 Camphor N-acylhydrazones showed promising antiviral activity towards vaccinia and influenza viruses.
Camphor N-acylhydrazones showed promising antiviral activity towards vaccinia and influenza viruses.
Abstract
The design and synthesis of a series of novel d-(+)-camphor N-acylhydrazones exhibiting inhibitory activity against vaccinia and influenza viruses are presented. An easy pathway to camphor-based N-acylhydrazones containing in their structure aliphatic, aromatic, and heterocyclic pharmacophore scaffolds has been developed. The conformation and configuration of the synthesized hydrazones were thoroughly characterized by a complete set of spectral characterization techniques, including 2D NMR spectroscopy, mass spectrometry, and X-ray diffraction analysis. In vitro screening for activity against vaccinia virus (VV) and influenza H1N1 virus was carried out for the obtained compounds. It was revealed that the derived N-acylhydrazones exhibited significant antiviral activity with a selectivity index >280 against VV for the most promising compound.
Introduction
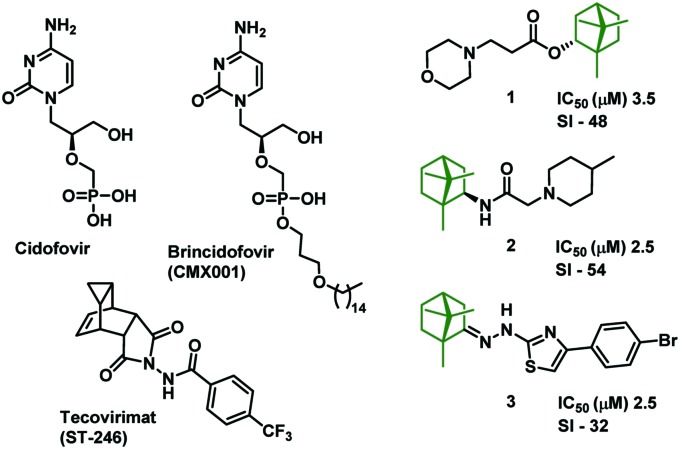
Variola virus (VARV) belongs to the family Poxviridae, the subfamily Chordopoxvirinae, and the genus Orthopoxvirus, and is highly contagious by transmission of airborne droplets causing smallpox. Usually, two subspecies are distinguished: V. major, which causes disease with a mortality rate of 5 to 40%, and V. minor, leading to a fatal outcome in less than 2% of cases. In 1999, the Centers for Disease Control and Prevention, USA classified the variola virus as category A hazard, along with the viruses of anthrax, plague, and tularemia, and a group of viruses causing hemorrhagic fever. In 1980, the World Health Organization announced the eradication of smallpox, and thereafter mandatory vaccination was abolished.1 Today, 90% of the population lacks immunity to smallpox, which poses a serious threat to new outbreaks of the disease. Therefore, the search for new antiviral drugs and the development of effective therapies are especially urgent. It is worth noting that currently only two laboratories in the world i.e., the Centers for Disease Control and Prevention, USA and the State Research Center of Virology and Biotechnology “VECTOR”, Russian Federation, officially possess variola virus strains and carry out research for antiviral agents.2 Chemotherapy drugs may be important for the treatment of human Orthopoxvirus infections, the search for which has been relatively successful in the past 20 years. The nucleotide analog cidofovir (produced under the name Vistide)3 and its lipid conjugate CMX001 (brincidofovir), which are inhibitors of viral DNA polymerase, became the first intensively studied anti-orthopoxviral agents (Fig. 1).4 Among the anti-orthopoxviral agents the compound ST-246 (tecovirimat), which blocks the last stage of the assembly of intracellular enveloped virions and prevents the virus from escaping from the infected cell, has received the greatest interest.5 ST-246 showed low toxicity and high antiviral efficacy using animal models in vivo. Although variola virus no longer circulates amongst the human population, there has recently been heightened concern that smallpox may be used as an agent for bioterrorism.6 Vaccinia virus (VV) is a member of the Poxviridae family and is closely related to variola virus, the causative agent of smallpox.7 VV is a useful proxy for in vitro and in vivo study when evaluating therapeutic potential of prospective variola virus treatments.8
Fig. 1. Chemical structure of inhibitors of Orthopoxvirus replication: cidofovir, brincidofovir, tecovirimat and inhibitors containing 1,7,7-trimethylbicyclo[2.2.1]heptane scaffolds 1–3.
Influenza virus is one of the most dangerous respiratory viral pathogens of humans. It causes annual epidemics and sporadic pandemics that involve large parts of the population and result in high morbidity and mortality.9 Drug resistance was detected for both groups of currently available anti-influenza drugs, i.e. derivatives of adamantane, amantadine, and rimantadine10 and the most widely used neuraminidase inhibitor oseltamivir phosphate.11 The search and development of novel antivirals against influenza virus is, therefore, a high priority goal of medical science and health care systems worldwide. It is desirable that these new antivirals should be directed against alternative viral targets, from the specific proteins of influenza A, i.e. M2 protein and neuraminidase. These targets should be highly conserved among related viruses, essential for the viral life cycle, and acquiring resistance to them should result in non-viable or non-pathogenic viral progeny.12
Natural compounds, in particular natural bicyclic monoterpenoids, carrying the bicyclo[2.2.1]heptane moiety have been used as starting molecules for the development of antiviral drugs.13 Thus, the substituted 1-norbornylamines synthesized from (±)-camphor exhibited high activity against influenza virus A and moderate activity against the African swine fever virus.14 The ability to effectively inhibit influenza A viruses (H1N1) has been demonstrated by imino and diimine derivatives of (+)-camphor.15–17 Compounds based on another natural monoterpenoid, (–)-borneol, with a similar skeleton have shown antiviral activity against influenza18 and Marburg viruses.19 Considering the importance of searching for new agents that are effective against orthopoxviruses, our group has begun the synthesis of libraries of new compounds based on monoterpenoids and the detection of agents with high antiviral activity against VV. As a result, we have shown that combinations of a saturated N-heterocycle, such as morpholine or 4-methylpiperidine, and a 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold from (–)-borneol 1 or (+)-isobornylamine 2 have favorable antiviral activity against orthopoxviruses.20 A series of nitrogen–sulphur-containing heterocycles, such as 1,3-thiazolidin-4-one and other thiazoles, and a 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold were synthesized on (+)-camphor and screened for their antiviral activity.21 The bioassay results showed that thiazole 3, which contained a substituted benzene ring, was able to inhibit VV with IC50 values in the micromolar range whilst exhibiting moderate cytotoxicity. The aim of this work was to synthesize N-acylhydrazones based on (+)-camphor bearing also some alkaloid nitrogen heterocycles as privileged fragments22 and to study their antiviral properties. The choice of heterocyclic substituents in the compounds was due to the availability of the isoindole scaffold and its high biochemical potential. A large number of isoindoles and their heteroannelated derivatives exhibit various biological activities.23–25 For example, an excellent inhibitory activity of isoindole derivatives against Gram-positive and Gram-negative bacteria,26,27 as well as high antiviral properties, has been reported.28,29
Results and discussion
Chemistry
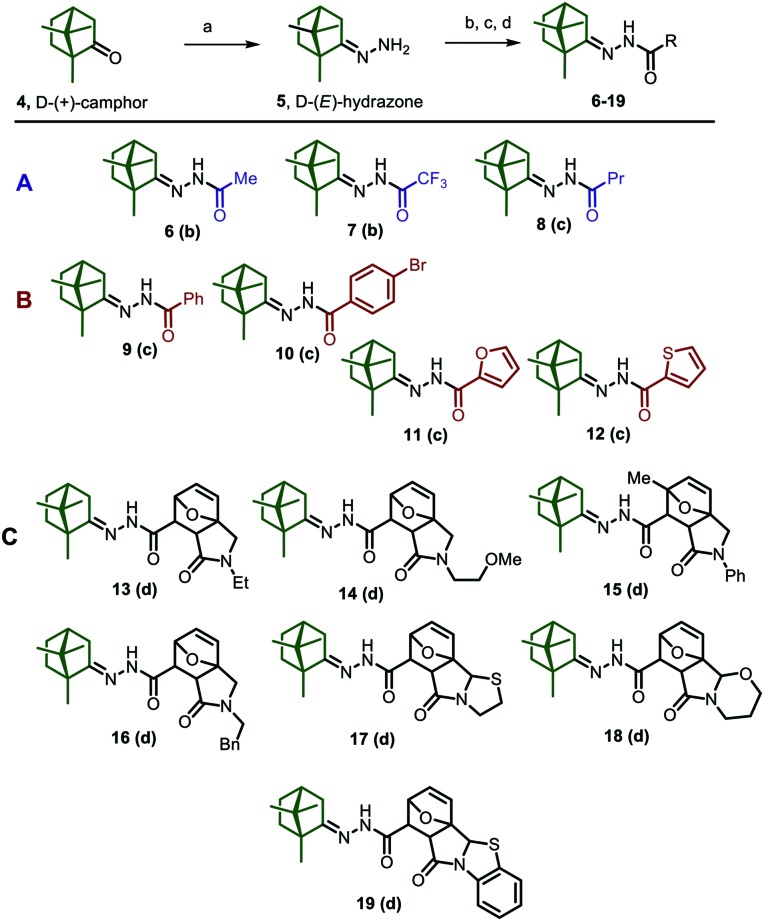
The target camphor derivatives in this study were prepared according to the general synthetic route depicted in Scheme 1, based on optically active d-(+)-camphor 4. In the first stage of our work, camphor hydrazone 5 was obtained by the interaction of (+)-camphor 4 with hydrazine hydrate in the presence of AcOH, according to a procedure described earlier.30 Subsequent N-acylation of compound 5 with various acid anhydrides and acid chlorides leads to a library of N-acylhydrazones 6–19, containing in their structure a chiral 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold, an acyl hydrazone linker, and various alkyl, aryl, or heterocyclic substituents (Schemes 1–3). Compounds 6 and 9, bearing acetyl and benzoyl substituents, have been previously synthesized. Camphor acetohydrazide 6 was previously obtained by treating camphor semicabazone with Ac2O/ZnCl2 (ref. 31) or by the treatment of camphor hydrazone 5 with acetic acid.32 For the synthesis of camphor benzohydrazide 9, two approaches were realized i.e., the reaction of camphor hydrazone with benzoyl chloride33 and the reaction between camphor and benzohydrazide.34 The first approach led to higher yields. Compounds 7, 8, and 10–19 were new synthetic derivatives not previously described in the literature.
