Abstract
Objective
We aimed to evaluate the effect of different timing of initiation of low-molecular-weight heparin (LMWH) administration on the pregnancy outcomes in women with antiphospholipid syndrome (APS).
Materials and methods
A randomized controlled study was conducted on women with obstetrical APS. All participants were randomly divided at documentation of positive pregnancy test into two groups; early initiation group in which LMWH therapy was started once positive pregnancy test was established (in the fifth week of gestation), and later initiation group in which LMWH therapy was started after sonographic confirmation of fetal cardiac pulsation (in the seventh week of gestation). In both groups, LMWH (enoxaparin) was given at a dose of 40 mg/day subcutaneously and the therapy continued until end of pregnancy. The primary outcome measure was ongoing pregnancy rate and the secondary outcome measures were fetal loss, live birth rate, preterm labor before 34 weeks of gestation, intrauterine growth restriction (IUGR), and congenital fetal malformations.
Results
Ninety-four women (48 in the early initiation group and 46 in the later initiation group) were subjected to final analysis. The ongoing pregnancy rate was significantly higher in the early initiation group than in the later initiation group (81.2% vs 60.9%; P=0.040). However, both groups were similar in the incidences of fetal loss, preterm labor before 34 weeks of gestation, and IUGR, and live birth rate. No recorded congenital fetal malformations in both groups.
Conclusion
Early administration of LMWH for pregnant women with obstetrical APS reduces early pregnancy loss, but does not affect the incidence of late obstetrical complications.
Keywords: APS, LMWH, fetal loss
Introduction
Obstetrical antiphospholipid syndrome (APS) is an autoimmune disease characterized by presence of characteristic auto-antibodies that jeopardize pregnancy with recurrent miscarriages, intrauterine growth restriction (IUGR), still birth, or severe preeclampsia with or without arterial and/or venous thrombosis. Antiphospholipid antibodies could be detected as normal variants in 1%–5% of population, this incidence is increased dramatically tô15% in women with recurrent miscarriages, and 40% in women with systemic lupus erythematosus (SLE).1
As an old concept, the pathogenesis of APS was attributed to thrombosis in vascular beds either arterial, venous, or in placental circulation, which results in placental insufficiency,2 but this hypothesis was questioned in the obstetrical APS due to absence of pathological evidence of vascular thrombosis or infarction in the placental components of some cases, permitting the refinement of obstetrical APS as an autoimmune inflammatory disease.3 The APS auto-antibodies can bind to negatively charged macromolecules, such as phospholipids in the cell membranes initiating endothelial cell activation,4 and defective placentation or activation of the complement system triggering cascade of reactions, which in role produce pro-inflammatory substances that damage the growing products of conception, either the placenta or the fetus itself.3
Management of obstetrical APS, specially the pure category with no history of thrombosis, is based mainly on a combination between low-dose aspirin and unfractionated heparin (UFH).5 There is no evidence of superiority of low-molecular-weight heparin (LMWH) over UFH if both were combined with low-dose aspirin in treatment of obstetrical APS,6,7 but LMWH may be preferable to UFH to decrease the risk of heparin-induced thrombocytopenia and osteopenia.8,9
Several mechanisms have been proposed for heparin role in treatment of obstetrical APS, including prevention of thrombosis in placental vasculature,10 prevention of deposition of anti-beta-2 glycoprotein I antibodies into the trophoblastic phospholipids,11 attenuation of trophoblastic apoptosis,12 anti-complementary effect,13 and immunomodulatory action.14,15
From all the previous data, treatment of patients with obstetrical APS using heparin therapy is mandatory, but the timing of its initiation is still debatable and is according to the treatment protocol for each institute, some support initiation of heparin therapy as early as positive urine pregnancy test, while others prefer to wait until documentation of the fetal cardiac activity. This work tried to identify the proper timing of initiation of heparin therapy in cases of obstetrical APS.
