of the morphological index (MI) and a 2 log increase in the bacteriological index (BI) after ≥ 12 months of WHO-MDT-MBR. In view of the opinion that these patients might represent “late responders” rather than an actual treatment failure, 11 initial patients had been continued on 24 months of MDT-MBR. During this extended 12-month period, these 11 patients also received four doses of MIP/BCG vaccine according to our leprosy clinic protocol. Although MI decreased in some of these patients, it never became zero. These 11 patients were further followed up for another 2 years during which almost all of them continued to develop recurrent reactions. Although reactional episodes were adequately managed, the patients became steroid dependent. In addition, no improvement was observed in the clinical lesions (that had become persistent) and bacteriology. Therefore, these patients were started on ALT.
Therefore, in the next 24 patients, ALT was started when the patient had minimal clinical and microbiological response to conventional WHO-MDT-MBR (and MDT MBR was not extended beyond 12 months). In subsequent sections, we describe the clinical and microbiological response of these patients to ALT.
Anti-leprosy therapy comprised minocycline 100 mg/day, clofazimine 50 mg/day, and ofloxacin 400 mg/day for 6 months (intensive phase), and ofloxacin 400 mg/day and clofazimine 50 mg/day for the next 18 months (maintenance phase).7 At baseline, complete blood cell count, liver and renal function tests, antinuclear antibody profile, chest radiography, and ultrasonography of the abdomen were performed in all patients. Complete blood cell count, and liver and renal function tests were subsequently repeated at an interval of 3 months. In an event of transaminitis, viral and alcoholic hepatitis was ruled out and a more frequent monitoring (twice weekly) of liver function tests was performed till the hepatitis resolved. In none of the patients did the levels of the liver enzymes rise above two times the normal. Written informed consent was obtained from the patients before photography.
Results