Scheme 1. Reagents and conditions: (a) NH2NH2·H2O (4 equiv.), CH3CO2H (1 equiv.), EtOH, reflux 4 h; (b) Et3N (1.2 equiv.), Ac2O or TFA (1 equiv.), CHCl3; (c) Et3N (1.2 equiv.), carboxylic acid chloride (1 equiv.), CHCl3; (d) carboxylic acid (1 equiv.), EtO2CCl/Et3N, CHCl3.
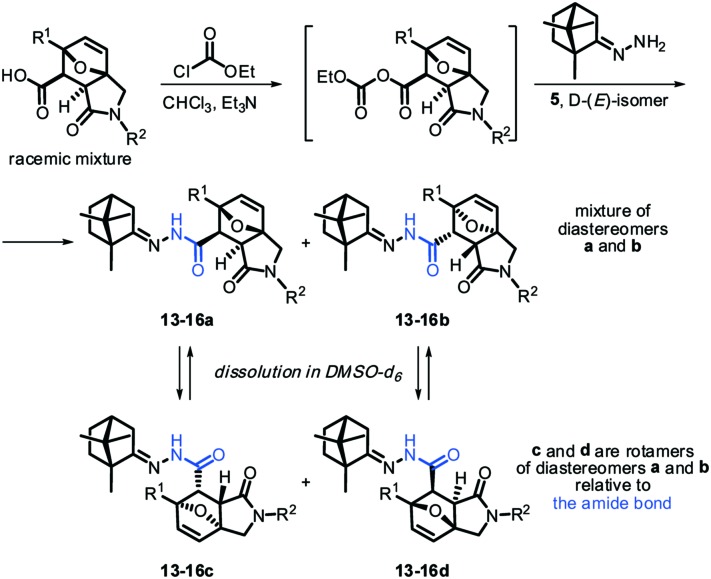
Scheme 2. Synthesis of compounds 13–16 (group C).
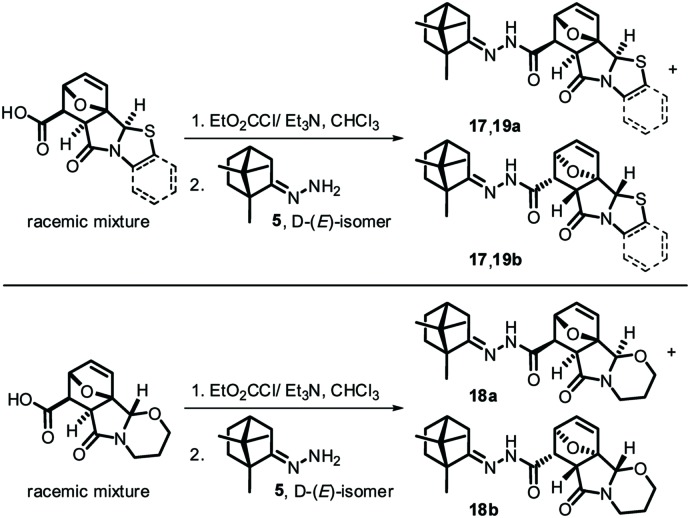
Scheme 3. Synthesis of compounds 17–19 (group C).
Formally, the synthesized compounds could be subdivided into three groups. Group A combined substances 6–8 with aliphatic substituents on the N-acylhydrazone fragment; group B included compounds 9–12 having aryl or heteroaromatic structural blocks; and group C contained compounds 13–19 containing polyheterocyclic scaffolds which were synthesized in previous work.35–37
The compounds of groups A and B were obtained by the same synthetic route, by treatment of camphor hydrazone with an equimolar amount of the appropriate carboxylic acid anhydride (for 6 and 7) or carboxylic acid chloride (for 8–12). Reactions were carried out at room temperature in the presence of triethylamine.
The synthetic sequence depicted in Schemes 2 and 3 was successfully applied to obtain acylhydrazones 13–19 containing polyheterocyclic substituents in their frame. It should be mentioned here that the initial heterocyclic acids were solids, sparingly soluble in most organic solvents (almost insoluble in CHCl3 and Me2CO); therefore, they reluctantly interacted with oxalyl chloride, SOCl2 or POCl3 under the classical conditions. For the same reason, we were forced to abandon the methods of peptide chemistry, including application of N,N′-dicyclohexylcarbodiimide (DCC), 1,1′-carbonyldiimidazole (CDI), and others as condensing agents. The most successful method for the synthesis of N-acylhydrazones 13–19 turned out to be the reaction of the corresponding carboxylic acid with ethyl chloroformate in the presence of triethylamine and the subsequent in situ reaction of the resulting mixed anhydride with camphor d-(E)-hydrazone 5. This approach facilitated the isolation of the target products and allowed achievement of good yields of N-acetyl derivatives (up to 70–83%). Since the acylation was carried out with optically active camphor hydrazone 5 and the racemic mixture of the corresponding acid, in all cases mixtures of diastereomers 13–16a/13–16b were isolated in ratios from 1 : 1 to 1 : 2 (Scheme 2). Attempts to separate these mixtures by preparative column chromatography failed due to close retention factors. Therefore, the diastereomeric mixtures were used for subsequent biological tests.
The remaining compounds of group C (17–19) were prepared analogously, and they also represented mixtures of diastereomers a/b (Scheme 3).
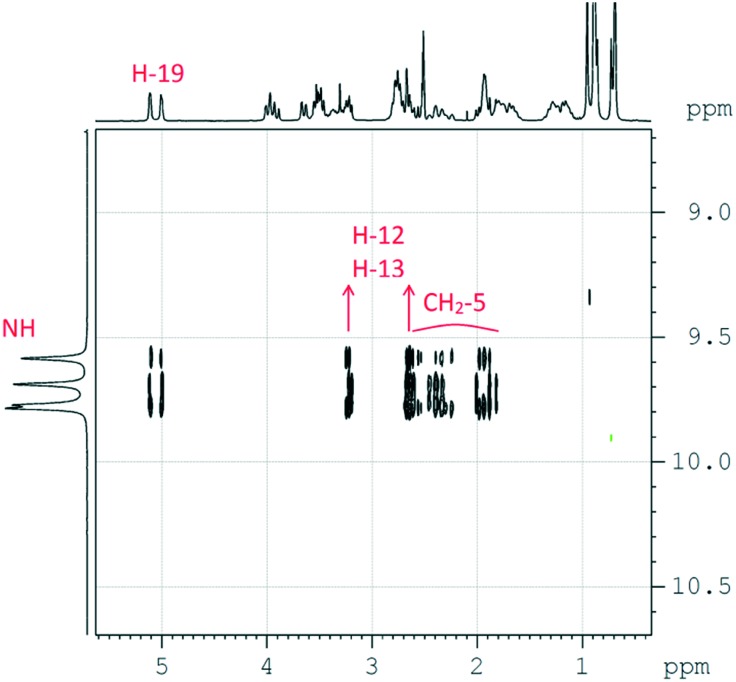
The structures of compounds 13–19 were elucidated using the combination of 1D and 2D methods of NMR spectroscopy (COSY, TOCSY, NOESY, HSQC, HMBC, and HSQC-COSY) and in the case of 11, 14 and 16, their structures were determined by X-ray analysis (for details, see the ESI†). The 1H NMR spectra of the derived N-acylhydrazones were extremely complicated. In DMSO-d6 at room temperature, solutions of 13–19 represented mixtures of four isomers, two of which (a and b, see Scheme 2) were diastereomers due to chiral carbons 6 and 7 and the two others (c and d) were diastereomers due to the slow rotation of the C–N amide bond. The (E)-configuration of the hydrazone fragment of all four isomers was established based on 2D NOESY experiments (see the ESI†). In the corresponding NOESY spectra, intense nOe cross-peaks between the NH-group and the CH2-group of the camphor fragment were present, and vice versa, cross peaks between the NH-group and camphor methyl groups were absent (Fig. 2).
Fig. 2. A fragment of 2D NOESY spectra for compound 16. The nOe cross-peaks between the NH-groups (on the left projection) and the CH2-groups of the camphor fragments (on the top projection) for a mixture of four isomers (16a, 16b, 16c, and 16d) are presented.
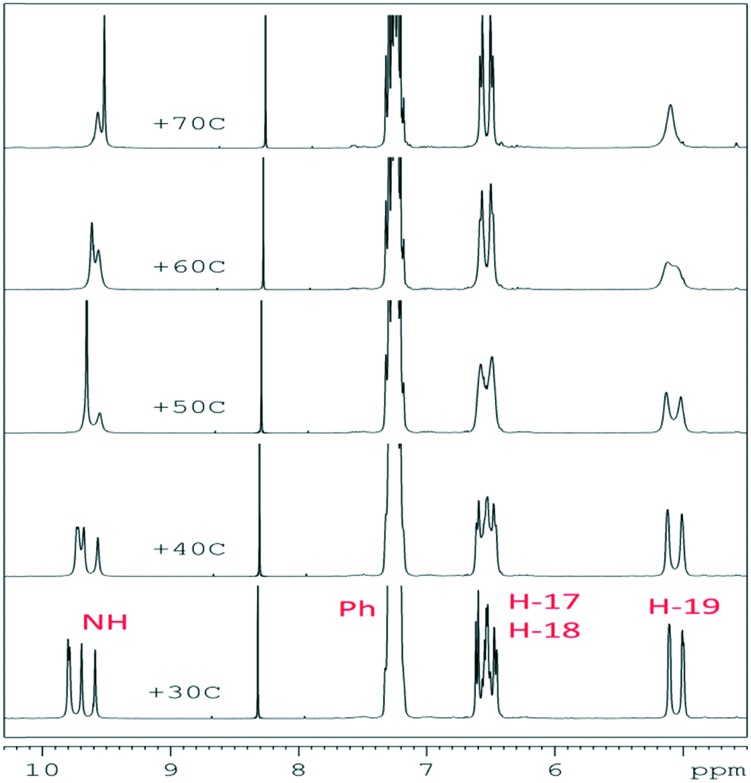
As was mentioned above, at room temperature DMSO solutions of hydrazones 13–19 exhibited two pairs of amide rotamers a ⇆ c and b ⇆ d, which were in dynamic equilibrium with each other (Scheme 2). This fact was confirmed by intense exchange cross-peaks, which were observed for each pair of diastereomers in their NOESY spectra. Additionally, the presence of the rotamers c and d for each of the diastereomers a and b in a solution was confirmed by conversion of a quadruple set of signals into a double one in the 1H NMR spectra under heating of the samples above 50–60 °C in an NMR tube (Fig. 3).