Materials and methods
This was a prospective, randomized, controlled, parallel-group study conducted during the period from January 2015 through May 2017 in Mansoura University Hospital and private practice settings in Mansoura, Egypt. The study protocol was reviewed and approved by the Mansoura Faculty of Medicine Institutional Research Board (Code No R/15.08.15) and the trial was registered with ClinicalTrials.gov, identifier NCT02326051. All procedures performed in the study were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Women with obstetrical APS, diagnosed according to the revised classification criteria for APS in 2006,16 were selected for participation in our study. All the potential participants were interviewed, informed about the study, and counseled for participation. They then were evaluated regarding the inclusion and exclusion criteria.
The inclusion criteria were as follows: 1) history of ≥3 consecutive early miscarriages (before the tenth week of gestation), and/or ≥1 unexplained death of a morphologically normal fetus at or beyond the tenth week of gestation, and/or ≥1 premature birth of a morphologically normal neonate before 34 weeks of gestation because of severe preeclampsia or eclampsia or placental insufficiency; and 2) anticardiolipin antibodies (immunoglobulin M and/or immunoglobulin G) present in serum or plasma in medium or high titer (>40 mL/L or >99 percentile) on ≥2 occasions at least 12 weeks apart, and/or lupus anticoagulant present in plasma on ≥2 occasions at least 12 weeks apart. Anticardiolipin antibodies titer was measured by a standardized ELISA and the presence of lupus anticoagulant was detected using the dilute Russell viper venom time.
Women with any of the following criteria were excluded from the study: 1) age <20 or >38 years; 2) early pregnancy body weight <50 or >90 kg; 3) SLE; 4) active thromboembolic disorders; 5) history of previous thromboembolic disorders; 6) coexisting hereditary thrombophilia; 7) genetic or hormonal cause for pregnancy loss; 8) uterine anomaly or malformation that could induce defective placentation, early pregnancy loss, or preterm labor, such as uterine septum, bicornuate uterus, or unicornuate uterus; 9) uterine myoma that could lead to adverse pregnancy events; 10) cervical insufficiency; 11) medical condition affecting the pregnancy outcome; or 12) known allergy or contraindication to LMWH or aspirin.
All eligible participants were requested to report once positive pregnancy test was documented. Biochemical pregnancy was then confirmed by performing quantitative assay of serum beta subunit of human chorionic gonadotropin. A written informed consent was taken from each woman selected to participate before enrollment. All women participating in the study were randomly divided at documentation of positive pregnancy test into two groups; early LMWH initiation group and later LMWH initiation group. The randomization was simple and balanced (1:1) and was carried out by a nurse through sealed, unlabeled, opaque envelopes containing computer-generated random numbers. The study was open label (the participants, caregivers, and investigators were not blinded to group assignment).
Women in both groups started aspirin (81 mg/day) before conception (in the luteal phase) and continued till 37 weeks of gestation. Women in the early LMWH initiation group started LMWH therapy once positive pregnancy test was established (in the fifth week of gestation) while women in the later LMWH initiation group started LMWH therapy after sonographic confirmation of fetal cardiac pulsation (in the seventh week of gestation). In both groups, LMWH (enoxaparin; Clexane®; Sanofi-Aventis, Paris, France) was given in a dose of 40 mg/day subcutaneously and the therapy continued until end of pregnancy.
All participants were examined by transvaginal sonography (TVS) 2 weeks after positive pregnancy test to document clinical intrauterine pregnancy, which was defined as presence of at least one intrauterine gestational sac with fetal pole and cardiac activity on TVS scan at 6–7 weeks of gestation. All women received routine antenatal care and supplementations, and were followed up every 2–3 weeks. During each follow-up visit, women were interviewed to determine the occurrence of vaginal bleeding or any adverse event. Ultrasonography was also performed at each follow-up visit to document fetal viability and monitor fetal growth and well-being. Doppler ultrasound examinations were used for fetal surveillance in pregnancies complicated by IUGR, gestational hypertension, or preeclampsia. Patients who developed gestational hypertension or preeclampsia were managed accordingly and antihypertensive drugs were administered to control blood pressure. Compliance with LMWH was calculated for each participant by dividing the number of ampoules actually used by the number of ampoules that should have been used.
