Abstract
Objective
To characterize continuous EEG (cEEG) use patterns in the critically ill and to determine the association with hospitalization outcomes for specific diagnoses.
Methods
We performed a retrospective cross-sectional study with National Inpatient Sample data from 2004 to 2013. We sampled hospitalized adult patients who received intensive care and then compared patients who underwent cEEG to those who did not. We considered diagnostic subgroups of seizure/status epilepticus, subarachnoid or intracerebral hemorrhage, and altered consciousness. Outcomes were in-hospital mortality, hospitalization cost, and length of stay.
Results
In total, 7,102,399 critically ill patients were identified, of whom 22,728 received cEEG. From 2004 to 2013, the proportion of patients who received cEEG increased from 0.06% (95% confidence interval [CI] 0.03%–0.09%) to 0.80% (95% CI 0.62%–0.98%). While the cEEG cohort appeared more ill, cEEG use was associated with reduced in-hospital mortality after adjustment for patient and hospital characteristics (odds ratio [OR] 0.83, 95% CI 0.75–0.93, p < 0.001). This finding held for the diagnoses of subarachnoid or intracerebral hemorrhage and for altered consciousness but not for the seizure/status epilepticus subgroup. Cost and length of hospitalization were increased for the cEEG cohort (OR 1.17 and OR 1.11, respectively, p < 0.001).
Conclusions
There was a >10-fold increase in cEEG use from 2004 to 2013. However, this procedure may still be underused; cEEG was associated with lower in-hospital mortality but used for only 0.3% of the critically ill population. While administrative claims analysis supports the utility of cEEG for critically ill patients, our findings suggest variable benefit by diagnosis, and investigation with greater clinical detail is warranted.
Continuous EEG (cEEG), a valuable tool to assess brain function, is increasingly used for critically ill patients. Unlike neurologic examination, intracranial pressure monitoring, and many imaging modalities, cEEG provides noninvasive, real-time information about brain activity.1,2 The American Clinical Neurophysiology Society has specified cEEG indications: seizures and/or status epilepticus diagnosis, cerebral ischemia identification, level of consciousness assessment, and post–cardiac arrest prognostication.3 However, the effect of cEEG on outcomes for critically ill patients over the last decade has not been well characterized.4,5
cEEG is particularly advantageous for identifying seizures in patients who lack overt clinical symptoms.2,6,7 Subclinical or nonconvulsive seizures have been associated with both signs of neuronal injury (hippocampal atrophy,8 elevated intracranial pressure, increased lactate/pyruvate ratio9) and neurologic decline.10,11 Therefore, cEEG frequently leads to antiepileptic therapy administration and informs other diagnostic decisions.5,12–14 However, there is great practice variability in cEEG indication, initiation timing, length of use, and ensuing therapeutic interventions.15–17 Furthermore, cEEG is a resource-intensive endeavor.18,19 Understanding cEEG utility in clinical practice will help define its optimal use and thereby reduce variability in care and outcomes.
Moreover, as alternative payment models become increasingly prevalent, establishing the value of cEEG is critical.20,21 While the examination of cEEG value is complex and warrants multipronged inquiry, it is important to first characterize current use patterns and the association with outcomes. Insurance data are commonly and readily used in the measurement of value; therefore, we investigated the use and utility of cEEG for critically ill patients with administrative claims from 2004 to 2013.
Methods
Data
We used data from the National Inpatient Sample (NIS), the largest all-payer inpatient health care national database, made available by the Agency for Healthcare Research and Quality. Before 2012, the NIS included all discharges from a 20% sample of hospitals in the United States; starting in 2012, the NIS estimates a 20% sample of discharges from all hospitals in the United States. The NIS database contains deidentified information on patient demographics, measures of comorbidity, diagnoses, procedures, admission and disposition details, payer information, hospital characteristics, and total hospital charges. More than 7 million hospital discharges are reported annually in this database.22 Our study included discharges from 2004 to 2013.
