Abstract
Asbestos-induced diseases like fibrosis and mesothelioma are very aggressive, without any treatment options. These diseases are diagnosed only at the terminal stages due to lack of early stage biomarkers. The recent discovery of exosomes as circulating biomarkers led us to look for exosomal biomarkers of asbestos exposure in mouse blood. In our model, mice were exposed to asbestos as a single bolus dose by oropharyngeal aspiration. Fifty six days later blood was collected, exosomes were isolated from plasma and characterized and subjected to proteomic analysis using Tandem Mass Tag labelling. We identified many proteins, some of which were more abundant in asbestos exposed mouse serum exosomes, and three selected proteins were validated by immunoblotting. Our study is the first to show that serum exosomal proteomic signatures can reveal some important proteins relevant to asbestos exposure that have the potential to be validated as candidate biomarkers. We hope to extrapolate the positive findings of this study to humans in future studies.
Keywords: Exosomes, Asbestos, Proteomics, Mesothelioma
The causative factor leading to the development of malignant mesothelioma (MM) is exposure to asbestos fibers (Berman and Crump, 2008). Mesothelioma is a fatal cancer arising on the mesothelial cell lining of the pleura, most commonly, but can also present itself on the peritoneal lining, pericardium, and rarely the testicular tunica vaginalis. A recent CDC report states that there are a substantial number of MM cases, which are increasing in numbers (Mazurek et al., 2017). The median lifespan, once diagnosed with MM, is 6–10 months and there are currently no successful treatment options. Further, there are no standard biomarkers for early diagnosis of the disease, neither are there any biomarkers to indicate harmful levels of asbestos exposure. It is therefore a highly valuable public health endeavor to identify signatures of asbestos exposure in order to more adequately detect harmful levels exposure before an individual develops MM.
The field of biomarker discovery has found its trajectory leading to the field of extracellular vesicle investigations, particularly exosomes. Exosomes are 30–140 nm membrane bound vesicles derived from endocytic origin, which are now known to be major players in transmitting biological content between cells and tissues (Colombo et al., 2014; Munson and Shukla, 2015). Their content is remarkable in that it can be utilized for discovering unique biomarkers of disease states, such as cancer. Beyond biomarker discovery, exosome research has furthered the understanding of myriad biological mechanisms. Therefore, this avenue of discovery is fitting to make the necessary strides in being able to one day diagnose dangerous exposure to asbestos and pre-empt the development of MM.
To date there is no study of exosomes proteomic signature in relationship to asbestos exposure, making our paper the first of its kind. There is mounting evidence, however, of the relevance and toxicology of exosomes released after exposure to chemicals and environmental toxins (Harischandra et al., 2017). Asbestos itself is one of the most well-known environmental toxins and is currently classified as a Class I Carcinogen. Furthermore, the use of exosomes as cancer and disease biomarkers is now very common place, with serum exosomal proteomic signature being a particular focus for many studies on elucidating unique signatures (Colletti et al., 2017; Sun et al., 2017). One of the most exciting uses of serum exosomes for biomarker discovery was by Melo et al. in 2015 indicating that exosomal Glipican-1 was a discriminate factor for pinpointing pancreatic cancer (Melo et al., 2015).
The current study presented focuses on describing the exosomal proteomic analysis of an asbestos exposure mouse model. Our preliminary goal was to quarry for differential abundances of proteins in exosomes derived from asbestos exposed animals, particularly proteins in increased abundance, or unique, as those would more likely lead to future biomarker identification studies. The mouse model used here, exposes animals to asbestos via oropharyngeal aspiration (OA) in a bolus dose. OA is a well-established mode of exposing mice to asbestos fibers by deposition in the airway and lungs (Teeguarden et al., 2011) and is closest to an inhalation model. Serum was collected and exosomes were isolated for proteomic profiling.
Although the full nature of exosome cargo packaging is not fully understood, it is known that exosomes become specifically enriched in certain proteins and molecules from the producer cell cytoplasm in ratios not directly parallel to the cytosolic fraction or freely secreted portion of those same molecules (given that all of them are freely secreted from the cell in the first place). Hence, isolating exosomes provides a specific vantage point that avoids much of the other potentially unimportant molecules that are secreted into the circulation or into cavity spaces.
Materials and Methods
Oropharyngeal Aspiration of asbestos
C57/Bl6 mice (5/group) were exposed to crocidolite asbestos (NIEHS reference sample) or saline (50 μL) as a single bolus dose by oropharyngeal aspiration (OA) as described (Teeguarden et al., 2011). After 56 days of exposure, whole blood was collected via cardiac puncture and serum was collected using Microtainer Serum Separating Tubes (BD, Franklin Lakes, NJ) following the manufacturer’s protocol. Serum was frozen at −80°C until exosome collection. All experiments using mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Vermont, Larner College of Medicine (Burlington, VT).
