The American Diabetes Association’s (ADA’s) Standards of Medical Care in Diabetes is updated and published annually in a supplement to the January issue of Diabetes Care. The ADA’s Professional Practice Committee, which includes physicians, diabetes educators, registered dietitians (RDs), and public health experts, develops the Standards. The Standards include the most current evidence-based recommendations for diagnosing and treating adults and children with all forms of diabetes. ADA’s grading system uses A, B, C, or E to show the evidence level that supports each recommendation.
A—Clear evidence from well-conducted, generalizable randomized controlled trials that are adequately powered
B—Supportive evidence from well-conducted cohort studies
C—Supportive evidence from poorly controlled or uncontrolled studies
E—Expert consensus or clinical experience
This is an abridged version of the 2019 Standards containing the evidence-based recommendations most pertinent to primary care. The tables and figures have been renumbered from the original document to match this version. The complete 2019 Standards of Care document, including all supporting references, is available at professional.diabetes.org/standards.
1. IMPROVING CARE AND PROMOTING HEALTH IN POPULATIONS
Diabetes and Population Health
Recommendations
Ensure treatment decisions are timely, rely on evidence-based guidelines, and are made collaboratively with patients based on individual preferences, prognoses, and comorbidities. B
Align approaches to diabetes management with the Chronic Care Model, emphasizing productive interactions between a prepared proactive care team and an informed activated patient. A
Care systems should facilitate team-based care, patient registries, decision support tools, and community involvement to meet patient needs. B
Population health is defined as “the health outcomes of a group of individuals, including the distribution of health outcomes within the group”; these outcomes can be measured in terms of health outcomes (mortality, morbidity, health, and functional status), disease burden (incidence and prevalence), and behavioral and metabolic factors (exercise, diet, A1C, etc.). Clinical practice recommendations for health care providers are tools that can ultimately improve health across populations; however, for optimal outcomes, diabetes care must also be individualized for each patient. Thus, efforts to improve population health will require a combination of system-level and patient-level approaches.
The proportion of patients with diabetes who achieve recommended A1C, blood pressure, and LDL cholesterol levels has increased in recent years. Nevertheless, a 2013 report found that 33–49% of patients still did not meet general targets for glycemic, blood pressure, or cholesterol control, and only 14% met targets for all three measures while also avoiding smoking.
Diabetes poses a significant financial burden to individuals and society. After adjusting for inflation, economic costs of diabetes increased by 26% from 2012 to 2017. This is attributed to the increased prevalence of diabetes and the increased cost per person with diabetes.
The Chronic Care Model (CCM) is an effective framework for improving the quality of diabetes care and includes six core elements:
Delivery system design (moving from a reactive to a proactive care delivery system where planned visits are coordinated through a team-based approach)
Self-management support
Decision support (basing care on evidence-based, effective care guidelines)
Clinical information systems (using registries that can provide patient-specific and population-based support to the care team)
Community resources and policies (identifying or developing resources to support healthy lifestyles)
Health systems (to create a quality-oriented culture)
Redefining the roles of the health care delivery team and empowering patient self-management are fundamental to the successful implementation of the CCM. Collaborative, multidisciplinary teams are best suited to provide care for people with chronic conditions such as diabetes and to facilitate patients’ self-management.
Tailoring Treatment for Social Context
Recommendations
Providers should assess social context, including potential food insecurity, housing stability, and financial barriers, and apply that information to treatment decisions. A
Refer patients to local community resources when available. B
Provide patients with self-management support from lay health coaches, navigators, or community health workers when available. A
Health inequities related to diabetes and its complications are well documented and are heavily influenced by social determinants of health. Social determinants of health are defined as the economic, environmental, political, and social conditions in which people live and are responsible for a major part of health inequality worldwide.
Food insecurity (FI) is the unreliable availability of nutritious food and the inability to consistently obtain food without resorting to socially unacceptable practices. FI affects more than 14% of the U.S. population, with higher rates in some racial/ethnic minority groups, in low-income households, and in homes headed by a single mother. FI is associated with increased risk for type 2 diabetes, suboptimal glycemic control, psychosocial conditions, and low treatment adherence.
Community health workers (CHWs), peer supporters, and lay leaders may assist in the delivery of diabetes self-management education and support (DSMES) services, particularly in underserved communities. CHWs can be part of a cost-effective, evidence-based strategy to improve the management of diabetes and cardiovascular risk factors in underserved communities and health care systems.
2. CLASSIFICATION AND DIAGNOSIS OF DIABETES
Diabetes can be classified into the following general categories:
Type 1 diabetes (due to autoimmune β-cell destruction, usually leading to absolute insulin deficiency)
Type 2 diabetes (due to a progressive loss of β-cell insulin secretion frequently on the background of insulin resistance)
Gestational diabetes mellitus (GDM) (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation)
Specific types of diabetes due to other causes, e.g., monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young), diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis), and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS, or after organ transplantation)
Diagnostic Tests for Diabetes
Recommendations
Testing for prediabetes and type 2 diabetes in asymptomatic people should be considered in adults of any age who are overweight or obese (BMI ≥25 kg/m2 or ≥23 kg/m2 in Asian Americans) and who have one or more additional risk factors for diabetes (Table 1). B
For all people, testing should begin at age 45 years. B
If tests are normal, repeat testing carried out at a minimum of 3-year intervals is reasonable. C
In patients with prediabetes and type 2 diabetes, identify and, if appropriate, treat other cardiovascular disease risk factors. B
Risk-based screening for prediabetes and/or type 2 diabetes should be considered after the onset of puberty or after 10 years of age, whichever occurs earlier, in children and adolescents who are overweight (BMI ≥85th percentile) or obese (BMI ≥95th percentile) and who have additional risk factors for diabetes. See Table 2 for evidence grading of risk factors.
TABLE 1.
Criteria for Testing for Diabetes or Prediabetes in Asymptomatic Adults
| 1. Testing should be considered in overweight or obese (BMI ≥25 kg/m2 or ≥23 kg/m2 in Asian Americans) adults who have one or more of the following risk factors: |
| • First-degree relative with diabetes |
| • High-risk race/ethnicity (e.g., African American, Latino, Native American, Asian American, Pacific Islander) |
| • History of CVD |
| • Hypertension (≥140/90 mmHg or on therapy for hypertension) |
| • HDL cholesterol level <35 mg/dL (0.90 mmol/L) and/or a triglyceride level >250 mg/dL (2.82 mmol/L) |
| • Women with polycystic ovary syndrome |
| • Physical inactivity |
| • Other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans) |
| 2. Patients with prediabetes (A1C ≥5.7% [39 mmol/mol], IGT, or IFG) should be tested yearly. |
| 3. Women who were diagnosed with GDM should have lifelong testing at least every 3 years. |
| 4. For all other patients, testing should begin at age 45 years. |
| 5. If results are normal, testing should be repeated at a minimum of 3-year intervals, with consideration of more frequent testing depending on initial results and risk status. |
IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
TABLE 2.
Risk-Based Screening for Type 2 Diabetes or Prediabetes in Asymptomatic Children and Adolescents in a Clinical Setting
| Testing should be considered in youth* who are overweight (≥85% percentile) or obese (≥95 percentile) A and who have one or more additional risk factors based on the strength of their association with diabetes: |
| • Maternal history of diabetes or GDM during the child’s gestation A |
| • Family history of type 2 diabetes in first- or second-degree relative A |
| • Race/ethnicity (Native American, African American, Latino, Asian American, Pacific Islander) A |
| • Signs of insulin resistance or conditions associated with insulin resistance (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome, or small-for-gestational-age birth weight) B |
After the onset of puberty or after 10 years of age, whichever occurs earlier.
If tests are normal, repeat testing at a minimum of 3-year intervals, or more frequently if BMI is increasing, is recommended.
Diabetes and prediabetes may be screened based on plasma glucose criteria, either the fasting plasma glucose (FPG) or the 2-h plasma glucose (2-h PG) value during a 75-g oral glucose tolerance test (OGTT), or A1C criteria (Table 3).
TABLE 3.
Criteria for the Screening and Diagnosis of Diabetes
| Prediabetes | Diabetes | |
|---|---|---|
| A1C | 5.7–6.4%* | ≥6.5%† |
| FPG | 100–125 mg/dL (5.6–6.9 mmol/L)* | ≥126 mg/dL (7.0 mmol/L)† |
| OGTT | 140–199 mg/dL (7.8–11.0 mmol/L)* | ≥200 mg/dL (11.1 mmol/L)† |
| RPG | ≥200 mg/dL (11.1 mmol/L)‡ |
For all three tests, risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at the higher end of the range.
In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate samples.
Only diagnostic in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis.
RPG, random plasma glucose.
There is incomplete concordance between A1C, FPG, and 2-h PG, and the 2-h PG value diagnoses more people with prediabetes and diabetes than the FPG or A1C cut points. Marked discrepancies between measured A1C and plasma glucose levels should prompt consideration that the A1C assay may not be reliable for that individual, since a relatively small percentage of patients have conditions such as sickle cell trait or hemoglobinopathies that skew A1C results. See “6. Glycemic Targets” in the complete 2019 Standards of Care for conditions causing discrepancies. Unless there is a clear clinical diagnosis based on overt signs of hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples. If using two separate test samples, it is recommended that the second test, which may either be a repeat of the initial test or a different test, be performed without delay. If patients have test results near the margins of the diagnostic threshold, the health care professional should follow the patient closely and repeat the test in 3–6 months.
3. PREVENTION OR DELAY OF TYPE 2 DIABETES
Recommendation
At least annual monitoring for the development of type 2 diabetes in those with prediabetes is suggested. E
“Prediabetes” is the term used for individuals whose glucose levels do not meet the criteria for diabetes but are too high to be considered normal. (See Table 3.) Prediabetes should not be viewed as a clinical entity in its own right but rather as an increased risk for diabetes and cardiovascular disease (CVD).
Screening for prediabetes and type 2 diabetes risk through an informal assessment of risk factors or with an assessment tool such as the ADA risk test is recommended to guide providers on whether to perform a diagnostic test for prediabetes (Table 3) and previously undiagnosed type 2 diabetes.
Lifestyle Interventions
Recommendations
Refer patients with prediabetes to an intensive behavioral lifestyle intervention program modeled on the Diabetes Prevention Program to achieve and maintain 7% loss of initial body weight and increase moderate-intensity physical activity (such as brisk walking) to at least 150 min/week. A
Based on patient preference, technology-assisted diabetes prevention interventions may be effective in preventing type 2 diabetes and should be considered. B
Several major randomized controlled trials, including the Diabetes Prevention Program (DPP), have demonstrated that an intensive lifestyle intervention can reduce the incidence of type 2 diabetes. In the DPP, diabetes incidence was reduced by 58% over 3 years. Follow-up in the Diabetes Prevention Program Outcomes Study has shown sustained reduction in the rate of conversion to type 2 diabetes of 34% at 10 years and 27% at 15 years.
The DPP’s 7% weight loss goal was selected because it was feasible to achieve and maintain and likely to lessen the risk of developing diabetes.
