Abstract
Anti-programmed cell death protein 1 (PD-1) agents have become the standard of care for platinum-refractory recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) and are currently being evaluated in various disease settings. However, despite the gain in overall survival seen in some of the clinical trials, the majority of patients display primary resistance and do not benefit from these agents. Taking into consideration the potentially severe immune-related toxicities and their high cost, the search for predictive biomarkers of response is crucial. Besides Programmed death ligand-1 (PD-L1) expression, other biomarkers such as immune infiltration, tumor mutational burden or immune-gene expression profiling have been explored, but none of them has been validated in this disease. Among these, the microbiota has recently garnered tremendous interest since it has proven to influence the efficacy of PD-1 blockade in some tumor types. With the accumulating evidence on the effect of the microbiota in HNSCC tumorigenesis and progression, the study of its potential role as a predictive immune biomarker is warranted. This review examines the available evidence on emerging immune predictive biomarkers of response to anti-PD-1/PD-L1 therapy in HNSCC, introducing the microbiota and its potential use as a predictive immune biomarker in this disease.
Keywords: head and neck squamous cell carcinoma, immune checkpoint inhibitors, anti-PD-1/PD-L1, biomarkers, microbiota
Key Message
Anti-PD-1 agents have become an important therapeutic backbone in R/M HNSCC. Several biomarkers have shown promising results in predicting response to these agents, although none have yet been validated to be uniformly applicable to all HNSCC patients. This comprehensive review provides the latest update of the available biomarker data for immuno-oncology treatment in HNSCC.
Introduction
Immune-checkpoint inhibitors (ICI) targeting cytotoxic T-lymphocyte antigen 4 and programmed cell death protein-1 (PD-1) and its ligands, programmed death ligand-1 (PD-L1)/2, have shown a significant and consistent benefit in survival when compared with standard therapies in prospective randomized clinical trials, leading to their regulatory approval in multiple tumor types [1–5]. In head and neck squamous cell carcinoma (HNSCC), anti-PD-1 antibodies are the first immunotherapeutic agents to demonstrate evidence of response durability and survival benefit in platinum-pretreated recurrent and metastatic (R/M) disease [6–9]. However, despite the encouraging results which led to the approval of nivolumab and accelerated approval of pembrolizumab by the US Food and Drug Administration (FDA) for platinum-refractory R/M HNSCC, the overall response rates (ORRs) of these agents ranged from only ∼13%–18% [9, 10].
Up to 60% of patients across different tumor types, including HNSCC, display primary resistance to anti-PD-1/PD-L1 agents [11]. Several mechanisms have been suggested such as poor tumor immunogenicity, limited intratumoral immune cell infiltration, coexpression of multiple inhibitory receptors, and induction of immunosuppressive pathways within the tumor microenvironment (TME) [12–14]. To overcome this resistance, many ongoing clinical trials are evaluating combination strategies with other immunotherapies, targeted agents, chemotherapy and radiotherapy, not only in R/M HNSCC, but also in the locoregionally advanced setting (NCT02952586, NCT03040999) [15]. This is of particular relevance as a proportion of patients with R/M HNSCC might experience rapid progression and decreased survival when treated with single-agent anti-PD-1/PD-L1 [16].
However, the potential immune-related toxicities of ICI and their high cost have urged the search for prospectively validated predictive biomarkers of response including PD-L1 protein expression, intratumoral immune cell infiltration, immune-gene expression profiling, and tumor mutational burden (TMB) [13, 14, 17]. Specifically, in HNSCC, none of them have been validated and ongoing exploration continues [9, 18].
Recently, the immunomodulatory role of the gut microbiota, defined as the collective microorganisms inhabiting the gastrointestinal tract, has raised a special interest, since its composition has proven to influence anti-PD-1 efficacy in preclinical models and has been associated with treatment responsiveness in patients with melanoma and some epithelial-derived tumors [19–22]. Interestingly, many retrospective studies in HNSCC have suggested that the oral microbiota might also be crucial for tumor development and progression, treatment-related toxicity and disease recurrence [23–25].
This review examines the available evidence on emerging immune predictive biomarkers of response to ICI in HNSCC, introducing the microbiota and its potential use as a predictive immune biomarker in this disease (Table 1).
Table 1.
Emerging immune biomarkers of response to anti-PD-1/PD-L1 agents in HNSCC
| Immune biomarkers | Assay | Predictive value in HNSCCa |
Evidence available | |
|---|---|---|---|---|
| HPV– | HPV+ | |||
| PD-L1 expression | PD-L1 staining by immunohistochemistry in tumor cells/immune cells (different cut-offs) | Positiveb | Positiveb | Prospective randomized clinical trials (Table 2). |
| Smoking |
|
|
|
|
| Tumor immune-cell infiltration |
|
|
Retrospective analysis of noncontrolled cohorts [73]. | |
| Circulating immune cells |
|
|
Prospective analysis in a randomized clinical trial[102]. | |
| Tumor mutational burden | Number of somatic coding missense mutations.
|
|
|
|
| T-cell-inflamed phenotype | Immune-related gene expression signatures | Positive | Positive | Retrospective analysis of prospective clinical trial [74, 75, 104]. Retrospective analysis from a noncontrolled cohort [73]. |
| Microbiota | 16S rRNA high throughput sequencing of saliva and stool |
|
Retrospective analysis of prospective randomized clinical trial [133]. | |
Predictive values in HPV– and HPV+ subgroups were defined positive or negative if a statistically significant correlation between response and the immune biomarker was described in the referenced studies; uncertain if no significant correlation was found; no data if no studies had evaluated the role of the biomarker in this setting at the time of this publication.
The positive correlation between PD-L1 expression and treatment response was not consistent across the studies.
Overview of emerging immune biomarkers in HNSCC
Is PD-L1 expression a reliable biomarker of response in HNSCC?
PD-L1+ tumors in general tend to demonstrate improved response rates to anti-PD-1/PD-L1 therapies, in comparison to PD-L1– tumors [26]. This correlation has been consistent with different anti-PD-1/PD-L1 drugs across many tumor types [5, 27, 28]. Most clinical trials evaluating ICI in R/M HNSCC suggested a similar pattern [29–31], and data from phase III randomized trials investigating pembrolizumab in the R/M setting (KEYNOTE-040 and KEYNOTE-048) endorsed this trend by demonstrating significantly increased survival in PD-L1+ patients [8, 32, 33]. However, CHECKMATE-141 failed to show a significant correlation between PD-L1 expression and tumor response or survival when evaluating nivolumab in the platinum-refractory R/M setting [9, 34] (Table 2).