Fig. 3. Fragments of the 1H NMR spectra for compound 16 at different temperatures.
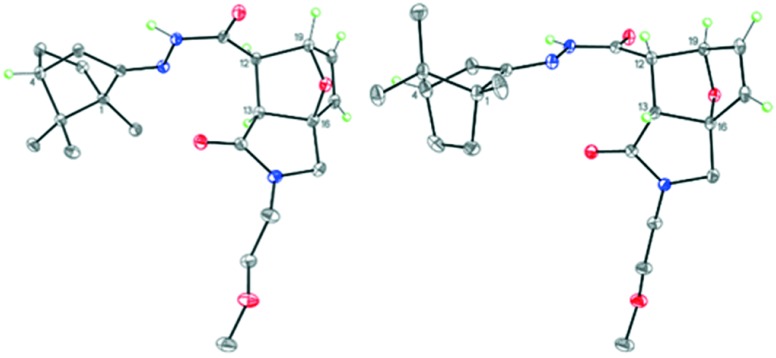
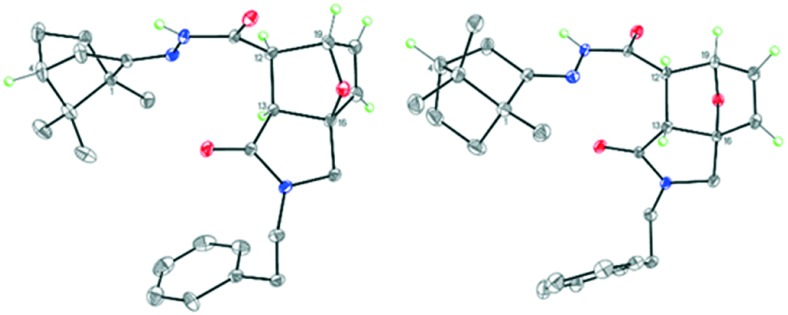
Some of the target N-acyl derivatives 13–19 were readily crystallized solids; therefore, a configuration of hydrazones belonging to group C was elucidated by X-ray analysis. As an illustration, the exact molecular structures of amides 14 and 16 are given in Fig. 4 and 5.
Fig. 4. Molecular structure of isomers 14a (on the left) and 14b (on the right). Displacement ellipsoids are depicted at the 50% probability level. Only hydrogen atoms at the asymmetric centers and at the double bonds are presented.
Fig. 5. Molecular structure of isomers 16a (on the left) and 16b (on the right). Displacement ellipsoids are depicted at the 50% probability level. Only hydrogen atoms at the asymmetric centers and at the double bonds are presented.
Despite the different substituents at the cyclic nitrogen atom, compounds 14 and 16 were isostructural and crystallized in the chiral monoclinic space group P21 with the four crystallographically independent molecules in the unit cell. However, these four independent molecules represented the two different 1R, 4R,3aR,6S,7R,7aS and 1R,4R,3aS,6R,7S,7aR diastereomers in the ratio of 2 : 2. Hence, the crystals of 14 and 16 were chiral conglomerates. Apparently, the aggregation was facilitated by the formation of robust H-bonded dimers from the different diastereomers. Moreover, all isomers of compounds 14 and 16 adopted the E-configuration relative to the N C double bond.
The chiral conglomerate formation allows explanation for the fact that we were not able to isolate the individual diastereomers a or b by recrystallization of 13–19a/13–19b mixtures from hexane–ethyl acetate. The structural information obtained above will be necessary for detailed analysis and explanation of biological activity in subsequent work.
Study of antiviral activity
The antiviral activity and cytotoxicity of the synthesized derivatives against VV were evaluated using an adapted method.38 The commercially available agent cidofovir was used as a positive control, and the results are shown in Table 1.
Table 1. Antiviral activity of target derivatives 6–19 against vaccinia virus.
| Compound | CC50, a , e μM | IC50, b , e μM | SI c |
| 6 | >250 | NA d | — |
| 7 | >250 | NA | — |
| 8 | 854.3 ± 131.3 | 69.8 ± 9.4 | 12 |
| 9 | 1430.1 ± 112.6 | 5.1 ± 0.9 | 280 |
| 10 | 287.8 ± 56.0 | 11.1 ± 2.4 | 26 |
| 11 | 285.0 ± 34.1 | 38.7 ± 7.1 | 7 |
| 12 | 362.5 ± 59.6 | NA | — |
| 13 | >250 | NA | — |
| 14 | >250 | NA | — |
| 15 | 600.2 ± 82.4 | 6.2 ± 1.6 | 97 |
| 16 | >250 | NA | — |
| 17 | >250 | NA | — |
| 18 | >250 | NA | — |
| 19 | 67.2 ± 13.7 | NA | — |
| Cidofovir | 475.3 ± 75.0 | 40.0 ± 2.9 | 12 |
aCC50 – 50% cytotoxic concentration; the concentration resulting in death of 50% of cells; M ± I95, n = 3.
bIC50 – 50% inhibitory concentration; the concentration leading to 50% inhibition of virus replication; M ± I95, n = 3.
cSI – selectivity index, ratio CC50/IC50.
dNA – not active.
e M – mean value; I95 – 95% confidence interval; n – the number of repeats of measurement of CC50 and IC50.
Various derivatives of monoterpenoids, in particular compounds including a 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold and N-heterocyclic fragment, exhibit antiviral properties against the influenza virus.39 In this regard, the obtained N-acylhydrazone camphor derivatives were screened for their inhibitory activity against influenza virus A H1N1. The results are shown in Table 2. Adamantane- and norbornane-based derivatives were used as reference compounds due to their close similarity to the compounds under investigation in having rigid cage fragments in their structures.
Table 2. Antiviral activity of camphor-based compounds 6–19 against influenza virus A/Puerto Rico/8/34 (H1N1) in MDCK cells.
| Compound | CC50, a μM | IC50, b μM | SI c |
| 6 | NT d | NT | — |
| 7 | >1079 | NA e | — |
| 8 | >1190 | 794 ± 92 | 2 |
| 9 | >1049 | 201 ± 26 | 5 |
| 10 | >822 | 219 ± 18 | 4 |
| 11 | 1087 ± 50 | 325 ± 41 | 3 |
| 12 | 1027 ± 71 | NA | — |
| 13 | 775 ± 48 | NA | — |
| 14 | 719 ± 55 | NA | — |
| 15 | 277 ± 16 | 22 ± 4 | 12 |
| 16 | 227 ± 11 | >72 | 3 |
| 17 | NT | NT | — |
| 18 | >721 | 481 ± 56 | 2 |
| 19 | 123 ± 6 | 36 ± 6 | 3 |
| Rimantadine | 335 ± 27 | 67 ± 5 | 5 |
| Amantadine | 284 ± 21 | 64 ± 5 | 4 |
| Deitiforine | 1266 ± 82 | 209 ± 15 | 6 |
| Ribavirin | >2130 | 30 ± 4 | 71 |
aCC50 – 50% cytotoxic concentration; the concentration resulting in death of 50% of cells.
bIC50 – 50% inhibitory concentration; the concentration leading to 50% inhibition of virus replication.
cSI – selectivity index, ratio CC50/IC50.
dNT – not tested.
eNA – not active.
The data presented are the mean of three independent experiments. Values of CC50 and IC50 are presented as mean ± error of the experiment.
An important characteristic of the biological activity of the studied compounds is their therapeutic index or selectivity index (SI). This ratio reflects the effectiveness and safety of the compounds under investigation. It is considered that compounds whose selectivity index exceeds 10 are safe.40 The presented biological data showed that among the compounds synthesized in this study were newly discovered agents having important activity against VV.
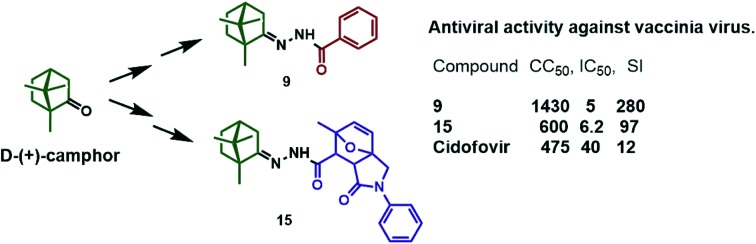
It was found that compounds 9, 10, and 15 possessing aromatic substituents were the most effective inhibitors of the smallpox virus. Their IC50 values were in the range of 5.1–11.1 μM, and their selectivity indices ranged from 26 to 280. The remaining compounds either showed higher inhibitory concentrations or did not show antiviral activity. It is important that the initial camphor (4), camphor hydrazone 5, and the acids used for the synthesis of N-derivatives did not exhibit antiviral activity at all. It should be noted that all the target compounds had optical activity and were levorotatory. In order to determine the effect of the stereochemistry of the 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold on the antiviral effect, we synthesized (+)-N-acetamide 9 starting from l-(–)-camphor. Biological testing of the agent (+)-9 against VV showed that this substance exhibited the same antiviral activity at a concentration of 5 μM and was nontoxic. Thus, probably, the stereochemical configuration of the camphor fragment does not affect the antiviral activity of all compounds 6–19.
We have previously shown that compounds containing a monoterpene fragment showed activity against influenza viruses at an early stage of viral replication.41–43 The presented biological data show that the agents 9 and 15 were active against VV and were, practically, not active against the influenza virus. The structures of surface viral proteins in influenza and vaccinia viruses differ significantly. The mechanism of membrane fusion of poxviruses is distinct from that of other viruses and requires not one but four attachment proteins and at least 11 viral proteins that form a fusion complex.44 Since compound 15 demonstrated the high activity against both vaccinia virus and influenza virus (although in the latter case the activity was low), one could suggest that it targets proteins with similar functions, like fusion proteins, and that it exhibits higher affinity for the vaccinia virus protein. Further studies can, therefore, optimize the structure of compounds of this class to result, in turn, in the development of broad-range antiviral activity. The identification of the target for the action of the most active compounds, the testing of these agents against variola virus, and experiments using animal models will be the focus of our further studies.