Nutrition
Structured behavioral weight loss therapy, including a reduced calorie meal plan and physical activity, is of paramount importance for those at high risk for developing type 2 diabetes who have overweight or obesity. Based on intervention trials, the eating patterns that may be helpful for those with prediabetes include a Mediterranean eating plan and a low-calorie, low-fat eating plan. Additional research is needed regarding whether a low-carbohydrate eating plan is beneficial for persons with prediabetes. In addition, evidence suggests that the overall quality of food consumed (as measured by the Alternative Healthy Eating Index), with an emphasis on whole grains, legumes, nuts, fruits, and vegetables and minimal refined and processed foods, is also important.
Whereas overall healthy low-calorie eating patterns should be encouraged, there is also some evidence that particular dietary components impact diabetes risk in observational studies. Higher intakes of nuts, berries, yogurt, coffee, and tea are associated with reduced diabetes risk. Conversely, red meats and sugar-sweetened beverages are associated with an increased risk of type 2 diabetes.
Cost-Effectiveness
A cost-effectiveness model suggested that the lifestyle intervention used in the DPP was cost-effective. The use of CHWs to support DPP efforts has been shown to be effective with cost savings.
The Centers for Medicare & Medicaid Services has expanded Medicare reimbursement coverage for the Centers for Disease Control and Prevention (CDC)-coordinated National DPP lifestyle intervention to CDC-recognized organizations that become Medicare suppliers for this service.
Pharmacologic Interventions
Recommendation
Metformin therapy for prevention of type 2 diabetes should be considered in those with prediabetes, especially for those with BMI ≥35 kg/m2, those aged <60 years, and women with prior GDM. A
Several pharmacologic agents have been shown to decrease the incidence of diabetes, although none are approved by the U.S. Food and Drug Administration (FDA) specifically for diabetes prevention. Metformin has the strongest evidence base and demonstrated long-term safety as pharmacologic therapy for diabetes prevention. For other drugs, cost, side effects, and durable efficacy require consideration.
Prevention of Cardiovascular Disease
Recommendation
Prediabetes is associated with heightened cardiovascular risk; therefore, screening for and treatment of modifiable risk factors for cardiovascular disease is suggested. B
People with prediabetes often have other cardiovascular risk factors, including hypertension and dyslipidemia, and are at increased risk for CVD. Although treatment goals for people with prediabetes are the same as for the general population, increased vigilance is warranted to identify and treat these and other cardiovascular risk factors.
4. COMPREHENSIVE MEDICAL EVALUATION AND ASSESSMENT OF COMORBIDITIES
Patient-Centered Collaborative Care
Recommendations
A patient-centered communication style that uses person-centered and strength-based language and active listening, elicits patient preferences and beliefs, and assesses literacy, numeracy, and potential barriers to care should be used to optimize patient health outcomes and health-related quality of life. B
Diabetes care should be managed by a multidisciplinary team that may draw from primary care physicians, subspecialty physicians, nurse practitioners, physician assistants, nurses, dietitians, exercise specialists, pharmacists, dentists, podiatrists, and mental health professionals. E
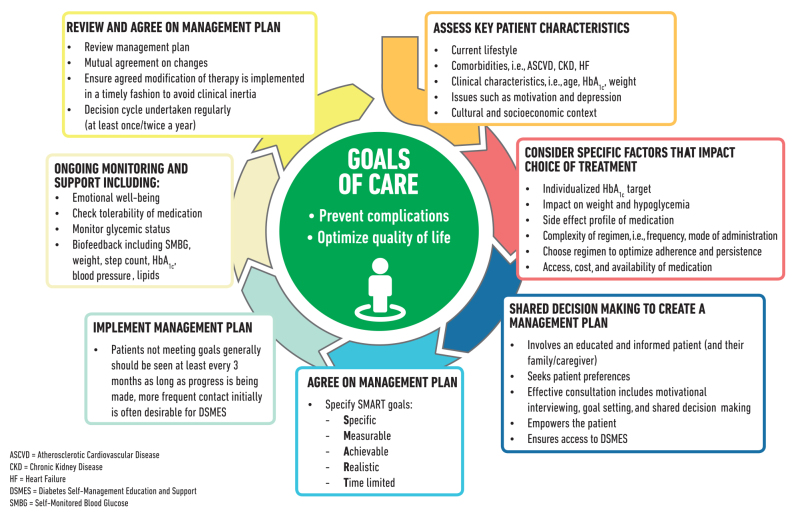
Individuals with diabetes must assume an active role in their care. The goals of treatment for diabetes are to prevent or delay complications and maintain quality of life. Treatment goals and plans for meeting them should be created collaboratively with patients (Figure 1).
FIGURE 1.
Decision cycle for patient-centered glycemic management in type 2 diabetes. Adapted from Davies MJ, D’Alessio DA, Fradkin J, et al. Diabetes Care 2018;41:2669–2701.
Comprehensive Medical Evaluation
Recommendations
-
A complete medical evaluation should be performed at the initial visit to:
◦ Confirm the diagnosis and classify diabetes. B
◦ Evaluate for diabetes complications and potential comorbid conditions. B
◦ Review previous treatment and risk factor control in patients with established diabetes. B
◦ Begin patient engagement in the formulation of a care management plan. B
◦ Develop a plan for continuing care. B
A follow-up visit should include most components of the initial comprehensive medical evaluation including: interval medical history, assessment of medication-taking behavior and intolerance/side effects, physical examination, laboratory evaluation as appropriate to assess attainment of A1C and metabolic targets, and assessment of risk for complications, diabetes self-management behaviors, nutrition, psychosocial health, and the need for referrals, immunizations, or other routine health maintenance screening. B
The risk assessment of acute and chronic diabetes complications and treatment planning are key components of initial and follow-up visits. The risk of atherosclerotic CVD (ASCVD) and heart failure, chronic kidney disease (CKD) staging, and treatment-associated hypoglycemia should be used to individualize targets for glycemia, blood pressure, and lipids and to select specific glucose-lowering medication, antihypertension medication, or statin treatment intensity.
Immunizations
Children and adults with diabetes should receive vaccinations according to age-specific recommendations. See the CDC website for current recommendations.
Assessment of Comorbidities
Besides assessing diabetes-related complications, clinicians and their patients need to be aware of common comorbidities that may complicate diabetes management.
Autoimmune Diseases
Recommendation
Consider screening patients with type 1 diabetes for autoimmune thyroid disease and celiac disease soon after diagnosis. B
Cancer
Diabetes is associated with increased risk of cancers of the liver, pancreas, endometrium, colon/rectum, breast, and bladder. The association may result from shared risk factors between type 2 diabetes and cancer (older age, obesity, and physical inactivity) but may also be due to diabetes-related factors, such as underlying disease physiology or diabetes treatments. Patients with diabetes should be encouraged to undergo recommended age- and sex-appropriate cancer screenings and to reduce their modifiable cancer risk factors (obesity, physical inactivity, and smoking). New onset of atypical diabetes (lean body type, negative family history) in a middle-aged or older patient may precede the diagnosis of pancreatic adenocarcinoma. However, in the absence of other symptoms (e.g., weight loss, abdominal pain), routine screening of all such patients is not currently recommended.
Cognitive Impairment/Dementia
Recommendation
In people with a history of cognitive impairment/dementia, intensive glucose control cannot be expected to remediate deficits. Treatment should be tailored to avoid significant hypoglycemia. B
See Sec. 5 “Lifestyle Management” and Sec. 12 “Older Adults” below for more discussion of this topic.
Other Conditions
Nonalcoholic fatty liver disease, hepatocellular carcinoma, hearing impairment, psychosocial/emotional disorders, hip fractures, low testosterone in men, obstructive sleep apnea, and periodontal disease are all more common in persons with diabetes. See “4. Comprehensive Medical Evaluation and Assessment of Comorbidities” in the complete 2019 Standards of Care for discussion on these topics.
5. LIFESTYLE MANAGEMENT
Lifestyle management is a fundamental aspect of diabetes care and includes DSMES, medical nutrition therapy (MNT), physical activity, smoking cessation counseling, and psychosocial care. Patients and providers should focus together on how to optimize lifestyle from the time of the initial comprehensive medical evaluation, throughout all subsequent evaluations and follow-up, and during the assessment of complications and management of comorbid conditions in order to enhance diabetes care.
Diabetes Self-Management Education and Support
Recommendations
In accordance with the national standards for DSMES, all people with diabetes should participate in diabetes self-management education to facilitate the knowledge, skills, and ability necessary for diabetes self-care. Diabetes self-management support is additionally recommended to assist with implementing and sustaining skills and behaviors needed for ongoing self-management. B
There are four critical times to evaluate the need for DSMES: at diagnosis, annually, when complicating factors arise, and when transitions in care occur. E
DSMES should be patient centered, may be given in group or individual settings or using technology, and should be communicated with the entire diabetes care team. A
Nutrition Therapy
For many individuals with diabetes, the most challenging part of the treatment plan is determining what to eat and following a meal plan. Each person with diabetes should be actively engaged in developing an individualized eating plan. All individuals with diabetes should be offered a referral for individualized MNT provided by an RD who is knowledgeable and skilled in providing diabetes-specific MNT.
Eating Patterns, Macronutrient Distribution, and Meal Planning
Evidence suggests that there is not an ideal percentage of calories from carbohydrate, protein, and fat for all people with diabetes. Therefore, macronutrient distribution should be based on an individualized assessment of current eating patterns, personal preferences (e.g., tradition, culture, religion, health beliefs and goals, economics), and metabolic goals.
The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based eating plans are examples of healthful eating patterns that have shown positive results in research. In addition, research indicates that low-carbohydrate eating plans may result in improved glycemia and have the potential to reduce antihyperglycemic medications for individuals with type 2 diabetes. There is inadequate research in type 1 diabetes to support one eating plan over another at this time.
A simple approach to glycemia and weight management emphasizing portion control and healthy food choices, such as the diabetes plate method, should be considered for those with type 2 diabetes who are not taking insulin, who have limited health literacy or numeracy, or who are older and prone to hypoglycemia. This visual guide shows how to control calories (by featuring a smaller plate) and carbohydrates (by limiting them to what fits in one-quarter of the plate) and puts an emphasis on low-carbohydrate (or nonstarchy) vegetables.
Alcohol
Moderate alcohol intake does not have major detrimental effects on long-term blood glucose control in people with diabetes. Risks associated with alcohol consumption include hypoglycemia (particularly for those using insulin or insulin secretagogue therapies), weight gain, and hyperglycemia (for those consuming excessive amounts). People with diabetes can follow the same guidelines as those without diabetes if they choose to drink. For women, no more than one drink per day, and two for men, is recommended. (One drink is equal to a 12-oz beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits.)
Nonnutritive Sweeteners
For some people with diabetes who are accustomed to sugar-sweetened products, nonnutritive sweeteners (containing few or no calories) may be an acceptable substitute for nutritive sweeteners when consumed in moderation. While use of nonnutritive sweeteners does not appear to have a significant effect on glycemic control, they can reduce overall calorie and carbohydrate intake. Most systematic reviews and meta-analyses show benefits for nonnutritive sweetener use in weight loss; however, some research suggests an association with weight gain. Overall, people are encouraged to decrease both sweetened and nonnutritive-sweetened beverages and use other alternatives, with an emphasis on water intake.
Physical Activity
Recommendations
Children and adolescents with type 1 or type 2 diabetes or prediabetes should engage in 60 min/day or more of moderate- or vigorous-intensity aerobic activity, with vigorous muscle-strengthening and bone-strengthening activities at least 3 days/week. C
Most adults with type 1 C and type 2 B diabetes should engage in 150 min or more of moderate-to-vigorous intensity aerobic activity per week, spread over at least 3 days/week, with no more than 2 consecutive days without activity. Shorter durations (minimum 75 min/week) of vigorous intensity or interval training may be sufficient for younger and more physically fit individuals.
