Abstract.
Zika virus (ZIKV) had emerged as a global arboviral concern since late 2015. In this study, we describe the results of ZIKV testing in returning Israeli travelers from Zika-endemic countries. We conducted a nation-wide prospective observational study, including all ZIKV tests during January 2016–June 2017. Zika virus infection was defined as confirmed, if diagnosed by polymerase chain reaction (PCR) or serology confirmed by neutralization, and as possible if diagnosed by serology alone. During the study period, 1,188 travelers were tested: 66.7%, 30.5%, 1.6%, and 1.2% had returned from the Americas, Asia, Africa, and Oceania, respectively. Thirty persons were diagnosed with ZIKV. Most travelers tested were women of reproductive age; the gender ratio among infected travelers however was 1.0. During 2016, 19/20 (95%) ZIKV cases were acquired in the Americas; in 2017, however, 6/10 (60%) cases were acquired in Asia. Of 248 symptomatic travelers, 28 (11.3%) were diagnosed with ZIKV infection, whereas only 2/940 (0.2%) of asymptomatic travelers were diagnosed with ZIKV infection Odds ratio = 59.7 (95% confidence interval: 14.1–252.5, P < 0.0001). Our findings suggest that although women are more likely to be referred for ZIKV testing, gender does not affect the likelihood of ZIKV infection and that asymptomatic ZIKV infection appears to be rare in travelers. Furthermore, it appears that in 2017, Southeast Asia emerges as the leading source of travel-related ZIKV infection.
Introduction
Since 2014, the current Zika virus (ZIKV) outbreak had progressed throughout South, Central, and North America and the Caribbean; ZIKV activity had been documented in nearly all countries and territories in the tropics, and also in subtropical regions such as Florida and Texas.1 Close to 600,000 confirmed and suspected cases had been reported in the Americas as of December 2017.2 In the past decades, ZIKV infection had been documented intermittently in returning travelers—sometimes as the only evidence of ZIKV activity in some countries.3,4 Concomitant with the current epidemic, thousands of travelers were diagnosed with ZIKV infection2; in Israel, ZIKV had become, for a period, the most frequently diagnosed arbovirus in travelers.4 Increased demand for ZIKV screening among travelers led to the establishment of a designated Zika clinic.
The aim of the present study was to describe epidemiological and clinical features of ZIKV tests and ZIKV cases in returning travelers during the first 18 months of testing.
Methods
This was a prospective observational study. The study period was 18 months: from January 2016 through June 2017.
Study population.
All persons referred for ZIKV testing that had traveled to a country with active ZIKV transmission, in accordance with the World Health Organization and Centers for Disease Control (CDC) updates were included. In addition, ZIKV tests were approved for two women that had not traveled abroad and had miscarried microcephalic fetuses.
In Israel, ZIKV diagnostic tests had become available in December 2015 and all tests were performed in the Central Virology Laboratory of the Israeli Ministry of Health at the Sheba Medical Center. All samples submitted for ZIKV evaluation were requested to be accompanied by a prestructured questionnaire with demographic data, travel history, and clinical details.
From each patient serum, urine and whole blood were requested.
Case definition.
A case of confirmed ZIKV infection was defined as either positive PCR result or positive serology confirmed by neutralization assay (NA); A case of possible ZIKV infection was defined as positive serology, without NA confirmation.
Diagnostic tests.
All submitted samples were tested regardless of the time elapsed since symptoms onset and return from travel. Zika virus reverse transcription polymerase chain reaction (RT-PCR) methods were performed as described previously.5–7 Serology was performed on sera by using an enzyme-linked immunosorbent assay (ELISA) IgM and IgG kit (Euroimmun AG, Lübeck, Germany), which detects antibodies against ZIKV nonstructural protein 1 (NS-1) protein and is considered very specific for ZIKV infection.8,9 Evaluation of other arboviral infections was achieved by performing serology tests against dengue virus (DENV) (Panbio NS1 early antigen, IgM and IgG ELISA; Alere (Abbott), Waltham, MA), West Nile virus (IgM and IgG ELISA Focus Diagnostics, Cypress, CA), and Chikungunya virus (CHIKV) (Immunofluorescence assay, [Euroimmun AG]). Positive and equivocal ZIKV serology was confirmed by neutralization assay (NA), as described elsewhere.10
Evaluation of ZIKV incidence in travelers.