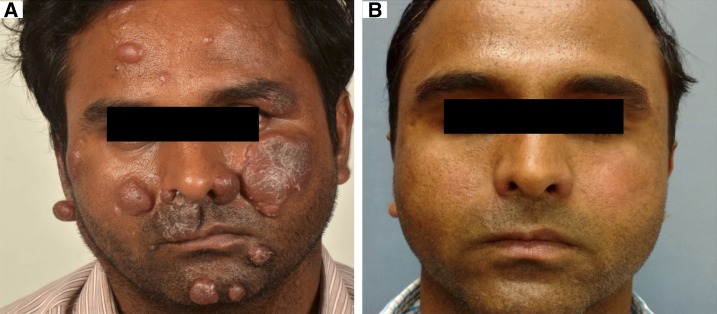
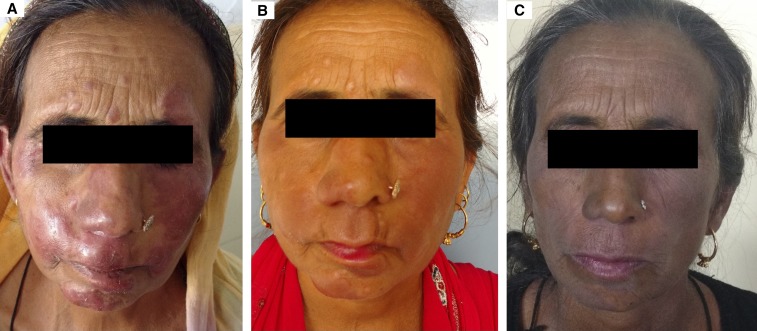
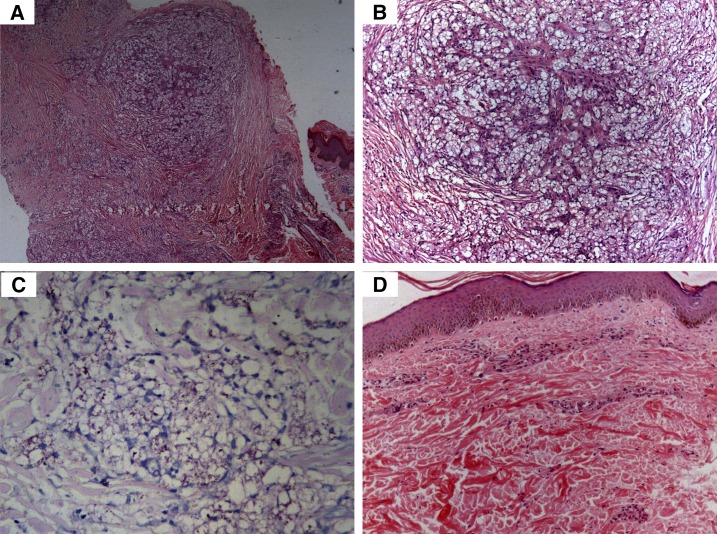
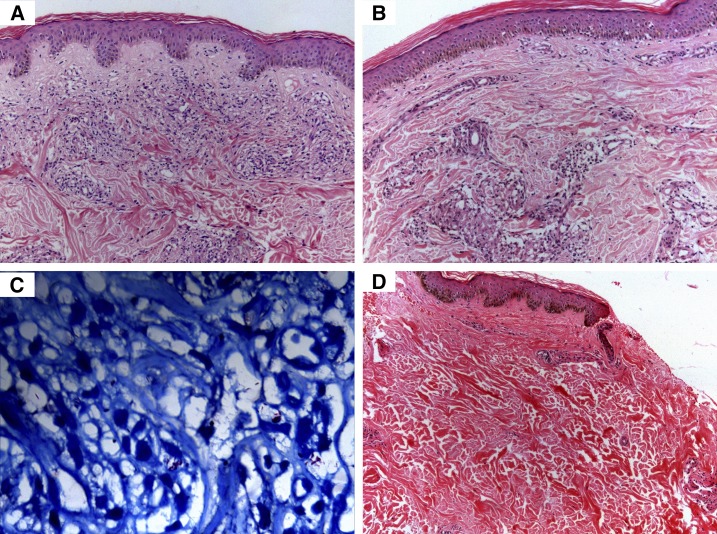
Of 556 total leprosy cases registered during the study-period, 40.3% (224) were SSS positive and 59.7% (332) were SSS negative. Of all, 35 patients (6.3%) satisfied the criteria for “nonresponsiveness.” Of the 224 SSS-positive patients, these 35 patients amounted to 15.6%. There were 28 males and seven females. The average age was 33.7 ± 11.8 years (Table 1). Clinically, these patients manifested with persistent and/or new-onset non-tender, non-ulcerated infiltrated plaques and nodules on the face, upper limbs, and trunk (Figures 1A, 2A, and 2B). Those with ENL presented with associated tender evanescent nodules and ulcers. Histopathologically, all cases showed diffuse dermal infiltration with foamy macrophages containing multiple solid-staining acid-fast bacilli (Figures 3A–C and 4A–C).
Table 1.
Basic clinical and demographical data of leprosy patients studied and screened for the present study
| Multidrug therapy multibacillary regimen nonresponsive patients who were administered alternate anti-leprosy treatment (n = 35) | All patients registered in the clinic (n = 556) | All smear-positive patients (n = 224) | |
|---|---|---|---|
| Mean age (years) | 33.7 ± 11.8 | 36.5 ± 14.7 | 36.8 ± 13.8 |
| Male:female | 4:1 | 4.1:1.5 | 162:62 |
| Mean bacteriological index (log) | 5.3 ± 0.6 | 1.5 ± 2.0 | 3.8 ± 1.3 |
| Mean morphological index (%) | 13.7 ± 6.8 | 2.2 ± 4.3 | 3.1 ± 4.9 |
| Mean duration of disease (years) | 6.4 ± 2.7 | 2.5 ± 3.5 | 2.2 ± 2.9 |
| Diagnosis | |||
| Tuberculoid tuberculoid | 0 | 2 (0.4%) | 0 |
| Borderline tuberculoid | 0 | 298 (53.6%) | 24 (10.7%) |
| Borderline borderline | 0 | 1 (0.2%) | 1 (0.4%) |
| Borderline lepromatous | 6 (17.1%) | 47 (8.5%) | 47 (21.0%) |
| Lepromatous lepromatous | 26 (74.2%) | 142 (25.5%) | 142 (63.5%) |
| Histoid leprosy | 3 (8.5%) | 10 (1.8%) | 10 (4.5%) |
| Indeterminate leprosy | 0 | 10 (1.8%) | 0 |
| Pure neuritic leprosy | 0 | 46 (8.3%) | 0 |
Figure 1.