Exosome Isolation
Exosomes were collected from serum using ExoQuick (System BioSciences, Palo Alto, CA) according to the manufacturer’s protocol (Eitan et al., 2017; Helwa et al., 2017). Precipitation method was used for serum exosome isolation, because of very low volume (200 μL) of the serum sample availability.
Transmission Electron Microscopy (TEM)
The membranous structure and size of exosomes was assessed by TEM. Formvar/carbon coated nickel 200 mesh grids were glow discharged for 60 seconds, and 5µL of sample was placed on grid and incubated for 1 minute. Excess sample was wicked and the grids were touched to 30µL water drops with wicking performed between each rinse. Grids were touched to 2 sequential 30µL drops of 2% aqueous uranyl acetate, excess was wicked, and grids were air dried. Grids were imaged under transmission electron microscope for exosomes using a JEOL 1400 TEM (JEOL, Peabody, MA).
NanoParticle Tracking Analysis
Number and size of exosomes were further assessed by nanoparticle tracking analysis (NTA) using the ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and Software ZetaView 8.02.31.
Exosome marker characterization
Exosome purity was characterized by assessment of exosome specific markers, CD9 and CD81 by using specific antibodies (Sigma Aldrich, St. Louis MO) in immunoblot analysis as described below (Thompson et al., 2017).
Sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and trypsin digestion
The extracted exosomal proteins (maximum amounts less than 100 µg) were loaded onto SDS-PAGE. Equal amounts (100 ng) of gylceraldehyde-3-phosphate dehydrogenase (Sigma Aldrich) were added to each sample to control for digestion and labeling efficiencies. The proteins were allowed to migrate 3 to 5-mm into the separating gel, and then the gels were stained with Coomassie Brilliant Blue. The gel lanes were excised into 3 slices according to their molecular weights (I- upper, II-mid, III-lower). (Figure 2A) The slices were destained with 50% acetonitrile (ACN)/50 mM NH4HCO3 and subjected to trypsin digestion protocols, as described previously (Hristova et al., 2014).
Figure 2.
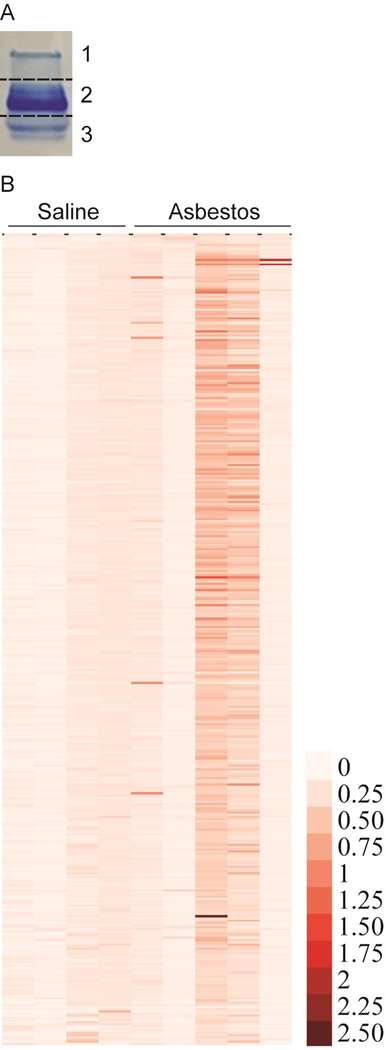
Depiction of gel cuts into three bands prior to in-gel trypsin digestion (A) and heatmap showing differential abundance of mouse serum exosomal proteins in response to asbestos exposure. Mice were exposed to saline (n=4) or asbestos (n=5) by oropharyngeal aspiration. Eight weeks later serum was collected, exosomes were isolated and proteomic analysis was performed as described in material and method section (B).
After our initial pilot experiments, we have optimized this gel based separation strategy to allow the high abundant proteins to be confined to gel slice II-mid, whereas the relatively low abundant proteins were localized in gel slices I-upper and III-lower. Gel slices I, II, II were analyzed separately in three mass spectrometry runs to increase the proteome coverage.