Adults with type 1 C and type 2 B diabetes should engage in 2–3 sessions/week of resistance exercise on nonconsecutive days.
All adults, and particularly those with type 2 diabetes, should decrease the amount of time spent in daily sedentary behavior. B Prolonged sitting should be interrupted every 30 min for blood glucose benefits, particularly in adults with type 2 diabetes. C
Flexibility training and balance training are recommended 2–3 times/week for older adults with diabetes. Yoga and tai chi may be included based on individual preferences to increase flexibility, muscular strength, and balance. C
The ADA position statement “Physical Activity/Exercise and Diabetes” reviews the evidence for the benefits of exercise in people with type 1 and type 2 diabetes and offers specific recommendations.
Exercise in the Presence of Microvascular Complications
Retinopathy
If proliferative diabetic retinopathy or severe nonproliferative diabetic retinopathy is present, then vigorous-intensity aerobic or resistance exercise may be contraindicated because of the risk of triggering vitreous hemorrhage or retinal detachment. Consultation with an ophthalmologist prior to engaging in an intense exercise regimen may be appropriate.
Diabetic Kidney Disease
Physical activity can acutely increase urinary albumin excretion. However, there is no evidence that vigorous-intensity exercise increases the rate of progression of diabetic kidney disease (DKD), and there appears to be no need for specific exercise restrictions for people with DKD in general.
Neuropathy
Decreased pain sensation and a higher pain threshold in the extremities result in an increased risk of skin breakdown, infection, and Charcot joint destruction with some forms of exercise, so assessment is key, although moderate-intensity walking with proper footwear may not increase risk.
Smoking Cessation: Tobacco and e-Cigarettes
Recommendations
Advise all patients not to use cigarettes and other tobacco products A or e-cigarettes. B
Include smoking cessation counseling and other forms of treatment as a routine component of diabetes care. A
Psychosocial Issues
Recommendations
Psychosocial care should be integrated with a collaborative, patient-centered approach and provided to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. A
Psychosocial screening and follow-up may include, but are not limited to, attitudes about diabetes, expectations for medical management and outcomes, affect or mood, general and diabetes-related quality of life, available resources (financial, social, and emotional), and psychiatric history. E
Providers should consider assessment for symptoms of diabetes distress, depression, anxiety, disordered eating, and cognitive capacities using patient-appropriate standardized and validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Including caregivers and family members in this assessment is recommended. B
Consider screening older adults (aged ≥65 years) with diabetes for cognitive impairment and depression. B
The ADA position statement “Psychosocial Care for People With Diabetes” provides a list of assessment tools and additional details.
Diabetes Distress
Recommendation
Routinely monitor people with diabetes for diabetes distress, particularly when treatment targets are not met and/or at the onset of diabetes complications. B
Diabetes distress (DD) is very common and is distinct from other psychological disorders. DD refers to significant negative psychological reactions related to emotional burdens and worries specific to an individual’s experience in having to manage a severe, complicated, and demanding chronic disease such as diabetes. The constant behavioral demands (medication dosing, frequency, and titration; monitoring blood glucose, food intake, eating patterns, and physical activity) of diabetes self-management and the potential or actuality of disease progression are directly associated with reports of DD. The prevalence of DD is reported to be 18–45%, with an incidence of 38–48% over 18 months. High levels of DD significantly impact medication-taking behaviors and are linked to higher A1C, lower self-efficacy, and poorer dietary and exercise behaviors. DSMES has been shown to reduce DD.
Referral to a Mental Health Specialist
Indications for referral to a mental health specialist familiar with diabetes management may include positive screening for overall stress related to work-life balance, DD, diabetes management difficulties, depression, anxiety, disordered eating, and cognitive dysfunction, among other issues. It is preferable to incorporate psychosocial assessment and treatment into routine care rather than waiting for a specific problem or deterioration in metabolic or psychological status to occur. The ADA provides an online Mental Health Provider Directory of mental health providers who have received additional education in diabetes.
6. GLYCEMIC TARGETS
Assessment of Glycemic Control
Glycemic management is primarily assessed with the A1C test, the primary measure studied in clinical trials demonstrating the benefits of improved glycemic control. Self-monitoring of blood glucose (SMBG) may help with self-management and medication adjustment, particularly in individuals taking insulin. Continuous glucose monitoring (CGM) also has an important role in assessing the effectiveness and safety of treatment in many patients with type 1 diabetes, and limited data suggest it may also be helpful in selected patients with type 2 diabetes, such as those on intensive insulin regimens.
A1C Testing
Recommendations
Perform the A1C test at least two times a year in patients who are meeting treatment goals (and who have stable glycemic control). E
Perform the A1C test quarterly in patients whose therapy has changed or who are not meeting glycemic goals. E
Point-of-care testing for A1C provides the opportunity for more timely treatment changes. E
Glucose Assessment
Glucose monitoring is key for the achievement of glycemic targets for most people with diabetes. SMBG is an integral component of effective therapy of patients taking insulin. CGM has emerged as a complementary method for the assessment of glucose levels. Glucose monitoring allows patients to evaluate their individual response to therapy and assess whether glycemic targets are being safely achieved. Integrating results into diabetes management can be a useful tool for guiding MNT and physical activity, preventing hypoglycemia, and adjusting medications (particularly prandial insulin doses). The patient’s specific needs and goals should dictate SMBG frequency and timing or the consideration of CGM use. See “7. Diabetes Technology” in the complete Standards of Care for more discussion of the use of SMBG and CGM.
A1C Goals
Recommendations
A reasonable A1C goal for many nonpregnant adults is <7% (53 mmol/mol). A
Providers might reasonably suggest more stringent A1C goals (such as <6.5% [48 mmol/mol]) for selected individual patients if this can be achieved without significant hypoglycemia or other adverse effects of treatment (i.e., polypharmacy). Appropriate patients might include those with short duration of diabetes, type 2 diabetes treated with lifestyle or metformin only, long life expectancy, or no significant cardiovascular disease. C
Less stringent A1C goals (such as <8% [64 mmol/mol]) may be appropriate for patients with a history of level 3 hypoglycemia (altered mental and/or physical state requiring assistance), limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing diabetes in whom the goal is difficult to achieve despite diabetes self-management education, appropriate glucose monitoring, and effective doses of multiple glucose-lowering agents including insulin. B
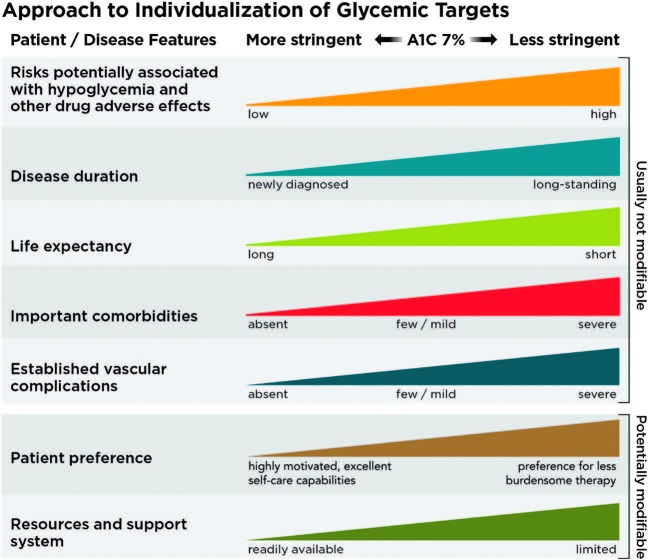
Reassess glycemic targets over time based on the criteria in Figure 2 or, in older adults, Table 12.1 [in the complete Standards of Care]. E
FIGURE 2.
Depicted are patient and disease factors used to determine optimal A1C targets. Characteristics and predicaments toward the left justify more stringent efforts to lower A1C; those toward the right suggest less stringent efforts. A1C 7% = 53 mmol/mol. Adapted with permission from Inzucchi SE, Bergenstal RM, Buse JB, et al. Diabetes Care 2015;38:140–149.
See “6. Glycemic Targets” in the complete 2019 Standards of Care for the justification for current glycemic control recommendations. See Sec. 13 “Children and Adolescents” and Sec. 14 “Management of Diabetes in Pregnancy” below for A1C goals for these populations. Table 4 summarizes glycemic recommendations for many nonpregnant adults. Figure 2 depicts factors used to determine A1C targets for individual patients.
TABLE 4.
Summary of Glycemic Recommendations for Many Nonpregnant Adults With Diabetes
| A1C | <7.0% (53 mmol/mol)* |
| Preprandial capillary plasma glucose | 80–130 mg/dL* (4.4–7.2 mmol/L) |
| Peak postprandial capillary plasma glucose† | <180 mg/dL* (10.0 mmol/L) |
More or less stringent glycemic goals may be appropriate for individual patients. Goals should be individualized based on duration of diabetes, age/life expectancy, comorbid conditions, known CVD or advanced microvascular complications, hypoglycemia unawareness, and individual patient considerations.
Postprandial glucose may be targeted if A1C goals are not met despite reaching preprandial glucose goals.
Postprandial glucose measurements should be made 1–2 h after the beginning of the meal, generally peak levels in patients with diabetes.
Hypoglycemia
Level 1 hypoglycemia is defined as a measurable glucose concentration <70 mg/dL (3.9 mmol/L). Level 2 hypoglycemia (defined as a blood glucose concentration <54 mg/dL [3.0 mmol/L]) is the threshold at which neuroglycopenic symptoms begin to occur and requires immediate action to resolve the hypoglycemic event. Level 3 hypoglycemia is defined as a severe event characterized by altered mental and/or physical functioning that requires assistance from another person for recovery.
Recommendations
Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter. C
Glucose (15–20 g) is the preferred treatment for the conscious individual with blood glucose <70 mg/dL (3.9 mmol/L), although any form of carbohydrate that contains glucose may be used. Fifteen minutes after treatment, if SMBG shows continued hypoglycemia, the treatment should be repeated. Once SMBG returns to normal, the individual should consume a meal or snack to prevent recurrence of hypoglycemia. E
Glucagon should be prescribed for all individuals at increased risk of level 2 hypoglycemia, defined as blood glucose <54 mg/dL (3.0 mmol/L), so it is available should it be needed. Caregivers, school personnel, or family members of these individuals should know where it is and when and how to administer it. Glucagon administration is not limited to health care professionals. E
Hypoglycemia unawareness or one or more episodes of level 3 hypoglycemia should trigger reevaluation of the treatment regimen. E
Insulin-treated patients with hypoglycemia unawareness or an episode of level 2 hypoglycemia should be advised to raise their glycemic targets to strictly avoid hypoglycemia for at least several weeks in order to partially reverse hypoglycemia unawareness and reduce risk of future episodes. A
Ongoing assessment of cognitive function is suggested with increased vigilance for hypoglycemia by the clinician, patient, and caregivers if low cognition or declining cognition is found. B
7. DIABETES TECHNOLOGY
“Diabetes technology” is the term used to describe the hardware, devices, and software that people with diabetes use to help manage blood glucose levels, stave off diabetes complications, reduce the burden of living with diabetes, and improve quality of life. Historically, diabetes technology has been divided into two main categories: insulin administered by syringe, pen, or pump, and blood glucose monitoring as assessed with a meter or CGM system. More recently, diabetes technology has expanded to include hybrid devices that both monitor glucose and deliver insulin, some automatically, as well as software that serves as a medical device, providing diabetes self-management support. Diabetes technology, when applied appropriately, can improve the lives and health of people with diabetes; however, the complexity and rapid change of the diabetes technology landscape can also be a barrier to patient and provider implementation. Patient interest will certainly be a driver for more widespread use of diabetes technology, and this may include primary care practices caring for those with diabetes.