(A) Persistent erythematous, infiltrated nodules and plaques of lepromatous leprosy despite 12 months of multidrug therapy multibacillary regimen. (B) Resolution of the plaques and improvement in infiltration after alternate anti-leprosy treatment. This figure appears in color at www.ajtmh.org.
Figure 2.
(A) Infiltrated facial plaques of lepromatous leprosy on the first visit of the patient. (B) Minimal improvement after completion of standard World Health Organization multidrug therapy multibacillary regimen. (C) Complete resolution after completion of alternate anti-leprosy treatment. This figure appears in color at www.ajtmh.org.
Figure 3.
Histopathology of the patient depicted in Figure 1. (A) Diffuse dermal infiltration by foamy macrophages (hematoxylin and eosin [H&E], 100×). (B) Higher power view of A (H&E, 200×). (C) Acid-fast bacilli are seen arranged in globi on Fite-Faraco staining (Fite, 400×). (D) Resolution of foamy macrophages after alternate anti-leprosy treatment (H&E, 200×). This figure appears in color at www.ajtmh.org.
Figure 4.
Histopathology of the patient depicted in Figure 2. (A) Dermal infiltration by foamy macrophages on first visit (H&E, 200×). (B) Histopathology after completion of standard 12 months multidrug therapy multibacillary regimen (H&E, 200×). (C) Acid-fast bacilli are seen on Fite-Faraco staining (Fite, 400×). (D) Resolution after alternate anti-leprosy treatment (H&E, 200×). This figure appears in color at www.ajtmh.org.
Of all, 10 patients had received prior MDT-MBR from centers other than ours. They were either referred by the treating staff or sought consultation themselves. Their clinical details and compliance with the treatment were confirmed from their booklets listing dates of dispensing medications and return of the empty blister packs. Wherever doubt existed, compliance to MDT-MBR and a nonresponse were confirmed from the medical officer in charge of the concerned leprosy treatment center/district hospital. Compliance to conventional MDT-MBR was also ensured for the patients who had received treatment from our center.
Eleven (31.4%) of 35 patients had received 24 months of MDT along with immunotherapy (as described previously). The mean BI and MI of these 35 patients after completion of 12/24 months of WHO-MDT-MBR or before initiating ALT were 5.3 ± 0.6 and 14 ± 6.8%, respectively. The MI had remained the same as baseline in 19 (54.3%), had increased in nine (25.7%), and had decreased (but not reaching zero) in seven (20%) patients. Of 35, 26 (74.2%) were classified as having lepromatous (LL) leprosy, and histoid leprosy was diagnosed in three patients. Rest six were classified as having borderline lepromatous (BL) leprosy (Table 1).
In total, 26 of 35 (74.2%) patients had ENL. Recurrent/chronic steroid-dependent ENL was diagnosed in 16 of 35 (45.7%, six—chronic ENL, 10—recurrent ENL) patients (Table 2), who had received one or more of the following agents: clofazimine 100 mg three times a day; pentoxifylline 800 mg three times a day; colchicine 1.5 mg/day; azathioprine 100–150 mg/day, and hydroxychloroquine 400 mg/day for variable durations in addition to prednisolone. The mean number of episodes of ENL/year in patients having recurrent ENL was 8.6 ± 0.7. The mean prednisolone dosage received by 16 patients having recurrent/chronic disease during 1 year preceding the initiation of ALT was 12,882.5 ± 5,130.3 mg.
Table 2.