Conclusions
In summary, a series of N-acylhydrazones containing a monoterpenoid fragment and different substituents were synthesized and their antiviral activity was studied in vitro against vaccinia virus and influenza virus. Compounds 9, 10, and 15, containing in their structure a monoterpene moiety, N-acylhydrazone linker, and aromatic substituent, demonstrated low toxicity and the best activity against vaccinia virus. Only compound 15 showed moderate activity against influenza virus. The structures of all synthesized camphor derivatives were confirmed by NMR, HRMS, and X-ray analysis.
Experimental
Chemistry
The reagents were obtained from Acros Organics and Alfa Aesar and were used without additional purification, while the solvents were distilled prior to the syntheses. Melting points were determined on an SMP 30 apparatus in open capillaries and were not corrected. TLC analysis was performed on Sorbfil PTSKh-AF-A plates and visualization was carried out with UF-light, iodine vapor, or KMnO4 solution.
1H and 13C NMR spectra were acquired on a Bruker DRX-500 spectrometer (500 and 125 MHz, respectively) and on a Bruker AVANCE-III-HD-300 spectrometer (300 and 75 MHz, respectively) in CDCl3 or DMSO-d6, with residual solvent signals used as internal standards (7.26 ppm for 1H nuclei and 76.9 ppm for 13C nuclei in CDCl3 or 2.50 ppm for 1H nuclei and 39.5 ppm for 13C nuclei in DMSO-d6). High-resolution mass spectra were recorded on a Thermo Scientific DFS instrument in full scan mode over the m/z range of 0–500, by ionization with an electron impact of 70 eV, and direct introduction of samples. The reaction products were separated by column chromatography (60–200 μm silica gel, Masherey-Nagel). GC-MS analysis was performed on an Agilent 7890 A gas chromatograph with an Agilent 5975C quadrupole mass spectrometer as a detector and an HP-5MS 30 000 × 0.25 mm fused silica column with helium as carrier gas. The identification of compounds was performed by comparing the retention times with a series of authentic samples and comparing the complete experimentally obtained mass spectra with a mass spectral database.
The structures of the obtained compounds were established from 1H and 13C NMR spectra, two-dimensional homonuclear 1H–1H COSY, TOCSY, and NOESY experiments, two-dimensional heteronuclear 1H–13C HSQC, HMBC, and HSQC-COSY experiments, and also 1H–15N HSQC and HMBC experiments. Dynamic NMR experiments were performed using the recording of the conventional 1H NMR spectra in DMSO-d6 at different temperatures in the interval of 30–110 °C with a step of 10 °C. The atom numbering in the compounds is intended for the assignment of NMR signals and does not match the IUPAC nomenclature (see the ESI†).
Synthesis of d-(+)-camphor hydrazone 5
d-(+)-Camphor (20.0 g, 0.13 mol), hydrazine hydrate (26 mL, 0.52 mol), and glacial acetic acid (7.4 mL, 0.13 mol) were dissolved in ethanol (300 mL). The reaction mixture was heated at reflux for 4 h and then cooled to room temperature (r.t.). The solvent was evaporated under reduced pressure. The residue was dissolved in chloroform (50 mL), washed with a saturated solution of NaHCO3 (2 × 10 mL), and finally with water (10 mL). The organic phase was separated, dried over anhydrous Na2SO4, and evaporated under reduced pressure to yield the target hydrazone 5 as a white powder. The yield was 98% (0.128 mol, 21.4 g).
General procedure for synthesis of compounds 6–12
d-(+)-Camphor hydrazone 5 (0.50 g, 3.1 mmol) was dissolved in a mixture of chloroform (50 mL) and triethylamine (0.44 mL, 3.2 mmol). Equimolar amounts of appropriate carboxylic acid anhydride or carboxylic acid chloride were added dropwise, after that the reaction mixture was stirred for 4 h at r.t. The chloroform solution was washed with water (3 × 10 mL) and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum and the resulting solids were purified by recrystallization from a hexane/ethyl acetate mixture to give products 6–12 as white powders.
General procedure for synthesis of compounds 13–19
The carboxylic acid (3.0 mmol) was dissolved in a mixture of chloroform (100 mL) and triethylamine (0.44 mL, 3.2 mmol), then a solution of ethyl chloroformate (0.28 mL, 3.0 mmol) in chloroform (30 mL) was added dropwise. The reaction mixture was stirred for 15 min at r.t, then a solution of d-(+)-camphor hydrazone (5) in chloroform (30 mL) was added dropwise. The solution was stirred for 4 h and washed with water (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The obtained products were purified by silica gel column chromatography to give the title compounds as white or light-yellow solids.
N′-[(1R,2E,4R)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]acetohydrazide (–)-6
White solid (79% yield); 1H NMR (400 MHz, CDCl3): 0.71 (3H, s, Me-9), 0.90 (3H, s, Me-8), 0.96 (3H, s, Me-10), 2.22 (3H, s, Me-12), 1.36 (H-2), 1.18 (1H, m, H-3), 1.80 (1H, d, J = 16.8, H-5), 2.30 (1H, dt, J = 16.8, J = 3.6, H-5), 1.69 (1H, m, H-2), 1.83 (1H, m, H-3), 1.96 (1H, m, H-4), 8.39 (1H, s, NH). 13C NMR (100 MHz, CDCl3): 173.15 (C-11), 164.79 (C-6), 10.90 (Me-10), 18.48 and 19.33 (Me-8 and Me-9), 20.08 (Me-12), 43.92 (C-4), 27.13 (C-3), 32.44 (C-2), 33.17 (C-5), 47.71 (C-7), 52.35 (C-1). Found, m/z: 208.1574 [M]+. C12H20ON2. Calculated, m/z: 208.1570. [α]25D = –36.0 (CHCl3, c = 0.32).
2,2,2-Trifluoro-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]acetohydrazide (–)-7
White solid (80% yield); 1H NMR (400 MHz, CDCl3 + DMSO-d6): 0.70 (3H, s, Me-9), 0.85 (3H, s, Me-8), 0.96 (3H, s, Me-10), 1.34 (H-2), 1.14 (1H, m, H-3), 1.76 (1H, d, J = 16.8, H-5), 2.47 (1H, dt, J = 16.8, J = 3.6, H-5), 1.66 (1H, m, H-2), 1.83 (1H, m, H-3), 1.95 (1H, m, H-4), 10.48 (1H, s, NH). 13C NMR (100 MHz, CDCl3 + DMSO-d6): 181.05 (C-11), 169.99 (C-6), 10.91 (Me-10), 18.40 and 19.29 (Me-8 and Me-9), 43.52 (C-4), 26.80 (C-3), 32.15 (C-2), 35.14 (C-5), 47.95 (C-7), 52.52 (C-1), 112.49, 114.77, 117.06, 119.35 (C-12). Found, m/z: 262.1286 [M]+. C12H17ON2F3. Calculated, m/z: 262.1288. [α]25D = –59.3 (CHCl3, c = 0.18).
N′-[(1R,2E,4R)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]butanohydrazide (–)-8
White solid (59% yield); 1H NMR (400 MHz, CDCl3): 0.71 (3H, s, Me-9), 0.90 (3H, s, Me-8), 0.97 (3H, s, Me-10), 0.95 (3H, t, J13–14 = 7.4, Me-14), 1.36 (H-2), 1.18 (1H, m, H-3), 2.60 (2H, t, H-12), 1.68 (2H, m, H-13) 1.77 (1H, d, J = 16.5, H-5), 2.27 (1H, dt, J = 16.5, J = 3.6, H-5), 1.70 (1H, m, H-2), 1.84 (1H, m, H-3), 1.97 (1H, m, H-4), 8.09 (1H, s, NH). 13C NMR (100 MHz, CDCl3): 175.53 (C-11), 164.31 (C-6), 10.91 (Me-10), 18.48 and 19.31 (Me-8 and Me-9), 13.83 (Me-14), 43.94 (C-4), 27.15 (C-3), 32.45 (C-2), 34.41 (C-5), 47.68 (C-7), 52.31 (C-1), 18.06 (C-13), 33.04 (C-12). Found, m/z: 236.1884 [M]+. C14H24ON2. Calculated, m/z: 236.1883. [α]25D = –36.9 (CHCl3, c = 0.42).
N′-[(1R,2E,4R)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]benzohydrazide (–)-9
White solid (70% yield); 1H NMR (400 MHz, DMSO-d6): 0.76 and 0.91 (3H, s, H-8 and H-9), 0.99 (3H, s, H-10), 1.21 (1H, m, H-3), 1.35 (1H, m, H-2), 2.61 (1H, m, H-5), 2.10 (1H, d, J = 17.5, H-5), 1.94 (1H, m, H-4), 1.73 (1H, m, H-2), 1.81 (1H, m, H-3), 7.80 (2H, m, H-13, H-17), 7.47 (2H, m, H-14, H-16), 7.54 (1H, m, H-15). 13C NMR (100 MHz, CDCl3): 11.28 (C-10), 18.71 and 19.73 (C-8 and C-9), 44.08 (C-4), 33.81 (C-5), 27.37 (C-3), 32.54 (C-2), 53.26 (C-1), 48.27 (C-7), 127.23 (C-13, C-17), 128.78 (C-14, C-16), 131.74 (C-15), 134.13 (C-12), 163.75 (C-6), 171.51 (C-11). Found, m/z: 270.1729 [M]+. C17H22ON2. Calculated, m/z: 270.1727. [α]25D = –57.5 (CHCl3, c = 0.32).
