Abstract
Background
Experimental evidence suggests that ranolazine decreases susceptibility to ischemia‐induced arrhythmias independent of effects on coronary artery blood flow.
Objective
In symptomatic diabetic patients with non–flow‐limiting coronary artery stenosis with diffuse atherosclerosis and/or microvascular dysfunction, we explored whether ranolazine reduces T‐wave heterogeneity (TWH), an electrocardiographic (ECG) marker of arrhythmogenic repolarization abnormalities shown to predict sudden cardiac death.
Methods
We studied all 16 patients with analyzable ECG recordings during rest and exercise tolerance testing before and after 4 weeks of ranolazine in the double‐blind, crossover, placebo‐controlled RAND‐CFR trial (NCT01754259). TWH was quantified without knowledge of treatment assignment by second central moment analysis, which assesses the interlead splay of T waves in precordial leads about a mean waveform. Myocardial blood flow (MBF) was measured by positron emission tomography.
Results
At baseline, prior to randomization, TWH during rest was 54 ± 7 μV and was not altered following placebo (47 ± 6 μV, p = .47) but was reduced by 28% (to 39 ± 5 μV, p = .002) after ranolazine. Ranolazine did not increase MBF at rest. Exercise increased TWH after placebo by 49% (to 70 ± 8 μV, p = .03). Ranolazine did not reduce TWH during exercise (to 75 ± 16 μV), and there were no differences among the groups (p = .95, ANOVA). TWH was not correlated with MBF at rest before (r 2 = .07, p = .36) or after ranolazine (r 2 = .23, p = .06).
Conclusions
In symptomatic diabetic patients with non‐flow‐limiting coronary artery stenosis with diffuse atherosclerosis and/or microvascular dysfunction, ranolazine reduced TWH at rest but not during exercise. Reduction in repolarization abnormalities appears to be independent of alterations in MBF.
Keywords: diabetes, late sodium current, microvascular dysfunction, non–flow‐limiting coronary artery stenosis, ranolazine, T‐wave heterogeneity
1. INTRODUCTION
Ranolazine is an antianginal agent with a relatively unique mode of action. Specifically, its anti‐ischemic effects are mediated via inhibition of the late sodium current (INaL), which in turn through reverse sodium–calcium exchange improves diastolic compliance (Antzelevitch, Burashnikov, Sicouri, & Belardinelli, 2011). The Metabolic Efficiency with Ranolazine for Less Ischemia in Non‐ST‐Elevation Acute Coronary Syndrome: Thrombolysis in Myocardial Infarction (MERLIN TIMI) 36 trial (NCT00099788), which enrolled 6,560 patients, showed the drug to be safe and effective as antianginal therapy (Morrow et al., 2007). Subanalyses of the results revealed that ranolazine suppressed nonsustained ventricular tachycardia (Scirica et al., 2007) and reduced mortality among patients with elevated T‐wave alternans (Nieminen et al., 2014), a marker of risk for lethal arrhythmia (Verrier et al., 2011). These conclusions are consistent with preclinical studies demonstrating potent antiarrhythmic properties (Kumar et al., 2008; Nieminen et al., 2011). In a large animal model of partial coronary artery stenosis, Nieminen et al. (2011) reported that ranolazine significantly blunted the ischemia‐induced fall in ventricular fibrillation threshold and reduced T‐wave alternans, indicating the drug's capacity to protect against repolarization heterogeneity, which underlies propensity for reentrant arrhythmias.
Recently, a noninvasive method has been developed to assess repolarization heterogeneity using second central moment analysis of the interlead splay of T waves in precordial leads about a mean waveform as the central axis. This parameter, termed “T‐wave heterogeneity” (TWH), was shown to predict sudden cardiac death in over 5,600 subjects enrolled in Health Survey 2000, which was designed to provide a cross‐section of the entire Finnish population (Kenttä et al., 2016).