Clinical, demographical, and follow-up data of 35 World Health Organization multidrug therapy multibacillary regimen refractory patients
| Age (years) | Sex | Diagnosis | Duration of disease (in years, from first symptom to initiation of ALT) | BI baseline (before initiating ALT) | MI baseline (%, before initiating ALT) | BI (after ALT completion) | MI (after ALT completion) | Number of reactional episodes (ENL) before ALT | Number of reactional episodes (ENL) after initiating ALT | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | BL | 4 | 5 | 10 | 2 | 0 | Recurrent | 0 |
| 2 | 50 | M | LL | 5 | 6 | 20 | 3 | 0 | 0 | 0 |
| 3 | 30 | M | HISTOID | 4 | 5 | 25 | 2 | 0 | 0 | 0 |
| 4 | 35 | F | LL | 6 | 6 | 5 | 3 | 0 | Recurrent | 0 |
| 5 | 34 | M | LL | 4 | 5 | 10 | 1 | 0 | Recurrent | 0 |
| 6 | 25 | M | BL | 3 | 4 | 5 | 1 | 0 | Chronic | 0 |
| 7 | 28 | M | BL | 8 | 4 | 5 | 1 | 0 | Chronic | 0 |
| 8 | 20 | M | BL | 5 | 5 | 10 | 2 | 0 | Recurrent | 0 |
| 9 | 28 | F | LL | 6 | 4 | 20 | 1 | 0 | 3 | 0 |
| 10 | 28 | M | BL | 4 | 5 | 10 | 2 | 0 | Chronic | 0 |
| 11 | 28 | M | LL | 4 | 6 | 20 | 3 | 0 | Recurrent | 0 |
| 12 | 26 | M | BL | 4 | 5 | 5 | 2 | 0 | Recurrent | 0 |
| 13 | 45 | M | LL | 7 | 5 | 10 | 2 | 0 | 4 | 0 |
| 14 | 25 | M | LL | 3 | 6 | 15 | 3 | 0 | Chronic | 1 |
| 15 | 24 | M | HISTOID | 8 | 6 | 30 | 3 | 0 | 0 | 0 |
| 16 | 24 | M | LL | 5 | 6 | 15 | 2 | 0 | 2 | 0 |
| 17 | 42 | M | LL | 7 | 5 | 10 | 1 | 0 | Recurrent | 1 |
| 18 | 27 | F | LL | 3 | 5 | 20 | 1 | 0 | Chronic | 0 |
| 19 | 28 | M | LL | 6 | 5 | 10 | 1 | 0 | 4 | 0 |
| 20 | 22 | M | LL | 4 | 6 | 20 | 2 | 0 | Recurrent | 0 |
| 21 | 35 | M | LL | 12 | 6 | 15 | 3 | 0 | 6 | 2 |
| 22 | 21 | M | LL | 3 | 5 | 5 | 1 | 0 | Chronic | 1 |
| 23 | 24 | M | LL | 4 | 5 | 15 | 1 | 0 | 3 | 0 |
| 24 | 20 | M | LL | 4 | 5 | 20 | 2 | 0 | 0 | 0 |
| 25 | 42 | M | LL | 9 | 5 | 20 | 1 | 0 | 2 | 0 |
| 26 | 45 | F | LL | 7 | 6 | 15 | 2 | 0 | 4 | 0 |
| 27 | 42 | M | LL | 10 | 6 | 20 | 2 | 0 | 0 | 0 |
| 28 | 41 | M | LL | 9 | 6 | 15 | 2 | 0 | 0 | 0 |
| 29 | 27 | M | LL | 4 | 5 | 10 | 1 | 0 | Recurrent | 0 |
| 30 | 42 | M | HISTOID | 6 | 6 | 30 | 2 | 0 | 0 | 0 |
| 31 | 55 | F | LL | 12 | 5 | 10 | 1 | 0 | 0 | 0 |
| 32 | 22 | F | LL | 7 | 6 | 5 | 1 | 0 | 3 | 0 |
| 33 | 30 | F | LL | 6 | 6 | 15 | 1 | 0 | 2 | 0 |
| 34 | 43 | M | LL | 11 | 5 | 10 | 1 | 0 | Recurrent | 0 |
| 35 | 65 | M | LL | 9 | 6 | 10 | 2 | 0 | 0 | 0 |
ALT = alternate anti-leprosy treatment; BI = bacteriological index; BL = borderline lepromatous; ENL = erythema nodosum leprosum; F = female; LL = lepromatous lepromatous; M = male; MI = morphological index. Recurrent ENL – ENL recurring within 6 weeks of stopping treatment for ENL. Chronic ENL – ENL lasting > 24 weeks.