Table 2.
| Agents | Target | Phase/study | N | PD-L1 expression Location | Cut-off | ORR (%) |
OS (HR)a |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | PD-L1+ | PD-L1- | Overall | PD-L1+ | PD-L1– | ||||||
| Nivolumab | PD-1 | III (CHECKMATE-141) | 240 | TCs | >1% | 13.3% | 17% | 11.8% | 0.68 | 0.55 | 0.73 |
| Pembrolizumab | PD-1 |
|
132 | TCs+ICs TCs only |
|
|
|
|
|
|
|
|
|
TCs+ICs(CPS) TCs (TPS) |
|
|
|
|
|
|
|
||
|
|
TCs+ ICs (CPS) |
|
|
|
|
|
|
|
||
| Durvalumab | PD-L1 |
|
|
TCs TCs TCs |
|
|
|
|
|
NA | |
| Atezolizumab | PD-L1 | I (GO27831) | 32 | ICs |
|
22% | 24% | 14% | NA | NA | |
HR for OS resulting from: nivolumab and pembrolizumab versus investigator’s choice of chemotherapy (Docetaxel, Methotrexate and Cetuximab) in the CHECKMATE-141 and KEYNOTE-040 studies, respectively; pembrolizumab monotherapy versus EXTREME regimen in the KEYNOTE-048 study; durvalumab versus tremelimumab plus durvalumab in the CONDOR study.
ORR, overall response rate; OS, overall survival; HR, hazard ratio; TCs, tumor cells; ICs, immune cells; CPS, number of PD-L1-positive cells (tumor cells, lymphocytes, macrophages) divided by total number of tumor cells × 100; TPS, percentage of tumor cells with membranous PD-L1 expression; NA, not applicable; Ø, no data available.
The discordance of the results across studies might be explained by several reasons. One of the most relevant is the lack of uniformity in the assays and the variability in the thresholds used to define PD-L1 positivity, which have led to the launch of harmonization projects on PD-L1 assays by the scientific community and regulatory agencies [28, 35, 36]. This inconsistency is evident in the development of anti-PD-1/PD-L1 agents investigated to date in R/M HNSCC, including pembrolizumab, nivolumab, atezolizumab, durvalumab and avelumab, thus impairing cross-study comparisons and undermining the value of PD-L1 as a biomarker [6, 9, 30–32, 37, 38]. Importantly, PD-L1 expression seems to be regulated by multiple signaling pathways, including MAPK, PI3K and Akt/PKB that are commonly altered in HNSCC [39–41]. As a consequence of these molecular crosstalks, PD-L1 is a dynamic biomarker that is subject to temporal variations and spatial heterogeneity. Its expression may change from the point of initial diagnosis to recurrence or progression, and may differ between primary and coexisting metastatic lesions [42–45]. Published reports on the intratumoral heterogeneity of PD-L1 expression in HNSCC demonstrate conflicting results [46, 47].
In HNSCC, PD-L1 is highly expressed not only by tumor cells, but also by immune cells present in the TME, including regulatory T cells (Tregs), natural killer (NK) cells and antigen presenting cells (APCs) [18, 48–51]. Across various cancer types, it remains unclear whether PD-L1 expression and thresholds should take into consideration all or only selected cell populations. Both pembrolizumab and atezolizumab used combined scores based on the ratio between tumor cells and immune cells expressing PD-L1 to define tumor PD-L1 positivity, and pembrolizumab did show a positive correlation with response and survival in the phase III KEYNOTE-040 study when using the combined positive score (CPS) [52]. Recently, the results from the phase III KEYNOTE-048 study in first line R/M HNSCC revealed that pembrolizumab monotherapy improved OS when compared with the EXTREME regimen in patients whose tumors had PD-L1 expression ≥1% and ≥20% by CPS [hazard ratio (HR) 0.78 (0.64–0.96), P = 0.0086 and HR 0.61 (0.45–0.83), P = 0007, respectively] [33]. However, in KEYNOTE-040, the correlation with clinical outcome was also strongly positive when using PD-L1 expression in tumor cells only (TPS ≥ 50%), congruent with the experience in non-small-cell lung cancer (NSCLC) in KEYNOTE-010 [53, 54]. In contrast, there was no correlation in the nivolumab CHECKMATE-141 study where PD-L1 expression was exclusively determined in tumor cells, although the thresholds used were different (>1%, 5% and 10%) [9]. These divergent results and the limited data available suggest no firm conclusion can be made in this regard, although CPS seems to be more predictive than TPS in HNSCC, and the required cut-off for the latter appears to be higher in the mentioned studies.
Nonetheless, it is noteworthy that, although relevant in a smaller percentage, PD-L1– tumors also benefit from ICI [9]. Therefore, additional factors beyond PD-L1 expression, such as human papillomavirus (HPV) status, tumor immune infiltration or TMB, might also contribute to treatment response.
Are HPV+ tumors more responsive to immunotherapy?
HPV+ oropharyngeal squamous cell carcinoma (OPSCC) is a biologically distinct disease with better prognosis and improved treatment responsiveness when compared with HPV– disease at the same or similar stage [55–57]. Virus-related tumor types are postulated to be more responsive to ICI due to intrinsic characteristics including baseline tumor immunogenicity, increased immune infiltration and increased PD-L1 expression [58, 59]. HPV+ OPSCC have been shown to have a less immunosuppressive TME when compared with HPV– HNSCC, as it harbors greater infiltration by tumor infiltrating lymphocytes (TILs), higher proportion of CD8+ T cells, increased levels of interferon gamma (IFN-γ), decreased CD4+/CD8+ ratio, and lower numbers of Tregs [60–64]. These findings can be explained by a preexisting adaptive host immune response against viral and tumor-specific antigens, which may in turn lead to PD-L1 expression in immune cells. Indeed, a recent retrospective study showed that not only CD8+ TILs (≥30%) but also high PD-L1 expression in immune cells (≥5%) were both favorable prognostic factors in HPV+ disease regardless of stage [65, 66].
Altogether these findings suggest a potentially higher sensitivity of HPV+ disease to immune-checkpoint blockade. This hypothesis was initially supported by the results from the HNSCC cohort of the multibasket phase I KEYNOTE-012 trial in which HPV+ tumors had increased ORR to pembrolizumab compared with those that were HPV– (25%–32% versus 14%) [6, 7]. However, these results were not reproduced in the phase III KEYNOTE-040 trial, and further studies investigating other anti-PD-1/PD-L1 agents have reported mixed results. For instance, increased response rates were observed among HPV+ patients treated with durvalumab while no differences were seen with atezolizumab [30, 31]. In the CHECKMATE-141 study, nivolumab did not yield significant differences in ORR or OS between HPV+ and HPV– patients [HR for OS 0.60 (0.37–0.97) versus 0.59 (0.38–0.92), respectively] [9, 32, 34].