Population
We identified the population of patients with critical illness, as defined by the ICD-9 procedure code for mechanical ventilation (x96.7).23 Exclusion criteria were patients admitted electively (to eliminate epilepsy monitoring unit admissions) and patients <18 years of age. A discharge diagnosis of cardiac arrest (427.5) also led to exclusion because that diagnosis warrants unique outcome considerations; specifically, cEEG is more commonly used in this population to inform prognosis rather than to aid diagnosis and management. Within our patient population, we defined the study sample as those patients who received cEEG (89.19) at some point during their hospitalization; the control sample comprised those critically ill patients who never received cEEG during hospitalization. Exclusive use of cEEG on the day of discharge led to exclusion from the study because in such cases cEEG use was less likely to change the course of the hospitalization (figure 1). Corresponding to the American Clinical Neurophysiology Society–proposed cEEG indications, we analyzed subgroups of patients by the following discharge diagnoses categories: seizure/status epilepticus (345.x, 780.3x),24 subarachnoid or intracerebral hemorrhage (430, 431),25 and altered consciousness (altered mental status 780.97; coma 780.0x, selected codes within 800.xx–854.xx; delirium 291.0, 292.81, 293.0, 293.1; encephalopathy 348.3x, 394.82, 437.2, 572.2, 768.7x). These subcategories were not mutually exclusive.
Figure 1. Study population flow diagram.
Application of inclusion and exclusion criteria. cEEG = continuous EEG; NIS = National Inpatient Sample.
Outcomes and variables
The main independent variable was use of cEEG, and the primary outcome was in-hospital mortality. Secondary outcomes were hospitalization cost and length of stay.
We considered patient characteristics of age group (18–39, 40–49, 50–59, 60–69, 70–79, ≥80 years), sex, race (white, black, Hispanic, Asian or Pacific Islander, Native American, other), primary payment source (Medicare, Medicaid, private insurance, self-pay, no charge, other), household income by ZIP code, Elixhauser comorbidity score (0–1, 2, 3, ≥4),26 palliative care consultation (V66.7), and year of discharge. Presence of routine EEG (rEEG) procedure code (89.14) was also noted. Hospital characteristics included geographic region (Northeast, Midwest, South, West), bed size, rural/urban location, and teaching status. Annual cEEG procedure volume and annual critically ill patient volume were collected for hospitals sampled from 2004 to 2011.
Statistical analysis
All statistics accounted for the complex survey design of the NIS to ensure proper confidence intervals (CIs). Descriptive statistics using χ2 tests were applied to assess the use of cEEG over time and to compare patient-related and hospital-related characteristics of the 2 cohorts. Multivariable logistic regression was performed to assess the association of patient-specific and hospital-specific characteristics with cEEG use. The associations of cEEG use with in-hospital mortality and cEEG use with hospitalization cost and length of stay were evaluated with multivariable regression analysis, which was adjusted for patient characteristics (age, sex, race, primary payer, household income, comorbidity score, palliative care consultation), hospital characteristics (geographic region, bed size, rural/urban location, teaching status), and year of discharge. Additional multivariable regression analysis was performed to investigate the effect of annual hospital cEEG volume on in-hospital mortality, which was adjusted for patient characteristics, hospital characteristics, and year of discharge. For binary outcomes, unconditional logistic regression was used. Because of violations of normality, we applied log transformation to cost and length-of-stay data. Statistical analysis was performed with Statistical Analysis System version 9.4 (SAS Institute Inc, Cary, NC). Hypothesis testing was 2 sided, and statistical significance was defined as p < 0.05.
Standard protocol approvals, registrations, and patient consents
The University of Pennsylvania Institutional Review Board approved this study.
Data availability
The full dataset is publicly available as the NIS via the Agency for Healthcare Research and Quality as part of the Healthcare Utilization and Cost Project.