After completion of ALT, the mean BI decreased from 5.3 ± 0.6 to 1.7 ± 0.7 (P < 0.0001, Mann–Whitney test). The mean MI decreased from 14 ± 6.8% to 0% (P < 0.0001, Mann–Whitney test). The infiltrated plaques and nodules resolved in all patients (Figures 1B and 2C) and ulcerative lesions of ENL resolved with atrophic scarring. Histopathologically, resolution of granulomas and foamy macrophages was observed (Figures 3D and 4D).
All patients with recurrent/chronic ENL responded (Table 2). Compared with mean prednisolone received over the last 1 year, the mean dose of prednisolone required after the initiation of ALT (over next 12 months) was 2,505 ± 614 mg (P < 0.0001, Mann–Whitney test). The mean number of episodes of ENL in the year following the initiation of ALT in patients having recurrent ENL reduced to 0.1 ± 0.3 (P < 0.0001, Mann–Whitney test). The duration of follow-up after the completion of ALT ranged from 4 months to 3 years.
There was no case of clinical relapse or worsening of nerve function during this follow-up period. Nine patients who had not experienced reactional episodes before initiating ALT did not develop new reactional episodes after the introduction of ALT.
Adverse effects.
All 35 patients developed some hyperpigmentation. Gastrointestinal adverse effects in the form of nausea, vomiting, and epigastric discomfort were seen in 12 (34.2%) patients. Transient transaminitis was noted in five (14.2%, < 2 times the normal levels) patients that resolved spontaneously on further monitoring. None of the patients developed any adverse effects serious enough to warrant discontinuation of therapy.
Discussion
By virtue of its land and population size, ecology, and socioeconomic factors, India contributes maximally to the global burden of leprosy. In the post-elimination era, we were expecting fewer new cases of leprosy and more of the rehabilitation work. Rather, the current statistics show an alarming picture with the number of newly detected adult and child cases remaining the same over the last decade. It suggests that despite attaining elimination, the transmission of leprosy continues uninterrupted and we are still very far from realizing the dream of a leprosy-free world.8 There is also concern about the increase in the number of new cases presenting with histoid leprosy, smear positivity, grade 2 disability,8 and treatment refractory disease not responding to the current WHO-MDT-MBR.2
Previously, Gupta et al.9 found viable bacilli in 23.5%, 7.1%, and 3.84% patients by mouse footpad inoculation and 29.4%, 10.7%, and 3.84% patients by ATP assay, respectively, after 1, 2, and 3 years of treatment with MDT-MBR; but no viable bacilli could be identified in the group that had received minocycline and ofloxacin for 1 year. Shetty et al.10 also demonstrated viable bacilli in 14% and 16% of BL and LL patients at the end of 12 months of MDT-MBR. Other previous works have also proposed the persistence of live bacilli in a significant number of patients, even after the completion of more than stipulated duration of MDT-MBR.11,12 Higher relapse rates have been previously reported in a subgroup of patients having a high baseline BI (≥ 4+), even after treatment with 24 months of WHO-MDT-MBR.13,14
A significant 15.6% of the smear-positive MB leprosy patients in our study were seen harboring viable bacilli at the end of 12 months of MDT-MBR. Prolonged treatment with the same regimen has been previously proposed for nonresponders (treating them as late-responders), and we indeed administered 24 months of MDT-MBR in 11 of 35 patients. But, these patients continued to harbor viable bacilli even after 24 months of MDT-MBR administration.
Our observation coupled with the previously discussed works strongly suggests that FD-MDT-MBR may not be effective anymore in a subset of MB patients. It is important that such “nonresponders” are identified earlier in the course because they are highly infectious and would pose an enhanced disease transmission risk, if they were released from treatment after 12 months of MDT-MBR (in accordance with current WHO recommended practice). In our study, these patients were characterized by lepromatous spectrum of the disease, and a high BI (> 4+) and MI (> 5%) at baseline.