The goal of the present secondary analysis was to evaluate the effect of ranolazine on TWH in patients participating in the Effects of Ranolazine on Coronary Flow Reserve in Symptomatic Patients with Diabetes and Suspected or Known Coronary Artery Disease (RAND‐CFR) trial (NCT01754259). Diabetic patients are at risk for mortality in large part due to cardiac electrical instability that results from autonomic neuropathy resulting in adrenergic predominance (Carnethon, Golden, Folsom, Haskell, & Liao, 2003) and from repolarization abnormalities reflected in increased QT dispersion (Arildsen, May, Christiansen, & Damsgaard, 1999; Cardoso, Salles, Bloch, Deccache, & Siqueira‐Filho, 2001; Wei, Dorian, Newman, & Langer, 1995). As experimental studies indicate that ranolazine can protect against these adverse factors, we hypothesized that ranolazine will suppress TWH in RAND‐CFR patient cohort, who exhibited non–flow‐limiting coronary artery stenosis with diffuse atherosclerosis and/or microvascular dysfunction. Furthermore, based on findings in large animals with concurrent measurement of coronary artery blood flow and repolarization abnormalities (Nieminen et al., 2011), we postulated that alterations in TWH will occur despite the absence of effects on myocardial blood flow (MBF), which was shown in the parent study (Shah et al., 2017).
2. METHODS
2.1. Study design
We studied all 16 patients from the double‐blind, cross‐over, placebo‐controlled RAND‐CFR study with analyzable ECG recordings during both rest and exercise in three settings: baseline (before randomization), following placebo, and following 4 weeks of ranolazine (Shah et al., 2017). The study and medical records review were approved by the Partners Healthcare Institutional Review Board. The ECG recordings were scanned and converted into digital files. TWH was quantified by second central moment analysis without knowledge of treatment assignment.
2.2. RAND‐CFR trial design
A complete description of the methodology of the RAND‐CFR study has been published (Shah et al., 2017). Briefly, study patients had diabetes, baseline stable angina and/or exertional dyspnea, and exercise tolerance testing (ETT) of ≥3 metabolic equivalents. Patients with obstructive coronary artery disease (defined as ≥50% stenosis on invasive coronary angiography within the past year or on study protocol‐mandated screening by coronary computer tomography angiography) and patients with a stress perfusion deficit >8.8% (suggestive of clinically significant ischemia and/or scar) on baseline myocardial perfusion positron emission tomography (PET) were excluded.
The order of ranolazine and placebo exposure was randomly assigned. During the 4‐week treatment periods, ranolazine (Gilead Sciences, Foster City, CA, USA) and matching placebo were administered as 500 mg by mouth twice daily for 1 week and increased to 1,000 mg by mouth twice daily for 3 weeks, as tolerated. Treatment compliance was measured by pill count. Patients and study investigators were blinded to treatments and treatment order throughout the study protocol.
MBF was measured at rest and in response to symptom‐limited supine bicycle exercise using 13N‐ammonia as a PET flow tracer. Heart rate, blood pressure, and 12‐lead electrocardiogram (ECG) were recorded at baseline and every minute during and after ETT. An identical ETT protocol and workload were used for PET scans after each treatment arm. Absolute left ventricular MBF (ml/g/min) was computed from the dynamic rest and ETT imaging series using commercially available software and previously validated methods (Murthy et al., 2011). Per‐patient global coronary flow reserve (CFR) was calculated as the ratio of absolute MBF at ETT over rest for the entire left ventricle. Quantitation of MBF was performed by two operators blinded to the patient, treatment, and treatment order. To account for differences in resting cardiac workload, which can affect global rest left ventricular MBF, “corrected” CFR was calculated: corrected CFR = peak global LV left ventricular MBF/[(rest MBF/rest rate – pressure product) × 10,000].
2.3. Electrocardiogram recordings
Recordings of standard 12‐lead analog ECGs (25 mm/s, 10 mV/mm) were printed for 12 s at rest and during ETT. The printed 12‐lead ECG recordings were scanned with a high‐resolution scanner. Patients without a complete set of V4, V5, or V6 ECG leads, which are essential for TWH calculation, or whose tracings had significant noise artifact or baseline wander were excluded. The image‐processing software, “ECGScan” (AMPS‐LLC, New York, NY), was used to extrapolate the ECG waveforms using an active modeling technique (Badilini, Erdem, Zareba, & Moss, 2005). The resulting digital waveforms were converted into .txt files that could be imported into spreadsheets for subsequent analyses of TWH.