The primary outcome measure of this study was ongoing pregnancy rate and the secondary outcome measures were the fetal loss, live birth rate, preterm labor before 34 weeks of gestation, IUGR, gestational hypertension, preeclampsia, and congenital fetal malformations. Ongoing pregnancy was defined as pregnancy that progressed beyond the critical first trimester (12 weeks of gestation). Fetal loss was defined as unexplained fetal death of morphologically normal fetus after the first trimester. Live birth was defined as birth of a living fetus after 24 weeks of gestation regardless of gestational age. The IUGR was defined as estimated fetal weight less than the 10th percentile for gestational age. Gestational hypertension was defined as development of hypertension (SBP >140 mmHg and/or DBP >90 mmHg on two occasions at least 6 hours apart) without proteinuria after 20 weeks of gestation. Preeclampsia was defined as development of hypertension with pathological proteinuria (protein loss in urine of at least 0.3 g/24 hours) after 20 weeks of gestation.
The sample size was calculated using the computer statistical software G*Power 3.1.9.2 (Fisher’s exact test, two-tailed significance, alpha error probability =0.05, power =80%, allocation ratio for groups =1). The estimation of the sample size was based on the previously reported live birth rates, which were 84% with administration of LMWH as soon as the serum pregnancy test became positive6 and 78% with later administration of LMWH (starting before 12 weeks of gestation).17 For detection of a small difference (6%) between our study groups, a sample size of at least 438 women (219 per group) was needed, but recruiting such number of participants was time-consuming (~8 years); therefore, we decided to enroll 100 participants (50 per group) in our study.
The IBM® SPSS® Statistics, version 20.0 for Windows was used for statistical analysis. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to test normality distribution of continuous variables. Normally distributed continuous variables were presented as mean ± SD and the Student’s t-test was used to compare between the two studied groups. Continuous variables without normal distribution were presented as median (minimum and maximum) and the Mann–Whitney U test was used to compare between the two studied groups. Categorical variables were presented as frequencies and percentages and the Fisher’s exact test was used to compare between the two studied groups. P-values ≤0.05 were considered statistically significant.
Results
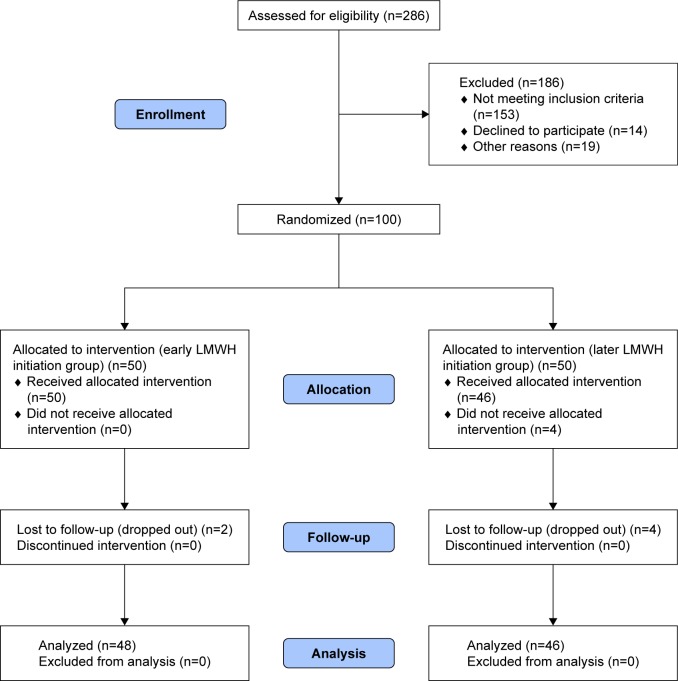
During the period of the study, 286 pregnant women with obstetrical APS were assessed for eligibility to participate in the study and 100 of them were randomized. Of the 100 women who were randomized, 4 women in the later initiation group did not receive the allocated intervention due to absence of fetal cardiac pulsation. These patients, however, were included in the analysis as results were reported on intention-to-treat basis. There were six women who were lost to follow-up from both groups. Data on all relevant outcomes were available for 94 women and data were analyzed from 48 women in the early initiation group and 46 in the later initiation group (Figure 1). The demographic characteristics and antiphospholipid antibodies status of both groups are shown in Table 1.