Operationally and clinically acceptable relapse rates with FD-MDT-MBR, observed by us in a previous retrospective study (1999–2010) with no MDT nonresponders,15 suggest that this phenomenon of “nonresponsiveness” to MDT-MBR is relatively recent (in previous 6–7 years). Persistence of symptoms despite continued treatment adversely affects the compliance of the patients and is proving to be a cause of trepidation for treating dermatologists and patients alike. A significant proportion of our MDT-MBR “nonresponders” had concomitant ENL. Moreover, ENL was chronic and recurrent in more than half of them. Multiple courses of prednisolone were administered to these patients without much response, and many eventually became steroid dependent despite adequate trials of adjuvants, including pentoxifylline, colchicine, hydroxychloroquine, clofazimine, and thalidomide. The serious implication of administering corticosteroids in such “nonresponders” lies in the fact that corticosteroid-induced immunosuppression might further enhance the persistence of bacilli, further predisposing to reactional episodes, thus ending in a vicious cycle.10 Workdays lost with subsequent economic impact cannot be overstated because leprosy primarily affects the socioeconomically weaker subgroup of the population.
We observed complete clearance of the persisting lesions, which was substantiated by values of MI falling to 0% after completion of 6 months of ALT and stayed the same till the end of the study period. All patients having steroid-dependent recurrent/chronic ENL started responding and corticosteroids could be tapered off within the next 6–12 months, with almost no reactional episodes observed after the completion of ALT. This suggests the plausible role played by ALT in causing effective clearance of bacilli and antigenic load in these patients, which probably contributed to its steroid-sparing action. Apart from transient transaminitis that resolved spontaneously, no serious adverse effects were noted in the present study, although hyperpigmentation was seen in all 35 patients and almost one-third developed mild gastrointestinal adverse effects.
Minocycline is a bactericidal drug and has additional anti-inflammatory and anti-apoptotic properties. It has been shown to cause inhibition of proteolysis, angiogenesis, and collagenases.16 Similar immunomodulatory and anti-mycobacterial actions have been proposed for clofazimine and ofloxacin.17,18 The addition of minocycline to MDT has been shown to be more efficacious than the MDT alone9 and has been found to impart a better control of reactional episodes.19,20 There is a dearth of studies assessing the effectiveness of alternative/newer regimens in the management of leprosy in the last few decades. In a prospective study on 21 MB patients using the same alternative regimen as used in the present study, Maia et al.21 found that all patients tolerated the drugs well, with satisfactory compliance, and no events adverse enough to warrant discontinuation of the treatment were noted.
Completion of FD-MDT-MBR or release from treatment is not equivalent to the cure for an infection like leprosy because of the unique nature of M. leprae. In the post-elimination era, SSS is not recommended at peripheral centers and is undertaken only in some research institutes. Therefore, such “nonresponsders” are released from treatment (especially in the peripheral and remote areas) until they eventually present with clinical deterioration or reactions to the referral centers. Mere dissipation of free-of-cost MDT-MBR to patients without keenly assessing the subsequent changes in bacillary indices and degree of clinical improvement may lead to a situation similar to that of multidrug-resistant tuberculosis.
Limitation.
Retrospective nature is the chief limitation of this study, and future studies should be carried out prospectively to assess the efficacy of ALT in WHO-MDT-MBR refractory patients. The present case series comprises patients having variable prior treatment histories. During evaluation of initial 11 patients, we had waited for two more years after the completion of extended 24 months WHO-MDT-MBR and immunization schedule, but the patients had positive MI even after that and were presenting with recurrent reactional episodes. In the absence of a control group, the confounding factor of an enduring albeit delayed beneficial effect of immunotherapy cannot be conclusively ruled out. Still, regardless of the prior treatments, all recruited patients had demonstrated a positive and high lesional MI (≥ 5%) at the time of initiating ALT, which reflected a highly bacilliferous state, rendered the patients infectious, and justified the attempt to initiate ALT. Not treating patients having highly positive MI (to formulate a control group), who can potentially infect multiple individuals who they come in contact with, seemed unethical. Moreover, this study represents a retrospective evaluation of all such patients encountered over a period of 6 years who were treated using ALT and, therefore, lacks an obvious control group.