4-Bromo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]benzohydrazide (–)-10
White solid (56% yield); 1H NMR (400 MHz, DMSO-d6): 0.75 (3H, s, Me-9), 0.90 (3H, s, Me-8), 0.98 (3H, s, Me-10), 1.33 (1H, m, H-2), 1.20 (1H, m, H-3), 1.67–1.85 (2H, m, H-2, H-3), 1.93 (1H, m, H-4), 2.59 (1H, m, H-5), 2.09 (1H, d, J = 17.7, H-5), 7.64–7.77 (4H, m, H-13, H-14, H-16, H-17), 10.29 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6): 174.31 (C-11), 162.26 (C-6), 11.47 (Me-10), 18.54 and 19.34 (Me-8 and Me-9), 43.32 (C-4), 26.84 (C-3), 32.34 (C-2), 34.82 (C-5), 47.64 (C-7), 52.72 (C-1), 124.92 (C-15), 129.80 (C-13, C-17), 131.28 (C-14, C-16), 133.33 (C-12). Found, m/z: 348.0834 [M]+. C17H21ON279Br1. Calculated, m/z: 348.0832.
N′-[(1R,2E,4R)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]-2-furohydrazide (–)-11
White solid (56% yield); 1H NMR (400 MHz, CDCl3): 0.75 (3H, s, Me-9), 0.92 (3H, s, Me-8), 1.10 (3H, s, Me-10), 1.18–1.24 (1H, m, H-2), 1.39–1.50 (1H, m, H-3), 1.70–1.76 (1H, m, H-2), 1.90–1.97 (1H, d, J = 17.0, H-5), 2.46 (1H, d, J = 16.0, H-5), 2.02 (1H, t, H-4), 1.83–1.90 (1H, m, H-3), 6.49 (1H, s, H-14), 7.41 (1H, d, J = 4.6, H-15), 7.20 (1H, d, J = 2.9, H-13), 8.68 (1H, s, NH). 13C NMR (100 MHz, CDCl3): 11.01 (Me-10), 18.46 and 19.43 (Me-8 and Me-9), 43.84 (C-4), 27.12 (C-3), 32.30 (C-2), 33.42 (C-5), 48.00 (C-7), 52.96 (C-1), 170.78 (C-11), 153.77 (C-6), 112.19 (C-14), 115.27 (C-13), 143.75 (C-15), 147.15 (C-12). Found, m/z: 260.1527 [M]+. C15H20O2N2. Calculated, m/z: 260.1519. [α]25D = –47.2 (CHCl3, c = 0.14)
N′-[(1R,2E,4R)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]thiophene-2-carbohydrazide (–)-12
White solid (68% yield); 1H NMR (400 MHz, CDCl3): 0.80 (3H, s, Me-9), 0.96 (3H, s, Me-8), 1.18 (3H, s, Me-10), 7.12 (1H, dd, H-14), 7.62 (1H, d, J = 4.6, H-15), 8.18 (1H, d, J = 2.9, H-12), 9.10 (1H, s, NH), 2.49 (1H, dt, J = 17.0, J = 3.8, H-5), 2.01 (1H, d, J = 17.0, H-5), 2.06 (1H, m, H-4), 1.48 (1H, m, H-2), 1.27 (1H, m, H-3), 1.79 (1H, m, H-2), 1.90 (1H, m, H-3). 13C NMR (100 MHz, CDCl3): 168.88 (C-11), 162.70 (C-6), 11.84 (Me-10), 18.75 and 19.67 (Me-8 and Me-9), 44.29 (C-4), 27.38 (C-3), 32.69 (C-2), 33.61 (C-5), 48.07 (C-7), 53.33 (C-1), 126.32 (C-14), 134.32 (C-13), 135.15 (C-15), 133.57 (C-12). Found, m/z: 276.1298 [M]+. C15H20O2N32S. Calculated, m/z: 276.1291. [α]25D = –52.7 (CHCl3, c = 0.26).
Mixture of (3aR,6S,7R,7aS)-2-ethyl-1-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (a) and (3aS,6R,7S,7aR)-2-ethyl-1-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (b) (–)-13
White solid (76% yield); 1H NMR (400 MHz, CDCl3): 10.24, 10.38, 8.11 and 8.13 (2H, NH-a, NH-b); 6.41–6.53 (4H, m, H-17a, H-18a, H-17b, H-18b); 5.53 and 5.18 (2H, d, J = 20.4, J = 4.5, H-19a, H-19b); 2.18–2.50 (2H, m, H-5a, H-5b); 0.70 and 0.72 (6H, s, Me-9a, Me-9b); 0.86–0.92 (9H, s, Me-8a, Me-10a, Me-8b); 1.02–1.20 (11H, m, Me-10b, Me-21a, Me-21b, H-3a, H-3b); 3.86–3.96 (2H, m, H-15a, H-15b), 3.12–3.21 and 3.23–3.33 (2H, m, H-12a, H-12b), 1.29–1.49 (2H, m, H-2a, H-2b); 3.61–3.71 (2H, m, H-15a, H-15b); 1.58–1.96 (8H, m, H-2a, H-3a, H-4a, H-5a, H-2b, H-3b, H-4b, H-5b); 2.65–2.81 (2H, m, H-13a, H-13b), 3.36–3.51 (4H, m, H-20a, H-20b). 13C NMR (100 MHz, CDCl3): 10.94, 11.03, 11.05 (C-10a, C-10b); 18.43, 18.51 (C-8a, C-8b); 19.30, 19.38, 19.44 (C-9a, C-9b); 12.19, 12.26 (C-21a, C-21b); 134.59, 134.61, 134.88, 134.99 (C-18a, C-18b); 137.52, 137.57 (C-17a, C-17b); 80.51, 80.75, 83.15, 83.24 (C-19a, C-19b); 49.88, 50.01, 50.39, 50.61 (C-12a, C-12b); 42.00, 42.26, 43.83, 43.92 (C-13a, C-13b); 26.97, 26.12, 27.17 (C-3a, C-3b); 32.18, 32.28, 32.35 (C-2a, C-2b); 32.76, 33.01, 34.34, 34.68 (C-5a, C-5b); 52.21, 52.68 (C-1a, C-1b); 47.57, 47.65, 47.72, 47.78, 47.87, 48.03, 48.13 (C-7a, C-7b, C-15a, C-15b, C-4a, C-4b); 88.25, 89.20 (C-16a, C-16b); 37.21, 37.24, 37.55, 37.60 (C-20a, C-20b); 164.31, 164.75, 169.90, 170.11, (C-6a, C-6b); 170.30, 170.55, 171.34, 171.46, (C-14a, C-14b) 166.06, 166.10, 172.95 (C-11a, C-11b). Found, m/z: 371.2205 [M]+. C21H29O3N3. Calculated, m/z: 371.2203. [α]25D = –21.3 (CHCl3, c = 0.31).
Mixture of (3aR,6S,7R,7aS)-2-(2-methoxyethyl)-1-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (a) and (3aS,6R,7S,7aR)-2-(2-methoxyethyl)-1-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (b) (–)-14
White solid (81% yield); 1H NMR (400 MHz, CDCl3): 10.24, 10.38, 8.11 and 8.13 (2H, NH-a, NH-b); 6.41–6.53 (4H, m, H-17a, H-18a, H-17b, H-18b); 5.53 (1H, d, J = 20.4, H-19a); 5.18 (1H, d, J = 4.5, H-19b); 2.18–2.50 (2H, m, H-5a, H-5b), 0.70 and 0.73 (6H, s, Me-9a, Me-9b); 0.88, 0.91, 1.06 (12H, m, Me-8a, Me-10a, Me-8b, Me-10b); 1.13–1.22 (2H, m, H-3a, H-3b); 1.29–1.49 (2H, m, H-2a, H-2b); 1.58–2.00 (8H, m, H-2a, H-3a, H-5a, H-4a, H-2b, H-3b, H-5b, H-4b); 2.68–2.81 (2H, m, H-13a, H-13b); 4.00–4.09 (2H, m, H-15a, H-15b); 3.15–3.36 (6H, m, 2H-22a, H-12a, 2H-22b, H-12b); 3.43–3.55 (6H, m, H-15a, 2H-20a, H-15b, 2H-20b), 3.62–3.84 (4H, m, 2H-21a, 2H-21b). 13C NMR (100 MHz, CDCl3): 10.96, 11.06 (C-10a, C-10b); 18.45, 18.53 (C-8a, C-8b); 19.33, 19.36, 19.60 (C-9a, C-9b); 134.67, 134.89, 135.02 (C-18a, C-18b); 137.52, 137.48 (C-17a, C-17b); 80.49, 80.76, 83.17, 83.24 (C-19a, C-19b); 26.99, 27.15, 27.19 (C-3a, C-3b); 32.21, 32.28, 32.37 (C-2a, C-2b); 32.81, 33.04, 34.35, 34.69 (C-5a, C-5b); 58.46, 58.51, 58.63 (C-22a, C-22b); 70.51, 70.61, 70.92 (C-21a, C-21b); 88.58, 89.49 (C-16a, C-16b); 49.63, 49.77, 50.10, 50.34 (C-12a, C-12b); 42.08, 42.40, 43.88, 43.94 (C-13a, C-13b); 52.25, 52.72 (C-1a, C-1b); 164.38, 164.86, 166.06, 166.10 (C-6a, C-6b); 170.37, 170.41, 170.62, 171.86, 172.01, 173.02 (C-11a, C-11b, C-14a, C-14b); 49.90, 50.00, 50.24 (C-15a, C-15b); 47.63, 47.72, 47.82, 47.91 (C-4a, C-4b, C-7a, C-7b); 42.59, 42.67, 42.91 (C-20a, C-20b). Found, m/z: 401.2305 [M]+. C22H31O4N3. Calculated, m/z: 401.2309. [α]25D = –16.3 (CHCl3, c = 0.59).