SMBG
Recommendation
When prescribed as part of a broad educational program, SMBG may help to guide treatment decisions and/or self-management for patients taking less frequent insulin injections. B
In people with type 2 diabetes not using insulin, routine glucose monitoring may be of limited additional clinical benefit. For some individuals, glucose monitoring can provide insight into the impact of diet, physical activity, and medication management on glucose levels. Glucose monitoring may also be useful in assessing hypoglycemia, glucose levels during intercurrent illness, or discrepancies between measured A1C and glucose levels when there is concern that an A1C result may not be reliable in specific individuals. However, several randomized trials have called into question the clinical utility and cost-effectiveness of routine SMBG in noninsulin-treated patients. The ongoing need for and frequency of SMBG should be reevaluated at each routine visit to avoid overuse, particularly if SMBG is not being used effectively for self-management.
Due to the newness and complexity of this topic, readers are referred to the discussion in “7. Diabetes Technology” in the complete 2019 Standards of Care.
8. OBESITY MANAGEMENT FOR THE TREATMENT OF TYPE 2 DIABETES
There is strong and consistent evidence that obesity management is beneficial in the treatment of type 2 diabetes. In patients with type 2 diabetes who are overweight or obese, modest and sustained weight loss has been shown to improve glycemic control and to reduce the need for glucose-lowering medications.
Assessment
Recommendation
At each patient encounter, BMI should be calculated and documented in the medical record. B
Providers should advise patients who are overweight or obese that, in general, higher BMIs increase the risk of CVD and all-cause mortality. Providers should assess each patient’s readiness to achieve weight loss and jointly determine weight-loss goals and intervention strategies.
Diet, Physical Activity, and Behavioral Therapy
Recommendations
Diet, physical activity, and behavioral therapy designed to achieve and maintain >5% weight loss should be prescribed for patients with type 2 diabetes who are overweight or obese and ready to achieve weight loss. A
Such interventions should be high intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500–750 kcal/day energy deficit. A
Diets should be individualized, as those that provide the same caloric restriction but differ in protein, carbohydrate, and fat content are equally effective in achieving weight loss. A
For patients who achieve short-term weight-loss goals, long-term (≥1 year) comprehensive weight maintenance programs should be prescribed. Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently) and/or other self-monitoring strategies, such as tracking intake, steps, etc.; continued consumption of a reduced-calorie diet; and participation in high levels of physical activity (200–300 min/week). A
To achieve weight loss of >5%, short-term (3-month) interventions that use very low-calorie diets (≤800 kcal/day) and total meal replacements may be prescribed for carefully selected patients by trained practitioners in medical care settings with close medical monitoring. To maintain weight loss, such programs must incorporate long-term comprehensive weight-maintenance counseling. B
Pharmacotherapy
Recommendations
When choosing glucose-lowering medications for overweight or obese patients with type 2 diabetes, consider their effect on weight. E
Whenever possible, minimize medications for comorbid conditions that are associated with weight gain. E
Weight-loss medications are effective as adjuncts to diet, physical activity, and behavioral counseling for selected patients with type 2 diabetes and BMI ≥27 kg/m2. Potential benefits must be weighed against the potential risks of the medications. A
If a patient’s response to weight-loss medications is <5% weight loss after 3 months or if there are significant safety or tolerability issues at any time, the medication should be discontinued and alternative medications or treatment approaches should be considered. A
The FDA has approved medications for both short-term and long-term weight management as adjuncts to diet, exercise, and behavioral therapy. Nearly all FDA-approved medications for weight loss have been shown to improve glycemic control in patients with type 2 diabetes and delay progression to type 2 diabetes in patients at risk. Table 8.2 in the complete 2019 Standards of Care lists the currently available obesity drugs.
Metabolic Surgery
Recommendations
Metabolic surgery should be recommended as an option to treat type 2 diabetes in appropriate surgical candidates with BMI ≥40 kg/m2 (BMI ≥37.5 kg/m2 in Asian Americans) and in adults with BMI 35.0–39.9 kg/m2 (32.5–37.4 kg/m2 in Asian Americans) who do not achieve durable weight loss and improvement in comorbidities (including hyperglycemia) with reasonable nonsurgical methods. A
Metabolic surgery may be considered as an option for adults with type 2 diabetes and BMI 30.0–34.9 kg/m2 (27.5–32.4 kg/m2 in Asian Americans) who do not achieve durable weight loss and improvement in comorbidities (including hyperglycemia) with reasonable nonsurgical methods. A
Metabolic surgery should be performed in high-volume centers with multidisciplinary teams that understand and are experienced in the management of diabetes and gastrointestinal surgery. C
Long-term lifestyle support and routine monitoring of micronutrient and nutritional status must be provided to patients after surgery, according to guidelines for postoperative management of metabolic surgery by national and international professional societies. C
People presenting for metabolic surgery should receive a comprehensive readiness and mental health assessment. B
People who undergo metabolic surgery should be evaluated to assess the need for ongoing mental health services to help them adjust to medical and psychosocial changes after surgery. C
A substantial body of evidence has now been accumulated, including data from numerous randomized controlled clinical trials, demonstrating that metabolic surgery achieves superior glycemic control and reduction of cardiovascular risk factors in patients with type 2 diabetes and obesity compared with various lifestyle/medical interventions.
9. PHARMACOLOGIC APPROACHES TO GLYCEMIC TREATMENT
Pharmacologic Therapy for Type 1 Diabetes
Recommendations
Most people with type 1 diabetes should be treated with multiple daily injections of prandial and basal insulin, or continuous subcutaneous insulin infusion. A
Most individuals with type 1 diabetes should use rapid-acting insulin analogs to reduce hypoglycemia risk. A
Consider educating individuals with type 1 diabetes on matching prandial insulin doses to carbohydrate intake, premeal blood glucose levels, and anticipated physical activity. E
Insulin Therapy
Generally, insulin requirements can be estimated based on weight, with typical doses ranging from 0.4 to 1.0 units/kg/day. Higher amounts are required during puberty, pregnancy, and illness. A typical starting dose is 0.5 units/kg/day in patients with type 1 diabetes who are metabolically stable, with half administered as prandial insulin given to control blood glucose after meals and the other half as basal insulin to control glycemia in the periods between meal absorption.
Physiologic insulin secretion varies with glycemia, meal size, and tissue demands for glucose. To approach this variability in people using insulin treatment, strategies have evolved to adjust prandial doses based on predicted needs. Thus, education of patients on how to adjust prandial insulin to account for carbohydrate intake, premeal glucose levels, and anticipated activity can be effective and should be considered.
Postprandial glucose excursions may be better controlled by adjusting the timing of prandial insulin dose administration. The optimal time to administer prandial insulin varies, based on the type of insulin used (regular, rapid-acting analog, inhaled, etc.), measured blood glucose level, timing of meals, and carbohydrate consumption. Recommendations for prandial insulin dose administration should therefore be individualized. Insulin pumps and CGM systems may provide advantages in reducing hypoglycemia.
Pharmacologic Therapy for Type 2 Diabetes
Recommendations
Metformin is the preferred initial pharmacologic agent for the treatment of type 2 diabetes. A
Once initiated, metformin should be continued as long as it is tolerated and not contraindicated; other agents, including insulin, should be added to metformin. A
Long-term use of metformin may be associated with biochemical vitamin B12 deficiency, and periodic measurement of vitamin B12 levels should be considered in metformin-treated patients, especially in those with anemia or peripheral neuropathy. B
The early introduction of insulin should be considered if there is evidence of ongoing catabolism (weight loss), if symptoms of hyperglycemia are present, or when A1C levels (>10% [86 mmol/mol]) or blood glucose levels (≥300 mg/dL [16.7 mmol/L]) are very high. E
Consider initiating dual therapy in patients with newly diagnosed type 2 diabetes who have A1C ≥1.5% (12.5 mmol/mol) above their glycemic target. E
A patient-centered approach should be used to guide the choice of pharmacologic agents. Considerations include comorbidities (ASCVD, heart failure, CKD), hypoglycemia risk, impact on weight, cost, risk for side effects, and patient preferences. E
Among patients with type 2 diabetes who have established ASCVD, sodium–glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide 1 (GLP-1) receptor agonists with demonstrated CVD benefit (Table 5) are recommended as part of the antihyperglycemic regimen. A
Among patients with ASCVD at high risk of heart failure or in whom heart failure coexists, SGLT2 inhibitors are preferred. C
For patients with type 2 diabetes and CKD, consider use of an SGLT2 inhibitor or GLP-1 receptor agonist shown to reduce risk of DKD progression, cardiovascular events, or both. C
In most patients who need the greater glucose-lowering effect of an injectable medication, GLP-1 receptor agonists are preferred to insulin. B
Intensification of treatment for patients with type 2 diabetes not meeting treatment goals should not be delayed. B
The medication regimen should be reevaluated at regular intervals (every 3–6 months) and adjusted as needed to incorporate new patient factors (Table 5). E
TABLE 5.
Drug-Specific and Patient Factors to Consider When Selecting Antihyperglycemic Treatment in Adults With Type 2 Diabetes
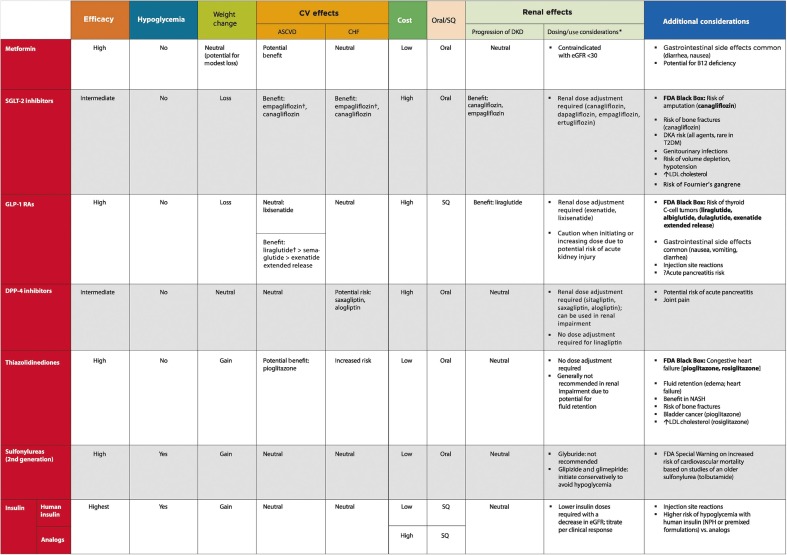 |
*For agent-specific dosing recommendations, please refer to the manufacturers’ prescribing information.
†FDA approved for CVD benefit.