Results
Demographics and use patterns
We identified a total of 7,102,399 critically ill patients discharged from 2004 to 2013, of whom 22,728 (0.3%) received cEEG. During this period, the proportion of mechanically ventilated patients who received cEEG increased from 0.06% (95% CI 0.03%–0.09%) to 0.80% (95% CI 0.62%–0.98%); similar increases were observed across all subgroups (figure 2).
Figure 2. Use of cEEG in critically ill discharges from 2004 to 2013 by diagnostic subcategory.
Numerator is the number of patients who underwent continuous EEG (cEEG); denominator is the total discharges with a particular diagnosis.
Despite this growth, cEEG was applied in the small minority of critically ill patients. Over the entire study period, only 1.71% (13,576 of 792,645) of patients with seizure/status epilepticus diagnosis, 0.94% (2,953 of 315,020) of patients with subarachnoid or intracerebral hemorrhage diagnosis, and 0.69% (12,393 of 1,806,218) of patients with altered consciousness diagnosis were assessed with cEEG.
Patient demographics and hospital characteristics of discharges who received cEEG and those who did not are presented in table 1. Patients who underwent cEEG were more likely to be younger (18–39 years: 19.4% vs 12.7%, ≥80 years: 10.8% vs 17.8%, p < 0.001), to have a higher median income (76th–100th percentile: 23.2% vs 19.3%, p = 0.045), and to be covered by Medicaid (19.8% vs 13.8%, p < 0.001) or private health insurance (25.7% vs 20.8%, p < 0.001). The cEEG cohort had higher comorbidity scores (score ≥4: 65.8% vs 56.4%, p < 0.001) and more frequent palliative care consultation (12.1% vs 5.3%, p < 0.001). A larger proportion of the cEEG cohort was located in the Northeast (33.3% vs 20.8%, p < 0.001) and Midwest regions (30.2% vs 21.2%, p < 0.001). Lastly, cEEG was more commonly performed in large hospitals (86.5% vs 67.8%, p < 0.001) and in urban teaching hospitals (94.9% vs 53.6%, p < 0.001) compared to nonteaching or rural hospitals.
Table 1.
Patient and hospital characteristics


Predictors of cEEG use
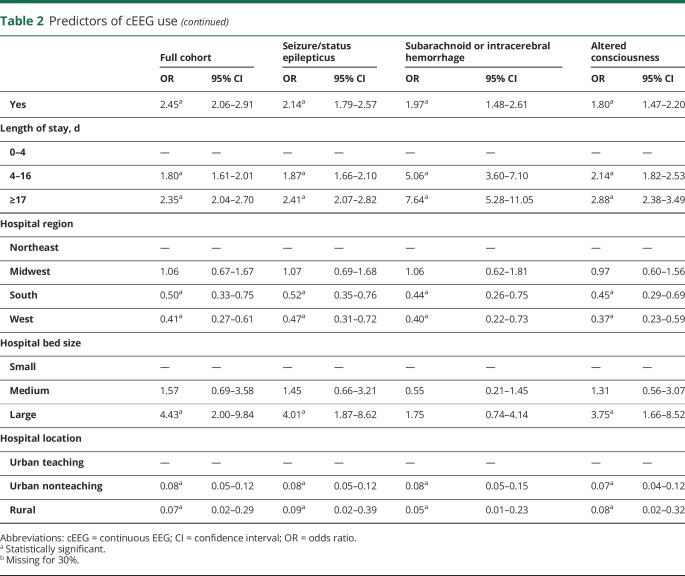
In multivariable logistic regression, predictors of cEEG use included younger age, female sex, private insurance, higher median income, greater comorbidity score, palliative care consultation, longer length of stay, Northeast or Midwest location, larger bed size, and urban teaching setting (table 2).
Table 2.