Mixture of (3aR,6S,7R,7aS)-6-methyl-1-oxo-2-phenyl-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (a) and (3aS,6R,7S,7aR)-6-methyl-1-oxo-2-phenyl-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (b) (–)-15
White solid (74% yield); 1H NMR (400 MHz, CDCl3): 8.30, 8.70, 8.82 (2H, NH-a, NH-b); 7.03–7.16 (2H, m, H-24a, H-24b); 7.50–7.62 (4H, m, H-23a, H-23b, H-25a, H-25b); 7.25–7.35 (4H, m, H-22a, H-22b, H-26a, H-26b); 6.56–6.65 (2H, m, H-17a, H-17b); 6.17–6.32 (2H, m, H-18a, H-18b); 3.89–4.46 (4H, m, 2H-15a, 2H-15b); 0.54–1.09 (18H, s, Me-8a, Me-9a, Me-10a, Me-8b, Me-9b, Me-10b); 2.77–3.14 (4H, m, H-12a, H-12b, H-13a, H-13b); 1.08–1.51 (6H, m, 2H-2a, 2H-2b, H-3a, H-3b); 1.75–2.40 (6H, m, H-4a, H-4b, 2H-5a, 2H-5b); 1.59–1.74 (8H, m, Me-20a, Me-20b, H-3a, H-3b). 13C NMR (100 MHz, CDCl3): 11.00 (C-10a, C-10b), 18.37, 18.41, 18.45 (C-8a, C-8b); 19.25, 19.34, 19.37, 19.40 (C-9a, C-9b); 15.80, 15.90, 16.07, 16.11 (C-20a, C-20b); 26.62, 26.98, 27.05, 27.08 (C-3a, C-3b); 32.14, 32.35, 32.49 (C-2a, C-2b); 33.02, 33.09, 33.17, 33.22 (C-5a, C-5b); 52.26, 52.30, 52.49, 52.51 (C-1a, C-1b); 47.70, 47.86 (C-7a, C-7b); 86.72, 87.00, 87.11 (C-19a, C-19b); 89.50, 89.56, 89.95, 90.08 (C-16a, C-16b); 138.77, 139.11 (C-21a, C-21b); 164.60, 164.73, 168.73, 169.06 (C-6a, C-6b); 165.01, 171.06, 170.98 (C-11a, C-11b); 169.54, 169.62, 170.88, 171.01 (C-14a, C-14b); 139.45, 139.67, 140.43, 140.55 (C-18a, C-18b); 135.74, 136.26, 136.38 (C-17a, C-17b); 124.76, 124.67, 124.31, 124.29 (C-24a, C-24b); 119.38, 119.46, 120.08, 120.18 (C-22a, C-22b, C-26a, C-26b); 128.43, 128.61, 128.74, 128.80 (C-23a, C-23b, C-25a, C-25b); 50.14, 50.17, 49.83, 49.87 (C-15a, C-15b); 50.47, 50.50 (C-12a, C-12b); 54.92, 55.02, 55.30, 56.05 (C-13a, C-13b); 43.57, 43.75, 43.82, 43.85 (C-4a, C-4b). Found, m/z: 433.2361 [M]+. C26H31O3N3. Calculated, m/z: 433.2360. [α]25D = –22.9 (CHCl3, c = 0.15).
Mixture of (3aR,6S,7R,7aS)-1-oxo-2-(2-phenylethyl)-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (a) and (3aS,6R,7S,7aR)-1-oxo-2-(2-phenylethyl)-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carbohydrazide (b) (–)-16
White solid (76% yield); 1H NMR (400 MHz, DMSO-d6): 5.11 (1H, d, J = 1.5, H-19a); 5.00 (1H, d, J = 2.5, H-19b); 6.41–6.63 (4H, m, H-17a, H-17b, H-18a, H-18b); 7.15–7.34 (10H, m, H-23a, H-23b, H-24a, H-24b, H-25a, H-25b, H-26a, H-26b, H-27a, H-27b); 0.69 and 0.73 (6H, s, Me-9a, Me-9b); 0.86–0.95 (12H, s, Me-8a, Me-10a, Me-8b, Me-10); 9.59, 9.69, 9.77 and 9.79 (2H, NH-a, NH-b); 1.08–1.37 (4H, m, H-2a, H-2b, H-3a, H-3b); 1.57–1.85 (4H, m, H-2a, H-2b, H-3a, H-3b); 1.85–2.02 (4H, m, H-5a, H-5b, H-4a, H-4b); 2.21–2.47 (2H, m, H-5a, H-5b), 3.84–4.04 (2H, m, H-15a, H-15b), 3.16–3.70 (8H, m, H-15a, H-15b, 2H-20a, 2H-20b, H-12a, H-12b), 2.54–2.84 (6H, m, H-13a, H-13b, 2H-21a, 2H-21b). 13C NMR (100 MHz, DMSO-d6): 80.11, 80.35, 82.18, 82.30 (C-19a, C-19b); 135.43, 135.60, 136.52, 136.58 (C-18a, C-18b); 136.88, 138.27, 138.21 (C-17a, C-17b); 126.61, 126.69 (C-25a, C-25b); 128.80, 128.86, 129.03 (C-23a, C-23b, C-24a, C-24b, C-26a, C-26b, C-27a, C-27b); 139.35, 139.48 (C-22a, C-22b); 11.65, 11.86 (C-10a, C-10b); 18.96, 19.12 (C-8a, C-8b); 19.66, 19.78 (C-9a, C-9b); 88.80, 89.02 (C-16a, C-16b); 27.37 (C-3a, C-3b); 32.65, 32.87 (C-2a, C-2b); 34.30, 34.51, 34.61 (C-5a, C-5b); 52.27, 52.34, 52.54 (C-1a, C-1b); 47.70, 47.87 (C-7a, C-7b); 48.31, 48.64 (C-15a, C-15b); 43.18, 43.39 (C-12a, C-12b); 43.80, 43.99, 44.08 (C-20a, C-20b, C-4a, C-4b); 163.98, 164.33, 167.78, 168.05 (C-6a, C-6b); 166.39, 166.50, 172.75 (C-11a, C-11b); 170.27, 170.40, 170.97 (C-14a, C-14b); 33.41, 33.51 (C-21a, C-21b); 49.75, 49.97, 50.72, 50.78 (C-13a, C-13b). Found, m/z: 447.2520 [M]+. C27H33O3N3. Calculated, m/z: 447.2516. [α]25D = –11.2 (CHCl3, c = 0.39).
Mixture of (5aS,6R,7S,9aR)-5-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-2,3,5,5a,6,7-hexahydro-7,9a-epoxy[1,3]thiazolo[2,3-a]isoindole-6-carbohydrazide (a) and (5aR,6S,7R,9aS)-5-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-2,3,5,5a,6,7-hexahydro-7,9a-epoxy[1,3]thiazolo[2,3-a]isoindole-6-carbohydrazide (b) (–)-17
White solid (70% yield); 13C NMR (100 MHz, DMSO-d6): 52.27, 52.36 (C-1a); 52.52 (C-1b); 32.90 (C-2a); 32.61 (C-2b); 27.42 (C-3a, C-3b); 43.96 (C-4a); 43.80 (C-4b); 34.44, 34.61 (C-5a, C-5b); 161.03, 161.23 (C-6a); 167.64, 167.85 (C-6b); 47.69 (C-7a); 47.83 (C-7b); 18.96, 19.06 (C-8a, C-8b); 19.58, 19.72 (C-9a, C-9b); 11.69, 11.82 (C-10a, C-10b); 172.59, 172.64 (C-11a); 166.32, 166.49 (C-11b); 45.59 (C-12a); 45.75 (C-12b); 52.43, 52.62 (C-13a); 53.69, 53.41 (C-13b); 170.71, 170.78 (C-14a); 170.42, 170.50 (C-14b); 63.99, 64.06 (C-15a, C-15b); 92.62 (C-16a); 92.08 (C-16b); 139.15 (C-17a); 137.77, 137.86 (C-17b); 133.76 (C-18a); 135.08 (C-18b); 79.40, 79.56 (C-19a); 81.24, 81.47 (C-19b); 45.50, 45.43 (C-20a); 45.25, 45.14 (C-20b); 32.19 (C-21a); 32.55 (C-21b). 1H NMR (400 MHz, DMSO-d6): 1.57–1.72 (2H, m, H-2a, H-2b); 1.09–1.36 (4H, m, H-2a, H-2b, H-3a, H-3b); 1.72–1.85 (2H, m, H-3a, H-3b); 1.87–2.00 (4H, m, H-4a, H-4b, H-5a, H-5b); 2.22–2.47 (2H, m, H-5a, H-5b); 0.83–0.96 (12H, s, Me-8a, Me-8b, Me-10a, Me-10b); 0.66, 0.69 (3H, s, Me-9a); 0.71, 0.73 (3H, s, Me-9b); 5.62–5.68 (2H, m, H-15a, H-15b); 6.47–6.66 (4H, m, H-17a, H-17b, H-18a, H-18b); 5.07 (2H, m, H-19a, H-19b); 3.97–4.10 (2H, m, H-20a, H-20b); 2.85–3.17 (8H, m, H-12a, H-12b, H-13a, H-13b, H-21a, H-21b, H-20a, H-20b); 2.60–2.83 (2H, m, H-21a, H-21b); 9.77 and 9.79 (1H, NH-a); 9.50 and 9.56 (1H, NH-b). Found, m/z: 401.1770 [M]+. C21H27O3N332S. Calculated, m/z: 401.1768.