CHF, congestive heart failure; CV, cardiovascular; DPP-4, dipeptidyl peptidase 4; DKA, diabetic ketoacidosis; GLP-1 RAs, GLP-1 receptor agonists; NASH, nonalcoholic steatohepatitis; SQ, subcutaneous; T2DM, type 2 diabetes.
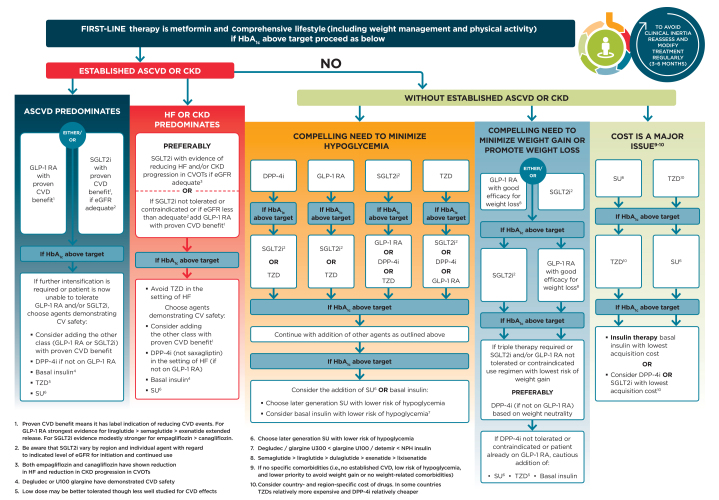
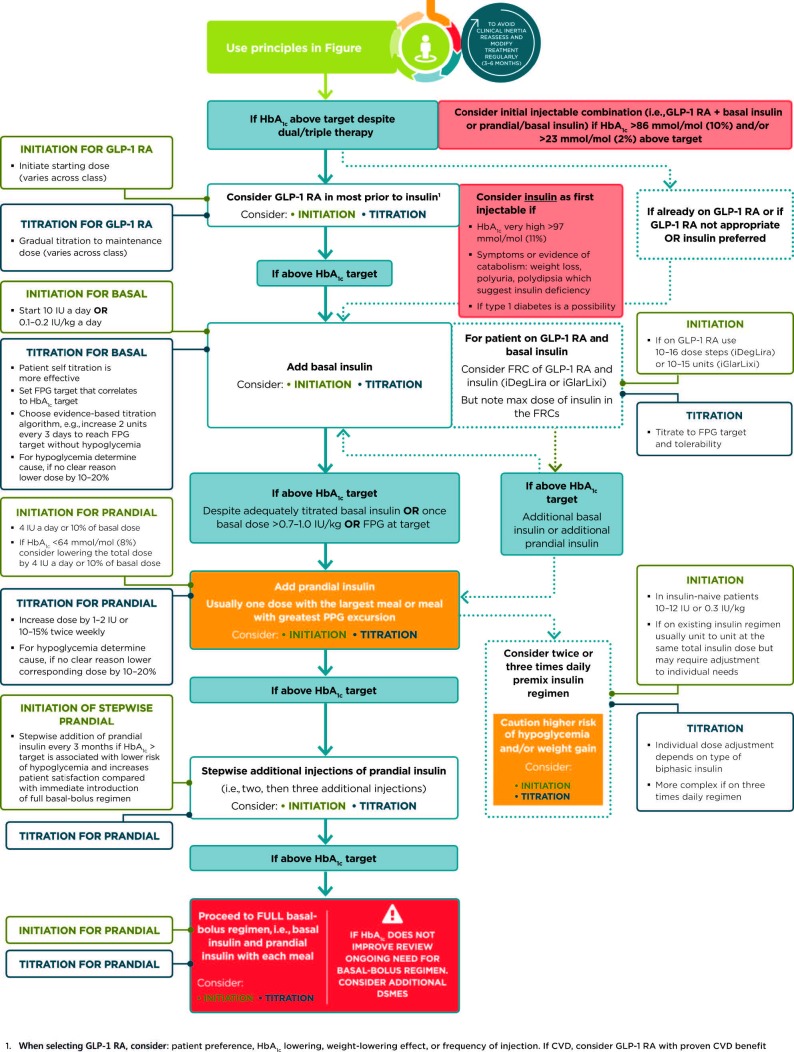
Table 5 highlights considerations for a patient-centered approach to choosing appropriate pharmacologic treatment of blood glucose. Figures 3 and 4 outline monotherapy and combination therapy, including initiating and intensifying injectable therapies, emphasizing drugs commonly used in the United States and/or Europe.
FIGURE 3.
Glucose-lowering medication in type 2 diabetes: overall approach. For appropriate context, see Figure 1. CV, cardiovascular; CVOTs, cardiovascular outcomes trials; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, GLP-1 receptor agonist; HbA1c, glycated hemoglobin; HF, heart failure; SGLT2i, SGLT2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione. Adapted from Davies MJ, D’Alessio DA, Fradkin J, et al. Diabetes Care 2018;41:2669–2701.
FIGURE 4.
Intensifying to injectable therapies. FRC, fixed-ratio combination; GLP-1 RA, GLP-1 receptor agonist; Hba1c, glycated hemoglobin; iDegLira, insulin degludec/liraglutide; iGlarLixi; insulin glargine/lixsenatide; max, maximum; PPG, postprandial glucose. Adapted from Davies MJ, D’Alessio DA, Fradkin J, et al. Diabetes Care 2018;41:2669–2701.
10. CARDIOVASCULAR DISEASE AND RISK MANAGEMENT
ASCVD—defined as coronary heart disease, cerebrovascular disease, or peripheral arterial disease (PAD) presumed to be of atherosclerotic origin—is the leading cause of morbidity and mortality for individuals with diabetes. Heart failure is another major cause of morbidity and mortality from CVD.
For prevention and management of both ASCVD and heart failure, cardiovascular risk factors should be systematically assessed at least annually in all patients with diabetes. These risk factors include obesity/overweight, hypertension, dyslipidemia, smoking, a family history of premature coronary disease, CKD, and the presence of albuminuria. The American College of Cardiology/American Heart Association ASCVD risk calculator (Risk Estimator Plus) is generally a useful tool to estimate 10-year ASCVD risk.
Hypertension/Blood Pressure Control
Recommendations
Screening and Diagnosis
Blood pressure should be measured at every routine clinical visit. Patients found to have elevated blood pressure (≥140/90 mmHg) should have blood pressure confirmed using multiple readings, including measurements on a separate day, to diagnose hypertension. B
All hypertensive patients with diabetes should monitor their blood pressure at home. B
Treatment Goals
For patients with diabetes and hypertension, blood pressure targets should be individualized through a shared decision-making process that addresses cardiovascular risk, potential adverse effects of antihypertensive medications, and patient preferences. C
For individuals with diabetes and hypertension at higher cardiovascular risk (existing ASCVD or 10-year ASCVD risk >15%), a blood pressure target of <130/80 mmHg may be appropriate, if it can be safely attained. C
For individuals with diabetes and hypertension at lower risk for cardiovascular disease (10-year ASCVD risk <15%), treat to a blood pressure target of <140/90 mmHg. A
Treatment Strategies
For patients with blood pressure >120/80 mmHg, lifestyle intervention consists of weight loss if overweight or obese, a DASH-style dietary pattern including reducing sodium and increasing potassium intake, moderation of alcohol intake, and increased physical activity. B
Patients with confirmed office-based blood pressure ≥140/90 mmHg should, in addition to lifestyle therapy, have prompt initiation and timely titration of pharmacologic therapy to achieve blood pressure goals. A
Patients with confirmed office-based blood pressure ≥160/100 mmHg should, in addition to lifestyle therapy, have prompt initiation and timely titration of two drugs or a single-pill combination of drugs demonstrated to reduce cardiovascular events in patients with diabetes. A
Treatment for hypertension should include drug classes demonstrated to reduce cardiovascular events in patients with diabetes (ACE inhibitors, angiotensin receptor blockers [ARBs], thiazide-like diuretics, or dihydropyridine calcium channel blockers). A
Multiple-drug therapy is generally required to achieve blood pressure targets. However, combinations of ACE inhibitors with ARBs and combinations of ACE inhibitors or ARBs with direct renin inhibitors should not be used. A
An ACE inhibitor or ARB, at the maximum tolerated dose indicated for blood pressure treatment, is the recommended first-line treatment for hypertension in patients with diabetes and urinary albumin-to-creatinine ratio ≥300 mg/g creatinine A or 30–299 mg/g creatinine. B If one class is not tolerated, the other should be substituted. B
For patients treated with an ACE inhibitor, ARB, or diuretic, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored at least annually. B
Patients with hypertension who are not meeting blood pressure targets on three classes of antihypertensive medications (including a diuretic) should be considered for mineralocorticoid receptor antagonist therapy. B
Lipid Management
Recommendations
Lifestyle Intervention
Lifestyle modification focusing on weight loss (if indicated); application of a Mediterranean eating plan or DASH dietary pattern; the reduction of saturated fat and trans fat; increase of dietary n-3 fatty acids, viscous fiber, and plant stanols/sterols intake; and increased physical activity should be recommended to improve the lipid profile and reduce the risk of developing ASCVD in patients with diabetes. A
Intensify lifestyle therapy and optimize glycemic control for patients with elevated triglyceride levels (≥150 mg/dL [1.7 mmol/L]) and/or low HDL cholesterol (<40 mg/dL [1.0 mmol/L] for men, <50 mg/dL [1.3 mmol/L] for women). C
Ongoing Therapy and Monitoring With Lipid Panel
In adults not taking statins or other lipid-lowering therapy, it is reasonable to obtain a lipid profile at the time of diabetes diagnosis, at an initial medical evaluation, and every 5 years thereafter if under the age of 40 years, or more frequently if indicated. E
Obtain a lipid profile at initiation of statins or other lipid-lowering therapy, 4–12 weeks after initiation or a change in dose, and annually thereafter as it may help to monitor the response to therapy and inform medication adherence. E
Statin Treatment
For patients of all ages with diabetes and ASCVD or 10-year ASCVD risk >20%, high-intensity statin therapy should be added to lifestyle therapy. A
For patients with diabetes aged <40 years with additional ASCVD risk factors, the patient and provider should consider using moderate-intensity statin in addition to lifestyle therapy. C
For patients with diabetes aged 40–75 years A and >75 years B without ASCVD, use moderate-intensity statin in addition to lifestyle therapy.
In patients with diabetes who have multiple ASCVD risk factors, it is reasonable to consider high-intensity statin therapy. C
For patients who do not tolerate the intended intensity, the maximally tolerated statin dose should be used. E
For patients with diabetes and ASCVD, if LDL cholesterol is ≥70 mg/dL on maximally tolerated statin dose, consider adding additional LDL-lowering therapy (such as ezetimibe or PCSK9 inhibitor). A Ezetimibe may be preferred due to lower cost.