Predictors of cEEG use
Association with hospitalization outcomes
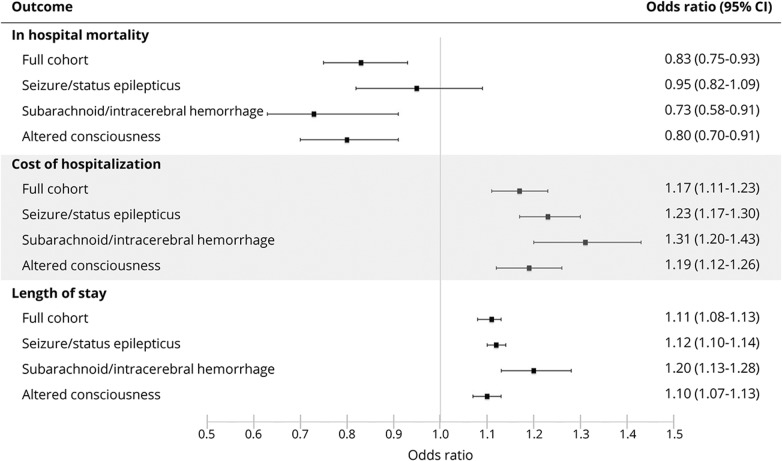
Mortality in the cEEG cohort was 22.8% vs 27.8% in the cohort without cEEG. The odds of in-hospital mortality were decreased for the cEEG cohort compared to controls (odds ratio [OR] 0.83, 95% CI 0.75–0.93, p < 0.001) in multivariable regression analysis adjusted for patient-specific and hospital-specific characteristics listed in table 1 and year of discharge (figure 3). A similar relationship was observed with use of cEEG in patients with subarachnoid or intracerebral hemorrhage (mortality 26.3% vs 54.0%, OR 0.72, 95% CI 0.58–0.91, p = 0.005) and patients with altered consciousness (mortality 21.5% vs 28.3%, OR 0.80, 95% CI 0.70–0.91, p < 0.001). However, cEEG use was not associated with a decreased risk of mortality in the seizure/status epilepticus subgroup (18.2% vs 19.8%, OR 0.95, 95% CI 0.82–1.09, p = 0.467). A closer look at this subgroup, in which patients with a seizure diagnosis (684,469 in total) were separated from patients with a status epilepticus diagnosis (108,176 in total), revealed lower mortality for patients with seizure who received cEEG compared to no cEEG (16.7% vs 20.2%) and higher mortality for patients with status epilepticus who received cEEG compared to no cEEG (20.6% vs 17.2%). Still, in our multivariate analysis, cEEG use was not associated with decreased mortality for either seizure or status epilepticus diagnosis (OR 0.87, 95% CI 0.74–1.02, p = 0.085 and OR 0.98, 95% CI 0.78–1.24, p = 0.868, respectively).
Figure 3. Hospitalization outcomes with cEEG use.
The reference group is no continuous EEG (cEEG). CI = confidence interval.
Use of cEEG was associated with an increase in median total hospitalization charges (OR 1.17, 95% CI 1.11–1.23, p < 0.001) and in median length of stay (OR 1.11, 95% CI 1.01–1.13, p < 0.001) in multivariable regression analysis adjusted for patient-specific and hospital-specific characteristics and year of discharge (figure 3). Median total hospital charges were $141,892 (interquartile range [IQR] $70,486–$273,564) for the cEEG cohort and $77,536 (IQR $37,557–$157,037) for the no cEEG cohort. Median hospital length of stay was 12.8 (IQR 6.2–23.6) and 8.7 (IQR 3.7–16.5) days, respectively. Similar relationships in hospitalization cost and length of stay were observed across all subgroups. The addition of rEEG as a covariate in the model resulted in no change in ORs or CIs for in-hospital mortality, hospitalization cost, and length of stay (table 3).
Table 3.