2.4. Measurement of T‐wave heterogeneity
The .txt files were imported to spreadsheets and leads V4, V5, and V6 were plotted and superimposed. Second central moment analysis (Nearing & Verrier, 2003, 2015, Verrier & Huikuri, 2017) was performed on the JT interval to measure TWH for every beat. The maximum splay in microvolts about the mean ECG waveform from the J point to end of the T wave for each patient during rest and ETT before and after ranolazine was reported. Since TWH is measured over the entire JT waveform, it does not depend on the specific T‐wave endpoint as do time‐dependent indices of dispersion of repolarization such as Tpeak‐Tend or QTc intervals.
2.5. Statistics
Data are reported as means ± standard error of the mean (SEM). Statistical differences were calculated using standard t‐tests. Results within each group were compared using paired t‐tests. Bonferroni correction was performed for multiple comparisons within or between groups, and 2‐tailed p < .05 was considered significant.
3. RESULTS
3.1. Clinical characteristics
The age of the subjects in this study was 64 ± 1.6 years. All major clinical characteristics are presented in Table 1, and exercise hemodynamics are summarized in Table 2.
Table 1.
Patient characteristics at baseline (n = 16)
| Age (years) | 64 ± 1.6 |
| Sex (M/F) | 9/7 |
| BMI | 32 ± 1.8 |
| Cardiovascular risk factors | |
| Diabetes, n (%) | 16 (100) |
| Hypertension, n (%) | 13 (81) |
| Smoking, n (%) | 9 (56) |
| Hyperlipidemia, n (%) | 16 (100) |
| Drug therapy | |
| Beta‐blockers, n (%) | 7 (44) |
| Calcium antagonists, n (%) | 3 (19) |
| ACE‐inhibitors and/or ARBs, n (%) | 12 (75) |
| Statins, n (%) | 15 (94) |
| Antiaggregants, n (%) | 4 (25) |
| Nitrates, n (%) | 4 (25) |
BMI, body mass index; ACE, angiotensin‐converting enzyme; ARB, angiotensin‐II receptor blocker.
Table 2.
Exercise tolerance test parameters (n = 16)
| Rest | |
| SBP (mm Hg) | 130 ± 3 |
| DBP (mm Hg) | 64 ± 1.4 |
| HR (bpm) | 70 ± 3 |
| RPP (mm Hg × bpm) | 9,098 ± 486 |
| Peak exercise | |
| SBP (mm Hg) | 177 ± 4 |
| DBP (mm Hg) | 79 ± 3.4 |
| HR (bpm) | 132 ± 5 |
| RPP (mm Hg × bpm) | 23,403 ± 1,161 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; RPP, rate pressure product.
3.2. Coronary flow reserve
The maximum corrected CFR value in the 16 patients in the current TWH substudy was 2.57 and the minimum was 1.13. The 75th percentile was 1.80. Thirteen (81.25%) of the 16 patients had CFR values <2, the threshold for hemodynamic significance (Kern et al., 2006). At rest, corrected MBF was 0.96 ± 0.4 before drug, 0.92 ± 0.04 after placebo, and 0.93 ± 0.04 after ranolazine (p = .81, ANOVA). There was no correlation between MBF and TWH at rest before (r 2 = .07, p = .36) or after ranolazine (r 2 = .23, p = .06).
3.3. T‐wave heterogeneity
At baseline before randomization, TWH during rest was 54 ± 7 μV and was not altered following placebo (47 ± 6 μV, p = .47). After treatment with ranolazine, TWH during rest was significantly reduced by 28% (to 39 ± 5 μV, p = .002), as depicted in a representative patient (Figure 1) and shown in the groups (Figure 2, left panel).
Figure 1.
