Abstract
Background
AML is a rapidly progressing bone marrow cancer, with poor survival rates compared to other types of leukemia. IC and NIC as well as BSC treatment options are available; however, there is scant published literature on the impact of disease and treatment on the HRQoL in patients receiving NIC.
Aim
This study determined the HRQoL among NIC AML patients.
Materials and methods
Embase, Medline, Cochrane database, and conference abstracts were searched using the prespecified PICOS criteria from January 2000 to November 2017 for studies reporting HRQoL and patient preference utilities in NIC AML. Studies on patients with RAEB-t MDS, randomized clinical trials (RCTs), prospective observational studies, and patient surveys were included, while systematic reviews and meta-analyses were used for bibliographic searching.
Results
Thirteen records from 12 original studies were identified. These included five records from four RCTs, three prospective studies, four patient survey studies, and one cost-effectiveness analysis. At baseline, NIC AML patients had poor HRQoL scores especially in fatigue (33) and GHS (50) on a 0–100 scale, with higher scores indicating better health. Low baseline HRQoL scores, especially PF and fatigue (<50) were shown to be significant independent predictors of poor survival. Clinical responders demonstrated meaningful improvements, especially in PF and fatigue, along with other health domains after being treated with NIC agents across several studies.
Conclusion
HRQoL is poor for patients with NIC AML; measures such as fatigue and PF at baseline have been identified as independent prognostic factors for overall survival with several studies showing improvement in both domains with treatment. RCTs should incorporate evaluation of treatment impact on patients’ PF and fatigue as important measures of effectiveness.
Keywords: acute myeloid leukemia, hematology, unfit, low intensity, hypomethylating agents, myelodysplastic syndrome
Introduction
AML is generally a disease of older people and is uncommon before the age of 45 years.1,2 Within USA, the average age of a patient with AML is 68 years, with about 19,520 new cases of AML patients and 10,670 deaths from AML.3 AML is an aggressive disease with an unfavorable prognosis and accounts for 25% of acute leukemias in adults worldwide, with an estimated 5-year survival of 26% in USA.3,4 Prior epidemiological research has shown that age >70 years was the strongest predictor to receive nonintensive treatment compared to IC in patients newly diagnosed with AML based in an academic population-based registry study, whereas younger age (<60 years) was inversely associated with IC.5 Treatments for these patients are limited, particularly for those with poor performance status, comorbidities, and unfavorable cytogenetic abnormalities.6–8
The standard of care for fit patients with AML is IC, which includes the “7+3” regimen, comprising 7 days of treatment with cytarabine combined with 3 days of treatment with an anthracycline.7,9,10 For unfit patients with AML who are not eligible for IC, the NCCN guidelines include therapy with HMA such as decitabine, azacitidine, and low-dose cytarabine (LDAC); similarly, the ELN guidelines state that treatment alternatives for unfit patients are limited to BSC, low-intensity treatment, or clinical trials with investigational drugs.11,12 Low-intensity options are either LDAC or therapy with HMA. LDAC is generally well tolerated and produces complete remission rates in the order of 15–25%; however, overall survival (median, 5–6 months) is unsatisfactory.12 Even though AML is primarily a disease of older adults,1,2,7,13 age is not the only factor for determining treatment with intensive or NIC or BSC. Treatment decisions for patients with AML may be impacted by patient-specific factors such as cytogenetics, initial blood counts, performance status, comorbidities, daily life activities that have led to the changes in chemotherapy regimens, treatment-related mortality, and toxicity risk.13
It has also become increasingly important to assess the impact of AML and its treatments on HRQoL since not all patients are eligible for IC. The awareness of HRQoL is a broad concept that covers different domains such as physical, mental, social, and role functioning.14,15 Information about the impact on HRQoL can be used for different purposes. First, this information is useful for treatment allocation; currently, treatment allocation in AML depends largely on the effectiveness of the different treatments in terms of survival.14,16,17 Furthermore, HRQoL information provides insight into specific health problems and treatment needs of patients with AML. The identification of these health problems can help in the effort to improve current treatments and develop new treatment modalities.14,18,19 For example, results from a prospective evaluation indicated that negative effects of treatment on MDS patient’s quality of life were limited to the time in the hospital, specifically intensively treated patients spent 79% of their remaining lifetime in hospitals, whereas nonintensively treated patients spent 14%.20,21 The HRQoL of these patients and their ability to function improved since they left the hospital and scores after discharge were similar as to pretreatment scores.20,21 The symptom burden for AML patients is significant and involves some of the following: general (weight loss/loss of appetite, fever); low count of red blood cells (anemia); tiredness/fatigue, weakness, and dyspnea (shortness of breath); low count of white blood cells (leukopenia/neutropenia): infections, fever; low count of blood platelet counts (thrombocytopenia): excess bruising/bleeding; increase in blast counts; disturbed sleep; and dry mouth.6–8 To the authors’ knowledge, there are no prior systematic reviews published evaluating the impact of disease and treatment on the HRQoL in AML patients who are not eligible for IC. The aim of this study was to conduct a SLR to determine the reported HRQoL among patients with AML receiving NIC.
Materials and methods
A SLR of evidence was conducted on HRQoL reported in patients with AML receiving NIC using matches on prespecified PICOS approach. In addition, the PRISMA was used as a guide to ensure that the current standard for systematic review methodology was met.22 In this review, Embase, Medline, and the Cochrane collaboration databases were searched using the Ovid platform, covering ~10 years, from January 2007 to November 2017 to identify relevant studies reporting HRQoL and patient preference utilities and to ensure that all relevant studies included the HMA regimens used in NIC AML. Systematic reviews and meta-analyses were utilized for bibliography searching to identify additional relevant studies. In addition, conference abstracts were searched to retrieve studies that had not yet been published as full-text articles and to supplement results of previously published studies. Abstracts from the American Society of Clinical Oncology, European Hematology Society, European Society of Medical Oncology, and American Society of Hematology for the period 2014–2017 were searched. The detailed search strategy is presented in Table S1. For disease, “LEUKEMIA, MYELOID”, “ACUTE/”, “LEUKEMIA, MYELOID/”, and ACUTE DISEASE/” were the MeSH terms we used. For the quality of life, we used “quality of life/” and “quality adjusted life year/” as MeSH terms.