The inconsistencies in the abovementioned trials might be explained by other coexisting factors beyond PD-L1 expression and immune infiltration. Smoking, mutational signatures and TMB are thought to influence response to ICI in HNSCC although their relevance differs between HPV+ and HPV– disease (Table 1).
Smoking seems to contribute to a more immunosuppressive TME and negatively impact on anti-PD-1/PD-L1 efficacy in HNSCC. In CHECKMATE-141 study, the subgroup analysis reported a trend toward decreased survival benefit from nivolumab among smokers when compared with nonsmokers [9]. Similarly, a retrospective analysis of 81 HNSCC patients treated with anti-PD-1/PD-L1 showed that former/current smokers were less responsive to these agents when compared with never smokers. However, this correlation only remained significant among HPV– patients, suggesting the immunosuppressive effects of smoking may not be as significant in HPV+ tumors [67]. In support of this, a genomic analysis of 287 HNSCC tumor samples revealed that smoking history and tumors with high smoking mutational signatures were correlated with decreased immune infiltration and downregulation of immune-signaling pathways in HPV– but not HPV+ tumors [67].
Conversely, the presence of other mutational signatures unrelated to smoking such as APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like) is of particular relevance in HPV+ disease. Reduced exposure to exogenous carcinogens such as tobacco seems to favor the emergence of tumors with APOBEC-driven mutations such as PI3KCA [68, 69]. Moreover, APOBEC activity is known to be crucial for innate and adaptive immune responses, and HPV infection is thought to enhance it in an attempted host immune response against the virus. In a study analyzing over 500 HNSCC tumor samples, APOBEC mutational signatures were associated with upregulation of immune-signaling pathways [69]. APOBEC-driven mutagenesis might alter tumor immunogenicity in HPV+ disease impacting on immune checkpoint efficacy. Parenthetically, the presence of APOBEC signatures has been associated with increased immune infiltration and PD-L1 expression in other tumor types [70–72].
Increased TMB and neoantigen load have been shown to correlate with response to ICI in HPV– HNSCC, whereas most of the studies conducted to date have refuted their predictive value in HPV+ patients [73–75]. TMB is a quantitative measure of the total number of coding mutations in the tumor genome. Theoretically, the higher the number of missense mutations, the higher expression of tumor neoantigens which can elicit the greatest antitumor immune response and increase sensitivity to ICI. A retrospective analysis from KEYNOTE-012 and -055 demonstrated a stronger correlation between response to pembrolizumab and high TMB and neoantigen load in the HPV– subgroup than HPV+ subgroup [75]. As a matter of fact, in virally induced tumors such as HPV+ tumors or Merkel-cell carcinoma, response rates to ICI are higher than expected when adjusted for TMB and compared with other tumors types, suggesting immune responses may also be triggered by virus-specific antigens rather than by tumor-neoantigens alone [39, 76–78]. In support of this, a retrospective study analyzing a cohort of 126 patients with R/M HNSCC treated with anti-PD-1/PDL-1 agents showed that HPV+ patients had, as expected, lower TMB (8.2 versus 4.7 mut/MB, P < 0.01) when compared with HPV– disease, while the number of responses was similar (7 versus 10 responses, P = 0.54) [73]. More importantly, among HPV+ patients, responders had increased CD8+ TILs regardless of TMB.
Overall, with the current available data, it is not possible to determine whether HPV+ OPSCC have higher (or lower) sensitivity to ICI when compared with HPV– disease. HPV positivity alone does not seem to be a reliable biomarker of response to ICI and needs to be interpreted along with other companion clinical and molecular biomarkers.
Is there a role for tumor immune infiltration and T-cell-inflamed phenotypes?
Tumor immune infiltration implies initial recognition by the immune system and might indicate an antitumor immune response [79]. Multiple immune cells coexist within the TME, including TILs (CD8+ T cells and Tregs), NK cells, macrophages, APC and myeloid-derived suppressor cells. The composition of these immune cells within TME, recently defined as immune contexture, has prognostic implications but can also be predictive of response to therapies [17, 61, 80]. For instance, CD8+ T-cell infiltration at baseline has been correlated with increased response to anti-PD-1/PD-L1 agents in melanoma [81, 82].
HNSCC tumors are highly immune-infiltrated but overall characterized by an immunosuppressive TME [48, 83]. Many retrospective studies have attempted to assess the prognostic and predictive value of tumor immune cell infiltration (supplementary Table S1, available at Annals of Oncology online) [18, 62, 63, 84–89]. Despite the heterogeneity of these studies, increased infiltration by CD8+ T cells is the only immune cell type in HNSCC consistently proven to be correlated with increased survival regardless of tumor location, stage and treatment [61, 65]. A retrospective evaluation of 126 patients diagnosed with R/M HNSCC treated with anti-PD-1/PD-L1 agents showed that increased tumoral infiltration by CD8+ T cells and an increased ratio CD8+ T cells/Tregs were positively correlated with treatment response, indicating their potential role as predictive biomarkers [73].
In addition, the relative proportion of the various immune cell subsets and their location within the TME may be of relevance in predicting response to ICI. The immunoscore (IS) is a tool quantifying the density of CD8+ T cells within the tumor center versus the invasive margin. Increased number of CD8+ T cells in the tumor center (high IS) is thought to indicate an effective antitumor immune response and has been proven to be an independent prognostic biomarker in early stage colorectal cancer, melanoma and NSCLC [80, 90–92]. In HNSCC, a high IS is associated with lower levels of Tregs, increased PD-L1 and MHC type I expressions in tumor cells [62, 93], suggesting its potential to identify a subset of tumors with increased sensitivity to anti-PD-1/PD-L1 therapy. However, the predictive role of IS in HNSCC has not been explored yet.
The coexpression of other inhibitory immune-checkpoint molecules such as TIM-3 (T-cell immunoglobulin and mucin domain-containing protein 3), lymphocyte-activating gene 3 (LAG-3) and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) has also shown to impair immune T-cell-mediated responses, conferring resistance to anti-PD-1/PD-L1 agents in preclinical models and in patients across different tumor types such as melanoma and NSCLC [15, 94–99]. In HNSCC, a recent study showed intratumoral exhausted PD-1+ CD8+ T cells expressing TIM-3 or LAG-3 were higher among nonresponders to anti-PD-1 therapy [73]. In this regard, the predictive value of response to ICIs offered by immunophenotyping of circulating T-cell subsets versus TILs has demonstrated relevance in melanoma and NSCLC but it is still unknown in HNSCC [100, 101]. In a substudy of CHECKMATE-141 evaluating treatment with nivolumab beyond progression, responders had significantly lower levels of circulating PD-1+ CD8+ T cells at baseline and lower levels of PD-1+ Tregs at day 43, indicating circulating exhausted T cells could be a negative predictive biomarker to anti-PD-1/PD-L1 agents [102]. Although the available data are still limited and should be interpreted with caution, determining the coexpression of inhibitory checkpoint molecules in intratumoral and/or circulating T-cell subsets could be predictive of resistance to anti-PD-1/PD-L1 agents and potentially indicate the need for ICI combinations in selected cases of HNSCC.