Figure 1.
Study flow diagram.
Abbreviation: LMWH, low-molecular-weight heparin.
Table 1.
Demographic characteristics and antiphospholipid antibodies status
| Early initiation group (n=48) | Later initiation group (n=46) | P-value | |
|---|---|---|---|
| Age (years)a | 27.77±3.72 | 28.28±4.32 | 0.539 |
| BMIb | 29.81 (22.94–32.77) | 28.51 (20.80–32.79) | 0.216 |
| Gravidityb | 6 (4–10) | 5 (4–9) | 0.115 |
| Nulliparous womenc | 21/48 (43.8%) | 28/46 (60.9%) | 0.105 |
| Previous first trimester miscarriagesb | 4 (3–8) | 3 (3–7) | 0.338 |
| Women with previous second trimester miscarriagesc | 8/48 (16.7%) | 6/46 (13.0%) | 0.774 |
| Women with previous preterm laborc | 4/48 (8.3%) | 4/46 (8.7%) | 1.000 |
| Women with previous fetal lossc | 8/48 (16.7%) | 5/46 (10.9%) | 0.553 |
| Type of positive antiphospholipid antibodiesc | |||
| ACL IgM only | 3/48 (6.3%) | 2/46 (4.3%) | 1.000 |
| ACL IgG only | 5/48 (10.4%) | 7/46 (15.2%) | 0.548 |
| LAC only | 22/48 (45.8%) | 25/46 (54.3%) | 0.536 |
| ACL IgM and IgG | 3/48 (6.3%) | 2/46 (4.3%) | 1.000 |
| ACL IgM and LAC | 4/48 (8.3%) | 2/46 (4.3%) | 0.678 |
| ACL IgG and LAC | 5/48 (10.4%) | 5/46 (10.9%) | 1.000 |
| ACL IgM and IgG and LAC | 6/48 (12.5%) | 3/46 (6.5%) | 0.487 |
Notes: Expressed as mean ± SD and P-value was calculated by the t-test.
Expressed as median and range and P-value was calculated by the Mann–Whitney U test.
Expressed as frequency and percentage and P-value was calculated by the Fisher’s exact test.
Abbreviations: ACL, anticardiolipin; BMI, body mass index; IgG, immunoglobulin G; IgM, immunoglobulin M; LAC, lupus anticoagulant.
As expected, the median gestational age at start of LMWH treatment was significantly different between the early and later initiation groups (4.57 vs 6.71 weeks; P<0.001). No significant difference between both groups in the compliance with treatment, rates of first and second trimesters bleeding, and clinical pregnancy rate. The first trimester miscarriage rate was significantly lower in the early initiation group than in the later initiation group (18.8% vs 39.1%; P=0.040) and consequently, the ongoing pregnancy rate was significantly higher in the early initiation group than in the later initiation group (81.2% vs 60.9%; P=0.040). No significant difference between both groups in the second trimester miscarriage rate, gestational age at miscarriage, fetal loss rate, and live birth rate (Table 2).
Table 2.