Data on the number of Israeli travelers to the ZIKV epidemic areas in the Americas are unavailable. Data on the numbers and duration of stay of Israeli travelers in Thailand for the study period were available, based on press communiques from the Thai Ministry of tourism.11,12 Data on the numbers of Israeli travelers to the Philippines were available from the Ministry of Tourism of the Philippines (E. Meltzer, personal communication). For average duration of stay in the Philippines, we have used 26 days: the average stay of patients with diagnosed ZIKV or DENV after travel to the Philippines.
Statistical analysis.
Fisher’s exact test and Student’s t-test were used to analyze categorical and continuous variables, respectively.
The study was approved by the institutional review board at the Sheba Medical Center.
Results
During the study period, 1,188 persons were referred for ZIKV testing. During January 2016 the number of tests performed was small (N = 5) and only symptomatic patients suspected of ZIKV disease were referred for evaluation. In late January 2016, the Israeli Ministry of Health issued a travel warning, stressing pregnancy-related risks of ZIKV; from February 2016 onward, demand for ZIKV tests increased markedly and averaged 70 ± 33 patients (mean ± standard deviation [SD]) per month.
Altogether, 30 cases of ZIKV infection were diagnosed in returning travelers; 20 ZIKV cases (19 confirmed and one possible) were diagnosed in 2016 and 10 cases (six confirmed and four possible) in 2017. Demographic and travel data are presented in Table 1. Most women travelers tested for ZIKV were between the ages of 25–40 years (84.4%) (Figure 1).
Table 1.
Demographic data and travel details of Israeli travelers tested for ZIKV
| ZIKV neg. (N = 1,158) | ZIKV pos. (N = 30) | P value | |
|---|---|---|---|
| Age (years, mean ± SD, IQR) | 31.6 ± 6.0, 29–34 | 29.9 ± 10.7, 27–31 | 0.19 |
| Gender (M/F) | 0.85 | 1.0 | 0.8 |
| Reason for travel (N = 981, including 30 ZIKV pos. cases) | |||
| Tourism | 88.2% | 90% | 0.76* |
| Business | 10.1% | 6.7% | |
| Visiting friends/relatives (VFRs) | 1.0% | 3.3% | |
| Humanitarian | 0.4% | 0.0% | |
| Other causes | 0.2% | 0.0% | |
| Travel region (N = 981, including 30 ZIKV pos. cases) | |||
| Americas (total) | 66.7% | 81.8% | 0.34† |
| Asia | 30.5% | 18.2% | |
| Africa | 1.6% | 0.0% | |
| Oceania | 1.2% | 0.0% | |
ZIKV = Zika virus.
For comparison of Business and tourism.
For comparison of travel to Asia and the Americas.
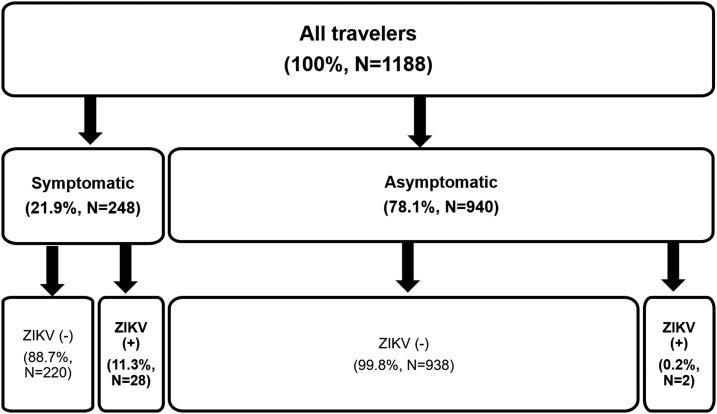
Figure 1.
Distribution of travelers tested for Zika virus (ZIKV) according to test result and symptoms.
Travel history and ZIKV epidemiology.
Analysis of travel parameters was performed on 981 (82.6%) persons, for whom these data were available. Most (88.2%) had traveled for recreational tourism, 10.1% for business, and 1.0% visiting friends and relatives. Most travelers (66.7%) had returned from the Americas, 30.5% from Asia, 1.6% from Africa, and 1.1% from Oceania.
Of 23 ZIKV cases acquired in the Americas, all had traveled to Latin America and/or the Caribbean except for one case acquired after a visit to Miami, FL. In 2016, 1/20 (5.0%) ZIKV cases were acquired in Asia (patient from Vietnam, as described previously4). In 2017, 6/10 (60.0%) cases were acquired in Asia: three from the Philippines, two from Thailand, and one had traveled to both countries. Among travelers from the Americas, ZIKV-positive cases represented 9.3% and 1.7% of tested patients returning from this region in 2016 and 2017, respectively. Among travelers from Asia, ZIKV-positive cases represented 1.3% and 2.9% in 2016 and 2017, respectively.