Analysis of hospitalization outcomes with cEEG use with rEEG covariate
cEEG volume
To examine the effect of hospital cEEG volume, we performed a subanalysis of data from 2004 to 2011 (during which time all discharges from a particular hospital were sampled). The proportion of cEEG use among critically ill patients was calculated for each year that a particular hospital was sampled. Of 3,054 unique hospitals, 93.9% (2,867) never used cEEG and were eliminated from further analysis. Remaining hospitals were organized into tertiles by cEEG volume, which resulted in 62 low-volume (>0%–0.2% of critically ill discharges), 63 medium-volume (>0.2%–0.8%), and 62 high-volume (>0.8%) hospitals. In a multivariable regression analysis adjusted for cEEG, patient-specific and hospital-specific characteristics, and year of discharge, the hospital cEEG volume was not associated with in-hospital mortality (figure 4). However, an association emerged when the interaction of cEEG and volume was included in the regression, which demonstrated decreased in-hospital mortality risk for patients receiving cEEG in high-volume hospitals (OR 0.78, 95% CI 0.66–0.92, p = 0.004).
Figure 4. Effect of hospital cEEG volume on in-hospital mortality (2004–2011).
The reference group is low volume. cEEG = continuous EEG; CI = confidence interval.
Discussion
In this study, we describe a >10-fold increase in cEEG use for critically ill patients from 2004 to 2013. However, cEEG may still be underused; although it was associated with lower odds of in-hospital mortality, only 0.3% of the critically ill population received cEEG. This benefit is particularly notable because, while the cEEG cohort was younger, these patients also appear to have been more ill, with higher comorbidity scores, more frequent palliative care consultation, and longer lengths of stay than the control cohort.
Our study uses diagnosis subcategories to better define who may benefit from cEEG. This subcategorization led to the unexpected finding that while cEEG was associated with decreased in-hospital mortality risk for the full cohort, the seizure/status epilepticus subgroup did not demonstrate this association. This subanalysis is most likely confounded by disease severity, which is suggested by the higher mortality observed in patients with status epilepticus who underwent cEEG compared to no cEEG. There are several important considerations. First, the diagnosis of seizure/status epilepticus identifies a highly heterogeneous group. Seizures are only a symptom of disease, and it has been well established in the status epilepticus literature that underlying pathology is the most critical determinant of outcome.27,28 Patients with lethal underlying etiologies of their seizures most likely do not benefit from further monitoring. In addition, there are a variety of seizure presentations in the critically ill, and it is not clear that all patients benefit from attempts at seizure treatment,29,30 although treatment often becomes inevitable once seizure is suggested by cEEG. This commitment to monitoring and prolonged therapy is particularly problematic in patients with EEG patterns on the ictal-interictal continuum (potentially harmful electrographic activity that does not meet strict seizure criteria), which may not have pathophysiology similar to more straightforward epileptic conditions.31 Further analysis of this subgroup in datasets with improved clinical resolution, particularly with regard to details of seizure presentation, illness severity, and underlying etiology, is necessary to elucidate for whom and by what mechanism cEEG is most beneficial. In addition, prospective studies that can assess a wider range of outcomes such as neurologic functional status and development of subsequent epilepsy are needed.
Not surprisingly, hospitals with larger bed size and urban teaching status were associated with cEEG use, as seen in prior studies.4 We also demonstrated variation across hospitals in the proportion of critically ill patients who undergo cEEG, which may indicate discrepancies in hospital resources, expertise, or medical sophistication. This raises the question of whether, despite adjustment for bed size and urban teaching status, unmeasured hospital characteristics of academic medical centers are driving the observed disparity in mortality between the cEEG and no cEEG cohorts. Notably, our analysis found that simply being at a medium-volume or high-volume hospital was not associated with improved survival; rather, it was necessary to additionally undergo cEEG in this setting to show associated benefit. Moreover, it appears that there may be associated survival advantage with receiving cEEG even at a medium-volume hospital.
Given our finding that cEEG was associated with lower risk of in-hospital death, we attempted to further investigate the value of cEEG by examining the costs associated with the procedure. While we found greater hospitalization costs and longer lengths of stay for the cEEG cohort (17% and 11% median increases, respectively), these may well be justifiable in the context of a procedure associated with substantial reduction in mortality risk (almost 20%). Explicit cost analysis, however, was not possible within the constraints of this dataset. With the rapid rise in use, further quantification of the value of cEEG in critically patients is imperative. This analysis would also be helpful in defining parameters for the application of automated EEG interpretation with seizure-detection algorithms, which may allow substantial cost and resource reduction.