Treatment characteristics and pregnancy outcomes
| Early initiation group (n=48) | Later initiation group (n=46) | P-value | |
|---|---|---|---|
| Gestational age at start of treatment (weeks)a | 4.57 (4.43–4.86) | 6.71 (6.29–6.86) | <0.001 |
| Compliance with LMWHa | 96.67% (93.18%–100%) | 98.46% (91.80%–100%) | 0.199 |
| First trimester bleedingb | 8/48 (16.7%) | 6/46 (13.0%) | 0.774 |
| Second trimester bleedingb | 3/48 (6.3%) | 5/46 (10.9%) | 0.481 |
| Clinical pregnancyb | 47/48 (97.9%) | 42/46 (91.3%) | 0.199 |
| Ongoing pregnancyb | 39/48 (81.2%) | 28/46 (60.9%) | 0.040 |
| First trimester miscarriageb | 9/48 (18.8%) | 18/46 (39.1%) | 0.040 |
| Second trimester miscarriageb | 4/48 (8.3%) | 2/46 (4.3%) | 0.678 |
| Gestational age at miscarriage (weeks)a | 9.43 (6.86–22.57) | 8.64 (6.43–21.29) | 0.237 |
| Fetal lossb | 4/48 (8.3%) | 2/46 (4.3%) | 0.678 |
| Live birthb | 34/48 (70.8%) | 26/46 (56.5%) | 0.198 |
Notes: Expressed as median and range and P-value was calculated by the Mann–Whitney U test.
Expressed as frequency and percentage and P-value was calculated by the Fisher’s exact test. Bold P-values are statistically significant.
Abbreviation: LMWH, low-molecular-weight heparin.
Sixty-one pregnancies progressed beyond 24 weeks of gestation (35 in the early initiation group and 26 in the later initiation group). No significant difference between both groups in these pregnancies in the gestational age at delivery, and the incidences of preterm delivery before 34 weeks, intrauterine fetal death, IUGR, gestational hypertension, preeclampsia, and placental abruption (Table 3). There were 60 live births (34 in the early initiation group and 26 in the later initiation group). The neonatal outcomes were similar among live births in both groups as shown by comparable birth weight, and comparable incidences of neonatal respiratory distress syndrome, admission to neonatal intensive care unit, the need for mechanical ventilation, and early neonatal death (Table 4).
Table 3.
Characteristics and complications among pregnancies progressed beyond 24 weeks
| Early initiation group (n=35) | Later initiation group (n=26) | P-value | |
|---|---|---|---|
| Gestational age at delivery (weeks)a | 37.71 (28.57–39.14) | 38.07 (29.14–39.29) | 0.321 |
| Delivery before 34 weeksb | 5/35 (14.3%) | 4/26 (15.4%) | 1.000 |
| IUFDb | 1/35 (2.9%) | 0/26 (0.0%) | 1.000 |
| IUGRb | 7/35 (20.0%) | 5/26 (19.2%) | 1.000 |
| Gestational hypertensionb | 1/35 (2.9%) | 2/26 (7.7%) | 0.570 |
| Preeclampsiab | 8/35 (22.9%) | 7/26 (26.9%) | 0.770 |
| Placental abruptionb | 3/35 (8.6%) | 1/26 (3.8%) | 0.629 |
Notes: Expressed as median and range and P-value was calculated by the Mann–Whitney U test.
Expressed as frequency and percentage and P-value was calculated by the Fisher’s exact test.
Abbreviations: IUFD, intrauterine fetal death; IUGR, intrauterine growth restriction.
Table 4.
Neonatal outcomes among live births
| Early initiation group (n=34) | Later initiation group (n=26) | P-value | |
|---|---|---|---|
| Birth weight (g)a | 2,950 (1,050–3,300) | 2,950 (1,150–3,400) | 0.535 |
| Birth weight <1,500 gb | 7/34 (20.6%) | 6/26 (23.1%) | 1.000 |
| Birth weight <2,500 gb | 9/34 (26.5%) | 8/26 (30.8%) | 0.777 |
| Congenital malformationb | 0/34 (0.0%) | 0/26 (0.0%) | 1.000 |
| Neonatal RDSb | 9/34 (26.5%) | 8/26 (30.8%) | 0.777 |
| Admission to NICUb | 11/34 (32.4%) | 10/26 (38.5%) | 0.785 |
| Mechanical ventilationb | 8/34 (23.5%) | 6/26 (23.1%) | 1.000 |
| Early neonatal deathb | 6/34 (17.6%) | 4/26 (15.4%) | 1.000 |
Notes: Expressed as median and range and P-value was calculated by the Mann–Whitney U test.