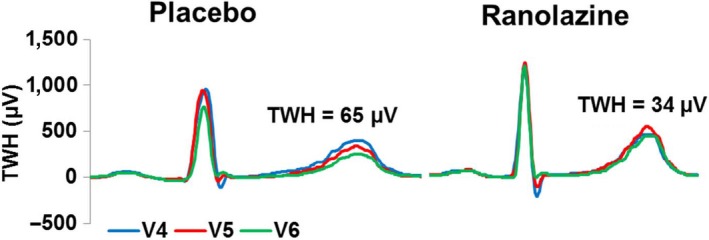
Digitized tracings from a representative patient illustrating the reduction in T‐wave heterogeneity (TWH) by ranolazine at rest compared with placebo. The complexes obtained from ECG leads V4, V5, and V6 are QRS aligned. Note that with placebo, TWH is markedly elevated at 65 μV while it is reduced to 34 μV following ranolazine administration
Figure 2.
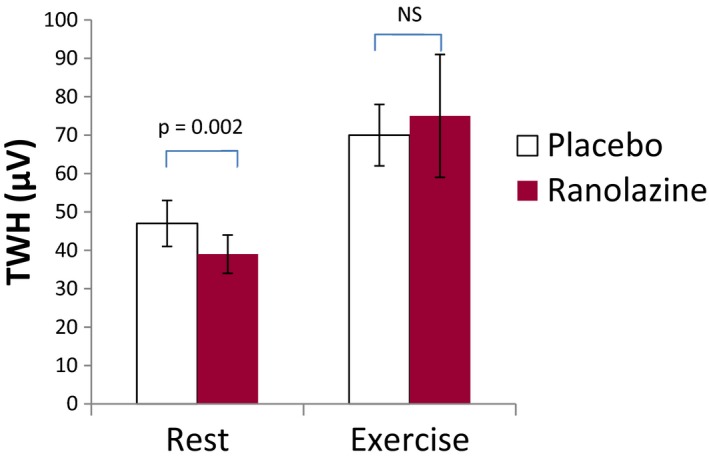
Group data showing decrease in T‐wave heterogeneity (TWH) at rest (left panel) by ranolazine compared to placebo and the exercise‐induced increase in TWH (right panel), which was not affected by ranolazine (both, n = 16)
Unlike in the resting condition, ranolazine did not alter elevated TWH induced by exercise. Specifically, exercise increased TWH before drug by 37% (to 74 ± 11 μV, p = .07) and after placebo by 49% (to 70 ± 8 μV, p = .03). Ranolazine did not reduce TWH during exercise (to 75 ± 16 μV) (Figure 2, right panel) and there were no differences among the groups in that setting (p = .95, ANOVA).
4. DISCUSSION
The present study uses a new noninvasive ECG‐based method to quantify the effects of ranolazine on repolarization heterogeneity in symptomatic diabetic patients with non–flow‐limiting coronary artery stenosis with diffuse atherosclerosis and/or microvascular dysfunction. We found ranolazine significantly reduced TWH in patients at rest, indicating a decrease in repolarization heterogeneity, a property linked to cardiovascular mortality and sudden cardiac death in a >5,600‐subject Health Survey (Kenttä et al., 2016). However, the drug was not capable of counteracting the increase in TWH associated with exercise.
4.1. Prior studies
Ranolazine was developed as an antianginal agent but proved to have important antiarrhythmic properties (Antzelevitch et al., 2011). Both cardioprotective effects appear to be related primarily to the drug's inhibition of INaL rather than through its other actions of inhibiting peak INa and IKr. INaL inhibition achieves its antiarrhythmic effects through reverse sodium–calcium exchange, ultimately preventing development of arrhythmogenic levels of cytosolic calcium. In preclinical studies, ranolazine was shown to reduce coronary artery stenosis‐induced repolarization heterogeneity as assessed by T‐wave alternans and by the threshold for vulnerability to ventricular fibrillation (Nieminen et al., 2011). INaL inhibition was implicated in the antiarrhythmic effect as the IKr inhibitor E4031 decreased the ventricular fibrillation threshold in the same model. Recently, Justo et al. (2016) demonstrated that the highly selective INaL inhibitor eleclazine significantly decreased stenosis‐induced TWH in intact porcines. Thus, the protective effect of ranolazine appears to be due to direct actions on myocardial electrical properties, as coronary artery blood flow was maintained constant.