Survival advantage associated with cEEG use was observed in a prior investigation that compared cEEG to rEEG use in critically ill patients using a smaller sample from the NIS (2005–2009).4 While it may be considered reassuring that both studies show a potential protective effect of cEEG in the critically ill, our study differs in several important ways. First, we chose not to define the control population as patients who received rEEG. While using rEEG suggests the need to assess brain function, desirable for the comparison group, a more concerning patient presentation would typically lead to the initiation of cEEG rather than rEEG. Therefore, the rEEG control group does not eliminate selection bias. To confirm this, we completed an additional regression analysis to assess the association of cEEG with outcome and added rEEG as a covariate, which did not change our results. Second, our study contains a larger sample size and includes the years during which there was a massive increase in cEEG use, suggesting that our results are more likely to reflect current use. The only other study to assess cEEG outcomes in critically ill patients was methodologically different: it prospectively recruited 234 critically ill patients who underwent cEEG compared to matched controls who did not undergo any EEG and found no differences in discharge disposition.5 This study, however, did not include admissions for seizure, which is a large proportion of cEEG use in the critically ill.
This study is limited by the typical challenges associated with administrative claims data: measuring codes rather than true clinical data. The ICD-9 procedure codes may not be the most comprehensive way to capture EEG use, leading to undersampling of the population of interest. In particular, we found that only 5.9% of the cEEG cohort had a procedure code for rEEG (and only 0.9% of the no cEEG cohort), which was lower than anticipated. We suspect that cEEG is also undercoded in this dataset; however, we believe procedure codes are most likely missing at random. Similarly, use of the procedure code for mechanical ventilation is specific but not sensitive in capturing critically ill patients.23 Diagnosis codes are associated with discharge and cannot be temporally related to procedure dates; furthermore, a diagnosis code could reflect a suspected diagnosis that was subsequently refuted (unfortunately, administration of antiepileptic medication, which would support a seizure diagnosis, is not captured in this dataset). In addition, because patients have multiple diagnosis codes, our subcategories are not mutually exclusive. There is, unfortunately, no perfect control group in which to retrospectively investigate the effect of cEEG in the critically ill population, and it is challenging to fully account for disease severity. While our control cohort is in several ways dissimilar to the case cohort, the measurable differences should bias toward the null hypothesis. As previously discussed, assessment of cost was greatly restricted by the absence of charge detail within this dataset and the limited estimate of total hospitalization cost (e.g., the reported charges do not include professional fees). Lastly, the assessment of the outcome of primary interest, mortality, was limited to the period of patient confinement; therefore, our results may describe only a fraction of the benefit of cEEG use that might be measured if data after hospital discharge were available. Furthermore, with this dataset, we were unable to assess change in functional status, which is arguably of even greater interest than survival.
Evaluation of cEEG use for critically ill patients with administrative claims data demonstrates an association with decreased risk of in-hospital mortality; therefore, continued expansion of cEEG may be warranted. However, the survival benefit appears to vary across subcategories of patients, and this particular methodologic approach is limited in its clinical resolution. Further investigations, both retrospective and prospective, will be crucial for developing a strong evidence base for cEEG practice to improve patient outcomes, to decrease variations in care, and to design informed payment structures.
Glossary
- cEEG
continuous EEG
- CI
confidence interval
- ICD-9
International Classification of Diseases, 9th revision
- IQR
interquartile range
- NIS
National Inpatient Sample
- OR
odds ratio
- rEEG
routine EEG
Footnotes
Podcast: NPub.org/rwghl3
Author contributions
Chloe Hill and Leah Blank contributed to study design, data analysis and interpretation, and manuscript drafting and revision. D. Thibault contributed to study design, data analysis, data interpretation, and statistical analysis. Kathryn Davis contributed to data interpretation and manuscript revision. N. Dahodwala contributed to study design, data interpretation, and manuscript revision. B. Litt contributed to study conceptualization, data interpretation, and manuscript revision. Allison Willis contributed to study design and conceptualization, data analysis and interpretation, and manuscript revision.