Peptide labeling by Tandem Mass Tags
The labeling procedures were performed according to the manufacturers’ protocols (Thermo Fisher Scientific, Waltham, MA, USA) with the following modifications. Briefly, dried peptides in gel slice II, and gel slices I and III from each sample were resuspended in 50 and 25 µL of triethyl ammonium bicarbonate, respectively. Twenty and ten µL of TMT reagents (0.8 mg dissolved in 41 µL of acetonitrile (CH3CN)) was added to gel slice II, and gel slices I and III, respectively, followed by briefly vortexing and an incubation for 1.5 h at room temperature. After incubation, 5% hydroxylamine was added to quench the reactions. One-third the reactions were combined, dried down and kept at −80o C until mass spectrometry analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The purified labeled peptides were resuspended in 5 µL of 2.5% acetonitrile CH3CN and 2.5% formic acid (FA) in water for subsequent LC-MS/MS based peptide identification and quantification. Analyses were performed on the Q-Exactive mass spectrometer coupled to an EASY-nLC (Thermo Fisher Scientific, Waltham, MA, USA). Samples were loaded onto a 100 μm x 120 mm capillary column packed with Halo C18 (2.7 μm particle size, 90 nm pore size, Michrom Bioresources, CA, USA) at a flow rate of 300 nl min-1. Peptides were separated using a gradient of 2.5–35% CH3CN/0.1% FA over 150 min, 35–100% CH3CN/0.1% FA in 1 min and then 100% CH3CN /0.1% FA for 8 min, followed by an immediate return to 2.5% CH3CN/0.1% FA and a hold at 2.5% CH3CN/0.1% FA. Peptides were introduced into the mass spectrometer via a nanospray ionization source and a laser pulled ~3 μm orifice with a spray voltage of 2.0 kV. Mass spectrometry data was acquired in a data-dependent “Top 10” acquisition mode with lock mass function activated (m/z 371.1012; use lock masses: best; lock mass injection: full MS), in which a survey scan from m/z 350–1600 at 70, 000 resolution (AGC target 1e6; max IT 100 ms; profile mode) was followed by 10 higher-energy collisional dissociation (HCD) tandem mass spectrometry (MS/MS) scans on the most abundant ions at 35,000 resolution (AGC target 1e5; max IT 100 ms; profile mode). MS/MS scans were acquired with an isolation width of 1.2 m/z and a normalized collisional energy of 35%. Dynamic exclusion was enabled (peptide match: preferred; exclude isotopes: on; underfill ratio: 1%; exclusion duration: 30 sec). Product ion spectra were searched using the SEQUEST and Mascot search engines on Proteome Discoverer 1.4 (Thermo Fisher Scientific, Waltham, MA, USA) against a curated Mouse Uniprot (Mus protein database; 3AUP000000589; downloaded Feb. 21, 2017) with sequences in forward and reverse orientations. Search Parameters were as follows: (1) full trypsin enzymatic activity; (2) maximum missed cleavages = 2; (3) minimum peptide length = 6, (4) mass tolerance at 20 ppm for precursor ions and 0.02 Da for fragment ions; (5) dynamic modifications on methionines (+15.9949 Da: oxidation), Dynamic TMT6plex modification (The TMT6plex and TMT10plex have the same isobaric mass) on N-termini and lysines (229.163 Da); (6) 4 maximum dynamic modifications allowed per peptide; and (7) static carbamidomethylation modification on cysteines (+57.021 Da). Percolator node was included in the workflow to limit the false positive (FP) rates to less than 1% in the data set. The TMT ratios were generated with a common denominator using the four controls. All the protein identification and quantification information (<1% FP; with protein grouping enabled) was exported from the msf result files to Excel spreadsheets for further statistical analyses. Identification of keratins were removed from the list. Average means and p-values were calculated in Excel (Supplementary Table. S 1, Excel spreadsheet).
Western blot analysis for validation of proteins:
Few selected high abundance proteins were validated by immunoblot analysis in exosomes isolated from serum of saline and asbestos exposed mice. Western blot analysis was performed on exosome samples from serum suspended in 4X lysis buffer and boiled for 5 min at 95 °C. Thereafter 10–15 μL of each sample was resolved on a 10% SDS PAGE for subsequent immunoblotting for selected proteins, ceruloplasmin (Abcam, Cambridge, MA), haptoglobin (Abcam) and fibulin-1 (Thermo Fisher Scientific) using specific antibodies as described before (Thompson et al., 2017).
Results
Characterization of exosomes
Mouse serum exosomes were purified by ExoQuick precipitation following the manufacturer’s protocol and characterized before proteomics analysis. Isolated exosomes from mouse serum was initially characterized by nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM) to assess the particle concentration, size distribution, and membrane bound nature, as exosomes under TEM have a characteristic cup-shaped morphology (Szatanek et al., 2017) (Figure 1A). NTA indicates particles directly the in size range of exosomes with a median size of 68.1nm (Figure 1B). Exosome preparation from serum also showed exosome specific markers, CD9 and CD81 (Figure 1C).
Figure 1.