Treatment of Other Lipoprotein Fractions or Targets
For patients with fasting triglyceride levels ≥500 mg/dL (5.7 mmol/L), evaluate for secondary causes of hypertriglyceridemia and consider medical therapy to reduce the risk of pancreatitis. C
In adults with moderate hypertriglyceridemia (fasting or nonfasting triglycerides 175–499 mg/dL), clinicians should address and treat lifestyle factors (obesity and metabolic syndrome), secondary factors (diabetes, chronic liver or kidney disease and/or nephrotic syndrome, hypothyroidism), and medications that raise triglycerides. C
Other Combination Therapy
Combination therapy (statin/fibrate) has not been shown to improve ASCVD outcomes and is generally not recommended. A
Combination therapy (statin/niacin) has not been shown to provide additional cardiovascular benefit above statin therapy alone, may increase the risk of stroke with additional side effects, and is generally not recommended. A
Antiplatelet Agents
Recommendations
Use aspirin therapy (75–162 mg/day) as a secondary prevention strategy in those with diabetes and a history of ASCVD. A
For patients with ASCVD and documented aspirin allergy, clopidogrel (75 mg/day) should be used. B
Dual antiplatelet therapy (with low-dose aspirin and a P2Y12 inhibitor) is reasonable for a year after an acute coronary syndrome A and may have benefits beyond this period. B
Aspirin therapy (75–162 mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased cardiovascular risk, after a discussion with the patient on the benefits versus increased risk of bleeding. C
Cardiovascular Disease
Recommendations
Screening
In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as ASCVD risk factors are treated. A
Consider investigations for coronary artery disease in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs or symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or peripheral arterial disease; or electrocardiogram abnormalities (e.g., Q waves). E
Treatment
In patients with known ASCVD, consider ACE inhibitor or ARB therapy to reduce the risk of cardiovascular events. B
In patients with prior myocardial infarction, β-blockers should be continued for at least 2 years after the event. B
In patients with type 2 diabetes with stable congestive heart failure, metformin may be used if eGFR remains >30 mL/min but should be avoided in unstable or hospitalized patients with congestive heart failure. B
Among patients with type 2 diabetes who have established ASCVD, SGLT2 inhibitors or GLP-1 receptor agonists with demonstrated cardiovascular disease benefit (Table 5) are recommended as part of the antihyperglycemic regimen. A
Among patients with ASCVD at high risk of heart failure or in whom heart failure coexists, SGLT2 inhibitors are preferred. C
See Figure 3 for additional recommendations on antihyperglycemic treatment in adults with type 2 diabetes.
11. MICROVASCULAR COMPLICATIONS AND FOOT CARE
Chronic Kidney Disease
Recommendations
Screening
At least once a year, assess urinary albumin (e.g., spot urinary albumin-to-creatinine ratio) and eGFR in patients with type 1 diabetes with duration of ≥5 years, in all patients with type 2 diabetes, and in all patients with comorbid hypertension. B
Treatment
Optimize glucose control to reduce the risk or slow the progression of CKD. A
For patients with type 2 diabetes and CKD, consider use of an SGLT2 inhibitor or a GLP-1 receptor agonist shown to reduce risk of CKD progression, cardiovascular events, or both (Table 5). C
Optimize blood pressure control to reduce the risk or slow the progression of CKD. A
For people with nondialysis-dependent CKD, dietary protein intake should be approximately 0.8 g/kg body weight per day (the recommended daily allowance). For patients on dialysis, higher levels of dietary protein intake should be considered. B
In nonpregnant patients with diabetes and hypertension, either an ACE inhibitor or an ARB is recommended for those with modestly elevated urinary albumin-to-creatinine ratio (30–299 mg/g creatinine) B and is strongly recommended for those with urinary albumin-to-creatinine ratio ≥300 mg/g creatinine and/or eGFR <60 mL/min/1.73 m2. A
Periodically monitor serum creatinine and potassium levels for the development of increased creatinine or changes in potassium when ACE inhibitors, ARBs, or diuretics are used. B
An ACE inhibitor or ARB is not recommended for the primary prevention of CKD in patients with diabetes who have normal blood pressure, normal urinary albumin-to-creatinine ratio (<30 mg/g creatinine), and normal eGFR. B
When eGFR is <60 mL/min/1.73 m2, evaluate and manage potential complications of CKD. E
Patients should be referred for evaluation for renal replacement treatment if they have an eGFR <30 mL/min/1.73 m2. A
Promptly refer to a physician experienced in the care of kidney disease for uncertainty about the etiology of kidney disease, difficult management issues, and rapidly progressing kidney disease. B
Epidemiology of Diabetes and Chronic Kidney Disease
CKD is diagnosed by the persistent presence of elevated urinary albumin excretion (albuminuria), low eGFR, or other manifestations of kidney damage. At any eGFR, the degree of albuminuria is associated with risk of CKD progression, CVD, and mortality. Among people with type 1 or type 2 diabetes, the presence of CKD markedly increases cardiovascular risk and health care costs. Table 6 summarizes the staging of CKD.
TABLE 6.
CKD Stages and Corresponding Focus of Kidney-Related Care
| CKD Stage† | Focus of Kidney-Related Care | |||||
|---|---|---|---|---|---|---|
| Stage | eGFR (mL/min/1.73 m2) | Evidence of kidney damage* | Diagnose cause of kidney injury | Evaluate and treat risk factors for CKD progression** | Evaluate and treat CKD complications*** | Prepare for renal replacement therapy |
| No clinical evidence of CKD | ≥60 | − | ||||
| 1 | ≥90 | + | ✓ | ✓ | ||
| 2 | 60–89 | + | ✓ | ✓ | ||
| 3 | 30–59 | +/− | ✓ | ✓ | ✓ | |
| 4 | 15–29 | +/− | ✓ | ✓ | ✓ | |
| 5 | <15 | +/− | ✓ | ✓ | ||
CKD stages 1 and 2 are defined by evidence of kidney damage (+), while CKD stages 3–5 are defined by reduced eGFR with or without evidence of kidney damage (+/−). At any stage of CKD, the degree of albuminuria, observed history of eGFR loss, and cause of kidney damage (including possible causes other than diabetes) may also be used to characterize CKD, gauge prognosis, and guide treatment decisions.
Kidney damage is most often manifest as albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g Cr) but can also include glomerular hematuria, other abnormalities of the urinary sediment, radiographic abnormalities, and other presentations.
Risk factors for CKD progression include elevated blood pressure, hyperglycemia, and albuminuria.
See Table 11.2 in the complete Standards of Care.
Interventions
Selection of Glucose-Lowering Medications for Patients With Chronic Kidney Disease
The FDA revised its guidance for the use of metformin in CKD in 2016, stating that metformin is contraindicated in patients with an eGFR <30 mL/min/1.73 m2, eGFR should be monitored while taking metformin, the benefits and risks of continuing treatment should be reassessed when eGFR falls to <45 mL/min/1.73 m2, metformin should not be initiated for patients with an eGFR <45 mL/min/1.73 m2, and metformin should be temporarily discontinued at the time of or before iodinated contrast imaging procedures in patients with eGFR 30–60 mL/min/1.73 m2.
See Sec. 9 “Pharmacologic Approaches to Glycemic Treatment” above for considerations regarding appropriate pharmacologic therapy for patients with type 2 diabetes and CKD.
Two clinical trials studied the combinations of ACE inhibitors and ARBs and found no benefits on CVD or CKD, and the drug combination had higher adverse event rates (hyperkalemia and/or acute kidney injury). Therefore, the combined use of ACE inhibitors and ARBs should be avoided.
Referral to a Nephrologist
Consider referral to a physician experienced in the care of CKD when there is uncertainty about the etiology of CKD, difficult management issues (anemia, secondary hyperparathyroidism, metabolic bone disease, resistant hypertension, or electrolyte disturbances), or stage 4 CKD (eGFR <30 mL/min/1.73 m2) requiring discussion of renal replacement therapy for end-stage renal disease. Consultation with a nephrologist when stage 4 CKD develops (eGFR <30 mL/min/1.73 m2) has been found to reduce cost, improve quality of care, and delay dialysis.
Diabetic Retinopathy
Recommendations
Optimize glycemic control to reduce the risk or slow the progression of diabetic retinopathy. A
Optimize blood pressure and serum lipid control to reduce the risk or slow the progression of diabetic retinopathy. A
Screening
Adults with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years after the onset of diabetes. B
Patients with type 2 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist at the time of the diabetes diagnosis. B
If there is no evidence of retinopathy for one or more annual eye exam and glycemia is well controlled, then exams every 1–2 years may be considered. If any level of diabetic retinopathy is present, subsequent dilated retinal examinations should be repeated at least annually by an ophthalmologist or optometrist. If retinopathy is progressing or sight-threatening, then examinations will be required more frequently. B
Telemedicine programs that use validated retinal photography with remote reading by an ophthalmologist or optometrist and timely referral for a comprehensive eye examination when indicated can be an appropriate screening strategy for diabetic retinopathy. B
Women with preexisting type 1 or type 2 diabetes who are planning pregnancy or who are pregnant should be counseled on the risk of development and/or progression of diabetic retinopathy. B
Eye examinations should occur before pregnancy or in the first trimester in patients with preexisting type 1 or type 2 diabetes, and then patients should be monitored every trimester and for 1-year postpartum as indicated by the degree of retinopathy. B
Treatment
Promptly refer patients with any level of macular edema, severe nonproliferative diabetic retinopathy (a precursor of proliferative diabetic retinopathy), or any proliferative diabetic retinopathy to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. A
The presence of retinopathy is not a contraindication to aspirin therapy for cardioprotection, as aspirin does not increase the risk of retinal hemorrhage. A
Neuropathy
Recommendations
Screening
All patients should be assessed for diabetic peripheral neuropathy starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes and at least annually thereafter. B
Assessment for distal symmetric polyneuropathy should include a careful history and assessment of either temperature or pinprick sensation (small-fiber function) and vibration sensation using a 128-Hz tuning fork (for large-fiber function). All patients should have annual 10-g monofilament testing to identify feet at risk for ulceration and amputation. B
Symptoms and signs of autonomic neuropathy should be assessed in patients with microvascular complications. E
Treatment
Optimize glucose control to prevent or delay the development of neuropathy in patients with type 1 diabetes A and to slow the progression of neuropathy in patients with type 2 diabetes. B
Assess and treat patients to reduce pain related to diabetic peripheral neuropathy B and symptoms of autonomic neuropathy and to improve quality of life. E
Pregabalin, duloxetine, or gabapentin are recommended as initial pharmacologic treatments for neuropathic pain in diabetes. A
Diabetic neuropathies are a heterogeneous group of disorders with diverse clinical manifestations. The early recognition and appropriate management of neuropathy in the patient with diabetes is important.
Diabetic neuropathy is a diagnosis of exclusion. Nondiabetic neuropathies may be present in patients with diabetes and may be treatable.
Numerous treatment options exist for symptomatic diabetic neuropathy.
Up to 50% of diabetic peripheral neuropathy (DPN) may be asymptomatic. If not recognized and if preventive foot care is not implemented, patients are at risk for injuries to their insensate feet.
Recognition and treatment of autonomic neuropathy may improve symptoms, reduce sequelae, and improve quality of life.
Specific treatment for the underlying nerve damage, other than improved glycemic control, is currently not available. Therapeutic strategies (pharmacologic and nonpharmacologic) for the relief of painful DPN and symptoms of autonomic neuropathy can potentially reduce pain and improve quality of life.
Neuropathic Pain
Neuropathic pain can be severe and can impact quality of life, limit mobility, and contribute to depression and social dysfunction. No compelling evidence exists in support of glycemic control or lifestyle management as therapies for neuropathic pain in diabetes or prediabetes, which leaves only pharmaceutical interventions.