Gene-expression profiling (GEP) signatures that identify tumors with a T-cell-inflamed phenotype have shown promising results in predicting response to anti-PD-1/PD-L1 agents [103, 104]. A 18-gene T-cell-inflamed signature including genes that reflect an ongoing adaptive Th1 and cytotoxic CD8+ T-cell response (including IFN-γ signaling, cytolytic activity, antigen presentation and T cell trafficking) has been tested in two HNSCC cohorts from prospective clinical trials (KEYNOTE-012 and KEYNOTE-055) treated with single-agent pembrolizumab showing a positive correlation with response and survival, regardless of HPV status [74, 75]. This signature has been recently validated in additional tumor cohorts from KEYNOTE-012 and -028 studies, including melanoma and HNSCC. The study confirmed its predictive value as a biomarker of response to pembrolizumab and also revealed a positive correlation with PD-L1 expression by CPS [105].
Despite the prognostic implications and early data suggesting a correlation between TILs and response to anti-PD-1/PD-L1 therapy, prospective validation is needed. Moreover, identifying a T-cell-inflamed phenotype and determining coexisting immune cells and coexpression of other inhibitory immune checkpoint molecules beyond PD-1/PD-L1 within the TME could be instrumental to differentiate tumors that will likely be responsive to anti-PD-1/PD-L1 antibodies as single agents from those that may benefit from combined ICI for efficacy.
Tumor mutational burden and HNSCC mutational landscape
TMB has been recently evaluated as a potential biomarker of response to immune checkpoint blockade in prospective clinical trials and across many tumor types [77, 106–109]. An initial retrospective analysis of 27 tumor types and subtypes among patients who received PD-1/PD-L1 inhibitors demonstrated a significant correlation between TMB and response rate to these agents [77]. In this study, TMB was reported as a median number of coding somatic mutations per megabase (N mut/MB). Melanoma and squamous cell carcinoma of the skin (15–50 mut/MB) followed by tobacco-related cancers including NSCLC, urothelial cell carcinoma and HNSCC (5–10 mut/MB) comprised malignancies with the highest TMB [77]. Retrospective subset analyses of clinical trials evaluating pembrolizumab, atezolizumab and nivolumab in metastatic melanoma, NSCLC, urothelial carcinoma and HNSCC have demonstrated not only increased ORR but also improved survival in patients with high TMB [75, 106–108, 110]. These results were consistent across the studies, tumor type and anti-PD-1/PD-L1 agents. However, the cut-off and measure used to define a high TMB differed between studies, thus precluding direct comparisons. These results were further supported by a retrospective analysis of 126 HNSCC patients treated with anti-PD-1/PD-L1 agents. TMB was found to be significantly higher among responders (21.3 versus 8.2 mut/MB, P < 0.01) and was correlated with increased median OS (20 months if TMB > 10 mut/MB versus 6 months if TMB < 5 mut/MB, P = 0.01) in HPV– disease [73]. A combined biomarker analysis of multiple studies evaluating the correlation between TMB, T-cell-inflamed GEP, PD-L1 expression by CPS and response to pembrolizumab in HNSCC showed no significant correlation between TMB and inflammatory biomarkers (i.e. GEP or PD-L1). While this analysis did not stratify by HPV status, it suggests TMB and inflammatory biomarkers have distinct and independent predictive values, and may be used orthogonally to identify responders to pembrolizumab [105].
In addition to TMB, the specific tumor mutational landscape might be of biological relevance. Tumors characterized by mutations affecting DNA damage response, such as those with microsatellite instability high (MSI-H) or mismatch repair deficiency (dMMR), have the highest mutational load [77, 111]. These tumors have been shown to be particularly sensitive to ICI in prospective clinical trials, leading to the FDA approval of pembrolizumab for patients with dMMR or MSI-H tumors, regardless of histology [112, 113]. The estimated incidence of MSI-H tumors among HNSCC has been reported to be about 8% [114]. However, a recent study identified a subgroup of HNSCC responders to anti-PD-1/PD-L1 whose tumors were enriched with somatic mutations derived from frameshift events in tumor suppression genes such as NOTCH and SMARCA4 [73]. These cases are similar to what has been described in tumors with dMMR, with baseline increased mutational burden and greater sensitivity to ICI. The authors suggested this finding might represent a novel mutational signature in HNSCC with potential predictive value, although further validation is warranted.
HNSCC genomic classification described by the TGCA might be considered as well [39]. Four subtypes have been defined on the basis of gene expression: atypical, mesenchymal, basal and classical. The mesenchymal subtype, e.g. characterized by alterations in genes related to innate immunity, downregulation of MHC type I expression and deficient antigen-presentation machinery, would unlikely respond to anti-PD-1/PD-L1 agents.
Overall, while the predictive role of the specific molecular subtypes is yet to be explored, TMB has shown promising results and might become a useful predictive biomarker of immune-checkpoint blockade efficacy in HNSCC. However, similar to what occurred with the PD-L1 assay, the lack of uniformity in the methods used to determine the mutational burden (e.g. measured in the tumor or in the blood) and the variability of the thresholds used across studies are hampering the interpretation and extrapolation of the results obtained. Thus, standardization should be pursued when designing biomarker-validating studies using TMB. Moreover, TMB has not shown to correlate with PD-L1 expression or GEP signatures [73, 75, 105], again indicating the interactions between the tumor, TME and the immune system are complex and dynamic.