4.2. Current investigation
In the baseline resting state, TWH was 54 ± 7 μV, an elevated level compared to 19 ± 2 μV obtained in a previous study of patients without apparent organic disease of the ventricular myocardium (Tan, Nearing, Josephson, & Verrier, 2013). Placebo did not exert a significant change in TWH level (p = .47), but ranolazine significantly reduced TWH at rest to 39 ± 5 μV (p = .002). Although the reduction in TWH by ranolazine was moderate at 28%, this effect may reflect an important antiarrhythmic benefit, given that in the MERLIN trial the same dose of ranolazine substantially reduced ventricular tachyarrhythmias (Scirica et al., 2007).
As cardiac metabolic demands are low at rest, and ST segments were not abnormal, it does not appear that ranolazine's reduction in TWH during rest was due to an anti‐ischemic action. The possibility that the drug can alter cardiac electrophysiologic properties independent of an influence on coronary artery blood flow is consistent with preclinical evidence from Nieminen et al. (2011) and Justo et al. (2016), who demonstrated protective antiarrhythmic effects of INaL inhibition with ranolazine and eleclazine, respectively, during flow‐regulated coronary artery stenosis.
An additional mechanism for ranolazine's capacity to suppress TWH resides in the fact that INaL inhibitors appear to be capable of reducing the arrhythmogenic effect of catecholamine‐induced increases in INaL. Specifically, two selective INaL inhibitors, GS‐967 (Bento et al., 2015) and eleclazine (Bacic et al., 2017), have been shown to suppress catecholamine‐induced VT and TWH.
Potentially relevant in this regard is evidence indicating significant autonomic dysfunction in patients with diabetes, with predominance in sympathetic tone (Carnethon et al., 2003). Diabetes is associated with a decrease in high‐frequency (HF) heart rate variability (HRV) and increase in low/high (LF/HF)‐frequency HRV ratio. Further relevant to the present study are the findings that QT dispersion, an indicator of heterogeneity of repolarization, is elevated during rest in patients with diabetes (Arildsen et al., 1999; Cardoso et al., 2001; Wei et al., 1995). Thus, a component of ranolazine's effects on TWH may have been related to its reduction in enhanced INaL due to hyperadrenergic activity associated with diabetes.
The inability of the drug to protect against the exercise‐induced rise in TWH is puzzling, given its robust effect at rest. While the exact explanation is unclear, a possibility is that the heightened metabolic demands and/or excessive surges in exercise‐related sympathetic activity overwhelmed the drug's effects in these patients with non–flow‐limiting stenosis with diffuse atherosclerosis and/or microvascular dysfunction due to diabetes. The finding that ranolazine did not improve MBF and cardiac function during exercise, as reported in the parent study (Shah et al., 2017), supports this surmise. Thus, it remains to be determined whether a longer period of ranolazine therapy might have been effective, given its success in improving CFR after 8 weeks (Tagliamonte et al., 2015) and in reducing angina after 8 weeks in the Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina trial (TERISA, NCT01425359) (Kosiborod et al., 2013).
5. CONCLUSION
Ranolazine reduces TWH, an index of proarrhythmic repolarization abnormalities, in symptomatic patients with diabetes and non–flow‐limiting stenosis <50% with diffuse atherosclerosis and/or microvascular dysfunction. The precise mechanisms involved remain to be determined but do not appear to be linked to changes in MBF.
ACKNOWLEDGMENTS
We are grateful to Fabio Badilini for making available the image processing software, “ECGScan” (AMPS‐LLC, New York, NY), for use in this study.
Evaristo E, Stocco FG, Shah NR, et al. Ranolazine reduces repolarization heterogeneity in symptomatic patients with diabetes and non–flow‐limiting coronary artery stenosis. Ann Noninvasive Electrocardiol. 2018;23:e12480 10.1111/anec.12480
Funding information
This study was supported by a grant from Gilead Sciences, Inc., to Beth Israel Deaconess Medical Center, RL Verrier, P.I.