Foot Care
Recommendations
Perform a comprehensive foot evaluation at least annually to identify risk factors for ulcers and amputations. B
Patients with evidence of sensory loss or prior ulceration or amputation should have their feet inspected at every visit. C
Obtain a prior history of ulceration, amputation, Charcot foot, angioplasty or vascular surgery, cigarette smoking, retinopathy, and renal disease and assess current symptoms of neuropathy (pain, burning, numbness) and vascular disease (leg fatigue, claudication). B
The examination should include inspection of the skin, assessment of foot deformities, neurological assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, vibration), and vascular assessment including pulses in the legs and feet. B
Patients with symptoms of claudication or decreased or absent pedal pulses should be referred for ankle-brachial index and for further vascular assessment as appropriate. C
A multidisciplinary approach is recommended for individuals with foot ulcers and high-risk feet (e.g., dialysis patients and those with Charcot foot or prior ulcers or amputation). B
Refer patients who smoke or who have histories of prior lower-extremity complications, loss of protective sensation, structural abnormalities, or peripheral arterial disease to foot care specialists for ongoing preventive care and lifelong surveillance. C
Provide general preventive foot self-care education to all patients with diabetes. B
The use of specialized therapeutic footwear is recommended for high-risk patients with diabetes including those with severe neuropathy, foot deformities, or history of amputation. B
Foot ulcers and amputation, which are consequences of diabetic neuropathy and/or PAD, are common and represent major causes of morbidity and mortality in people with diabetes. Early recognition and treatment of patients with diabetes and feet at risk for ulcers and amputations can delay or prevent adverse outcomes.
Clinicians are encouraged to review ADA screening recommendations in “11. Microvascular Complications and Foot Care” in the complete Standards of Care for further details and practical descriptions of how to perform components of the comprehensive foot examination.
Treatment
People with neuropathy or evidence of increased plantar pressures (e.g., erythema, warmth, or calluses) may be adequately managed with well-fitted walking shoes or athletic shoes that cushion the feet and redistribute pressure. People with bony deformities (e.g., hammertoes, prominent metatarsal heads, bunions) may need extra wide or deep shoes, and some will require custom-molded shoes. Use of custom therapeutic footwear can help reduce the risk of future foot ulcers in high-risk patients.
12. OLDER ADULTS
Diabetes is an important health condition for the aging population, as approximately one-quarter of people over the age of 65 years have diabetes and one-half of older adults have prediabetes. Older individuals with diabetes have higher rates of premature death, functional disability, and coexisting illnesses. They also have higher incidences of all-cause dementia, Alzheimer’s disease, and vascular dementia than people with normal glucose tolerance.
Recommendations
Consider the assessment of medical, psychological, functional (self-management abilities), and social geriatric domains in older adults to provide a framework to determine targets and therapeutic approaches for diabetes management. C
Screening for geriatric syndromes may be appropriate in older adults experiencing limitations in their basic and instrumental activities of daily living as they may affect diabetes self-management and be related to health-related quality of life. C
Screening for early detection of mild cognitive impairment or dementia and depression is indicated for adults 65 years of age or older at the initial visit and annually as appropriate. B
Hypoglycemia should be avoided in older adults with diabetes. It should be assessed and managed by adjusting glycemic targets and pharmacologic interventions. B
Older adults who are otherwise healthy with few coexisting chronic illnesses and intact cognitive function and functional status should have lower glycemic goals (such as A1C <7.5% [58 mmol/mol]), while those with multiple coexisting chronic illnesses, cognitive impairment, or functional dependence should have less stringent glycemic goals (such as A1C <8.0–8.5% [64–69 mmol/mol]). C
Glycemic goals for some older adults might reasonably be relaxed as part of individualized care, but hyperglycemia leading to symptoms or risk of acute hyperglycemia complications should be avoided in all patients. C
Screening for diabetes complications should be individualized in older adults. Particular attention should be paid to complications that would lead to functional impairment. C
Treatment of hypertension to individualized target levels is indicated in most older adults. C
Treatment of other cardiovascular risk factors should be individualized in older adults considering the time frame of benefit. Lipid-lowering therapy and aspirin therapy may benefit those with life expectancies at least equal to the time frame of primary prevention or secondary intervention trials. E
Optimal nutrition and protein intake is recommended for older adults; regular exercise, including aerobic activity and resistance training, should be encouraged in all older adults who can safely engage in such activities. B
In older adults at increased risk of hypoglycemia, medication classes with low risk of hypoglycemia are preferred. B
Overtreatment of diabetes is common in older adults and should be avoided. B
Deintensification (or simplification) of complex regimens is recommended to reduce the risk of hypoglycemia, if it can be achieved within the individualized A1C target. B
Older adults are at higher risk of hypoglycemia for many reasons, including insulin deficiency necessitating insulin therapy and progressive renal insufficiency. Hypoglycemic events should be diligently monitored and avoided, whereas glycemic targets and pharmacologic interventions may need to be adjusted to accommodate for the changing needs of the older adult. It is important to prevent hypoglycemia to reduce the risk of cognitive decline and other major adverse outcomes.
The care of older adults with diabetes is complicated by their clinical, cognitive, and functional heterogeneity. Providers caring for older adults with diabetes should prioritize treatment goals. For patients with complications and reduced functionality, it is reasonable to set less intensive glycemic goals. Patients with good cognitive and physical function may benefit from interventions and goals similar to younger adults. DSMES is vital to diabetes care for older adults and their caregivers.
Pharmacologic Therapy
Special care is required in prescribing and monitoring pharmacologic therapies in older adults. See Figure 3 for general recommendations regarding antihyperglycemia treatment for adults with type 2 diabetes and Table 5 for patient- and drug-specific factors to consider when selecting antihyperglycemia agents. Metformin is the first-line agent for older adults with type 2 diabetes.
The patient’s living situation must be considered because it may affect diabetes management and support. Cost may be an important consideration. Deintensification of regimens in patients taking noninsulin glucose-lowering medications can be achieved by either lowering the dose or discontinuing some medications, so long as the individualized A1C target is maintained. Simplification of insulin regimens may also be appropriate.
Older adults with diabetes are likely to benefit from control of other cardiovascular risk factors. Evidence is strong for treatment of hypertension. There is less evidence for lipid-lowering and aspirin therapy, although the benefits of these interventions are likely to apply to older adults whose life expectancies equal or exceed the time frames of clinical prevention trials.
Treatment in Skilled Nursing Facilities and Nursing Homes
Management of diabetes is unique in the long-term care (LTC) setting (i.e., nursing homes and skilled nursing facilities). Individualization of health care is important for all patients. Practical guidance is needed for medical providers as well as the LTC staff and caregivers. For patients in the LTC setting, special attention should be given to nutritional considerations, end-of-life care, and changes in diabetes management with respect to advanced disease. In some circumstances, withdrawal of medications may be appropriate.
13. CHILDREN AND ADOLESCENTS
Type 1 Diabetes
Recommendations
The majority of children and adolescents with type 1 diabetes should be treated with intensive insulin regimens, either via multiple daily injections or continuous subcutaneous insulin infusion. A
An A1C target of <7.5% (58 mmol/mol) should be considered in children and adolescents with type 1 diabetes but should be individualized based on the needs and situation of the patient and family. E
A multidisciplinary team of specialists trained in pediatric diabetes management and sensitive to the challenges of children and adolescents with type 1 diabetes and their families should provide care for this population.
See “13. Children and Adolescents” in the complete 2019 Standards of Care regarding the use of insulin pumps, blood glucose monitoring, and CGM in pediatric patients with type 1 diabetes.
Type 2 Diabetes
Screening and Diagnosis
Recommendations
Risk-based screening for prediabetes and/or type 2 diabetes should be considered in children and adolescents after the onset of puberty or ≥10 years of age, whichever occurs earlier, who are overweight (BMI ≥85th percentile) or obese (BMI ≥95th percentile) and who have one or more additional risk factors for diabetes (see Table 2 for evidence grading of other risk factors).
If tests are normal, repeat testing at a minimum of 3-year intervals E, or more frequently if BMI is increasing. C
Fasting plasma glucose, 2-h plasma glucose during a 75-g oral glucose tolerance test, and A1C can be used to test for prediabetes or diabetes in children and adolescents. B
Children and adolescents with overweight/obesity in whom the diagnosis of type 2 diabetes is being considered should have a panel of pancreatic autoantibodies tested to exclude the possibility of autoimmune type 1 diabetes. B
Management
Recommendations
Glycemic Targets
A reasonable A1C target for most children and adolescents with type 2 diabetes treated with oral agents alone is <7% (53 mmol/mol). More stringent A1C targets (such as <6.5%) may be appropriate for selected individual patients if this can be achieved without significant hypoglycemia or other adverse effects of treatment. Appropriate patients might include those with short duration of diabetes and lesser degrees of β-cell dysfunction and patients treated with lifestyle or metformin only who achieve significant weight improvement. E
Pharmacologic Management
Initiate pharmacologic therapy, in addition to lifestyle therapy, at diagnosis of type 2 diabetes. A
In incidentally diagnosed or metabolically stable patients (A1C <8.5% [69 mmol/mol] and asymptomatic), metformin is the initial pharmacologic treatment of choice if renal function is normal. A
If the A1C target is no longer met with metformin monotherapy, or if contraindications or intolerable side effects of metformin develop, basal insulin therapy should be initiated. B
Patients treated with basal insulin up to 1.5 units/kg/day who do not meet A1C target should be moved to multiple daily injections with basal and premeal bolus insulins. E
Use of medications not approved by the FDA for youth with type 2 diabetes is not recommended outside of research trials. B
See “13. Children and Adolescents” in the complete 2019 Standards of Care regarding the comprehensive treatment of children with type 2 diabetes.
14. DIABETES IN PREGNANCY
The prevalence of diabetes in pregnancy has been increasing in the United States. The majority is GDM with the remainder primarily preexisting type 1 diabetes and type 2 diabetes. The rise in GDM and type 2 diabetes in parallel with obesity both in the United States and worldwide is of particular concern.