Mixture of (6aS,7R,8S,10aR,10bR)-6-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-3,4,6,6a,7,8-hexahydro-2H-8,10a-epoxy[1,3]oxazino[2,3-a]isoindole-7(10bH)-carbohydrazide (a) and (6aR,7S,8R,10aS,10bS)-6-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-3,4,6,6a,7,8-hexahydro-2H-8,10a-epoxy[1,3]oxazino[2,3-a]isoindole-7(10bH)-carbohydrazide (b) (–)-18
White solid (75% yield); 1H NMR (400 MHz, DMSO-d6): 0.13–2.05 (12H, m, 2H-2a, 2H-2b, 2H-3a, 2H-3b, H-4a, H-4b, H-5a, H-5b); 2.17–2.52 (H-5a, H-5b); 0.90, 0.91, 0.98 (12H, s, Me-8a, Me-8b, Me-10a, Me-10b); 0.77, 0.71 (3H, s, Me-8a); 0.61, 0.76 (3H, s, Me-8b); 2.80–3.55 (4H, m, H-12a, H-12b, H-13a, H-13b); 6.36–6.80 (6H, m, H-17a, H-17b, H-18a, H-18b, H-15a, H-15b); 5.41 (1H, d, J = 4.3, H-19a); 5.37 (1H, d, J = 4.0, H-19b); 7.36–7.79 (4H, m, H-21a, H-21b, H-24a, H-24b); 6.90–7.21 (4H, m, H-22a, H-22b, H-23a, H-23b); 8.19, 8.23 (1H, s, NH-a); 8.88, 8.94 (1H, s, NH-b). 13C NMR (100 MHz, DMSO-d6): 52.30, 52.33 (C-1a); 52.32, 52.41 (C-1b); 32.42, 32.24 (C-2a); 32.15, 31.93 (C-2b); 27.11, 27.13 (C-3a); 26.90, 26.51 (C-3b); 43.92 (C-4a); 43.47, 43.65 (C-4b); 33.09, 32.87 (C-5a); 32.70, 32.36 (C-5b); 164.95, 165.12 (C-6a);167.93, 167.95 (C-6b); 47.73, 47.76 (C-7a); 47.79, 47.86 (C-7b); 18.57, 18.48 (C-8a); 18.42, 18.34 (C-8b); 19.32, 19.34 (C-9a); 19.41, 19.51 (C-9b); 11.17, 11.10 (C-10a); 10.93, 10.97 (C-10b); 172.05, 172.08 (C-11a); 165.52, 165.54 (C-11b); 42.05, 42.72 (C-12a, C-12b); 54.25, 54.01 (C-13a); 56.03, 56.27 (C-13b); 168.70, 168.92 (C-14a); 170.30, 170.38 (C-14b); 67.14, 67.06 (C-15a); 67.23, 67.26 (C-15b); 91.10, 91.08 (C-16a); 91.03 (C-16b); 139.36, 139.34 (C-17a); 139.61 (C-17b); 133.98, 133.87 (C-18a); 133.21, 133.18 (C-18b); 80.70, 80.94 (C-19a); 83.09, 83.01 (C-19b); 135.04, 135.10 (C-20a); 134.50, 134.43 (C-20b); 125.10, 125.12 (C-21a); 125.16, 125.13 (C-21b); 122.30, 122.32 (C-22a, C-22b); 116.66, 116.70(C-23a); 116.59, 116.87 (C-23b); 136.65, 136.52 (C-24a); 135.53, 135.46 (C-25b); 131.80, 131.86 (C-25a); 131.65, 131.64 (C-25b). Found, m/z: 449.1773 [M]+. C25H27O3N332S. Calculated, m/z: 449.1768. [α]25D = 10.0 (CHCl3, c = 0.12).
Mixture of (1R,2S,4aR,11aS)-11-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,11,11a-tetrahydro-2,4a-epoxyisoindolo[1,2-b][1,3]benzothiazole-1-carbohydrazide (a) and (1S,2R,4aS,11aR)-11-oxo-N′-[(1R,2E,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]-1,2,11,11a-tetrahydro-2,4a-epoxyisoindolo[1,2-b][1,3]benzothiazole-1-carbohydrazide (b) (–)-19
White solid (83% yield); 1H NMR (400 MHz, CDCl3): 1.23–1.36 (2H, m, H-2a, H-2b), 1.62–1.75 (2H, m, H-2a, H-2b), 1.07–1.23 (2H, m, H-3a, H-3b), 1.74–1.87 (2H, m, H-3a, H-3b), 1.85–2.01 (2H, m, H-4a, H-4b), 2.21–2.47 (2H, m, H-5a, H-5b), 1.82–1.99 (2H, m, H-5a, H-5b), 0.86–0.90 (6H, s, Me-8a, Me-8b), 0.69–0.72 (6H, s, Me-9a, Me-9b), 0.93 (6H, s, Me-10a, Me-10b), 9.56, 9.64, 9.86, 9.88 (2H, NH-a, NH-b), 2.52–2.57 (2H, m, H-12a, H-12b), 3.34–3.41 (2H, m, H-13a, H-13b), 2.99–3.16 (2H, m, H-15a, H-15b), 3.80–3.97 (2H, m, H-15a, H-15b), 1.44–1.71 (4H, m, H-16a, H-16b), 4.06–4.17 (2H, m, H-17a, H-17b), 3.80–3.97 (2H, m, H-17a, H-17b), 5.08, 5.09, 5.16, 5.19 (2H, s, H-18a, H-18b), 6.60–6.67 (2H, m, H-20a, H-20b), 6.48–6.57 (2H, m, H-21a, H-21b), 5.02–5.06, 5.12–5.15 (2H, m, H-22a, H-22b). 13C NMR (100 MHz, CDCl3): 51.81, 51.88, 52.03 (C-1a, C-1b); 32.14, 32.29 (C-2a, C-2b); 26.76, 26.84 (C-3a, C-3b); 43.25, 43.42, 43.65 (C-4a, C-4b); 33.90, 34.04, 34.24 (C-5a, C-5b); 164.07, 164.28, 168.04, 168.17 (C-6a, C-6b); 47.19, 47.23, 47.34 (C-7a, C-7b); 18.53, 18.41 (C-8a, C-8b); 19.10, 19.22 (C-9a, C-9b); 11.09, 11.21, 11.30 (C-10a, C-10b); 165.31, 165.43, 171.55 (C-11a, C-11b); 48.42, 48.58, 49.07, 49.27 (C-12a, C-12b); 41.45, 41.60 (C-13a, C-13b); 170.19, 170.32, 170.86 (C-14a, C-14b); 37.97 (C-15a, C-15b); 24.84 (C-16a, C-16b); 66.60 (C-17a, C-17b); 84.77, 84.82, 85.00 (C-18a, C-18b), 89.15, 89.19, 89.39, 89.44 (C-19a, C-19b); 133.91, 133.38, 133.20 (C-20a, C-20b); 137.45, 137.41, 136.44, 136.36 (C-21a, C-21b); 81.01, 81.23, 82.41, 82.58 (C-22a, C-22b). Found, m/z: 399.2154 [M]+. C22H29O4N3. Calculated, m/z: 399.2153. [α]25D = –12.9 (CHCl3, c = 0.34).
N′-[(1S,2E,4S)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene]benzohydrazide (+)-9
White solid (66% yield); 1H NMR (400 MHz, CDCl3): 0.75 and 0.90 (3H, s, H-8 and H-9), 0.98 (3H, s, H-10), 1.31–1.23 (1H, m, H-3), 1.30–1.38 (1H, m, H-2), 2.59 (1H, d, J = 17.4, H-5), 2.10 (1H, d, J = 17.4, H-5), 1.93 (1H, m, H-4), 1.65–1.75 (1H, m, H-2), 1.75–1.80 (1H, m, H-3), 7.74–7.82 (2H, m, H-12, H-16), 7.41–7.49 (2H, m, H-13, H-15), 7.50–7.56 (1H, m, H-14). 13C NMR (100 MHz, CDCl3): 11.64 (C-10), 18.74 and 19.54 (C-8 and C-9), 43.88 (C-4), 34.93 (C-5), 27.05 (C-3), 32.58 (C-2), 52.90 (C-1), 47.83 (C-7), 127.80 (C-12, C-16), 128.52 (C-13, C-15), 134.39 (C-17), 131.49 (C-14), 163.44 (C-6), 171.32 (C-11). Found, m/z: 270.1724 [M]+. C17H22ON2. Calculated, m/z: 270.1727 [α]25D = 53.8 (CHCl3, c = 0.15).
Biological assays
Screening for cytotoxicity and antiviral activity VV
A typical representative of orthopoxviruses, the vaccinia virus (strain Copenhagen), obtained from the State Collection of Virus Infection and Rickettsiosis Agents of VECTOR was used in this work. The virus was grown in a Vero cell culture. The virus concentration in the culture liquid was determined by plaque titration in the Vero cell culture, calculated and expressed in decimal logarithms of plaque-forming units in 1 mL (log10 PFU mL–1). The concentration of the virus in the samples used in this work was from 5.6 to 6.1 log10 PFU mL–1. The series of virus with the indicated titre was stored and used at work at –70 °C.
The antiviral efficacy of the compounds was evaluated as follows. In wells of 96-well plates containing a monolayer of Vero cells in 100 μl of DMEM medium with 2% fetal serum, 50 μl of serial dilutions of the test compounds were first introduced and then 50 μl of dilution of orthopoxvirus at a dose of 1000 PFU per well were added. The toxicity of the compounds was determined by the Vero cell death caused by the drug in the wells of the plate, into which the virus was not introduced. Monolayers of cells were used as controls in the wells of the plate, into which virus without compounds (virus control) was introduced, and monolayers of cells in wells, into which neither the virus nor the compound was introduced (cell culture control), were introduced. After incubation for 4 days, the monolayer of cells was stained with vital dye neutral red for 2 h. After removing the dye and washing the wells from its unbound fraction, a lysis buffer was added. The amount of dye adsorbed by the living cells of the monolayer was evaluated by optical density (OD), which is an indication of the number of cells undisturbed under the influence of a virus in a monolayer. The OD was measured on an Emax spectrophotometer (Molecular Devices, USA) at a wavelength of 490 nm. Results were processed using the Soft Max Pro 4.0 program, which computed the 50% toxic concentration (CC50 in μM) and 50% inhibitory concentration (IC50 in μM). The selectivity index (SI) was determined as SI = CC50/IC50 using the corresponding concentrations.
Screening for cytotoxicity and antiviral activity against influenza virus
Cytotoxicity assay
The microtetrazolium test (MTT) was used to study the cytotoxicity of the compounds. Briefly, a series of three-fold dilutions of each compound (300–400 μg mL–1) in Eagle's minimal essential medium (MEM) were prepared. MDCK cells were incubated for 48 h at 36 °C in 5% CO2 in the presence of the dissolved substances. The cells were washed twice with phosphate-buffered saline (PBS), and a solution of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (ICN Biochemicals Inc. Aurora, Ohio) (0.5 μg mL–1) in PBS was added to the wells. After 1 h incubation, the wells were washed and the formazan residue was dissolved in DMSO (0.1 mL per well). The optical density in the wells was then measured on a Victor2 1440 multifunctional reader (Perkin Elmer, Finland) at a wavelength of 535 nm and plotted against the concentration of the compounds. Each concentration was tested in three parallels. The 50% cytotoxic concentration (CC50) of each compound was calculated from the data obtained.