Another limitation shall be the lack of mouse footpad inoculation or polymerase chain reaction (PCR) studies to confirm drug resistance in these patients because of limitation of the resources. However, mouse footpad inoculation studies are cumbersome, take a long time before results are available, and are not available at most of the centers including ours. Through the WHO-initiated program for surveillance of antimicrobial resistance in leprosy, samples of suspected MDT-resistant leprosy patients can now be sent for further evaluation by PCR to either few national laboratories or four international reference laboratories situated in France, Switzerland, Japan, and the United States.22 In addition, SSS and MI might have been falsely negative in some of the nonresponsive patients, who were not administered ALT and were, therefore, not included in the present study. Morphological index is less sensitive in detecting the viability of bacilli than fluorescent dye assay and adenosine triphosphate metabolizing assays and, thus, can underestimate the number of total live bacilli.23 The facility to carry out these was not available at our center. However, in experienced hands, estimation of MI remains a standard method to evaluate the number of live and viable bacilli, and the same is reiterated by its prompt reduction (much faster than BI) after administration of MDT in those having responsive disease.24 Last, the follow-up was limited to 3–4 months in few cases.
The present case series is an attempt to present the phenomenon of clinical non-responsiveness that is being increasingly observed in leprosy patients at referral centers across India. It can prove to be a major threat for leprosy elimination campaign, by virtue of the sheer contribution that India makes toward the global leprosy burden. Suggestions of further reducing the duration of treatment as in uniform MDT can prove to be a recipe of disaster.
To conclude, we highlight the importance of a meticulous clinical examination and utilization of the available health-force and laboratory resources to identify treatment-refractory highly bacilliferous leprosy patients, so that a changeover from conventional MDT-MBR to alternative regimens can be carried out as earlier as possible. In the post-elimination era, an adequate change in the existing WHO guidelines (with special emphasis on such nonresponders) regarding a mandatory SSS, for at least clinically MB patients, and introduction of a robust MDT which can reduce the prolonged symptomology of the disease and reactions are the need of the hour and will help to restore patients’ confidence in the treatment. Future studies should try to define and refine the criteria for nonresponse/treatment failure after WHO-MDT and predict the factors that lead to nonresponse. A prospective comparison of prolongation of standard MDT-MBR25 and ALT in nonresponsive patients shall be interesting.
Acknowledgment:
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Smith CS, Aerts A, Saunderson P, Kawuma J, Kita E, Virmond M, 2017. Multidrug therapy for leprosy: a game changer on the path to elimination. Lancet Infect Dis 17: e293–e297. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization , 2017. Global leprosy update, 2016: accelerating reduction of disease burden. Wkly Epidemiol Rec 92: 501–519. [PubMed] [Google Scholar]
- 3.Liu D, et al. 2015. Drug resistance in Mycobacterium leprae from patients with leprosy in China. Clin Exp Dermatol 40: 908–911. [DOI] [PubMed] [Google Scholar]
- 4.Contreras Mejia Mdel C, Porto Dos Santos M, Villarouco da Silva GA, da Motta Passos I, Naveca FG, Souza Cunha Mda G, Moraes MO, de Paula L, 2014. Identification of primary drug resistance to rifampin in Mycobacterium leprae strains from leprosy patients in Amazonas state, Brazil. J Clin Microbiol 52: 4359–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavania M, et al. 2014. Drug resistance patterns in Mycobacterium leprae isolates from relapsed leprosy patients attending the leprosy mission (TLM) hospitals in India. Lepr Rev 85: 177–185. [PubMed] [Google Scholar]