Evaluation of ZIKV incidence in travelers.
In the absence of a reliable denominator, the incidence of ZIKV in travelers to the Americas cannot be established. During the study period, 239,712 Israelis traveled to Thailand, for a mean duration of 17 days, and 26,210 Israelis traveled to the Philippines. The estimated incidence of imported ZIKV was 17.9–26.8/100,000 travel-years for Thailand and 160.7–214.2/100,000 travel-years for the Philippines (according to whether the last Asian case was acquired in the Philippines or in Thailand). During the study period, 53 imported cases of DENV infection and 15 cases of CHIKV infection were diagnosed in Israel. Data on geographic source were available for 35/53 (64%) cases of DENV infection, of which 12 were acquired in Thailand and two in the Philippines. The minimal incidence of DENV cases was calculated at 107.5/100,000 travel-years for Thailand and at 91.6/100,000 travel-years for the Philippines.
Clinical aspects.
Two hundred forty-eight travelers (21.9%) were referred for ZIKV testing after having symptoms during or immediately after travel; the other travelers (78.1%) were asymptomatic. Symptoms among ZIKV-infected patients were usually mild, however, one child traveler with PCR-positive ZIKV infection presented with probable encephalitis as previously reported.13 The most frequent symptoms reported by symptomatic travelers were fever (48.4%) and rash (29.8%). No travelers were diagnosed with ZIKV-associated Guillain–Barré syndrome or ZIKV infection during pregnancy, and there were no cases of sexual transmission of ZIKV to a non-traveler spouse.
The leading reason for referral for testing was reproductive concern, including recent miscarriage (N = 5) and currently pregnant/spouse pregnant (N = 388). Three women had suffered miscarriage during travel and were tested for ZIKV after return—none were infected. Another two women who had miscarried microcephalic fetuses but had not traveled to ZIKV-endemic areas were also tested and were also negative.
Of 248 symptomatic travelers, 28 (11.3%) were diagnosed with ZIKV infection; of 940 asymptomatic travelers, two (0.2%) were diagnosed with ZIKV infection odds ratio = 59.7 (95% confidence interval: 14.1–252.5, P < 0.0001).
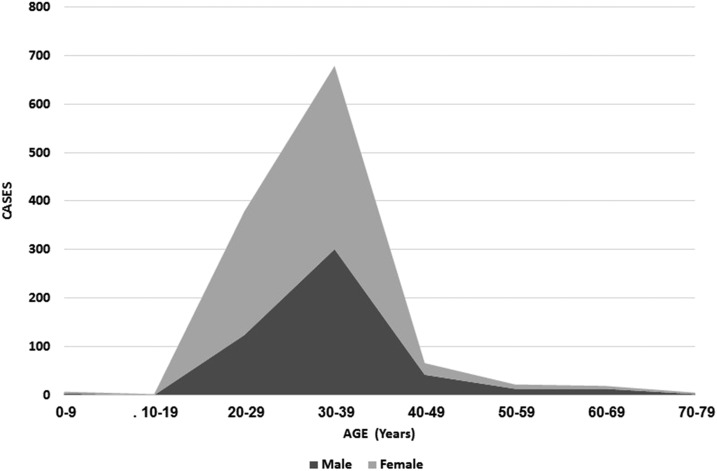
The age and gender distribution of the total population tested is presented in Figure 2, and demographics of ZIKV-infected patients in Table 1. Both age and gender were distributed unevenly, with most travelers being between the ages of 25–40. The male/female ratio ranged from as low as 0.49 in the 20–29 years age group, to as high as 2.1 in the 60–69 years age group. Male gender and travel to Asia were more frequent among ZIKV infected cases; this difference however did not reach statistical significance.
Figure 2.
Age and gender distribution of travelers referred for Zika virus testing.
Discussion
The ZIKV outbreak in the Americas had brought this once obscure Flavivirus to the forefront of clinicians’ awareness. In Israel, during the study period, ZIKV had emerged as the most tested for arboviral infection, and, after DENV, the second most diagnosed arbovirus in travelers.
We were unable to quantify the burden of ZIKV in Israeli travelers to the Americas, but it is likely to have been significant at the height of the outbreak. Indeed, some of our American cases were exposed to ZIKV transmission for as short as 2 days, signifying a very strong local force of transmission.