Introducing the microbiota as a potential immune biomarker for HNSCC
The microbiota in head and neck cancer
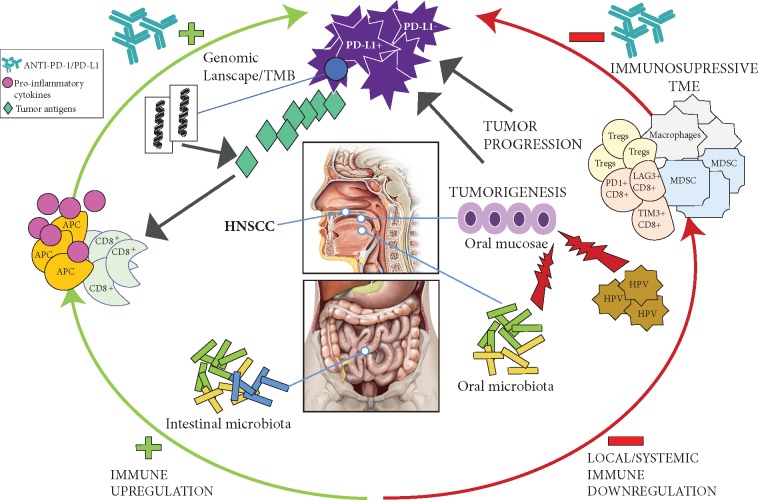
The composition of the microbiota present in the oro-gastrointestinal tract has been associated with immune dysregulation and initiation and progression of many cancers [23, 115–118]. The precise mechanisms of these associations are not known, but compositional and functional changes in the microbiota can induce or exacerbate chronic inflammation, resulting in cell damage and alteration of local and systemic immune homeostasis, which may affect local and distant carcinogenesis, ultimately dampening or enhancing antitumor immune responses [116, 119]. HNSCC arise from an epithelium and mucosae located in the oral cavity and the pharynx; both sites are constantly exposed to environmental factors that can alter the oral microbiota [120, 121]. Retrospective cohort studies have shown different microbiota composition in the saliva of HNSCC patients compared with healthy controls, while the presence of specific bacteria has been associated with reduced risk of developing HNSCC [23, 122–124]. Moreover, differentially enriched microbiota found in HPV+ and HPV– OPSCC and oral cavity SCC indicates the existence of specific microbiota according to tumor location and HPV status [24]. Nonetheless, some authors have underlined the challenge of distinguishing whether the changes observed in the oral microbiota from HNSCC patients are influenced by the TME and/or by local and systemic cancer therapies, since most of the studies to date have retrospectively evaluated small, heterogeneous and noncontrolled cohorts of patients comprising different tumor sites, variable disease stages, and treatment with multiple modalities [23]. In this regard, a study analyzing the oral microbiota present in the saliva of HNSCC patients before and after treatment [including surgery, chemoradiotherapy (CRT) and ICI] showed an association between specific oral bacteria composition (Fusobacterium and Lactobacillus), down-regulation of immune-signaling pathways and upregulation of oncogenic Wnt/Beta-catenin pathways [125]. Altogether these findings suggest that the oral microbiota might represent a promising prognostic and predictive biomarker in this disease (Figure 1).
Figure 1.
Interactions between the oral and intestinal microbiome, immune responses and the HNSCC TME. The composition of the oral microbiota alters the oral mucosae contributing to tumor development and progression in the context of other coexisting factors such as HPV infection. Intestinal and oral microbial composition and diversity regulate systemic and local immune responses modulating the TME along with other immune biomarkers such as TMB or immune checkpoint protein expression, ultimately dampening or enhancing antitumor immune responses.
Exploiting the microbiota as a biomarker of response to immunotherapy
Accumulating evidence has implicated that intestinal microbiota can modulate host anticancer immune responses and alter the efficacy of anticancer therapies, including immunotherapy [19, 126–131]. Two preclinical studies using mouse models of melanoma and lung cancer revealed a correlation between the presence of specific commensal intestinal bacteria (Bifidobacterium) and response to ICI [20, 132]. This was further supported by two recent publications evaluating the gut microbiome in patients with melanoma and epithelial-derived tumors, showing improved anti-PD-1/PD-L1 efficacy among patients harboring specific intestinal bacteria (the species of Akkermansia muciniphila and members of the Ruminococcaceae family) and higher microbial diversity [21, 22]. Remarkably, these microbiota were also correlated with enhanced local and systemic immune response, reduction in tumor growth and restoration of response to anti-PD-1/PD-L1 therapy in germ-free mice transplanted with fecal microbiota from responding patients. These latter findings indicate the potential modulation of the microbiota as a viable therapeutic target to increase response to ICI.
Whether the microbiota has a role in predicting response to immunotherapy in HNSCC is yet to be determined. Only one substudy from CHECKMATE-141 explored the role of the oral microbiota measured in the saliva as a predictive biomarker in patients with R/M HNSCC treated with nivolumab, showing no significant correlation with treatment efficacy or survival [9, 133]. However, the study had several limitations, including the lack of uniformity in sample collection, the small number of responses for correlation and importantly, the omission of intestinal microbiota. The predictive role of the oral microbiota was also investigated in melanoma patients treated with anti-PD-1/PD-L1 therapy, again reporting no association with treatment outcome, in contrast to the positive correlation observed with the intestinal microbiota composition [22]. Differential bacterial composition between these anatomical sites suggests oral and intestinal microbiota likely represent distinct entities with specific disease associations.
Considering the immunomodulatory effects of the intestinal microbiota and the growing evidence of the oral microbiota impacting HNSCC tumorigenesis and progression, the study of their role as a predictive biomarker of response to ICI in this disease is warranted. Hence, our group is currently conducting a research study at the Princess Margaret Cancer Centre to prospectively evaluate the oral and intestinal microbiota in a homogeneous cohort of patients diagnosed with locoregionally advanced OPSCC treated with definitive chemoradiotherapy. The overarching goal of this project is to characterize and explore the correlation with both oral and intestinal microbiota measured in the saliva and stool, respectively, by using 16S rRNA sequencing, in order to obtain a deeper understanding of their relationship with treatment response. The results of this ongoing study will serve as a fundamental basis to evaluate oral and intestinal microbiota signatures and their role as predictors of response to ICI in patients treated within the CCTG HN.9 clinical trial, a multicenter phase II noncomparative randomized study evaluating ICI plus RT followed by maintenance ICI versus standard chemoradiotherapy in intermediate-risk, HPV+ locoregionally advanced OPSCC (NCT034106615).
Discussion
Conclusion
Anti-PD-1 agents have become the standard of care for the platinum-refractory R/M HNSCC. Results from clinical trials evaluating their role in additional disease settings are pending, but clearly such compounds are already an important therapeutic backbone in this malignancy. As such, appropriate selection of patients who will benefit from these therapies is crucial. To date, there are no validated predictive biomarkers of response that are applicable uniformly to all HNSCC patients, although many candidate biomarkers with promising results are undergoing investigations. A systematic computational analysis of all clinically annotated biomarker data would be invaluable to further the knowledge in this field.