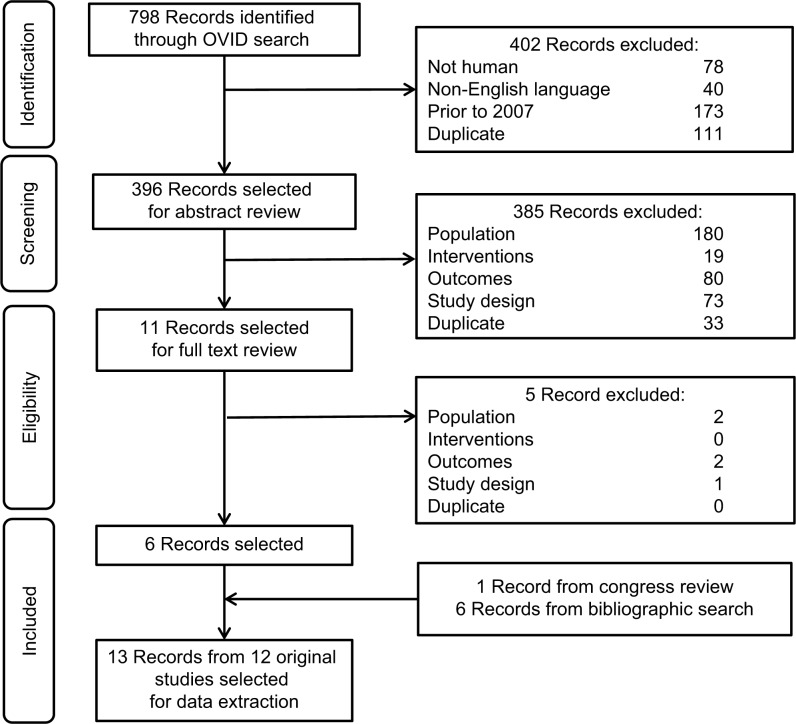
Study designs that were likely to report HRQoL and utility data for AML were included in this review. Based on the WHO AML criteria, studies on patients with RAEB-t MDS (≥20% bone marrow blast) also were included. Studies that did not have NIC AML populations and those not reporting HRQoL were excluded. Only publications written in English and published starting from January 2007 were considered since evidence older than 10 years may not be relevant as treatment practice may have changed. Shortlisted articles were initially assessed based on title and abstract. Publications not meeting inclusion criteria were excluded and listed along with the reason for study exclusion. Full-text publications were then retrieved and assessed based on the full text. Publications identified through the systematic review were evaluated in a three-step process (abstract review, full text review, and data extraction) to assess whether they should be included for data extraction. The inclusion and exclusion criteria used against the publications were developed using the PICOS format (Table 1). All steps were conducted by two independent reviewers, and any discrepancies in article selection were reassessed by a third reviewer. After the full-text review, all papers meeting inclusion criteria were retained for data extraction. Papers that were excluded in each step were listed, along with their reason for exclusion was documented for use in the PRISMA flow diagram (Figure 1).
Table 1.
Study eligibility criteria
| Element | Inclusion | Exclusion |
|---|---|---|
| Patient population | • Adults (≥18 years) • Newly diagnosed with AML or high risk MDS • Not eligible for IC |
• Nonhuman • Refractory/relapsed AML • AML treated with IC |
| Intervention and comparators | • Glasdegib • Azacitidine • Decitabine • Cytarabine (low dose) • Hydroxycarbamide • 6-Mercaptopurine • Etoposide • BSC |
• Studies not including any therapies of interest • Stem cell transplantation studies • Surgery studies • Radiotherapy studies |
| Outcomes measures | • Any HRQoL outcome • Utilities/disutilities/QALYs for health states or adverse events |
• Studies not including at least one of the outcomes listed in the inclusion criteria |
| Study design | • Reports of randomized clinical trials assessing HRQoL • Development and/or validation of HRQoL measures • Observational studies measuring PROs • Retrospective chart audits and database analyses reporting PROs • Patient surveys reporting PROs • Reports of mapping exercises for any outcome measure to utility • Reports of utility elicitation exercises • Reports of utility validation exercises • Reports of economic evaluations using utility measures elicited during studies • Studies that present data via a validated scale • Systematic reviews and meta-analyses (to be used for reference cross-checking only) |
• Reviews • Editorials • Notes/comments/letters |
| Restrictions | • Published in the English language • Year limitations: 2007 to present |
• Published in a non-English language |
Note: Utility studies were not limited to AML noneligible for IC.
Abbreviations: PROs, patient-reported outcomes; QALY, quality-adjusted life year.
Figure 1.
PRISMA flow diagram
The quality assessment for the HRQoL studies was assessed using the Efficace framework, which aims to determine the robustness, consistency, and relevance of such studies to support decision-making.23 The search for this SLR was conducted in December 2017, and the search strategy is provided in Table S1.
Results
A total of 13 records from 12 original studies were identified, which are listed in Table 2. These included five records from four original randomized clinical trials (RCTs), three prospective studies, four patient survey studies, and one cost-effectiveness analysis reporting utility values. Ten studies utilized the EORTC QLQ-C30 and five studies reported EQ-5D values. Other scales used included HRQoL questionnaire for patients with hematological diseases (QoL-E), QoL Cancer Survivor, FACT-leukemia, FACT-fatigue, global fatigue scale, FACIT Fatigue, activities of daily living index, and Hospital Anxiety and Depression Scale. The following four QLQ-C30 domains were considered most relevant: fatigue, PF, GHS, and dyspnea.9 A 10-point minimally important difference threshold on a 100-point scale was assumed by the majority of studies to represent meaningful change.9
Table 2.