Expressed as frequency and percentage and P-value was calculated by the Fisher’s exact test.
Abbreviations: NICU, neonatal intensive care unit; RDS, respiratory distress syndrome.
Discussion
The APS is one of the most common and most curable causes of recurrent miscarriage. Thrombosis in the placental bed explains late fetal loss but poorly correlates to the early pregnancy loss, which is explained by different mechanisms, including poor trophoblastic differentiation, invasion and migration, and complement-induced inflammatory response in the decidua.18 The later pathogenesis explains the success of heparin in treatment of recurrent early pregnancy loss as it was proved that heparin besides its antithrombotic effect, has an anti-inflammatory effect by inhibiting complement activation,19 and has a trophic effect on the trophoblast in vitro.20
This led to the hypothesis of our study that the earlier the start of heparin therapy, the more favorable outcome we can get in patients with APS associated with early pregnancy losses. In this study, we found a significant reduction in early pregnancy loss in the study group where we started LMWH therapy once positive pregnancy test was established than in women in the control group where LMWH therapy was started after sonographic confirmation of fetal cardiac pulsation.
There is a stronger association between the presence of lupus anticoagulant antibodies and early pregnancy loss.21 In our study, there was no significant difference in the frequency of lupus anticoagulant antibodies in the study and control groups, so we suppose that the significant reduction of early pregnancy loss in the study group could be attributed to the timing of initiation of LMWH therapy rather than to the incidence of lupus anticoagulant antibodies in both groups.
In agreement with our hypothesis that earlier intervention with LMWH is helpful in decreasing early pregnancy loss, Ismail et al22 tested the effect of preconceptional initiation of LMWH in patients with APS and recurrent pregnancy loss. They found significant reduction in early pregnancy loss when daily administration of 40 mg enoxaparin subcutaneously plus 81 mg aspirin was started after documentation of ovulation. However, preconceptional LMWH initiation has a drawback of administration of LMWH to a percentage of women who will not be pregnant therefore, we preferred to start LMWH administration with the onset of biochemical diagnosis of pregnancy to avoid unnecessary preconceptional LMWH administration to women who may not become pregnant.
We did not find significant difference in late obstetrical complications related to antiphospholipid antibodies, such as IUGR, intrauterine fetal death, preeclampsia, placental abruption, and preterm labor before 34 weeks of gestation. This can be attributed to the use of low-dose aspirin in both groups, which proved to be helpful in preventing IUGR and preeclampsia in patients with APS23 as low-dose aspirin stimulates the production of IL-3, a cytokine, which favors placental and fetal development.24
The main strength of our study was that it was a randomized study with robust methods of randomizations and allocation concealments. The results were also reported on intention-to-treat basis and the rate of loss to follow-up was within acceptable limits. A limitation of our study was that it was not done as a placebo-controlled trial with lack of blinding of participants and assessors. We believe, however, that this has not led to a significant degree of bias as the outcomes assessed were hard clinical outcomes with good compliance rates in both groups, which significantly rules out the potential for verification and performance bias. Another limitation of the study lies in the small sample size, which limits the study ability to detect significant differences in the outcome of live birth and may have limited the study’s power to detect other clinically significant differences in other outcomes as preeclampsia and IUGR. Nevertheless, despite this limitation, this study has detected a significant difference in effect size in relation to reduction of pregnancy losses and improvement of ongoing pregnancy with number needed to treat of 5. This will serve as proof of principle foundation for initiation of multicenter larger study with larger power.
Conclusion
Early administration of LMWH for pregnant women with obstetrical APS reduces early pregnancy loss, but does not affect the incidence of late obstetrical complications.