Most of the biomarkers in HNSCC have been explored retrospectively, often using baseline archival tumor samples at a single time point which may not reflect the impact of spatial and temporal intratumoral heterogeneity. Also, standalone evaluation of potential biomarkers without considering interactions with other factors is likely oversimplifying the complexity of immune response. The microbiota is a dynamic and complex ecosystem that interrelates the immune system and the TME, thus, potentially representing an ideal biomarker that reflects the interactions between these biological entities in totality. Both oral and intestinal microbiota may be important regulators of local and systemic immune responses induced by environmental factors, shaping the TME and ultimately modulating the efficacy of cancer therapies. Considering the emerging immunomodulatory effects of the microbiota, the study of its role as a predictive immune biomarker in HNSCC is of special interest and should be integrated into prospective clinical trials.
Supplementary Material
Acknowledgements
MO gratefully acknowledges the SEOM Foundation (Spanish Society of Medical Oncology) and Cris Cancer Foundation for their support of his fellowship program at Princess Margaret Cancer Center.
Funding
None declared.
Disclosure
MO: conference and personal fees from Merck. AS: consultant for: Merck (compensated), Bristol-Myers Squibb (compensated), Novartis (compensated), Oncorus (compensated). Grant/research support from (Clinical Trials): Novartis, Bristol-Myers Squibb, Symphogen AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson. MT: nonfinancial support from Merck and Astra Zeneca. Personal fees from Merck, Bristol-Myers, Astra Zeneca and Nanobiotics. LA: Grant/Research support from: Merck. BC: None. RM: consultant for: Merck, MSD, BMS, Roche. Conferences with fee: Merck, BMS, AZ, Roche. LLS: consultant for: Merck (compensated), Pfizer (compensated), Celgene (compensated), AstraZeneca/Medimmune (compensated), Morphosys (compensated), Roche (compensated), GeneSeeq (compensated), Loxo (compensated), Oncorus (compensated), Symphogen (compensated). Grant/research support from (Clinical Trials): Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, Regeneron, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca/Medimmune, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics. Stockholder in: Agios (spouse).
References
- 1. Hodi FS, O'Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8): 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellmunt J, de Wit R, Vaughn DJ. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376(11): 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373(2): 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seiwert TY, Burtness B, Mehra R. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17(7): 956–965. [DOI] [PubMed] [Google Scholar]
- 7. Chow LQM, Haddad R, Gupta S. et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016; 34(32): 3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soulieres D, Cohen E, Le Tourneau C. et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma. Abstract. Cancer Res 2018; CT115. [Google Scholar]
- 9. Ferris RL, Blumenschein G, Fayette J. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375(19): 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larkins E, Blumenthal GM, Yuan W. et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist 2017; 22(7): 873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topalian SL, Hodi FS, Brahmer JR. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366(26): 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Donnell JS, Long GV, Scolyer RA. et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017; 52: 71–81. [DOI] [PubMed] [Google Scholar]
- 13. Zou W, Wolchok JD, Chen L.. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8(328): 328rv4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schalper KA, Kaftan E, Herbst RS.. Predictive biomarkers for PD-1 axis therapies: the hidden treasure or a call for research. Clin Cancer Res 2016; 22(9): 2102–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melero I, Berman DM, Aznar MA. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015; 15(8): 457–472. [DOI] [PubMed] [Google Scholar]
- 16. Saâda-Bouzid E, Defaucheux C, Karabajakian A. et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017; 28(7): 1605–1611. [DOI] [PubMed] [Google Scholar]
- 17. Fridman WH, Zitvogel L, Sautès-Fridman C. et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 2017; 14(12): 717–734. [DOI] [PubMed] [Google Scholar]
- 18. Mandal R. et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016; 1(17): e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson MH, Diven MA, Huff LW. et al. Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res 2015; 2015: 368736.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sivan A, Corrales L, Hubert N. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350(6264): 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359(6371): 91–97. [DOI] [PubMed] [Google Scholar]
- 22. Gopalakrishnan V, Spencer CN, Nezi L. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359(6371): 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayes RB, Ahn J, Fan X. et al. Association of oral microbiome with risk for incident head and neck squamous cell cancer. JAMA Oncol 2018; 4(3): 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerrero-Preston R, Godoy-Vitorino R, Jedlicka A. et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016; 7(32): 51320–51334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guerrero-Preston R, White JR, Godoy-Vitorino F. et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget 2017; 8(67): 110931–110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen AR, Siu LL.. PD-L1 testing in cancer: challenges in companion diagnostic development. JAMA Oncol 2016; 2(1): 15–16. [DOI] [PubMed] [Google Scholar]
- 27. Taube JM, Klein A, Brahmer JR. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20(19): 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372(21): 2018–2028. [DOI] [PubMed] [Google Scholar]
- 29. Segal NH, Logan TF, Hodi FS. et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 2015; 33(Suppl 15): 3011–3011. [Google Scholar]
- 30. Zandberg D, Algazi A, Jimeno A. et al. Durvalumab of recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study. Ann Oncol 2017; 28(Suppl 5): v372–v394. [Google Scholar]
- 31. Bahleda R, Braither FS, Balmanoukian AS. et al. Long-term safety and clinical outcomes of atezolizumab in head and neck cancer: phase IA trial results. Ann Oncol 2017; 28(Suppl 5): v372–v394. [Google Scholar]
- 32. Cohen EE, Machiels JP, Harrington KJ. et al. KEYNOTE-040: a phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 2015; 33(Suppl 15): TPS6084–TPS6084. [Google Scholar]
- 33. Burtness B, Harrington KJ, Greil R. et al. KEYNOTE-048: phase 3 study of first-line pembrolizumab for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). ESMO Meeting 2018.