REFERENCES
- Antzelevitch, C. , Burashnikov, A. , Sicouri, S. , & Belardinelli, L. (2011). Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm, 8, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arildsen, H. , May, O. , Christiansen, E. H. , & Damsgaard, E. M. (1999). Increased QT dispersion in patients with insulin‐dependent diabetes mellitus. International Journal of Cardiology, 71, 235–242. [DOI] [PubMed] [Google Scholar]
- Bacic, D. , Carneiro, J. S. , Bento, A. A. , Nearing, B. D. , Rajamani, S. , Belardinelli, L. , & Verrier, R. L. (2017). Eleclazine, an inhibitor of the cardiac late sodium current, is superior to flecainide in suppressing catecholamine‐induced ventricular tachycardia and T‐wave alternans in an intact porcine model. Heart Rhythm, 14, 448–454. [DOI] [PubMed] [Google Scholar]
- Badilini, F. , Erdem, T. , Zareba, W. , & Moss, A. J. (2005). ECGScan: A method for conversion of paper electrocardiographic printouts to digital electrocardiographic files. Journal of Electrocardiology, 38, 310–318. [DOI] [PubMed] [Google Scholar]
- Bento, A. S. A. , Bacic, D. , Carneiro, J. S. , Nearing, B. D. , Fuller, H. , Justo, F. A. , … Verrier, R.L. (2015). Selective late INa inhibition by GS‐458967 exerts parallel suppression of catecholamine‐induced hemodynamically significant ventricular tachycardia and T‐wave alternans in an intact porcine model. Heart Rhythm, 12, 2508–2514. [DOI] [PubMed] [Google Scholar]
- Cardoso, C. , Salles, G. , Bloch, K. , Deccache, W. , & Siqueira‐Filho, A. G. (2001). Clinical determinants of increased QT dispersion in patients with diabetes mellitus. International Journal of Cardiology, 79, 253–262. [DOI] [PubMed] [Google Scholar]
- Carnethon, M. R. , Golden, S. H. , Folsom, A. R. , Haskell, W. , & Liao, D. (2003). Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: The Atherosclerosis Risk in Communities Study, 1987–1998. Circulation, 107, 2190–2195. [DOI] [PubMed] [Google Scholar]
- Justo, F. , Fuller, H. , Nearing, B. D. , Rajamani, S. , Belardinelli, L. , & Verrier, R. L. (2016). Inhibition of the cardiac late sodium current with eleclazine protects against ischemia‐induced vulnerability to atrial fibrillation and reduces atrial and ventricular repolarization abnormalities in the absence and presence of concurrent adrenergic stimulation. Heart Rhythm, 13, 1860–1867. [DOI] [PubMed] [Google Scholar]
- Kenttä, T. V. , Nearing, B. D. , Porthan, K. , Tikkanen, J. T. , Viitasalo, M. , Nieminen, M. S. , … Verrier, R.L. (2016). Prediction of sudden cardiac death with automated high throughput analysis of heterogeneity in standard resting 12‐lead electrocardiogram. Heart Rhythm, 13, 713–720. [DOI] [PubMed] [Google Scholar]
- Kern, M. J. , Lerman, A. , Bech, J. W. , De Bruyne, B. , Eeckhout, E. , Fearon, W. F. , … Spaan, J. A. (2006). Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation, 114, 1321–1341. [DOI] [PubMed] [Google Scholar]
- Kosiborod, M. , Arnold, S. V. , Spertus, J. A. , McGuire, D. K. , Li, Y. , Yue, P. , … Chaitman, B. R. (2013). Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: Results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects with Chronic Stable Angina). Journal of the American College of Cardiology, 61, 2038–2045. [DOI] [PubMed] [Google Scholar]
- Kumar, K. , Nearing, B. D. , Bartoli, C. R. , Kwaku, K. F. , Belardinelli, L. , & Verrier, R. L. (2008). Effect of ranolazine on ventricular vulnerability and defibrillation threshold in the intact porcine heart. Journal of Cardiovascular Electrophysiology, 19, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Morrow, D. A. , Scirica, B. M. , Karwatowska‐Prokopczuk, E. , Murphy, S. A. , Budaj, A. , Varshavsky, S. , … Braunwald, E. (2007). Effects of ranolazine on recurrent cardiovascular events in patients with non‐ST‐elevation acute coronary syndromes: The MERLIN‐TIMI 36 randomized trial. JAMA, 297, 1775–1783. [DOI] [PubMed] [Google Scholar]