Virus inhibition assay
The compounds in appropriate concentrations were dissolved in 0.1 mL DMSO and the final solutions were prepared by adding MEM with 1 μg mL–1 trypsin. The compounds were incubated with MDCK cells for 1 h at 36 °C. Each concentration of the compounds was tested in triplicate. The cell culture was then infected with influenza virus A/Puerto Rico/8/34 (H1N1) (moi 0.01) for 1 h, the unbound virions were removed by washing and cells were incubated for 24 h at 36 °C in the presence of 5% CO2. The virus titer in the supernatant was determined by a hemagglutination test after cultivation of the virus in MDCK cells for 48 h at 36 °C in the presence of 5% CO2. Rimantadine and oseltamivir carboxylate were used as reference drugs. For calculations, the virus titer was expressed as the percentage of the titer in control wells without compounds. The 50% inhibitory concentrations (IC50) and the selectivity indices (SI, the ratio of CC50 to IC50) were calculated from the data obtained.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
The synthesis of the target compounds 3–20 was supported by the Russian Foundation for Basic Research (grant No. 17-33-50110). The X-Ray and NMR analyses of the products were performed with financial support from the RUDN University program “5-100”. The virological study in the State Research Centre of Virology and Biotechnology VECTOR was conducted under state assignment GZ-8/18. The antiviral activity against influenza virus was determined with financial support from the Russian Scientific Foundation grant No. 15-13-00017. This research was partially carried out using the equipment of the Chemical Service Center of Collective Use of the SB RAS.
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1860266, 1860267 and 1873457. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8md00442k
References
- Fenner F., Henderson D. A., Arita I., Jezek Z. and Ladnyi I. D., Smallpox and its eradication, World Health Organization, Geneva, 1988. [Google Scholar]
- Shchelkunova G. A., Shchelkunov S. N. Acta Naturae. 2017;9:4. [PMC free article] [PubMed] [Google Scholar]
- DeClerc E. Antiviral Res. 2002;55:1. [Google Scholar]
- Parker S., Touchette E., Oberle C., Almond M., Robertson A., Trost L. C., Lampert B., Painter G., Buller R. M. Antiviral Res. 2008;77:39. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Pevear D. C., Davies M. H., Collett M. S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J. W., Baker R. O., Buller R. L. M., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. J. Virol. 2005;79:13139. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impelluso G., Lentzos F. Health Secur. 2017;15:1. doi: 10.1089/hs.2017.0045. [DOI] [PubMed] [Google Scholar]
- Moss B., Poxviridae, in, Fields virology, ed. D. M. Knipe and P. M. Howley, Lippincott Williams & Wilkins, Philadelphia, PA, 2013, vol. 2., p. 2130. [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research (CDER), Draft guidance for industry on smallpox (Variola) infection: developing drugs for treatment or prevention; availability. Doc. No. 2007D-0439, 2007.
- Krammer F., Smith G., Fouchier R., Peiris M., Kedzierska K., Doherty P., Palese P., Shaw M., Treanor J., Webster R., García-Sastre A. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyde V., Garten R., Sheu T., Smith C., Myrick A., Barnes J., Xu X., Shaw M., Klimov A., Gubareva L. Influenza Other Respir. Viruses. 2009;3:297. doi: 10.1111/j.1750-2659.2009.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Khandaker G., Ilgoutz S., Rashid H., Booy R. Infect. Disord.: Drug Targets. 2013;13:34. doi: 10.2174/18715265112129990006. [DOI] [PubMed] [Google Scholar]
- Boldescu V., Behnam M., Vasilakis N., Klein C. Nat. Rev. Drug Discovery. 2017;16:565. doi: 10.1038/nrd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salakhutdinov N., Volcho K., Yarovaya O. Pure Appl. Chem. 2017;89:1105. [Google Scholar]
- Martinez A. G., War E. T., Fraile A. G., Cerero S., Herrero M. E., Ruiz P. M., Subramanian L. R., Gancedo A. G. J. Med. Chem. 1995;38:4474. doi: 10.1021/jm00022a012. [DOI] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Shernyukov A., Gatilov Y., Razumova Y., Zarubaev V., Tretiak T., Kiselev O., Salakhutdinov N. Eur. J. Med. Chem. 2015;105:263. doi: 10.1016/j.ejmech.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Baev D., Shernyukov A., Shtro A., Zarubaev V., Salakhutdinov N. Eur. J. Med. Chem. 2017;127:661. doi: 10.1016/j.ejmech.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Sokolova A. S., Yarovaya O. I., Korchagina D. V., Zarubaev V. V., Tretiak T. S., Anfimov P. M., Kiselev O. I., Salakhutdinov N. F. Bioorg. Med. Chem. 2014;22:2141. doi: 10.1016/j.bmc.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Semenova M., Shtro A., Orshanskay Y., Zarubaev V., Salakhutdinov N. Med. Chem. Commun. 2017;8:960. doi: 10.1039/c6md00657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononova A., Sokolova A., Cheresiz S., Yarovaya O., Nikitina R., Chepurnov A., Pokrovsky A., Salakhutdinov N. Med. Chem. Commun. 2017;8:2233. doi: 10.1039/c7md00424a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Bormotov N., Shishkina L., Salakhutdinov N. Chem. Biodiversity. 2018:e1800153. doi: 10.1002/cbdv.201800153. [DOI] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Bormotov N., Shishkina L., Salakhutdinov N. Med. Chem. Commun. 2018;9:1746. doi: 10.1039/c8md00347e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch M. E., Snyder S. A., Stockwell B. R. Curr. Opin. Chem. Biol. 2010;14:347. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraja P., Patrizia D., Montalbano A., Carbone A., Viola G., Basso G., Salvador A., Vedaldi D., Dall'Acqua F., Cirrincione G. Bioorg. Med. Chem. 2011;19:2326. doi: 10.1016/j.bmc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Barraja P., Spanò V., Patrizia D., Carbone A., Cirrincione G., Vedaldi D., Salvador A., Viola G., Dall'Acqua F. Bioorg. Med. Chem. Lett. 2009;19:1711. doi: 10.1016/j.bmcl.2009.01.096. [DOI] [PubMed] [Google Scholar]
- Diana P., Martorana A., Barraja P., Montalbano A., Dattolo G., Cirrincione G., Dall'Acqua F., Salvador A., Vedaldi D., Basso G., Viola G. J. Med. Chem. 2008;51:2387. doi: 10.1021/jm070834t. [DOI] [PubMed] [Google Scholar]
- Khattab S. N., Hassan S. Y., El-Faham A., El Massry A. M. M., Amer A. J. Heterocycl. Chem. 2007;44:617. [Google Scholar]
- Lübbers T., Angehrn P., Gmünder H., Herzig S. Bioorg. Med. Chem. Lett. 2007;17:4708. doi: 10.1016/j.bmcl.2006.12.065. [DOI] [PubMed] [Google Scholar]
- Mertens A., Zilch H., König B., Schäfer W., Poll T., Kampe W., Seidel H., Leser U., Leinert H. J. Med. Chem. 1993;36:2526. doi: 10.1021/jm00069a011. [DOI] [PubMed] [Google Scholar]
- Zilch H., Poll T., Schaefer W., Koenig B. and Leser U., WO015310, 1992. Iino T., Bamba M., Eiki J. and Nagase T., WO066479, 2002.
- Mikle G., Boros B., Kollár L. Tetrahedron: Asymmetry. 2016;27:377. [Google Scholar]
- Somogyi L. Liebigs Ann. Chem. 1991;12:1267. [Google Scholar]
- Srivastava A., Verma S. M. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1995;34:550. [Google Scholar]
- Forster M., Trotter J., Weintroube J. J. Chem. Soc. 1911:1982. [Google Scholar]
- Filhjo J. M., Pinheiro S. M. Green Chem. 2017;19:2212. [Google Scholar]
- Varlamov A., Boltukhina E., Zubkov F., Sidorenko N., Chernyshev A., Grudinin D. Chem. Heterocycl. Compd. 2004;40:22. [Google Scholar]
- Zubkov F., Airiyan I., Ershova J., Galeev T., Zaytsev V., Nikitina E., Varlamov A. RSC Adv. 2012;2:4103. [Google Scholar]
- Zubkov F., Nikitina E., Galeev T., Zaytsev V., Khrustalev V., Novikov R. O., Orlova D., Varlamov A. Tetrahedron. 2014;70:1659. [Google Scholar]
- Selivanov B. A., Tikhonov A. Y., Belanov E. F., Bormotov N. I., Kabanov A. S., Mazurkov O. Y., Serova O. A., Shishkina L. N., Agafonov A. P., Sergeev A. N. Pharm. Chem. J. 2017;51:439. [Google Scholar]
- Artyushin O., Sharova E., Vinogradova N., Genkina G., Moiseeva A., Klemenkova Z., Orshanskaya I., Shtro A., Kadyrova R., Zarubaev V., Yarovaya O., Salakhutdinov N., Brel V. Bioorg. Med. Chem. Lett. 2017;27:2181. doi: 10.1016/j.bmcl.2017.03.051. [DOI] [PubMed] [Google Scholar]
- Smee D., Hurst B., Evans W., Clyde N., Wright S., Peterson C., Jung K., Day C. J. Virol. Methods. 2017;246:51. doi: 10.1016/j.jviromet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubaev V., Garshinina A., Tretiak T., Fedorova V., Shtro A., Sokolova A., Yarovaya O., Salakhutdinov N. Antiviral Res. 2015;120:126. doi: 10.1016/j.antiviral.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Khomenko T., Zarubaev V., Orshanskaya I., Kadyrova R., Sannikova V., Korchagina D., Volcho K., Salakhutdinov N. Bioorg. Med. Chem. Lett. 2017;27:2920. doi: 10.1016/j.bmcl.2017.04.091. [DOI] [PubMed] [Google Scholar]
- Ilyina I., Zarubaev V., Lavrentieva I., Shtro A., Esaulkova I., Korchagina D., Borisevich S., Volcho K., Salakhutdinov N. Bioorg. Med. Chem. Lett. 2018;28:2061. doi: 10.1016/j.bmcl.2018.04.057. [DOI] [PubMed] [Google Scholar]
- Moss B. Semin. Cell Dev. Biol. 2016;60:89. doi: 10.1016/j.semcdb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.