- 34. Ferris RL, Blumenschein G, Fayette J. et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018; 81: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Philip R, Carrington L, Chan M.. US FDA perspective on challenges in co-developing in vitro companion diagnostics and targeted cancer therapeutics. Bioanalysis 2011; 3(4): 383–389. [DOI] [PubMed] [Google Scholar]
- 36. Hirsch FR, McElhinny A, Stanforth D. et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017; 12(2): 208–222. [DOI] [PubMed] [Google Scholar]
- 37. Kotsakis A, Georgoulias V.. Avelumab, an anti-PD-L1 monoclonal antibody, shows activity in various tumour types. Lancet Oncol 2017; 18(5): 556–557. [DOI] [PubMed] [Google Scholar]
- 38. Heery CR, O'Sullivan-Coyne G, Madan RA. et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017; 18(5): 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517(7536): 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lui VWY, Hedberg ML, Li H. et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 2013; 3(7): 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skinner HD, Giri U, Yang LP. et al. Integrative analysis identifies a novel AXL-PI3 Kinase-PD-L1 signaling axis associated with radiation resistance in head and neck cancer. Clin Cancer Res 2017; 23(11): 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Darb-Esfahani S. et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016; 7(2): 1486–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cimino-Mathews A, Thompson E, Taube JM. et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016; 47(1): 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gadiot J, Hooijkaas AI, Kaiser ADM. et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011; 117(10): 2192–2201. [DOI] [PubMed] [Google Scholar]
- 45. Zhou J, Gong Z, Jia Q. et al. Programmed death ligand 1 expression and CD8. Biochem Biophys Res Commun 2018; 498(4): 751–757. [DOI] [PubMed] [Google Scholar]
- 46. Wildsmith S, Marietta S, Midhta A. et al. PD-L1 expression in patients screened for phase 2 head and neck squamous cell carcinoma clinical studies (HAWK and CONDOR). Cancer Res 2018; 78(Suppl 13): 5530–5530. [Google Scholar]
- 47. Okada S, Itoh K, Ishihara S. et al. Significance of PD-L1 expression in pulmonary metastases from head and neck squamous cell carcinoma. Surg Oncol 2018; 27(2): 259–265. [DOI] [PubMed] [Google Scholar]
- 48. Zandberg DP, Strome SE.. The role of the PD-L1: PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2014; 50(7): 627–632. [DOI] [PubMed] [Google Scholar]
- 49. Kansy BA, Concha-Benavente F, Srivastava RM. et al. PD-1 Status in CD8. Cancer Res 2017; 77(22): 6353–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jie H-B, Gildener-Leapman N, Li J. et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013; 109(10): 2629–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattox AK, Lee J, Westra WH. et al. PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4. Cancer Res 2017; 77(22): 6365–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen EEW. et al. KEYNOTE-040: a phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 2015; 33(Suppl 15): TPS6084–TPS6084. [Google Scholar]
- 53. Reck M, Rodríguez-Abreu D, Robinson AG. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375(19): 1823–1833. [DOI] [PubMed] [Google Scholar]
- 54. Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540–1550. [DOI] [PubMed] [Google Scholar]
- 55. Ang KK, Harris J, Wheeler R. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363(1): 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taberna M, Mena M, Pavón MA. et al. Human papillomavirus-related oropharyngeal cancer. Ann Oncol 2017; 28(10): 2386–2398. [DOI] [PubMed] [Google Scholar]
- 57. Huang SH, O'Sullivan B, Waldron J.. The current state of biological and clinical implications of human papillomavirus-related oropharyngeal cancer. Semin Radiat Oncol 2018; 28(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 58. Kanaan H, Kourie HR, Awada AH.. Are virus-induced cancers more sensitive to checkpoint inhibitors? Future Oncol 2016; 12(23): 2665–2668. [DOI] [PubMed] [Google Scholar]
- 59. Andersen AS, Koldjaer Sølling AS, Ovesen T. et al. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int J Cancer 2014; 134(12): 2755–2763. [DOI] [PubMed] [Google Scholar]
- 60. Matlung SE, Wilhelmina van Kempen PM, Bovenschen N. et al. Differences in T-cell infiltrates and survival between HPV+ and HPV– oropharyngeal squamous cell carcinoma. Future Sci OA 2016; 2(1): FSO88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Ruiter EJ, Ooft ML, Devriese LA. et al. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology 2017; 6(11): e1356148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lechner A, Schlößer H, Rothschild SI. et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017; 8(27): 44418–44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Partlová S, Bouček J, Kloudová K. et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015; 4(1): e965570.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lyford-Pike S, Peng S, Young GD. et al. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73(6): 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Solomon B, Young R, Bressel M. et al. Prognostic significance of PD-L1. Cancer Immunol Res 2018; 6(3): 295–304. [DOI] [PubMed] [Google Scholar]
- 66. Kim HR, Ha SJ, Hong MH. et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep 2016; 6: 36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Desrichard A, Kuo F, Chowell D. et al. Tobacco smoking-associated alterations in the immune microenvironment of squamous cell carcinomas. J Natl Cancer Inst 2018; doi: 10.1093/jnci/djy60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Henderson S, Chakravarthy A, Su X. et al. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep 2014; 7(6): 1833–1841. [DOI] [PubMed] [Google Scholar]
- 69. Faden DL, Thomas S, Cantalupo PG. et al. Multi-modality analysis supports APOBEC as a major source of mutations in head and neck squamous cell carcinoma. Oral Oncol 2017; 74: 8–14. [DOI] [PubMed] [Google Scholar]
- 70. Swanton C, McGranahan N, Starrett GJ. et al. APOBEC enzymes: mutagenic fuel for cancer evolution and heterogeneity. Cancer Discov 2015; 5(7): 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Glaser AP, Fantini D, Wang Y. et al. APOBEC-mediated mutagenesis in urothelial carcinoma is associated with improved survival, mutations in DNA damage response genes, and immune response. Oncotarget 2018; 9(4): 4537–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang S, Jia M, He Z. et al. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene 2018; 37(29): 3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hanna GJ, Lizotte P, Cavanaugh M. et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018; 3(4): e98811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haddad RI, Seiwert TY, Chow LQM. et al. Genomic determinants of response to pembrolizumab in head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2017; 35(Suppl 15): 6009. [Google Scholar]
- 75. Seiwert TY. et al. Biomarkers predictive of response to pembrolizumab in head and neck cancer (HNSCC). AACR meeting 2018. Abstract 339.