- 6.Lavania M, Nigam A, Turankar RP, Singh I, Gupta P, Kumar S, Sengupta U, John AS, 2015. Emergence of primary drug resistance to rifampicin in Mycobacterium leprae strains from leprosy patients in India. Clin Microbiol Infect 21: e85–e86. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization , 2010. WHO Expert Committee on Leprosy: Eighth Report. Report No.: WHO Technical Report Series; 968. Geneva, Switzerland: WHO. [Google Scholar]
- 8.National Leprosy Eradication Programme , 2016. NLEP—Progress Report for the Year 2015–16. New Delhi, India: Central Leprosy Division, DGHS, Govt of India. [Google Scholar]
- 9.Gupta UD, Katoch K, Singh HB, Natrajan M, Katoch VM, 2005. Persister studies in leprosy patients after multi-drug treatment. Int J Lepr Other Mycobact Dis 73: 100–104. [PubMed] [Google Scholar]
- 10.Shetty VP, Khambati FA, Ghate SD, Capadia GD, Pai VV, Ganapati R, 2010. The effect of corticosteroids usage on bacterial killing, clearance and nerve damage in leprosy; part 3—Study of two comparable groups of 100 multibacillary (MB) patients each, treated with MDT + steroids vs. MDT alone, assessed at 6 months post-release from 12 months MDT. Lepr Rev 81: 41–58. [PubMed] [Google Scholar]
- 11.Sharma A, Sharma VK, Rajwanshi A, Das A, Kaur I, Kumar B, 1999. Presence of M. leprae in tissues in slit skin smear negative multibacillary (MB) patients after WHO-MBR. Lepr Rev 70: 281–286. [DOI] [PubMed] [Google Scholar]
- 12.Lemaster JW, Shwe T, Butlin CR, Roche PW, 2001. Prediction of “highly skin smear positive” cases among MB leprosy patients using clinical parameters. Lepr Rev 72: 23–28. [DOI] [PubMed] [Google Scholar]
- 13.Girdhar BK, Girdhar A, Kumar A, 2000. Relapses in multibacillary leprosy patients: effect of length of therapy. Lepr Rev 71: 144–153. [DOI] [PubMed] [Google Scholar]
- 14.Jamet P, Ji B, 1995. Relapse after long-term follow up of multibacillary patients treated by WHO multidrug regimen. Marchoux Chemotherapy Study Group. Int J Lepr Other Mycobact Dis 63: 195–201. [PubMed] [Google Scholar]
- 15.Dogra S, Sendhil Kumaran MS, Narang T, Radotra BD, Kumar B, 2013. Epidemiological characteristics and clinical outcome in multibacillary leprosy patients treated with 12 months WHO MDT-MBR at a referral hospital in northern India: a retrospective analysis of 730 patients. Lepr Rev 84: 65–75. [PubMed] [Google Scholar]
- 16.Garrido-Mesa N, Zarzuelo A, Galvez J, 2013. Minocycline: far beyond an antibiotic. Br J Pharmacol 169: 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalhoff A, Shalit I, 2003. Immunomodulatory effects of quinolones. Lancet Infect Dis 3: 359–371. [DOI] [PubMed] [Google Scholar]
- 18.Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R, 2012. Clofazimine: current status and future prospects. J Antimicrob Chemother 67: 290–298. [DOI] [PubMed] [Google Scholar]
- 19.Narang T, Arshdeep, Dogra S, 2016. Minocycline in leprosy patients with recent onset clinical nerve function impairment. Dermatol Ther 30: e12404. [DOI] [PubMed] [Google Scholar]
- 20.Narang T, Sawatkar GU, Kumaran MS, Dogra S, 2015. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol 151: 1026–1028. [DOI] [PubMed] [Google Scholar]
- 21.Maia MV, Cunha Mda G, Cunha CS, 2013. Adverse effects of alternative therapy (minocycline, ofloxacin, and clofazimine) in multibacillary leprosy patients in a recognized health care unit in Manaus, Amazonas, Brazil. An Bras Dermatol 88: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A Guide for Surveillance of Antimicrobial Resistance in Leprosy, 2017 Update. Geneva, Switzerland: WHO; Available at: www.who.int/lep/resources/9789290226192/en/. Accessed August 20, 2018. [Google Scholar]
- 23.Odinsen O, Nilson T, Humber DP, 1986. Viability of Mycobacterium leprae: a comparison of morphological index and fluorescent staining techniques in slit-skin smears and M. leprae suspensions. Int J Lepr Other Mycobact Dis 54: 403–408. [PubMed] [Google Scholar]
- 24.Mahajan VK, 2013. Slit-skin smear in leprosy: lest we forget it! Indian J Lepr 85: 177–183. [PubMed] [Google Scholar]
- 25.Lastória JC, de Almeida TSC, Putinatti MSMA, Padovani CR, 2018. Effectiveness of the retreatment of patients with multibacillary leprosy and episodes of erythema nodosum leprosum and/or persistent neuritis: a single-center experience. Bras Dermatol 93: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]