Included studies
| References | Interventions | Type of study/study population | Scales used | QoL summary |
|---|---|---|---|---|
| Minden et al26 (abstract); Dombret et al9 | AZA vs CCR (standard induction chemotherapy, low-dose cytarabine, or supportive care only) | RCT, N=488; age ≥65 years, newly diagnosed AML, not eligible for HSCT | EORTC QLQ-C30 | 157 AZA patients and 134 CCR patients were evaluable for HRQoL. AZA or CCR showed general improvement in the four relevant domains. No HRQoL detriment was seen with AZA or CCR at the group level during treatment. “Few” statistically significant (P<0.05). “Fewer” met the MID threshold. CCR achieved meaningful improvement in fatigue (cycles 7 and 9) and GHS/QoL (cycle 9). Patients receiving AZA achieved meaningful improvement in fatigue (cycle 9). Scores varied substantially among individual patients in both treatment groups |
| Oliva et al24 (abstract) | AZA vs BSC after conventional induction (3+7) and consolidation chemotherapy | RCT, N=99; age >60 years, newly diagnosed or secondary AML (>30% myeloid marrow blasts), ECOG PS <3 | EORTC QLQ-C30, QoL-E (ver 3) | After first "3+7" regimen; QoL-E: no changes; QLQ-C30: deterioration in PF (median 80, IQR 60–93, to 67, IQR 52–87, P=0.008), role function (median 83, IQR 67–100, to 67, IQR 33–83, P=0.023), and GHS (median 50, IQR 33–69, to 67, IQR 50–75, P=0.002) and improvement in dyspnea (P=0.023). After consolidation therapy, among patients obtaining a CR QoL-E: improvement in median physical scores (56, IQR 41–72, to 63, IQR 50–84, P=0.033), disease-specific domain scores (59, IQR 48–67, to 74, IQR 67–85, P=0.003), and treatment outcome index scores (55, IQR 32–77, to 79, IQR 41–86, P=0.026); QLQ-C30: improvement in emotional function (83, IQR 67–92, to 92, IQR 77–100, P=0.015), GHS (median 50, IQR 33–65, to 67, IQR 58–83, P=0.002). Dyspnea and insomnia regressed while financial problems increased |
| Lübbert et al17 | DEC + BSC vs BSC | RCT, N=233; Age ≥60, MDS or CMML, int-1, int-2, or high-risk, ineligible for intensive treatment, ECOG ≤2 | EORTC QLQ-C30 | Patients on the DEC arm showed a significant improvement in their physical functioning and borderline improvement in GHS. No apparent effect was seen on dyspnea. Trends of most of QoL scales favors DEC |
| Sekeres et al25 | LDAC + lintuzumab vs LDAC | RCT, N=211; age ≥60 years, de novo AML, exposed to chemotherapy for different malignancies, ECOG PS ≤2 | FACT-Leu | No consistent pattern of change in FACT-Leu score was observed. The median change in FACT-Leu score was similar in both arms where the range of scores overlapped considerably |
| Tseng et al27 (abstract) | AZA | Prospective observational, N=56; MDS, treated with AZA | EORTC QLQ-C30, FACT-fatigue, EQ-5D, and a global fatigue scale | 50 were evaluable for QoL. Clinically important differences were seen in physical, role, cognitive, and social functioning, GHS between responders and nonresponders (all higher in responders). Responders had significantly superior GHS (P=0.001) and EQ-5D scores (P=0.0002) and lower levels of fatigue (P<0.0001) |
| Ingber et al28 (abstract) | AZA | Prospective observational, N=20; MDS, treated with AZA | EORTC QLQ-C30, EQ-5D, global fatigue scale | The only clinically significant improvements were observed with the EORTC physical functioning and fatigue subscales but constipation scores were higher and GHS/QoL deteriorated over time. |
| Oliva et al29 | Intensive therapy vs palliative treatment | Prospective observational, N=113; age ≥60, newly diagnosed de novo AML | EORTC QLQ-C30 (ver. 3) and QoL-E (ver. 2) | At diagnosis, the median QoL-E general standardized score 54 (IQR 46–70)/median EORTC QLQ-C30 global score decreased 50 (IQR 41–66) Fatigue in QoL-E median 45 (IQR 32–53)/QLQ-C30 median 33 (IQR 22–66) Loss of appetite was perceived by 75% of patients |
| Deschler et al30 | BSC vs (HMA vs IC + HCT | Patient survey, N=195 | EORT QLQ-C30 and ADL (Barthel index) | At baseline, median EORTC QLQ-C30 fatigue (BSC vs HMA vs IC/HCT vs total): 53.3 vs 66.6 vs 44.3 vs 53.3, median ADL (Barthel index) (BSC vs HMA vs IC/HCT vs total): 100 vs 100 vs 100 vs 100 |
| Pandya et al31 (abstract) | NA | Patient survey, N=75; AML, 1L or R/R (75% [n=56] first line vs 25% [n=19] relapsed/refractory AML) | FACT-Leu and EQ-5D-3L | First-line patients may have a directionally better QoL scores than those on later lines of therapy 1L vs R/R: EQ-5D =0.75 vs 0.71 (P=0.51) and the FACT-Leu =103.7 vs 92.5 (P=0.098). R/R patients were significantly more likely than first-line patients to be affected physically by their AML condition 1L vs R/R: FACT-Leu-physical well-being sub-domain =13.0 vs 17.6, P=0.005 |
| Cheng et al34 (abstract) | NA | Patient survey, N=18; AML, achieved the first CR | EORTC QLQ-C30, QoL-CS, FACIT-fatigue, and HADS | Participants scored well on the EORTC QLQ-C30. The FACIT-fatigue (worst 0 to best 52) mean score was 28.7 and the median score was 33.5 (normal ≥30). On the HADS anxiety scale, two participants scored in the abnormal range. On the QoL-CS, participants scored above 6/10 in all domains, with exceptions of the psychological subscales of distress and fear (physical 8.7/psychological 7.9/distress 4.7/fear 4.5/social 7.1/spiritual 7.4) |
| Leunis et al14 | NA | Patient survey, N=92; AML survivors vs general population | EQ-5D and EORTC QLQ-C30 | The majority of the patients with AML reported problems on the five functioning scales of the QLQ-C30. The average scores on all functioning scales were significantly lower in patients with AML compared to adjusted general population scores. The differences in physical, role, cognitive, and social functioning were also clinically relevant. Despite these differences, no significant difference was found for the global quality of life |
| Levy et al33 | AZA vs CCR (BSC alone, low-dose chemotherapy + BSC, and standard-dose chemotherapy + BSC) | Utility, CEA; AML survivors vs general population | EQ-5D mapped from EORTC QLQ-C30 and SF-6D mapped from SF-12 | The utility analysis results show that, compared with patients receiving BSC, patients treated with AZA had a better quality of life and the difference increased with increasing length of treatment |
Note: SF-6D and SF-12, short form health surveys.