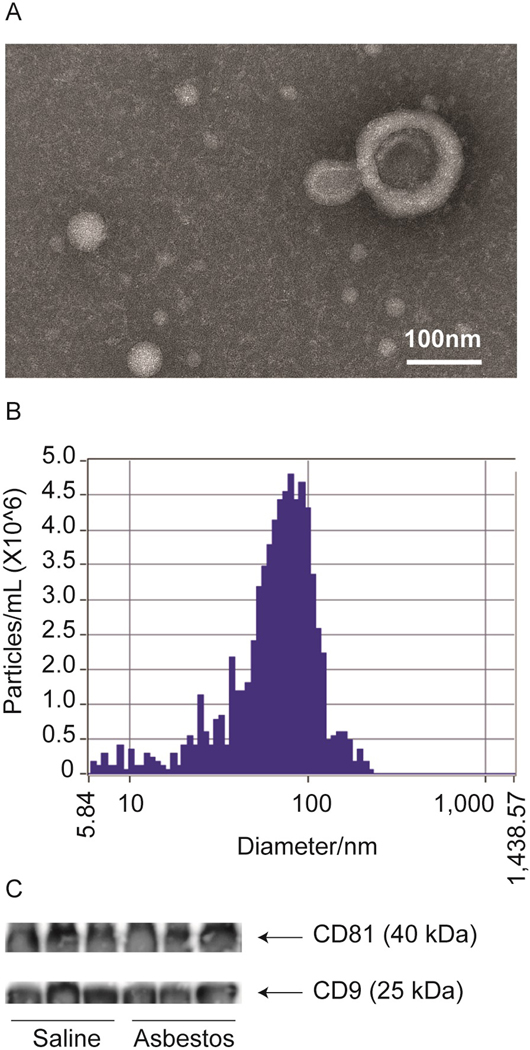
Characterization of exosomes derived from mouse serum by transmission electron microscopy (A), nanoparticle tracking analysis (B) and exosome specific marker immunoblotting (C).
Proteomic analysis of exosomes
The exosomes isolated from serum and analyzed by Tandem-Mass-Tag profiling yielded results of 376 quantifiable proteins (with less than 1% FP). The proteins were compared between the control, non-asbestos exposed mice (n=4), and mice exposed to asbestos via pharyngeal aspiration (n=5) and sorted by fold change (asbestos/control) and statistical significance (Supplementary Table S1). Although there were only a few proteins that had differentially abundance in the asbestos group with statistical significance (p < 0.05) due to biological variations among individual animals, proteins were clearly more abundant after asbestos exposure (Figure 2B). The proteins identified to be more abundant in the asbestos group were sorted through for biological significance, as well as if there was statistical significance, and the top 15 which met either or both criteria were compiled (Table 1).
Table 1:
Top 15 most biologically significant proteins from the most abundant exosomal proteins identified by proteomics analysis on asbestos exposed mice compared to non-exposed mice.
| Accession # | Description | Fold Change | p-value |
|---|---|---|---|
| Q61646 | Haptoglobin | 3.559 | 0.232 |
| O70456 | 14-3-3 protein sigma | 3.401 | 0.415 |
| Q5FW60 | Major urinary protein 20 | 3.075 | 0.188 |
| Q8K0E8 | Fibrinogen beta chain | 3.058 | 0.172 |
| Q91X70 | Complement component 6 | 3.014 | 0.151 |
| Q91X72 | Hemopexin | 2.592 | 0.235 |
| Q91V57-3 | Isoform 3 of N-chimaerin | 2.280 | 0.034 |
| Q08879-2 | Isoform C of Fibulin-1 | 2.248 | 0.160 |
| Q61147 | Ceruloplasmin (Splice Variant 2) | 2.169 | 0.199 |
| G3X9T8 | Ceruloplasmin (Splice Variant 1) | 2.166 | 0.198 |
| Q3V3K3 | Putative uncharacterized protein | 1.942 | 0.044 |
| Q08879 | Fibulin-1 | 1.801 | 0.227 |
| P19091 | Androgen receptor | 1.642 | 0.047 |
| E9Q5F6 | Polyubiquitin-C (Fragment) | 1.549 | 0.030 |
| Q5U405 | Transmembrane protease serine 13 | 1.493 | 0.020 |
Validation of selected proteins by immunoblot analysis:
Three of these exosomal proteins in greater abundance from the asbestos exposed animals, and of particular biological interest (excluding any contaminating serum proteins), were validated by Western blot analysis to confirm proteomics results (Figure 3A), and ponceau staining was used to ensure equal protein loading (Figure 3B) as no reliable standard exists for secreted proteins. Four of five asbestos exposed animals showed increased exosomal fibulin-1, all five animals showed increased exosomal ceruloplasmin, and three of five mice showed increased exosomal haptoglobin (Figure 3). Our protein validation was performed on the same group of mice and shows the same trends of abundance as indicated by our proteomics heatmap. The fact that the ratios determined by Western blot are in agreement with the TMT ratios for individual animals, demonstrates that our proteomics approach was robust and is applicable for these types of serum exosome proteomic profiling.