Data sharing statement
The authors intend to share individual deidentified participant data if required. The authors intend to share all collected dei dentified data in an excel sheet format. The study protocol, the patient consent form, and the Institutional Research Board approval document will be made available if required. The data will be available by contacting the corresponding author (msabdelhafez@gmail.com). The data will be available forever.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346(10):752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 2.Roubey RA. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39(9):1444–1454. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 3.Alijotas-Reig J, Vilardell-Tarres M. Is obstetric antiphospholipid syndrome a primary nonthrombotic, proinflammatory, complement-mediated disorder related to antiphospholipid antibodies? Obstet Gynecol Surv. 2010;65(1):39–45. doi: 10.1097/OGX.0b013e3181c97809. [DOI] [PubMed] [Google Scholar]
- 4.Meroni PL, Raschi E, Camera M, et al. Endothelial activation by aPL: a potential pathogenetic mechanism for the clinical manifestations of the syndrome. J Autoimmun. 2000;15(2):237–240. doi: 10.1006/jaut.2000.0412. [DOI] [PubMed] [Google Scholar]
- 5.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;2:CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble LS, Kutteh WH, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low-molecular-weight heparin versus unfractionated heparin. Fertil Steril. 2005;83(3):684–690. doi: 10.1016/j.fertnstert.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson MD, Ballem PJ, Tsang P, et al. Treatment of antiphospholipid antibody syndrome (APS) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. J Obstet Gynaecol Can. 2004;26(8):729–734. doi: 10.1016/s1701-2163(16)30644-2. [DOI] [PubMed] [Google Scholar]
- 8.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 9.Pettilä V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost. 2002;87(2):182–186. [PubMed] [Google Scholar]
- 10.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) BMJ. 1997;314(7076):253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerin J, Sheng Y, Reddel S, Iverson GM, Chapman MG, Krilis SA. Heparin inhibits the binding of beta 2-glycoprotein I to phospholipids and promotes the plasmin-mediated inactivation of this blood protein. Elucidation of the consequences of the two biological events in patients with the anti-phospholipid syndrome. J Biol Chem. 2002;277(4):2644–2649. doi: 10.1074/jbc.M110176200. [DOI] [PubMed] [Google Scholar]
- 12.Bose P, Black S, Kadyrov M, et al. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol. 2005;192(1):23–30. doi: 10.1016/j.ajog.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 14.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174(1):485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 15.Di Simone N, Di Nicuolo F, Sanguinetti M, et al. Low-molecular weight heparin induces in vitro trophoblast invasiveness: role of matrix metal-loproteinases and tissue inhibitors. Placenta. 2007;28(4):298–304. doi: 10.1016/j.placenta.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 17.Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100(3):408–413. doi: 10.1016/s0029-7844(02)02165-8. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti T, Cohen M, de Moerloose P. Obstetrical antiphospholipid syndrome: from the pathogenesis to the clinical and therapeutic implications. Clin Dev Immunol. 2013;2013(8):1–9. doi: 10.1155/2013/159124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simioni P, Sanson BJ, Prandoni P, et al. Incidence of venous thrombo-embolism in families with inherited thrombophilia. Thromb Haemost. 1999;81(2):198–202. [PubMed] [Google Scholar]
- 20.Salmon JE, Girardi G. The role of complement in the antiphospholipid syndrome. Curr Dir Autoimmun. 2004;7:133–148. doi: 10.1159/000075690. [DOI] [PubMed] [Google Scholar]
- 21.Opatrny L, David M, Kahn SR, Shrier I, Rey E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: a metaanalysis. J Rheumatol. 2006;33(11):2214–2221. [PubMed] [Google Scholar]
- 22.Ismail AM, Hamed AH, Saso S, Abu-Elhasan AM, Abu-Elghar MM, Abdelmeged AN. Randomized controlled study of pre-conception thromboprophylaxis among patients with recurrent spontaneous abortion related to antiphospholipid syndrome. Int J Gynaecol Obstet. 2016;132(2):219–223. doi: 10.1016/j.ijgo.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 24.Fishman P, Falach-Vaknin E, Sredni B, et al. Aspirin-interleukin-3 interrelationships in patients with anti-phospholipid syndrome. Am J Reprod Immunol. 1996;35(2):80–84. doi: 10.1111/j.1600-0897.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]