The signal among returning Israeli travelers for ZIKV transmission at the travel destination was sensitive and rapid, with documented cases in travelers being contemporaneous with local recognition, or sometimes preceding it, as was the case for Vietnam.4 Even small-scale outbreaks such as the Miami—Dade County outbreak resulted in a travel-related case. The first alert for ZIKV transmission in Dade County was released by the CDC in August 1, 2016; the Israeli case was acquired after a weeklong stay in mid-August 2016.
Our findings on the frequency of ZIKV among travel-related arboviral infections in Israel reflect the intensity of the ZIKV outbreak in the Americas and are in line with published data from epidemic countries. For example, in Puerto Rico, where a major ZIKV outbreak developed together with co-circulation of both DENV and CHIKV, during November 2015–July 2016, ZIKV was the most prevalent arbovirus diagnosed by more than 37:1 as compared with DENV diagnoses.14
However, the emerging picture during 2017 is that the number of cases acquired in the Americas is decreasing, probably reflecting the decreasing intensity of transmission there, whereas ZIKV acquired in Asia now forms the majority of diagnosed cases. Among Israeli travelers to Asia, where steady-state transmission exists rather than outbreak conditions, the incidence of ZIKV ranged from 17.9 to 26.8 cases/100,000 travel-years for Thailand, to 160.7–214.2/100,000 travel-years for the Philippines. To date, non-traveler data on autochthonous ZIKV in Southeast Asia are limited (as is the case for Thailand and Vietnam15,16) or completely unavailable (Philippines). It is likely that the true burden of ZIKV infection in these countries is underestimated: our data suggest that the burden of ZIKV in Southeast Asia is probably far greater than is currently recognized locally. The calculated incidence rates for ZIKV in Thailand and the Philippines were lower than the rates calculated for DENV but not dramatically so. If the epidemiological signal seen in travelers reflects the actual burden of infection in these locales, then the burden of ZIKV in Southeast Asia is significant. Clearly, further field studies in Asian countries are urgently needed to quantify the risk for locals and travelers alike.
The study of tropical diseases in travelers can also elucidate some clinical aspects of these diseases that are sometimes less recognized in endemic populations. Our data shed important light on the issues of gender and symptoms of ZIKV infection.
In our cohort, the age and gender distribution of persons tested for ZIKV was highly skewed, whereas among travelers to developed countries males usually predominate. In our series, the male/female ratio was reversed, with most travelers being in the age range most associated with reproduction. This gender imbalance mostly reflects referral bias, as reproductive concern was the main drive for screening. Among ZIKV-infected travelers, however, the gender ratio was 1.0—suggesting that referral bias rather than gender-based disease load may account for the increase in female over male patients seen in endemic countries. Thus, our data confirm reports from other series in travelers17 and is contrary to studies from endemic countries that reported gender inequality.18,19
Zika virus infection is mostly perceived to be a mild infection. We have previously reported on an infant that developed chronic seizure disorder after acute ZIKV infection, suggesting meningoencephalitis.13 It is interesting to note that the only other case of ZIKV encephalitis published to date had also involved a traveler, an 81-year-old, previously healthy man,20 but that no cases were reported from endemic countries. Whether infants and the elderly are at an increased risk for this complication is not known. The lack of cases with Guillain–Barré syndrome or intrauterine infections in our study probably reflects the small number (to date) of cases in Israel as both Guillain–Barré syndrome21 and adverse pregnancy outcomes22 have been described in travelers.
Asymptomatic returning travelers now constitute the predominant population tested for ZIKV in Israel, mostly due to concerns regarding reproduction. The percentage of subclinical infections varies in different members of the Flaviviridae, with up to 90% of cases of DENV for example, being asymptomatic.23,24 However, the exact percentage of asymptomatic cases for ZIKV is debatable, and a recent meta-analysis found reported studies to be too heterogeneous to allow a definite conclusion.25
We have shown that the likelihood of asymptomatic travelers to have been infected with ZIKV is very low (0.2%). Our data are corroborated by the fact that to date very few cases of asymptomatic ZIKV have been reported among travelers; a French couple retuning from Martinique were both diagnosed with ZIKV infection as part of evaluations before assisted reproduction: both were asymptomatic.26 Our study documented asymptomatic infection in only 2/30 (6.7%) of the ZIKV cases. Similarly, in metropolitan France in 2016, asymptomatic ZIKV (seven cases) represented just 1% of all ZIKV infections diagnosed in travelers.27
Our data are also in accord with recent studies from endemic countries; in Puerto Rico, for example, at the height of the outbreak, definite cases of ZIKV infection were diagnosed in less than 1% of 7,308 asymptomatic pregnant women.14
It is cited in the literature that up to 80% of cases of ZIKV infection are in fact asymptomatic.28 The source of this data is a study conducted during the 2009 Micronesian ZIKV outbreak in Yap Island.18 However, this study may have overestimated asymptomatic cases due to methodological limitations and recall bias. Such recall bias is less likely among returning travelers, who present early after travel.