Figure 3.
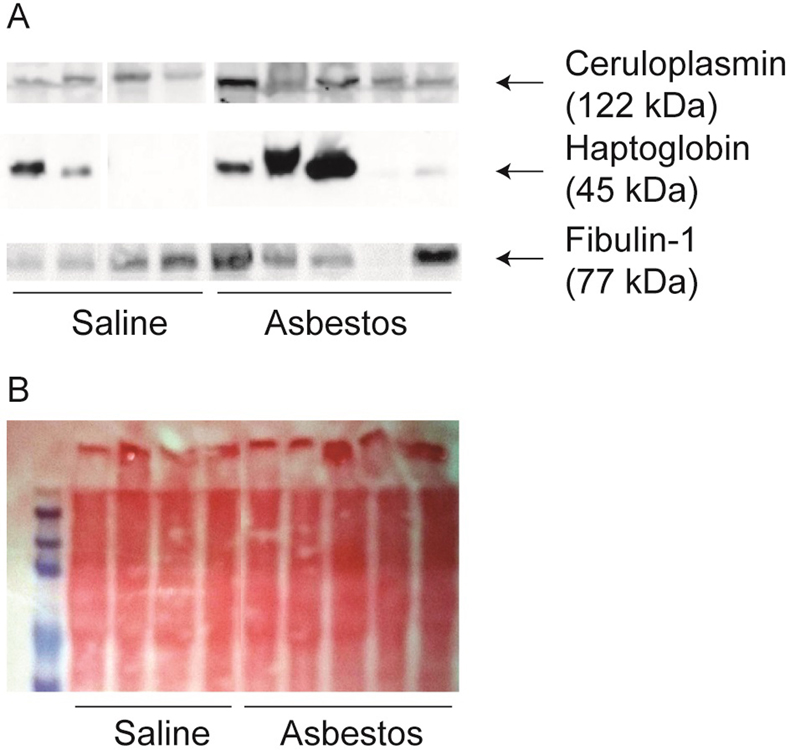
Proteomic results were validated by immunoblot assay. Three selected proteins, ceruloplasmin, haptoglobin, and fibulin, found in high abundance in asbestos group were validated by immunoblot analysis as described in materials and methods (A). As these are secreted proteins, loading controls are not available. Equal loading was achieved by keeping the starting and final volume same across the samples and was assessed by Ponceau staining after transferring proteins on membranes (B).
STRING Pathway Analysis
Pathway analysis was completed for the top 200 most abundant exosomal proteins in each group, based on fold change, using STRING functional protein-protein association network (https://string-db.org/) and the resulting top 10 Gene Ontology biological processes derived from the networking were compiled (Table 2).
Table 2:
Top 10 most significant gene ontology biological components in serum based on pathway analysis from the top 200 proteins of highest expression in exosomes from asbestos exposed mice.
| Pathway ID | Biological Process | Observed Gene Count | FDR |
|---|---|---|---|
| GO.0006952 | defense response | 22 | 4.45E-13 |
| GO.0006956 | complement activation | 9 | 1.55E-12 |
| GO.0006959 | humoral immune response | 10 | 7.91E-11 |
| GO.0006953 | acute-phase response | 8 | 1.85E-10 |
| GO.0045087 | innate immune response | 14 | 7.56E-10 |
| GO.0052547 | regulation of peptidase activity | 13 | 1.06E-08 |
| GO.0006950 | response to stress | 27 | 1.33E-08 |
| GO.0002253 | activation of immune response | 10 | 1.54E-08 |
| GO.0030162 | regulation of proteolysis | 15 | 4.33E-08 |
| GO.0052548 | regulation of endopeptidase activity | 12 | 5.87E-08 |
Discussion
This short study is the first of its kind to present data on the relevance of exosome-protein signature in regard to in vivo exposure to asbestos fibers. Asbestos exposure is the main causal factor of MM, the fatal cancer of the mesothelial lining of the pleura, peritoneum, and pericardium. There is no means of early diagnosis of MM, or capability to diagnose harmful exposure to asbestos fibers due to a lack of useful biomarkers. We conducted these studies to begin in an unexplored area of asbestos and exosome biomarker discovery. We chose to use a well-defined mouse model of asbestos exposure, OA. Studies from NIOSH and others have compared OA with inhalation exposure to asbestos and found these two very comparable ways to expose mice to asbestos (Mercer et al., 2011; Porter et al., 2010). OA is notably capable of eliciting the systemic effects of asbestos exposure from which we isolated serum exosomes.