Preconception Counseling
Recommendations
Starting at puberty and continuing in all women with reproductive potential, preconception counseling should be incorporated into routine diabetes care. A
Family planning should be discussed and effective contraception should be prescribed and used until a woman is prepared and ready to become pregnant. A
Preconception counseling should address the importance of glycemic management as close to normal as is safely possible, ideally A1C <6.5% (48 mmol/mol), to reduce the risk of congenital anomalies, preeclampsia, macrosomia, and other complications. B
Preconception Care
Recommendations
Women with preexisting type 1 or type 2 diabetes who are planning pregnancy or who have become pregnant should be counseled on the risk of development and/or progression of diabetic retinopathy. Dilated eye examinations should occur ideally before pregnancy or in the first trimester, and then patients should be monitored every trimester and for 1-year postpartum as indicated by the degree of retinopathy and as recommended by the eye care provider. B
Women with preexisting diabetes should ideally be managed in a multidisciplinary clinic including an endocrinologist, maternal-fetal medicine specialist, dietitian, and diabetes educator, when available. B
Glycemic Targets in Pregnancy
Recommendations
Fasting and postprandial self-monitoring of blood glucose are recommended in both GDM and preexisting diabetes in pregnancy to achieve glycemic control. Some women with preexisting diabetes should also test blood glucose preprandially. B
Due to increased red blood cell turnover, A1C is slightly lower in normal pregnancy than in normal nonpregnant women. Ideally, the A1C target in pregnancy is <6% (42 mmol/mol) if this can be achieved without significant hypoglycemia, but the target may be relaxed to <7% (53 mmol/mol) if necessary to prevent hypoglycemia. B
Similar to the targets recommended by the American College of Obstetricians and Gynecologists (the same as for GDM; described below), the ADA recommended targets for women with type 1 or type 2 diabetes are as follows:
Fasting <95 mg/dL (5.3 mmol/L) and either
One-hour postprandial <140 mg/dL (7.8 mmol/L) or
Two-hour postprandial <120 mg/dL (6.7 mmol/L)
Management of GDM
Recommendations
Lifestyle change is an essential component of management of GDM and may suffice for the treatment of many women. Medications should be added if needed to achieve glycemic targets. A
Insulin is the preferred medication for treating hyperglycemia in GDM as it does not cross the placenta to a measurable extent. Metformin and glyburide should not be used as first-line agents, as both cross the placenta to the fetus. All oral agents lack long-term safety data. A
Metformin, when used to treat polycystic ovary syndrome and induce ovulation, should be discontinued once pregnancy has been confirmed. A
Management of Preexisting Type 1 Diabetes and Type 2 Diabetes in Pregnancy
Insulin Use
Recommendation
Insulin is the preferred agent for management of both type 1 diabetes and type 2 diabetes in pregnancy because it does not cross the placenta and because oral agents are generally insufficient to overcome the insulin resistance in type 2 diabetes and are ineffective in type 1 diabetes. E
Due to the continuous change in insulin requirements during pregnancy, regular monitoring of blood glucose and insulin adjustments are necessary. Toward the end of the third trimester, it is common for there to be a greater need for prandial insulin (>50%) than for basal insulin (<50%). Referral to a specialized care team experienced in managing pregnancy in women with preexisting diabetes is recommended if this resource is available. None of the available human insulins have been found to cross the placenta. Use of a basal/bolus regimen versus continuous subcutaneous insulin infusion (insulin pump therapy) is to be individualized to patients’ needs.
Preeclampsia and Aspirin
Recommendation
Women with type 1 or type 2 diabetes should be prescribed low-dose aspirin 60–150 mg/day (usual dose 81 mg/day) from the end of the first trimester until the baby is born in order to lower the risk of preeclampsia. A
Pregnancy and Drug Considerations
Recommendations
In pregnant patients with diabetes and chronic hypertension, blood pressure targets of 120–160/80–105 mmHg are suggested in the interest of optimizing long-term maternal health and minimizing impaired fetal growth. E
Potentially teratogenic medications (i.e., ACE inhibitors, ARBs, statins) should be avoided in sexually active women of childbearing age who are not using reliable contraception. B
Postpartum Care
Postpartum care should include psychosocial assessment and support for self-care.
Because GDM may represent preexisting undiagnosed type 2 or even type 1 diabetes, women with GDM should be tested for persistent diabetes or prediabetes at 4–12 weeks postpartum with a 75-g OGTT using nonpregnancy criteria (Table 3).
Women should also be tested every 1–3 years thereafter if the 4- to 12-week postpartum 75-g OGTT is normal, with frequency of testing depending on other risk factors including family history, prepregnancy BMI, and need for insulin or oral glucose-lowering medication during pregnancy. Ongoing evaluation may be performed with any recommended glycemic test (e.g., A1C, FPG, or 75-g OGTT using nonpregnant thresholds).
In women taking insulin, particular attention should be directed to hypoglycemia prevention in the setting of breastfeeding and erratic sleep and eating schedules.
All women with diabetes of childbearing potential should have family planning options reviewed at regular intervals.
15. DIABETES CARE IN THE HOSPITAL
Hospitals should promote the shortest safe hospital stay, providing an effective transition out of the hospital that prevents acute complications and readmission. Prevention of hypoglycemia and hyperglycemia should be goals, since adverse outcomes are associated with both.
Hospital Care Delivery Standards
Recommendation
Perform an A1C on all patients with diabetes or hyperglycemia (blood glucose >140 mg/dL [7.8 mmol/L]) admitted to the hospital if not performed in the prior 3 months. B
Considerations on Admission
Initial orders should state the type of diabetes. For best practice, hospitals should establish protocols for structured patient care and structured order sets, which include computerized physician order entry.
Recommendation
Insulin should be administered using validated written or computerized protocols that allow for predefined adjustments in the insulin dosage based on glycemic fluctuations. E
Glycemic Targets in Hospitalized Patients
Recommendations
Insulin therapy should be initiated for treatment of persistent hyperglycemia starting at a threshold ≥180 mg/dL (10.0 mmol/L). Once insulin therapy is started, a target glucose range of 140–180 mg/dL (7.8–10.0 mmol/L) is recommended for the majority of critically ill patients and non-critically ill patients. A
More stringent goals, such as 110–140 mg/dL (6.1–7.8 mmol/L), may be appropriate for selected patients, if this can be achieved without significant hypoglycemia. C
Hyperglycemia in hospitalized patients is defined as blood glucose levels >140 mg/dL (7.8 mmol/L). An admission A1C value ≥6.5% (48 mmol/mol) suggests that diabetes preceded hospitalization. Hypoglycemia in the hospital is classified the same as in any setting. (See Sec. 6 “Glycemic Targets” above.)
Bedside Blood Glucose Monitoring
In the patient who is eating meals, glucose monitoring should be performed before meals. In the patient who is not eating, glucose monitoring is advised every 4–6 h. Testing every 30 min to every 2 h is required for intravenous insulin infusion.
Several inpatient studies have shown that CGM use did not improve glucose control but detected a greater number of hypoglycemic events than point-of-care (POC) glucose testing. However, a recent review has recommended against using CGM in adults in a hospital setting until more safety and efficacy data become available.
Antihyperglycemic Agents in Hospitalized Patients
Recommendations
Basal insulin or a basal plus bolus correction insulin regimen is the preferred treatment for non-critically ill hospitalized patients with poor oral intake or those who are taking nothing by mouth. An insulin regimen with basal, prandial, and correction components is the preferred treatment for noncritically ill hospitalized patients with good nutritional intake. A
Sole use of sliding scale insulin in the inpatient hospital setting is strongly discouraged. A
In most instances in the hospital setting, insulin is the preferred treatment for glycemic control.
Insulin Therapy
In the critical care setting, continuous intravenous insulin infusion has been shown to be the best method for achieving glycemic targets. Outside of critical care units, scheduled insulin regimens as described above are recommended.
If the patient is eating, insulin injections should align with meals. In such instances, POC glucose testing should be performed immediately before meals. Patients with type 1 diabetes should have basal-bolus insulin plus nutritional insulin if they are eating. A transition protocol from insulin infusion to subcutaneous insulin is recommended.
Noninsulin Therapies
The safety and efficacy of noninsulin antihyperglycemic therapies in the hospital setting is an area of active research. See “15. Diabetes Care in the Hospital” in the complete 2019 Standards of Care for a comprehensive review of the inpatient use of these medications.
Hypoglycemia
Recommendations
A hypoglycemia management protocol should be adopted and implemented by each hospital or hospital system. A plan for preventing and treating hypoglycemia should be established for each patient. Episodes of hypoglycemia in the hospital should be documented in the medical record and tracked. E
The treatment regimen should be reviewed and changed as necessary to prevent further hypoglycemia when a blood glucose value is <70 mg/dL (3.9 mmol/L). C
Patients with or without diabetes may experience hypoglycemia in the hospital setting. While hypoglycemia is associated with increased mortality, it may be a marker of underlying disease rather than the cause of increased mortality. However, until it is proven not to be causal, it is prudent to avoid hypoglycemia. Studies of “bundled” preventive therapies including proactive surveillance of glycemic outliers and an interdisciplinary data-driven approach to glycemic management showed that hypoglycemic episodes in the hospital could be prevented.
MNT in the Hospital
The goals of MNT in the hospital are to provide adequate calories to meet metabolic demands, optimize glycemic control, and address personal food preferences. The ADA does not endorse any single meal plan. An RD can serve as an inpatient team member.
Self-Management in the Hospital
Diabetes self-management in the hospital may be appropriate for select youth and adult patients. Sufficient cognitive and physical skills, adequate oral intake, proficiency in carbohydrate estimation, and knowledge of sick-day management are some of the requirements. Self-administered insulin with a multiple daily injection regimen or insulin pump therapy may be considered. A protocol should exist for these situations.
Standards for Special Situations
See “15. Diabetes Care in the Hospital” in the complete 2019 Standards of Care for guidance on enteral/parenteral feedings, diabetic ketoacidosis and hyperosmolar hyperglycemic state, perioperative care, and glucocorticoid therapy.
Transition From the Acute Care Setting
Recommendation
There should be a structured discharge plan tailored to the individual patient with diabetes. B
Transition from the acute care setting is a risky time for all patients A structured discharge plan tailored to the individual patient may reduce length of hospital stay and readmission rates and increase patient satisfaction. A structured discharge plan should be tailored to each patient and should include medication re-conciliation and structured discharge communication. Discharge planning should begin at admission and be updated as patient needs change. An outpatient follow-up visit 1 month after discharge is recommended.
16. DIABETES ADVOCACY
For a list of ADA advocacy position statements, including “Diabetes and Driving” and “Diabetes and Employment,” see “16. Diabetes Advocacy” in the complete Standards of Care.
Acknowledgments
Acknowledgments
This abridged version of the Standards of Medical Care in Diabetes—2019 was created by the ADA’s Primary Care Advisory Group (PCAG), with special thanks to PCAG chair Eric L. Johnson, MD, of Grand Forks, ND; vice-chair Hope Feldman, CRNP, FNP-BC, of Philadelphia, PA; Amy Butts, PA-C, MPAS, CDE, of Weirton, WV; CDR Billy St. John Collins, DHSc, MS, of Bethesda, MD; Joy Dugan, MPH, DHS(c), PA-C, of Vallejo, CA; Sandra Leal, PharmD, MPH, FAPhA, CDE, of Tucson, AZ; Andrew S. Rhinehart, MD, FACP, FACE, CDE, BC-ADM, CDTC, of Marco Island, FL; Jay H. Shubrook, DO, of Vallejo, CA; and Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC-ADM, of Aurora, CO; with ADA staff support from Sarah Bradley.
The complete Standards of Medical Care in Diabetes—2019 was developed by the ADA’s Professional Practice Committee: Joshua J. Neumiller, PharmD, CDE, FASCP (Chair); Christopher Cannon, MD; Ian de Boer, MD, MS; Jill Crandall, MD; David D’Alessio, MD; Mary de Groot, PhD; Judith Fradkin, MD; Kathryn Kreider, DNP, APRN, FNP-BC, BC-ADM; David Maahs, MD, PhD; Nisa Maruthur, MD, MHS; Melinda Maryniuk, MEd, RD, CDE; Medha N. Munshi, MD; Maria Jose Rdondo, MD, PhD, MPH; Guillermo E. Umpierrez, MD, CDE; and Jennifer Wyckoff, MD. ADA staff support includes Erika Berg, PhD; William T. Cefalu, MD; Matt Petersen; Shamera Robinson, MPH, RDN; Mindy Saraco, MHA; and Sacha Uelmen, RDN, CDE.
Footnotes
This is an abridged version of the American Diabetes Association’s Standards of Medical Care in Diabetes—2019. Diabetes Care 2018;42(Suppl. 1):S1–S194.
The complete 2019 Standards supplement, including all supporting references, is available at professional.diabetes.org/standards.