Abbreviations: ADL, activities of daily living; AZA, azacitidine; CEA, cost-effectiveness analysis; CCR, conventional care regimens; CMML, chronic myelomonocytic leukemia; CR, complete response; DEC, decitabine; ECOG PS, The Eastern Cooperative Oncology Group performance status; EQ-5D-3L, EuroQol 5-dimensions 3-levels; FACT-fatigue, Functional Assessment of Cancer Therapy-fatigue; FACT-Leu, Functional Assessment of Cancer Therapy-leukemia; HADS, Hospital Anxiety and Depression Scale; HCT, hematopoietic cell transplantation; HSCT, hematopoietic stem cell transplantation; MID, minimally important difference; NA, Not applicable; QoL, quality of life; QoL-CS, quality of life cancer survivor; RCT, randomized clinical trials; R/R, relapsed/refractory.
The HRQoL results from the four original RCTs informed the reviewers that some NIC treatments achieved a meaningful improvement in the fatigue of EORTC QLQ-C30, while other patients achieved meaningful improvement in both fatigue (cycles 7 and 9) and GHS.9,17,24–26 Patients aged ≥60 years with MDS or chronic myelomonocytic leukemia (intermediate 1/2, or high risk), who were ineligible for intensive treatment, showed a significant improvement in their PF and borderline improvement in GHS of EORTC QLQ-C30.17
There were similar findings from HRQoL prospective studies, of which one study conducted a longitudinal, observational prospective assessment of the EORTC QLQ-C30, FACT-fatigue, EQ-5D, and global fatigue scale in patients with MDS treated with azacitidine. The results informed the reviewer that responders to therapy had significantly superior EQ-5D scores (P=0.0002) and lower scores in FACIT-fatigue (P<0.0001) vs patients who did not respond to therapy.27 Another longitudinal study with MDS patients found that clinically significant improvements were achieved in the physical functioning and fatigue subscales of EORTC QLQ-C30.28 The final reviewed prospective study evaluated the relationship between HRQoL and survival where it was found that HRQoL scores at diagnosis discriminated patients according to overall survival.29 Patients with low scores including functional, PF, role function, and fatigue scores (<60) had shorter survival compared to those with higher scores: QOL-E functional score (median 15 weeks, 95% CI 12–17 weeks vs 55 weeks, 95% CI 42–69 weeks; P=0.002), QOL-E physical score (median 18 weeks, 95% CI 0–37 weeks vs 60 weeks, 95% CI 34–87 weeks; P=0.038), EORTC QLQ-C30 PF (median 14 weeks, 95% CI 5–24 weeks vs 60 weeks, 95% CI 44–77 weeks; P<0.0001), EORTC QLQ-C30 role function (median 21 weeks, 95% CI 7–36 weeks vs 55 weeks, 95% CI 32–79 weeks; P=0.015), and EORTC QLQ-C30 fatigue score (median 14 weeks, 95% CI 13–15 weeks vs 55 weeks, 95% CI 46–65 weeks; P=0.004).29
The HRQoL results from the four patient survey studies presenting the median fatigue scores of EORTC QLQ-C30 were 53.3, 66.6, and 44.3 in newly diagnosed MDS and AML patients (aged ≥60 years) receiving BSC, HMAs, and IC, respectively. The score in all patients was 53.3.30 Relapsed/refractory patients were significantly more likely to be affected physically than patients with first-line disease;31 the utility value for first-line patients were higher (EQ-5D =0.75) vs relapsed/refractory patients (EQ-5D =0.71) suggesting that first-line patients may have had better HRQoL scores than those on later therapies.31 Fatigue and distress (followed by pain) were the symptoms reported most often among patients with AML and MDS.32 According to Leunis et al,14 fatigue was the most frequently reported symptom in patients with AML (78%) and other frequently reported symptoms were pain, dyspnea, insomnia, and financial difficulties. Patients with AML had significantly more problems with fatigue, pain, dyspnea, and appetite loss than the general population.14 The utility value for the overall population was 0.82, patients with relapse had lower utility values vs those without a relapse (0.78 vs 0.83) and there was no much difference seen in the utility values between patients receiving high-dose chemotherapy plus hematopoietic stem cell transplantation (HSCT) vs HSCT alone.14 Overall, at baseline, NIC AML patients had poor HRQoL scores especially in fatigue (33) and GHS (50) on a 0–100 scale, with higher scores indicating better health. Low baseline HRQoL scores, especially PF and fatigue (<50) were shown to be significant independent predictors of poor survival. Clinical responders demonstrated meaningful improvements in QLQ-C30 physical, role, cognitive, and social functioning, GHS, fatigue, and EQ-5D scores from baseline after being treated with chemotherapy. Clinically meaningful and significant improvements in fatigue and PF were observed with nonintensive chemotherapeutic agents across several of these studies.