Use of mouse models to understand the systemic and/or local effect of asbestos exposure on exosomal protein candidates is the first step towards identifying biomarkers of asbestos exposure. Our mouse model allowed us an in vivo approach to describing the exosomal protein content of mouse serum, and any associated differences upon asbestos exposure. Currently, the 2 most common methods available to isolate exosomes from various samples, ultracentrifugation and ExoQuick precipitation. Comparative exosome isolation studies done using these two methods showed either comparable results (Helwa et al., 2017) or better (Eitan et al., 2017) results with ExoQuick. Due to very small volume (~200 μL) of serum availability from mouse, we used the ExoQuick precipitation method to isolate exosomes. Characterization of our exosome preparation showed a membranous structure in correct size range expressing specific markers. Proteomic analysis of our preparation identified a total of 376 proteins. Amongst these proteins we observed an increased abundance of multiple proteins of biological interest in the asbestos group. The heterogeneous effects observed in the asbestos group is not uncommon due to several reasons, 1) it is an insoluble (fibrous) agent and is not available to all cells (or all surfaces of tissues) uniformly, and 2) it is well known that only a small percentage of asbestos-exposed individuals develop mesothelioma suggesting a susceptibility issue (Mitchell Cheung, 2017; Roushdy-Hammady et al., 2001).
Moreover, as this study was intended to yield insight on potential exosomal protein biomarkers for asbestos exposure we believe the proteins with most increased abundance for asbestos exposed animals are of most interest. Within this subset of identified exosomal proteins there are some potential implications in the biology of asbestos exposure.
Increased proteins in the asbestos group that were validated by Western blot analysis were, ceruloplasmin, haptoglobin, and fibulin-1. These are acute phase proteins and shown previously to be upregulated in response to asbestos exposure (Afanas’eva et al., 1993; Ke et al., 1991; Shannahan et al., 2012; Spitsyn et al., 1992). Ceruloplasmin is a copper-carrying glycoprotein, plays an important role in iron metabolism (Frieden, 1971; Roeser et al., 1970). The toxicity of asbestos is in part due to iron metabolism dysregulation after exposure in the lung (Ghio et al., 2008). Ceruplasmin has been shown in a 2014 study to be increased in the serum of asbestos exposed individuals and even higher in those with mesothelioma (Sezgi et al., 2014). Use of postoperative tetrathiomolybdate to deplete copper and ceruloplasmin in mesothelioma patients has been shown to be beneficial (Pass et al., 2008), suggesting a strong role of ceruloplsmin in MM tumorigenesis.
The fibulin family of proteins (fibulin-1, −2, −3, −4, −5) are known to share extensive molecular functional similarities and sequence homology (Kobayashi et al., 2007; Timpl et al., 2003), and recent publications have indicated that fibulin-3 levels may be indicative markers of asbestos exposure and mesothelioma (Kirschner et al., 2015; Pass et al., 2012; Rapisarda et al., 2017). Our experiments have identified that fibulin-1 is increased in exosomes from asbestos exposed animals, which may indicate a role of fibulin family member in extracellular matrix remodeling after asbestos exposure. What is unique about fibulin-1 in this study is that it appears to be specifically enriched in exosomes of asbestos exposed animals, whereas the other studies indicate freely secreted fibulin-3.
Another protein of interest that was detected in increased abundance in the asbestos groups was ficolin-1, a protein involved in cell morphogenesis and known to target fibrinogen. Additionally, exosomal 14–3-3 protein sigma was increased in serum of asbestos exposed mice and it has been shown that this protein has extracellular functionality as an adaptor protein. The only known extracellular 14–3-3 proteins are secreted via exosomes, and have been shown to target the Wnt signaling pathway in target cells via the association of discheveled-2 (Dovrat et al., 2014). Interestingly, 14–3-3 protein sigma also plays roles in matrix metalloproteinase activity, activating fibroblast migration, and even reducing fibrosis and inflammation, both of which are hallmark effects of asbestos exposure (Kaplan et al., 2017). Interestingly, 14–3-3-theta levels were found to be upregulated in conditioned medium from MM cells as compared to mesothelial cells (Creaney et al., 2017), suggesting important role(s) of this group of proteins in mesothelioma tumorigenesis.