To avoid recall bias, prospective studies are required; however, prospective data are very limited. A small prospective study among viremic blood donors in Martinique, who were asymptomatic during blood donation, found that 54.7% developed symptoms later.29 Although the proportion of asymptomatic ZIKV found in Martinique is much lower than that described in Yap Island, it is higher than that found in Israeli and French tourists. Populations in endemic area may be less likely to develop symptomatic ZIKV—perhaps because of immune interference from seropositivity against other Flaviviridae (previous, repeated DENV, immunization against yellow fever etc.), however, this requires confirmation by larger studies.
All these data suggest that symptomatic infection is the rule in ZIKV rather than the exception and that the 4:1 proportion of asymptomatic/symptomatic cases in ZIKV may be incorrect.
Finally, our results may relate to the potential role of travelers as vectors for the further spread of ZIKV to new areas. In Israel, for example, as in many southern European Countries, Aedes aegypti—the main mosquito vector of ZIKV—is not present but Aedes albopictus, which may also act as vector for the disease, is prevalent; the potential of the introduction of arboviruses such as DENV and CHIKV is well recognized.30 Experience with CHIKV in Italy during the last decade has shown that, where travel-related cases coincide with a high mosquito burden (Emilia-Romagna in 2007 and Lazio in 201731,32), local outbreaks can occur.
If indeed asymptomatic ZIKV infections are rare among travelers, asymptomatic returnees may be reassured that the likelihood of harboring ZIKV is probably very small On the other hand, travelers with reproductive concerns may still opt for the further reassurance provided by negative blood tests.
Our study has several limitations. Geographical data were missing for 17.8% of persons referred for testing; however, we had complete data on all ZIKV infected cases. Recall bias cannot be completely ruled out and therefore symptoms may have been unrecorded. Also, association of reported symptoms with ZIKV in some patients seen after the acute illness is inferred rather than established. Incidence rates for both ZIKV and DENV relied on travel statistics that may be incomplete because some travelers may not have traveled by air—however, this number is probably negligible. Also, the incidence rates for DENV should be considered as the lowest likely estimate because some geographic data were missing.
Conclusion
In a nation-wide study spanning 18 months, among returning Israeli travelers, ZIKV was found to be the most frequently requested test for arboviral infection. There was a preponderance of females among persons tested, reflecting reproductive concern as the reason for referral. However, among infected travelers male/female ratio was 1.0. The proportion of cases acquired in Asia suggests that ZIKV circulation in Asia is much higher than currently perceived. Asymptomatic returning travelers appear to have a very low likelihood of being infected with ZIKV.
REFERENCES
- 1.WHO Zika Virus, Microcephaly and Guillain-Barré Syndrome Situation Report 20 January 2017. Available at: http://apps.who.int/iris/bitstream/10665/253604/1/zikasitrep20Jan17-eng.pdf?ua=1. Accessed January 28, 2017.
- 2.PAHO , 2017. Zika Cumulative Cases. Available at: http://www.paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090&lang=en. Accessed January 28, 2017.
- 3.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB, 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer E, et al. 2016. Zika virus disease in traveler returning from Vietnam to Israel. Emerg Infect Dis 22: 1521–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR, 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasi I, Cunio R, Warburg A, 2009. Identification of blood meals imbibed by Phlebotomine sand flies using cytochrome b PCR and reverse line blotting. Vector Borne Zoonotic Dis 9: 79–86. [DOI] [PubMed] [Google Scholar]
- 7.Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E, 2016. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill 21: pii=30269. [DOI] [PubMed] [Google Scholar]
- 8.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M, 2016. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill 21: pii=30203. [DOI] [PubMed] [Google Scholar]
- 9.Steinhagen K, et al. 2016. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21: pii=30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustig Y, Zelena H, Venturi G, Van Esbroeck M, Rothe C, Perret C, Koren R, Katz-Likvornik S, Mendelson E, Schwartz E, 2017. Sensitivity and kinetics of an NS1-based Zika virus enzyme-linked immunosorbent assay in Zika virus-infected travelers from Israel, the Czech Republic, Italy, Belgium, Germany, and Chile. J Clin Microbiol 55: 1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ynetnews , 2018. Major Increase in Israeli Tourism to Thailand (Hebrew). Available at: https://www.ynet.co.il/articles/0, 7340,L-4999047,00.html. Accessed March 11, 2018.