The single cost-effectiveness analysis compared an HMA with conventional care regimens, including BSC, and low or standard-dose chemotherapy plus BSC in the treatment of higher risk AML with 20–30% of blasts. The utility analysis results show that, compared with patients receiving BSC, patients treated with the HMA had a better quality of life with the utility values increasing from day 0 onward to day 183, and the difference increased with increasing length of treatment; the utility values for patients treated with the HMA were 0.67 at baseline and increased to 0.80 at day 182 vs BSC with the utility value of 0.67 at baseline and increased to 0.72 at day 182.33
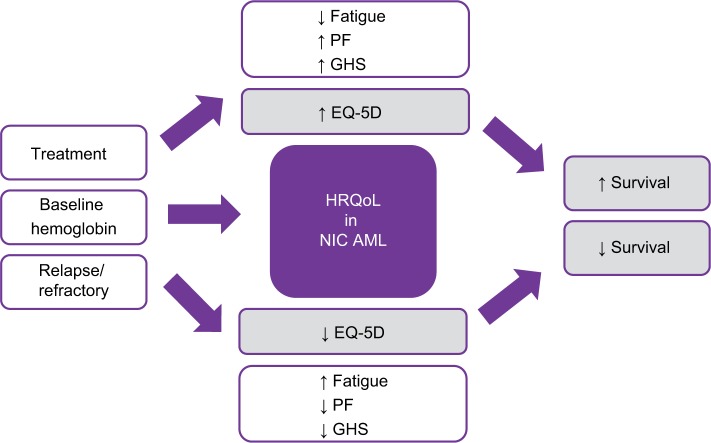
Interestingly, we found that baseline factors such as fatigue, gender, comorbidities, bone marrow blasts, and secondary AML were not related to HRQoL in one study.24 However, other studies showed these factors along with others to be correlated to HRQoL, such as baseline hemoglobin (Hg) levels and scores of QOL-E functional (r=0.0216, P=0.14), fatigue (r=0.256, P=0.002), and disease specific (r=0.247, P=0.010) were shown to be statistically correlated. Furthermore, scores from the EORTC QLQ-C30 of GHS (r=0.270, P=0.001), physical (r=0.304, P<0.0001), role (r=0.281, P=0.001), cognitive (r=0.262, P=0.003), social (r=0.229, P=0.010), functions and fatigue (r=−0.280, P=0.001), dyspnea (r=−0.287, P=0.001), and appetite loss (r=0.244, P=0.007) were statistically related to HRQoL. Age was also found to be correlated with QOL-E disease-specific scores (r=0.242, P=0.012). Finally, higher scores in HRQoL can be seen over time if transfusion dependence status is collected at the time of HRQoL assessments, displaying a possible strong HRQoL relationship between response and transfusion dependence, which could be a factor for NIC AML patients (Figure 2).27
Figure 2.
Impact of HRQoL in NIC AML
Discussion
HRQoL is a multi-dimensional concept encompassing the patient’s perception of functioning and well-being. To account for this complexity, HRQoL instruments capture certain domains, at a minimum, physical, emotional, and social functioning. Collecting and publishing HRQoL data are very important to both understand the patient’s perspective and evaluate any impact or correlation of treatment that may have on a patient’s condition. Currently, there are no prior SLRs focusing on HRQoL data in NIC AML patients; therefore, the need for this information is valuable. In this SLR, we identified only 12 studies reporting HRQoL and patient preference utilities in NIC AML patients; this clearly demonstrates that there is scant published literature on the impact of disease and treatment on the HRQoL in NIC AML patients. Having an instrument that succinctly and reliably captures the impact of AML disease and treatment in the NIC AML population would help investigators incorporate HRQoL endpoints into clinical trials and help inform health care decision makers to make better treatment plans. Helping people to maintain or have improvement in HRQoL and to live longer is clearly a goal of AML therapy, even for patients not eligible for IC. Failing to understand the HRQoL implications of different treatments may mean that it could be difficult to provide this information to future AML patients facing decisions who are not eligible for IC.
Although there is scarcity of published HRQoL reported data in the NIC AML patients, from the few studies that were reviewed, we found that HRQoL was correlated with better overall survival.9,17,26,33 Survival was independently predicted by the QoL-E scores of PF when controlling for factors such as age, concomitant diseases, and treatment options.29 Patients with short MDS duration had worse outcomes and was shown to be an independent adverse prognosticator.17 Even though HRQoL is highly subjective, this subjectivity can be alleviated since majority of these studies were randomized controlled trials. It has become clear that the role of HRQoL in the elderly AML patients has value at diagnosis as a prognostic factor for overall survival and, thus, a potential variable that may be integrated in the process of decision-making for treatment allocation.25
Understanding baseline factors can assist with individual patient treatment allocation and can provide additional information to a physician’s assessment. For example, older age, impairments in activities of daily living, Karnofsky index <80%, and HRQoL/fatigue ≥50 are likely to have poor outcomes.30 Also, NIC AML patients had poor HRQoL scores (<50) on a 0–100 scale, especially in fatigue (33) and GHS (50), which were shown to be significant independent predictors of poor survival.30 Prior studies, as Deschler et al, have found patient characteristics such as fatigue, PF, gender, comorbidities, bone marrow blasts, and secondary AML to be highly related to HRQoL outcomes; however, we found additional baseline HRQoL parameters to be possible independent prognostic factors in AML patients such as Hg level, age, and transfusion dependence. Including these additional baseline parameters when collecting HRQoL assessment could facilitate decision makers to assist with better treatment outcomes for NIC AML patients.
Patients diagnosed with AML are older and generally have poor prognosis. While treatment may extend overall survival for patients with AML, it may also cause significant toxicity and impairment of HRQoL;17,26,34 therefore, using less IC agents has shown to be associated with general improvement in HRQoL in the relevant domains of fatigue, PF, and GHS.14,24 Other studies observed clinically meaningful and significant improvements in fatigue and PF with nonintensive chemotherapeutic agents.17 These studies discussed how clinical responders demonstrated meaningful improvements in QLQ-C30 physical, role, cognitive, and social functioning, GHS, fatigue, and EQ-5D scores from baseline after being treated with nonintensive chemotherapeutic agents.
Finally, AML patients who have relapsed or become refractory to first-line treatment report worse HRQoL than those still on first-line treatments.31 These observational data showed a need for effective and tolerable treatments that can maintain or improve patients’ HRQoL, especially for patients with relapsed or refractory disease. Thus, it is important to understand the impact of AML on patients receiving first-line treatment vs those who were relapsed/refractory to first-line treatment.31 Although there is heterogeneity of data reporting across published studies, there is a consistent message that the HRQoL is poor, worsened by comorbidities, disease progression, or relapse in disease. Utility values and HRQoL have shown improvement with successful treatment; therefore, therapies that can help control symptom burden without negative adverse events and prevent relapses are needed.