The potential consequences of exosomal proteins listed above is speculative based on previous research and requires further study to elucidate mechanistic roles. However, our data is novel by being the first to indicate unique protein signatures of exosomes in response to asbestos exposure. Those exosomal proteins in greater abundance after asbestos exposure may lead to the identification of more useful biomarkers to diagnose and prevent asbestos related disease, as exosomal strategies are becoming convenient and common place.
The development of useful biomarker based diagnostic, and potentially therapeutic enterprises is of great public health concern for asbestos related diseases and beyond. We intend to continue our expedition to mine for exosomal biomarkers of asbestos exposure in future and ongoing experiments. Those include in vitro models of asbestos exposure and isolation of exosomes from the blood of individuals with known exposure to asbestos and mesothelioma patients. Our intentions are to provide new and useful exosome based strategies to identify asbestos exposure, and this study is the first of its kind in taking that initial step forward.
Supplementary Material
Acknowledgements
We would like to acknowledge Michele von Turkovich of the University of Vermont (UVM) Microscopy Imaging Core for TEM images and David Palmlund of Particle Metrix for NTA. The UVM Department of Pathology Graduate Student Fellowship, and financial support from NIH RO1 ES021110, DoD IDeA Award (W81XWH-13-PRCRP-IA) and UVMMC/LCCRO are greatly acknowledged. The Vermont Genetics Network Proteomics Facility is supported through NIH grant P20GM103449 from the INBRE Program of the National Institute of General Medical Sciences. All authors of this manuscript declare that they have no conflicts of interest to disclose.
Contract grant sponsor: The UVM Department of Pathology Graduate Student Fellowship
Contract grant sponsor: UVMMC/LCCRO
Contract grant sponsor: NIH
Contract grant number: RO1 ES021110
Contract grant sponsor: DOD
Contract grant number: IDeA Award W81XWH-13-PRCRP-IA
Contract grant sponsor: NIH from INBRE Program of the National Institute of General Medical Sciences
Contract grant number: P20GM103449
References
- Afanas’eva IS, Spitsyn VA, and Tsurikova GV. 1993. [Genetic polymorphism of haptoglobin and quantitative changes in its levels during exposure to asbestos]. Genetika 29:1895–1900. [PubMed] [Google Scholar]
- Berman DW, and Crump KS. 2008. Update of potency factors for asbestos-related lung cancer and mesothelioma. Critical reviews in toxicology 38 Suppl 1:1–47. [DOI] [PubMed] [Google Scholar]
- Colletti M, Petretto A, Galardi A, Paolo VD, Tomao L, Lavarello C, Inglese E, Bruschi M, Lopez AA, Pascucci L, Geoerger B, Peinado H, Franco L, and Angela DG. 2017. Proteomic analysis of neuroblastoma-derived exosomes: new insights into a metastatic signature. Proteomics [DOI] [PubMed]
- Colombo M, Raposo G, and Thery C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology 30:255–289. [DOI] [PubMed] [Google Scholar]
- Creaney J, Dick IM, Leon JS, and Robinson BW. 2017. A Proteomic Analysis of the Malignant Mesothelioma Secretome Using iTRAQ. Cancer genomics & proteomics 14:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovrat S, Caspi M, Zilberberg A, Lahav L, Firsow A, Gur H, and Rosin-Arbesfeld R. 2014. 14–3-3 and β-catenin are secreted on extracellular vesicles to activate the oncogenic Wnt pathway. Mol Oncol 8:894–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Green J, Bodogai M, Mode NA, Baek R, Jorgensen MM, Freeman DW, Witwer KW, Zonderman AB, Biragyn A, Mattson MP, Noren Hooten N, and Evans MK. 2017. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Scientific reports 7:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden E 1971. Ceruloplasmin, A Link Between Copper and Iron Metabolism. In Bioinorganic Chemistry Vol. 100 AMERICAN CHEMICAL SOCIETY; 292–321. [Google Scholar]
- Ghio AJ, Stonehuerner J, Richards J, and Devlin RB. 2008. Iron homeostasis in the lung following asbestos exposure. Antioxidants & redox signaling 10:371–377. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Rokad D, and Kanthasamy AG. 2017. Exosomes in Toxicology: Relevance to Chemical Exposure and Pathogenesis of Environmentally Linked Diseases. Toxicological sciences : an official journal of the Society of Toxicology [DOI] [PMC free article] [PubMed]
- Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, and Liu Y. 2017. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PloS one 12:e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova M, Veith C, Habibovic A, Lam YW, Deng B, Geiszt M, Janssen-Heininger YM, and van der Vliet A. 2014. Identification of DUOX1-dependent redox signaling through protein S-glutathionylation in airway epithelial cells. Redox biology 2:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Bueno M, and Fournier AE. 2017. Extracellular functions of 14–3-3 adaptor proteins. Cellular signalling 31:26–30. [DOI] [PubMed] [Google Scholar]