- 12.@globesnews , 2018. 161,000 Israelis Entered Thailand in 2016 (Hebrew). Available at: https://www.globes.co.il/news/article.aspx?fbdid=1001172287. Accessed March 11, 2018.
- 13.Meltzer E, Leshem E, Lustig Y, Gottesman G, Schwartz E, 2016. The clinical spectrum of Zika virus in returning travelers. Am J Med 129: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 14.Adams L, 2016. Update: ongoing Zika virus transmission—Puerto Rico, November 1, 2015–July 7, 2016. MMWR Morb Mortal Wkly Rep 65: 774–779. [DOI] [PubMed] [Google Scholar]
- 15.Ellison DW, et al. 2016. Complete genome sequences of Zika virus strains isolated from the blood of patients in Thailand in 2014 and the Philippines in 2012. Genome Announc 4: e00359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu DT, Ngoc VTN, Tao Y, 2017. Zika virus infection in Vietnam: current epidemic, strain origin, spreading risk, and perspective. Eur J Clin Microbiol Infect Dis 36: 2041–2042. [DOI] [PubMed] [Google Scholar]
- 17.Escutia G, McDonald E, Rodriguez-Lainz A, Healy J, 2018. Demographic and travel characteristics of travel-associated Zika virus infection case-patients in san Diego county, California (January 1, 2016–March 31, 2017). J Community Health 43: 566–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy MR, et al. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360: 2536–2543. [DOI] [PubMed] [Google Scholar]
- 19.Lozier M, et al. 2016. Incidence of Zika virus disease by age and sex–Puerto Rico, November 1, 2015–October 20, 2016. MMWR Morb Mortal Wkly Rep 65: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 20.Carteaux G, et al. 2016. Zika virus associated with meningoencephalitis. N Engl J Med 374: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 21.Beattie J, et al. 2018. Zika virus–associated Guillain-Barré syndrome in a returning US traveler. Infect Dis Clin Prac 2018 Jul 26 (Epub ahead of print).
- 22.Meaney-Delman D, 2016. Zika virus infection among US pregnant travelers—August 2015–February 2016. MMWR Morb Mortal Wkly Rep 65: 211–214. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer E, Heyman Z, Bin H, Schwartz E, 2012. Capillary leakage in travelers with dengue infection: implications for pathogenesis. Am J Trop Med Hyg 86: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratnam I, Leder K, Black J, Torresi J, 2013. Dengue fever and international travel. J Travel Med 20: 384–393. [DOI] [PubMed] [Google Scholar]
- 25.Haby MM, Pinart M, Elias V, Reveiz L, 2018. Prevalence of asymptomatic Zika virus infection: a systematic review. Bull World Health Organ 96: 402–413D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freour T, Mirallie S, Hubert B, Splingart C, Barriere P, Maquart M, Leparc-Goffart I, 2016. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill 21: pii=30254. [DOI] [PubMed] [Google Scholar]
- 27.Septfons A, et al. 2016. Travel-associated and autochthonous Zika virus infection in mainland France, 1 January to 15 July 2016. Euro Surveill 21: pii=30285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogoch, et al. 2016. Anticipating the international spread of Zika virus from Brazil. Lancet 387: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallian P, Cabié A, Richard P, Paturel L, Charrel RN, Pastorino B, Leparc-Goffart I, Tiberghien P, de Lamballerie X, 2017. Zika virus in asymptomatic blood donors in Martinique. Blood 129: 263–266. [DOI] [PubMed] [Google Scholar]
- 30.Leshem E, Bin H, Shalom U, Perkin M, Schwartz E, 2012. Risk for emergence of dengue and chikungunya virus in Israel. Emerg Infect Dis 18: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venturi G, et al. 2017. Detection of a chikungunya outbreak in central Italy, August to September 2017. Euro Surveill 22: pii=17-00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelini R, et al. 2007. An outbreak of chikungunya fever in the province of Ravenna, Italy. Euro Surveill 12: E070906.1. [DOI] [PubMed] [Google Scholar]