Conclusion
HRQoL plays a crucial role in the treatment of AML patients. Currently, there are no prior SLRs conducted in evaluating HRQoL within NIC AML patients and from this SLR exercise, we found very scant literature assessing this information. Fatigue and PF at baseline have been identified as independent prognostic factors for overall survival with several studies showing improvement in both domains with treatment. Alongside the evaluation of treatment-related efficacy and safety, randomized controlled studies should also incorporate and assess the impact of treatment on patient’s PF and fatigue with the aim to improve overall HRQoL.
Supplementary material
Table S1.
Search strategy
| 1 | exp LEUKEMIA, MYELOID, ACUTE/ | 79,787 |
| 2 | exp LEUKEMIA, MYELOID/ | 180,001 |
| 3 | exp ACUTE DISEASE/ | 323,400 |
| 4 | 2 and 3 | 9,745 |
| 5 | LEUKEMIA, MYELOID/ | 33,612 |
| 6 | ACUTE DISEASE/ | 323,400 |
| 7 | 5 and 6 | 7,711 |
| 8 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. | 2,725,487 |
| 9 | ((myelo$ or mielo$ or nonlympho$ or granulocytic$) and (leuk?em$ or leuc$)).tw,kf,ot. | 233,145 |
| 10 | 8 and 9 | 136,561 |
| 11 | aml.tw,kf,ot. | 82,861 |
| 12 | (Acute Myeloid Leukemia$ or Leukemia$, or Leukaemia$ or Acute Myeloid or Myeloid Leukemia$, Acute).af. | 787,634 |
| 13 | (Leukemia$, Laukaemia$, Myelo$, Acute or Myelo$ Leukemia$, Acute or Acute Myelo$ Leukemia$).af. | 100,183 |
| 14 | (Leukemia$, Nonlympho$, Acute or Nonlympho$ Leukemia$, Acute or Acute Nonlympho$ Leukemia$).af. | 5,775 |
| 15 | (Leukemia$, Leukaemia$, Granulo$, Acute or Granulo$ Leukemia$, Acute or Acute Granulo$ Leukemia$).af. | 53,759 |
| 16 | or/12–15 | 787,634 |
| 17 | 1 or 4 or 7 or 10 or 11 or 16 | 798,957 |
| 18 | (glasdegib or azacitidine or decitabine or low dose cytarabine or LDAC or hydroxycarbamide or 6-mercaptopurine | 72,222 |
| or etoposide or best supportive care).ti,ab | ||
| 19 | 17 and 18 | 16,693 |
| 20 | Quality of Life/ | 573,654 |
| 21 | (QOL or HRQL or HRQOL).ab,ti | 139,382 |
| 22 | (patient adj2 reported adj2 outcome adj2 measure$).ti,ab | 139,382 |
| 23 | (utility or utilities).ti,ab | 425,239 |
| 24 | utility measure$.ti,ab | 1,380 |
| 25 | “quality of life”.de | 573,654 |
| 26 | “quality adjusted life year”.de | 20,701 |
| 27 | quality adjusted life year/ | 36,275 |
| 28 | (quality adj4 life).ti,ab | 651,934 |
| 29 | (qol or “disability adjusted life”).ti,ab | 103,635 |
| 30 | (qaly* or qald* or qale* or qtime* or daly*).ti,ab | 32,294 |
| 31 | (euroqol or eq5d or “eq 5d” or “eq5d” or “euro qual” or “euro qol” or euroqual).ti,ab | 26,629 |
| 32 | (EORTC$ or European Organi$ation for Research and Treatment of Cancer).ti,ab | 7,383 |
| 33 | (willingness adj4 pay).ti,ab | 11,776 |
| 34 | (standard adj1 gamble*).ti,ab | 2,015 |
| 35 | ((“time trade” adj1 off*) or (time adj1 tradeoff*) or tto or timetradeoff).ti,ab | 4,369 |
| 36 | (TTO or SG or WTP).ti,ab | 27,036 |
| 37 | ((valu* or measur*) adj4 (health or outcome or outcomes or effect or effects or change* or state*)).ti,ab | 1,088,035 |
| 38 | (preference* adj4 (patient* or public or valu* or measur*)).ti,ab | 51,709 |
| 39 | (multiattribute* adj1 (health or theor* or analys* or utilit*)).ti,ab | 356 |
| 40 | ((multi adj1 attribute*) and (attribute* adj1 theor*)).ti,ab | 3 |
| 41 | ((multi adj1 attribute*) and (attribute* adj1 analys*)).ti,ab | 17 |
| 42 | ((multi adj1 attribute*) and (attribute* adj1 utilit*)).ti,ab | 391 |
| 43 | (utilit* adj4 (valu* or measur* or health or life or estimat* or elicit* or disease)).ti,ab | 32,190 |
| 44 | ((symptom or symptoms) adj5 (score* or scale* or instrument* or measur*)).ti,ab | 179,539 |
| 45 | (FACT-G or “EORTC QLQ-C-30” or FLIC or QLI-CV).tw. | 4,589 |
| 46 | functional assessment of cancer therapy.tw. | 5,456 |
| 47 | or/20–46 | 2,411,950 |
| 48 | 19 and 47 | 809 |
| 49 | (letter or editorial or comment or news or newspaper article).pt. | 3,485,218 |
| 50 | animals/not (humans/and animals/) | 35,974 |
| 51 | case reports/ | 2,029,566 |
| 52 | in vitro.pt | 3 |
| 53 | or/49–52 | 11,256,953 |
| 54 | 48 not 53 | 798 |
| 55 | limit 54 to Humans | 720 |
| 56 | limit 55 to English | 680 |
| 57 | limit 56 to 2007–present | 507 |
| 58 | Deduplicate | 396 |
Acknowledgments
Parts of this study were previously published in an abstract submitted to the 2018 Congress of the European Hematology Association (https://learningcenter.ehaweb.org/eha/2018/stockholm/215952/anna.forsythe.health.related.quality.of.life.28hrqol29.in.acute.myeloid.leukemia.html). Editorial support was provided by Nazia Merritt at Purple Squirrel Economics. This study was funded by Pfizer.