- Murthy, V. L. , Naya, M. , Foster, C. R. , Hainer, J. , Gaber, M. , Di Carli, G. , … Di Carli, M. F. (2011). Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation, 124, 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nearing, B. D. , & Verrier, R. L. (2003). Tracking heightened cardiac electrical instability by computing interlead heterogeneity of T‐wave morphology. Journal of Applied Physiology, 95, 2265–2272. [DOI] [PubMed] [Google Scholar]
- Nearing, B. D. , & Verrier, R. L. (2015). Multilead template‐derived residua of surface ECGs for quantitative assessment of arrhythmia risk. Annals of Noninvasive Electrocardiology, 20, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen, T. , Nanbu, D. Y. , Datti, I. P. , Vaz, G. R. , Tavares, C. A. M. , Pegler, J. R. M. , … Verrier, R. L. (2011). Antifibrillatory effect of ranolazine during severe coronary stenosis in the intact porcine model. Heart Rhythm, 8, 608–614. [DOI] [PubMed] [Google Scholar]
- Nieminen, T. , Scirica, B. M. , Pegler, J. R. , Tavares, C. , Pagotto, V. P. , Kanas, A. F. , … Verrier, R. L. (2014). Relation of T‐wave alternans to mortality and nonsustained ventricular tachycardia in patients with non‐ST‐segment elevation acute coronary syndrome from the MERLIN‐TIMI 36 trial of ranolazine versus placebo. American Journal of Cardiology, 114, 17–23. [DOI] [PubMed] [Google Scholar]
- Scirica, B. M. , Morrow, D. A. , Hod, H. , Murphy, S. A. , Belardinelli, L. , Hedgepeth, C. M. , … Braunwald, E. (2007). Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with nonST‐segment elevation acute coronary syndrome: Results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non ST‐Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation, 116, 1647–1652. [DOI] [PubMed] [Google Scholar]
- Shah, N. R. , Cheezum, M. K. , Veeranna, V. , Horgan, S. J. , Taqueti, V. R. , Murthy, V. L. , … Di Carli, M. F. (2017). Ranolazine in symptomatic diabetic patients without obstructive coronary artery disease: Impact on microvascular and diastolic function (RAND‐CFR). Journal of the American Heart Association, 6, e005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte, E. , Rigo, F. , Cirillo, T. , Astarita, C. , Quaranta, G. , Marinelli, U. , … Capuano, N. (2015). Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography, 32, 516–521. [DOI] [PubMed] [Google Scholar]
- Tan, A. Y. , Nearing, B. D. , Josephson, M. E. , & Verrier, R. L. (2013). Spatial heterogeneity of R and T waves derived from 12‐lead ECGs is associated with elevated risk of ventricular arrhythmia in patients with cardiomyopathy. Heart Rhythm, 10, S41. [Google Scholar]
- Verrier, R. L. , & Huikuri, H. V. (2017). Tracking interlead heterogeneity of R‐ and T‐wave morphology to disclose latent risk for sudden cardiac death. Heart Rhythm. [DOI] [PubMed] [Google Scholar]
- Verrier, R. L. , Klingenheben, T. , Malik, M. , El‐Sherif, N. , Exner, D. , Hohnloser, S. , … Rosenbaum, D.S. (2011). Microvolt T‐wave alternans: Physiologic basis, methods of measurement, and clinical utility. Consensus guideline by the International Society for Holter and Noninvasive Electrocardiology. Journal of the American College of Cardiology, 44, 1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, K. , Dorian, P. , Newman, D. , & Langer, A. (1995). Association between QT dispersion and autonomic dysfunction in patients with diabetes mellitus. Journal of the American College of Cardiology, 26, 859–863. [DOI] [PubMed] [Google Scholar]