- 76. Leemans CR, Snijders PJF, Brakenhoff RH.. The molecular landscape of head and neck cancer. Nat Rev Cancer 2018; 18(5): 269–282. [DOI] [PubMed] [Google Scholar]
- 77. Yarchoan M, Hopkins A, Jaffee EM.. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377(25): 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nghiem PT, Bhatia S, Lipson EJ. et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 2016; 374(26): 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Palucka AK, Coussens LM.. The basis of oncoimmunology. Cell 2016; 164(6): 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donnem T, Hald SM, Paulsen E-E. et al. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res 2015; 21(11): 2635–2643. [DOI] [PubMed] [Google Scholar]
- 81. Daud AI, Loo K, Pauli ML. et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 2016; 126(9): 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hamid O, Schmidt H, Nissan A. et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011; 9(1): 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gildener-Leapman N, Ferris RL, Bauman JE.. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol 2013; 49(12): 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Russell S, Angell T, Lechner M. et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol 2013; 5(3): 24.. [PMC free article] [PubMed] [Google Scholar]
- 85. Oguejiofor K, Galletta-Williams H, Dovedi SJ. et al. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV– oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017; 8(9): 14416–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karpathiou G, Casteillo F, Giroult JB. et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget 2017; 8(12): 19310–19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Molling JW, Langius JAE, Langendijk JA. et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol 2007; 25(7): 862–868. [DOI] [PubMed] [Google Scholar]
- 88. Wagner S, Wittekindt C, Reuschenbach M. et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer 2016; 138(9): 2263–2273. [DOI] [PubMed] [Google Scholar]
- 89. Davis RJ, Van Waes C, Allen CT.. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol 2016; 58: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mlecnik B, Bindea G, Angell HK. et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016; 44(3): 698–711. [DOI] [PubMed] [Google Scholar]
- 91. Galon J, Costes A, Sanchez-Cabo F. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313(5795): 1960–1964. [DOI] [PubMed] [Google Scholar]
- 92. Galon J, Fox BA, Bifulco CB. et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med 2016; 14: 273.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ishii H, Azuma K, Kawahara A. et al. Programmed cell death-ligand 1 expression and immunoscore in stage II and III non-small cell lung cancer patients receiving adjuvant chemotherapy. Oncotarget 2017; 8(37): 61618–61625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chauvin J-M, Pagliano O, Fourcade J. et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest 2015; 125(5): 2046–2058., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fourcade J, Sun Z, Benallaoua M. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207(10): 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kurtulus S, Sakuishi K, Ngiow S-F. et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest 2015; 125(11): 4053–4062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97. Jenkins RW, Barbie DA, Flaherty KT.. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118(1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Koyama S, Akbay EA, Li YY. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7: 10501.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thommen DS, Schreiner J, Müller P. et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3(12): 1344–1355., [DOI] [PubMed] [Google Scholar]
- 100. Zhuo M, Chen H, Zhang T. et al. The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer Biomark 2018; 22(3): 467–476. [DOI] [PubMed] [Google Scholar]
- 101. Martens A, Wistuba-Hamprecht K, Geukes Foppen M. et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016; 22(12): 2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Haddad RE, Blumenschein G, Fayette J Jr. et al. Treatment beyond progression with nivolumab in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the phase 3 checkmate 141 study: a biomarker analysis and updated clinical outcomes. Ann Oncol 2017; 28(Suppl 5):mdx374.001. [Google Scholar]
- 103. Jamieson NB, Maker AV.. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther 2017; 24(3): 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ayers M, Lunceford J, Nebozhyn M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017; 127(8): 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cristescu R, Mogg R, Ayers M. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018; 362(6411): eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rizvi NA, Hellmann MD, Snyder A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230): 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gandara D, Kowanetz M, Mok TSK. et al. Blood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann Oncol 2017; 28(Suppl 5): mdx380. [Google Scholar]
- 108. Galsky AS, Saci A, Szabo PM. et al. Impact of tumor mutation burden on nivolumab efficacy in second-line urothelial carcinoma patients: exploratory analysis of the phase II CheckMate 275 study. Ann Oncol 2017; 28(Suppl 5): v295–v329. [Google Scholar]
- 109. Snyder A, Makarov V, Merghoub T. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371(23): 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Goodman AM, Kato S, Bazhenova L. et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16(11): 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chalmers ZR, Connelly CF, Fabrizio D. et al. Analysis of 100, 000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9(1): 34., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372(26): 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Le DT, Durham JN, Smith KN. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357(6349): 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Malone E, Siu LL.. Precision medicine in head and neck cancer: myth or reality? Clin Med Insights Oncol 2018; 12: 1179554918779581.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Maynard CL, Elson CO, Hatton RD. et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012; 489(7415): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Garrett WS. Cancer and the microbiota. Science 2015; 348(6230): 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zitvogel L, Galluzzi L, Viaud S. et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med 2015; 7(271): 271ps1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Routy B, Gopalakrishnan V, Daillère R. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol 2018; 15(6): 382–396. [DOI] [PubMed] [Google Scholar]
- 119. Dzutsev A, Goldszmid RS, Viaud S. et al. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol 2015; 45(1): 17–31. [DOI] [PubMed] [Google Scholar]
- 120. Meurman JH. Oral microbiota and cancer. J Oral Microbiol 2010; 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Le Bars P, Matamoros S, Montassier E. et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can J Microbiol 2017; 63(6): 475–492. [DOI] [PubMed] [Google Scholar]
- 122. Pushalkar S, Ji X, Li Y. et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol 2012; 12: 144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hooper SJ, Wilson MJ, Crean SJ.. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck 2009; 31(9): 1228–1239. [DOI] [PubMed] [Google Scholar]
- 124. Wu J-Y, Yi C, Chung H-R. et al. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol 2010; 46(4): 226–231. [DOI] [PubMed] [Google Scholar]
- 125. Guerrero-Preston R, White JR, Godoy-Vitorino F. et al. High-resolution microbiome profiling and genome wide arrays uncover bacteria driven alterations of oncogenic and immune pathways in head and neck cancer patients treated with surgery, chemo-radiation and PD-1 checkpoint blockade therapy. Cancer Resh 2017; 77(Suppl 13): 1018–1018. [Google Scholar]
- 126. Roy S, Trinchieri G.. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 2017; 17(5): 271–285. [DOI] [PubMed] [Google Scholar]
- 127. Greenhill C. Anti-cancer therapies affected by gut microbiota. Nat Rev Gastroenterol Hepatol 2013; 11(1): 1. [DOI] [PubMed] [Google Scholar]
- 128. Garcia-Gonzalez AP. et al. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell 2017; 169(3): 431–441 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Montassier E, Gastinne T, Vangay P. et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther 2015; 42(5): 515–528. [DOI] [PubMed] [Google Scholar]
- 130. Kroemer G, Zitvogel L.. Cancer immunotherapy in 2017: the breakthrough of the microbiota. Nat Rev Immunol 2018; 18(2): 87–88. [DOI] [PubMed] [Google Scholar]
- 131. Yu TChung, Guo F, Yu Y. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017; 170(3): 548–563 e16., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vétizou M, Pitt JM, Daillère R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350(6264): 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ferris RL, Blumenschein G, Harrington K. et al. Evaluation of oral microbiome profiling as a response biomarker in squamous cell carcinoma of the head and neck: analyses from CheckMate 141. Cancer Res 2017; 77(Suppl 13): CT022–CT022. [Google Scholar]
- 134. Siu L, Even C, Mesia R. et al. A randomized, open-label, multicenter, global phase 2 study of durvalumab, tremelimumab, or durvalumab plus tremelimumab in patients with PD-L1 low/negative recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CONDOR. JAMA Oncol 2018. Nov 1 [Epub ahead of print], doi:10.1001/jamaoncol.2018.4628. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.