Study funding
The first authors were supported by the Mirowski Family Fund and the Family of Johnathan Rothberg (C.H.) and by the American Epilepsy Society/Epilepsy Foundation Research and Training Fellowship for Clinicians, as well as the Neurologic Clinical Epidemiology Training Grant (T32-NS-061779) (L.B.). Additional funding for this study came from the University of Pennsylvania Department of Neurology.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Scheuer ML. Continuous EEG monitoring in the intensive care unit. Epilepsia 2002;43(suppl 3):114–127. [DOI] [PubMed] [Google Scholar]
- 2.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg 2009;109:506–523. [DOI] [PubMed] [Google Scholar]
- 3.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I. J Clin Neurophysiol 2015;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005-2009: Neurology. Am Acad Neurol 2013;81:2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khawaja AM, Wang G, Cutter GR, Szaflarski JP. Continuous electroencephalography (cEEG) monitoring and outcomes of critically ill patients. Med Sci Monit 2017;23:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt SE. Utility of clinical features for the diagnosis of seizures in the intensive care unit. J Clin Neurophysiol 2017;34:158–161. [DOI] [PubMed] [Google Scholar]
- 7.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 8.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurol Am Acad Neurol 2010;75:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 10.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013;41:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol 2009;66:723–728. [DOI] [PubMed] [Google Scholar]
- 13.Abend NS, Topjian AA, Gutierrez-Colina AM, Donnelly M, Clancy RR, Dlugos DJ. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care 2011;15:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol 1999;16:1–13. [DOI] [PubMed] [Google Scholar]
- 15.Gavvala J, Abend N, LaRoche S, et al. Continuous EEG monitoring: a survey of neurophysiologists and neurointensivists. Epilepsia 2014;55:1864–1871. [DOI] [PubMed] [Google Scholar]
- 16.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care 2010;12:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez V, Rodriguez Ruiz AA, LaRoche S, et al. The use and yield of continuous EEG in critically ill patients: a comparative study of three centers. Clin Neurophysiol 2017;128:570–578. [DOI] [PubMed] [Google Scholar]
- 18.Abend NS, Topjian AA, Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures?. J Clin Neurophysiol 2015;32:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. Electroencephalogram monitoring in critically ill children: indications and strategies. Pediatr Neurol 2012;46:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelick PB. Adaptation of neurological practice and policy to a changing US health-care landscape. Lancet Neurol 2016;15:444–450. [DOI] [PubMed] [Google Scholar]
- 21.Committee on the Learning Health Care System in America; Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 22.HCUP-US NIS overview [online]. Available at: hcup-us.ahrq.gov/nisoverview.jsp. Accessed October 3, 2017.
- 23.Kerlin MP, Weissman GE, Wonneberger KA, et al. Validation of administrative definitions of invasive mechanical ventilation across 30 intensive care units. Am J Respir Crit Care Med 2016;194:1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid AY, St Germaine-Smith C, Liu M, et al. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res 2012;102:173–179. [DOI] [PubMed] [Google Scholar]
- 25.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 27.Sutter R, Kaplan PW, Rüegg S. Outcome predictors for status epilepticus: what really counts. Nat Rev Neurol 2013;9:525–534. [DOI] [PubMed] [Google Scholar]
- 28.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol 2015;14:615–624. [DOI] [PubMed] [Google Scholar]
- 29.Litt B, Wityk RJ, Hertz SH, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia 1998;39:1194–1202. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch LJ. Finding the lesser of two evils: treating refractory status epilepticus. Epilepsy Curr 2015;15:313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full dataset is publicly available as the NIS via the Agency for Healthcare Research and Quality as part of the Healthcare Utilization and Cost Project.