- Ke F, Zhang J, and Jin S. 1991. [The relationship of biochemical changes among bronchoalveolar lavage fluid serum and lung on dust-exposed rats]. Hua xi yi ke da xue xue bao = Journal of West China University of Medical Sciences = Huaxi yike daxue xuebao 22:292–295. [PubMed] [Google Scholar]
- Kirschner MB, Pulford E, Hoda MA, Rozsas A, Griggs K, Cheng YY, Edelman JJ, Kao SC, Hyland R, Dong Y, Laszlo V, Klikovits T, Vallely MP, Grusch M, Hegedus B, Dome B, Klepetko W, van Zandwijk N, Klebe S, and Reid G. 2015. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. British journal of cancer 113:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, and Sasaki T. 2007. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. The Journal of biological chemistry 282:11805–11816. [DOI] [PubMed] [Google Scholar]
- Mazurek JM, Syamlal G, Wood JM, Hendricks SA, and Weston A. 2017. Malignant Mesothelioma Mortality - United States, 1999–2015. MMWR. Morbidity and mortality weekly report 66:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, and Kalluri R. 2015. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, Castranova V, and Porter DW. 2011. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell Cheung C.W.M.a.J.R.T. 2017. Germline and somatic mutations in human mesothelioma and lessons from asbestos-exposed genetically engineered mouse models. In Asbestos and Mesothelioma Testa JR, editor. Springer; 175–196. [Google Scholar]
- Munson P, and Shukla A. 2015. Exosomes: Potential in Cancer Diagnosis and Therapy. Medicines (Basel) 2:310–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass HI, Brewer GJ, Dick R, Carbone M, and Merajver S. 2008. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. The Annals of thoracic surgery 86:383–389; discussion 390. [DOI] [PubMed] [Google Scholar]
- Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, Donington J, Huflejt M, Carbone M, Chia D, Goodglick L, Goodman GE, Thornquist MD, Liu G, de Perrot M, Tsao M-S, and Goparaju C. 2012. Fibulin-3 as a Blood and Effusion Biomarker for Pleural Mesothelioma. N Engl J Med 367:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, and Castranova V. 2010. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 269:136–147. [DOI] [PubMed] [Google Scholar]
- Rapisarda V, Caltabiano R, Musumeci G, Castrogiovanni P, Ferrante M, Ledda C, Lombardo C, Graziano ACE, Cardile V, and Loreto C. 2017. Analysis of fibulin-3 after exposure to asbestos-like fibers. Environmental research 156:381–387. [DOI] [PubMed] [Google Scholar]
- Roeser HP, Lee GR, Nacht S, and Cartwright GE. 1970. The role of ceruloplasmin in iron metabolism. Journal of Clinical Investigation 49:2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushdy-Hammady I, Siegel J, Emri S, Testa JR, and Carbone M. 2001. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet 357:444–445. [DOI] [PubMed] [Google Scholar]
- Sezgi C, Taylan M, Selimoglu Sen H, Evliyaoğlu O, Kaya H, Abakay O, Abakay A, Tanrıkulu AC, and Senyiğit A. 2014. Oxidative Status and Acute Phase Reactants in Patients with Environmental Asbestos Exposure and Mesothelioma. The Scientific World Journal 2014:902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Alzate O, Winnik WM, Andrews D, Schladweiler MC, Ghio AJ, Gavett SH, and Kodavanti UP. 2012. Acute phase response, inflammation and metabolic syndrome biomarkers of Libby asbestos exposure. Toxicology and applied pharmacology 260:105–114. [DOI] [PubMed] [Google Scholar]
- Spitsyn VA, Tsurikova GV, and Afanas’eva IS. 1992. [Gene manifestations in negative man-made environment: a selective genetically determined sensitivity to asbestos]. Vestnik Rossiiskoi akademii meditsinskikh nauk:46–52. [PubMed]
- Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu X, Qian L, Zhang Y, Fan L, Cao CX, and Xiao H. 2017. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Analytica chimica acta 982:84–95. [DOI] [PubMed] [Google Scholar]
- Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, and Baran J. 2017. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Webb-Robertson BJ, Waters KM, Murray AR, Kisin ER, Varnum SM, Jacobs JM, Pounds JG, Zanger RC, and Shvedova AA. 2011. Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Toxicological sciences : an official journal of the Society of Toxicology 120:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, MacPherson MB, Beuschel SL, and Shukla A. 2017. Asbestos-Induced Mesothelial to Fibroblastic Transition Is Modulated by the Inflammasome. The American journal of pathology 187:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, and Chu M-L. 2003. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4:479–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