Abbreviations
- AML
acute myeloid leukemia
- BSC
best supportive care
- ELN
European LeukemiaNet
- EORTC QLQ
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
- EQ-5D
EuroQOL-5 Dimensions
- FACIT
Functional Assessment of Chronic Illness Therapy
- FACT
Functional Assessment of Cancer Therapy
- GHS
global health status
- HMA
hypomethylating agents
- HRQoL
health-related quality of life
- IC
intensive chemotherapy
- LDAC
low-dose cytarabine
- MDS
myelodysplastic syndrome
- NCCN
National Comprehensive Cancer Network
- NIC
nonintensive chemotherapy
- PF
physical function
- PICOS
population, intervention, comparator, outcome and study design
- RAEB-t
refractory anemia with excess blasts in transformation
- SLR
systematic literature review
Footnotes
Author contributions
AF, CSK, and TB made substantial contributions to the conception and design of the study, analyzed and interpreted the data, and critically revised the article. TAS analyzed and interpreted the data and critically revised the article for important content. BA contributed to data interpretation and drafting of the article. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
TB, TAS, and BA are employees of Pfizer. AF and CSK are the employees of Purple Squirrel Economics who were paid consultants to Pfizer in connection with the conduct of the study and development of this article. The authors report no other conflicts of interest in this work.
References
- 1.Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol. 2008;35(4):430–438. doi: 10.1053/j.seminoncol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M, Swedish Acute Leukemia Registry Group Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119(17):3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute Myeloid Leukemia (AML) [homepage on the Internet] [Accessed April, 2018]. Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about.html.
- 4.Maynadié M, De Angelis R, Marcos-Gragera R, et al. HAEMA-CARE Working Group Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica. 2013;98(2):230–238. doi: 10.3324/haematol.2012.064014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel G, Weber D, Fromm E, et al. German-Austrian AML Study Group (AMLSG) Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO) Ann Hematol. 2017;96(12):1993–2003. doi: 10.1007/s00277-017-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SB, Park SW, Chung JS, et al. Hematology Association of South-East Korea (HASEK) study group Therapeutic decision-making in elderly patients with acute myeloid leukemia: conventional intensive chemotherapy versus hypomethylating agent therapy. Ann Hematol. 2017;96(11):1801–1809. doi: 10.1007/s00277-017-3104-9. [DOI] [PubMed] [Google Scholar]
- 8.Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–4424. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziogas DC, Voulgarelis M, Zintzaras E. A network meta-analysis of randomized controlled trials of induction treatments in acute myeloid leukemia in the elderly. Clin Ther. 2011;33(3):254–279. doi: 10.1016/j.clinthera.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . NCCN Guidelines for Acute Myeloid Leukemia Version 2 ed Plymouth Meeting. PA: National Comprehensive Cancer Network; 2016. [Accessed December 20, 2018]. Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. [Google Scholar]
- 12.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter RB, Estey EH. Management of older or unfit patients with acute myeloid leukemia. Leukemia. 2015;29(4):770–775. doi: 10.1038/leu.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leunis A, Redekop WK, Uyl-de Groot CA, Löwenberg B. Impaired health-related quality of life in acute myeloid leukemia survivors: a single-center study. Eur J Haematol. 2014;93(3):198–206. doi: 10.1111/ejh.12324. [DOI] [PubMed] [Google Scholar]
- 15.Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, Rothman M. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res. 2000;9(8):887–900. doi: 10.1023/a:1008996223999. [DOI] [PubMed] [Google Scholar]
- 16.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88(4):317–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 17.Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 18.Bevans M. Health-related quality of life following allogeneic hematopoietic stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010(1):248–254. doi: 10.1182/asheducation-2010.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efficace F, Novik A, Vignetti M, Mandelli F, Cleeland CS. Health-related quality of life and symptom assessment in clinical research of patients with hematologic malignancies: where are we now and where do we go from here? Haematologica. 2007;92(12):1596–1598. doi: 10.3324/haematol.11710. [DOI] [PubMed] [Google Scholar]
- 20.Deschler B, de Witte T, Mertelsmann R, Lübbert M. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: problems and approaches. Haematologica. 2006;91(11):1513–1522. [PubMed] [Google Scholar]
- 21.Pitako JA, Haas PS, van den Bosch J, et al. Quantification of outpatient management and hospitalization of patients with high-risk myelodysplastic syndrome treated with low-dose decitabine. Ann Hematol. 2005;84(Suppl 1):25–31. doi: 10.1007/s00277-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D’haese S, Zurlo A. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials-does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21(18):3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 24.Oliva EN, Salutari P, Candoni A. Quality of life in elderly patients with acute myeloid leukemia undergoing induction chemotherapy. Blood. 2015;126(23):2120–2120. [Google Scholar]
- 25.Sekeres MA, Lancet JE, Wood BL, Grove LE, Sandalic L, Sievers EL, Jurcic JG. Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica. 2013;98(1):119–128. doi: 10.3324/haematol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minden M, Dombret H, Seymour JF. The effect of azacitidine on health-related quality of life (HRQL) in older patients with newly diagnosed acute myeloid leukemia (AML): results from the AZA-AML-001 trial. Haematologica. 2015;22(100):40–41. [Google Scholar]
- 27.Tseng E, Wells RA, Alibhai SM. The effects of azacitidine on quality of life: a prospective longitudinal assessment. Blood. 2012;120(21):4938–4938. [Google Scholar]
- 28.Ingber SA, Thompson K, Lam A. The effects of azacitidine on quality of life measured longitudinally in MDS patients treated at a tertiary care center. Blood. 2010;116(21):2571–2571. [Google Scholar]
- 29.Oliva EN, Nobile F, Alimena G, et al. Quality of life in elderly patients with acute myeloid leukemia: patients may be more accurate than physicians. Haematologica. 2011;96(5):696–702. doi: 10.3324/haematol.2010.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–216. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya BJ, Hadfield A, Medeiros BC, et al. Quality of life of acute myeloid leukemia patients in a real-world setting. J Clin Oncol. 2017;35(15 Suppl):e18525. [Google Scholar]
- 32.Williams LA, Ahaneku H, Cortes JE. Comparison of symptom burden in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) Blood. 2014;124(21):2652–2652. [Google Scholar]
- 33.Levy AR, Zou D, Risebrough N, Buckstein R, Kim T, Brereton N. Cost-effectiveness in Canada of azacitidine for the treatment of higher-risk myelodysplastic syndromes. Curr Oncol. 2014;21(1):29–40. doi: 10.3747/co.21.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng MJ, Smith BD, Hourigan CS, et al. A single center survey of health-related quality of life among acute myeloid leukemia survivors in first complete remission. J Palliat Med. 2017;20(11):1267–1273. doi: 10.1089/jpm.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Search strategy
| 1 | exp LEUKEMIA, MYELOID, ACUTE/ | 79,787 |
| 2 | exp LEUKEMIA, MYELOID/ | 180,001 |
| 3 | exp ACUTE DISEASE/ | 323,400 |
| 4 | 2 and 3 | 9,745 |
| 5 | LEUKEMIA, MYELOID/ | 33,612 |
| 6 | ACUTE DISEASE/ | 323,400 |
| 7 | 5 and 6 | 7,711 |
| 8 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. | 2,725,487 |
| 9 | ((myelo$ or mielo$ or nonlympho$ or granulocytic$) and (leuk?em$ or leuc$)).tw,kf,ot. | 233,145 |
| 10 | 8 and 9 | 136,561 |
| 11 | aml.tw,kf,ot. | 82,861 |
| 12 | (Acute Myeloid Leukemia$ or Leukemia$, or Leukaemia$ or Acute Myeloid or Myeloid Leukemia$, Acute).af. | 787,634 |
| 13 | (Leukemia$, Laukaemia$, Myelo$, Acute or Myelo$ Leukemia$, Acute or Acute Myelo$ Leukemia$).af. | 100,183 |
| 14 | (Leukemia$, Nonlympho$, Acute or Nonlympho$ Leukemia$, Acute or Acute Nonlympho$ Leukemia$).af. | 5,775 |
| 15 | (Leukemia$, Leukaemia$, Granulo$, Acute or Granulo$ Leukemia$, Acute or Acute Granulo$ Leukemia$).af. | 53,759 |
| 16 | or/12–15 | 787,634 |
| 17 | 1 or 4 or 7 or 10 or 11 or 16 | 798,957 |
| 18 | (glasdegib or azacitidine or decitabine or low dose cytarabine or LDAC or hydroxycarbamide or 6-mercaptopurine | 72,222 |
| or etoposide or best supportive care).ti,ab | ||
| 19 | 17 and 18 | 16,693 |
| 20 | Quality of Life/ | 573,654 |
| 21 | (QOL or HRQL or HRQOL).ab,ti | 139,382 |
| 22 | (patient adj2 reported adj2 outcome adj2 measure$).ti,ab | 139,382 |
| 23 | (utility or utilities).ti,ab | 425,239 |
| 24 | utility measure$.ti,ab | 1,380 |
| 25 | “quality of life”.de | 573,654 |
| 26 | “quality adjusted life year”.de | 20,701 |
| 27 | quality adjusted life year/ | 36,275 |
| 28 | (quality adj4 life).ti,ab | 651,934 |
| 29 | (qol or “disability adjusted life”).ti,ab | 103,635 |
| 30 | (qaly* or qald* or qale* or qtime* or daly*).ti,ab | 32,294 |
| 31 | (euroqol or eq5d or “eq 5d” or “eq5d” or “euro qual” or “euro qol” or euroqual).ti,ab | 26,629 |
| 32 | (EORTC$ or European Organi$ation for Research and Treatment of Cancer).ti,ab | 7,383 |
| 33 | (willingness adj4 pay).ti,ab | 11,776 |
| 34 | (standard adj1 gamble*).ti,ab | 2,015 |
| 35 | ((“time trade” adj1 off*) or (time adj1 tradeoff*) or tto or timetradeoff).ti,ab | 4,369 |
| 36 | (TTO or SG or WTP).ti,ab | 27,036 |
| 37 | ((valu* or measur*) adj4 (health or outcome or outcomes or effect or effects or change* or state*)).ti,ab | 1,088,035 |
| 38 | (preference* adj4 (patient* or public or valu* or measur*)).ti,ab | 51,709 |
| 39 | (multiattribute* adj1 (health or theor* or analys* or utilit*)).ti,ab | 356 |
| 40 | ((multi adj1 attribute*) and (attribute* adj1 theor*)).ti,ab | 3 |
| 41 | ((multi adj1 attribute*) and (attribute* adj1 analys*)).ti,ab | 17 |
| 42 | ((multi adj1 attribute*) and (attribute* adj1 utilit*)).ti,ab | 391 |
| 43 | (utilit* adj4 (valu* or measur* or health or life or estimat* or elicit* or disease)).ti,ab | 32,190 |
| 44 | ((symptom or symptoms) adj5 (score* or scale* or instrument* or measur*)).ti,ab | 179,539 |
| 45 | (FACT-G or “EORTC QLQ-C-30” or FLIC or QLI-CV).tw. | 4,589 |
| 46 | functional assessment of cancer therapy.tw. | 5,456 |
| 47 | or/20–46 | 2,411,950 |
| 48 | 19 and 47 | 809 |
| 49 | (letter or editorial or comment or news or newspaper article).pt. | 3,485,218 |
| 50 | animals/not (humans/and animals/) | 35,974 |
| 51 | case reports/ | 2,029,566 |
| 52 | in vitro.pt | 3 |
| 53 | or/49–52 | 11,256,953 |
| 54 | 48 not 53 | 798 |
| 55 | limit 54 to Humans | 720 |
| 56 | limit 55 to English | 680 |
| 57 | limit 56 to 2007–present | 507 |
| 58 | Deduplicate | 396 |