Abstract
Objective
To assess white matter integrity in patients with essential tremor (ET) and Parkinson disease (PD) with moderate to severe motor impairment.
Methods
Sedated participants with ET (n = 57) or PD (n = 99) underwent diffusion tensor imaging (DTI) and fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity values were computed. White matter tracts were defined using 3 well-described atlases. To determine candidate white matter regions that differ between ET and PD groups, a bootstrapping analysis was applied using the least absolute shrinkage and selection operator. Linear regression was applied to assess magnitude and direction of differences in DTI metrics between ET and PD populations in the candidate regions.
Results
Fractional anisotropy values that differentiate ET from PD localize primarily to thalamic and visual-related pathways, while diffusivity differences localized to the cerebellar peduncles. Patients with ET exhibited lower fractional anisotropy values than patients with PD in the lateral geniculate body (p < 0.01), sagittal stratum (p = 0.01), forceps major (p = 0.02), pontine crossing tract (p = 0.03), and retrolenticular internal capsule (p = 0.04). Patients with ET exhibited greater radial diffusivity values than patients with PD in the superior cerebellar peduncle (p < 0.01), middle cerebellar peduncle (p = 0.05), and inferior cerebellar peduncle (p = 0.05).
Conclusions
Regionally, distinctive white matter microstructural values in patients with ET localize to the cerebellar peduncles and thalamo-cortical visual pathways. These findings complement recent functional imaging studies in ET but also extend our understanding of putative physiologic features that account for distinctions between ET and PD.
Essential tremor (ET) symptoms are hypothesized to localize to the cerebello-thalamo-motor cortical pathway,1 but the underlying pathology of ET is not well-understood. MRI studies employing diffusion tensor imaging (DTI) techniques have provided preliminary evidence for regionally altered white matter microstructural integrity in ET, indicating that ET symptoms may be related to white matter dysfunction.2,3 However, patients with ET present and progress in a heterogeneous manner,1 and limitations including small sample sizes and heterogeneous cohorts in these studies have led to disagreement as to whether ET should be considered a neurodegenerative disorder.4 In addition, healthy controls are often used as the comparator group, limiting the ability of these studies to detect white matter changes in regions that are uniquely affected by ET pathology from regions that may also be affected in other movement disorders, including Parkinson disease (PD).
To address these limitations, we obtained DTI scans from a cohort of sedated patients with ET and PD to study the differential effects on white matter microstructure of ET pathology compared to PD. Study participants presented for implantation of deep brain stimulation (DBS) devices and are clinically well-characterized as exhibiting moderate to severe motor symptoms. We measured fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity, and radial diffusivity (RD) to test the hypothesis that white matter microstructure along the cerebello-thalamo-motor cortical pathway differs in ET compared to PD.
Methods
Standard protocol approvals, registrations, and consent
All study participants provided informed, written consent for this prospective institutional review board–approved study.
Participant demographics
Study participants consisted of a cohort of patients evaluated at Vanderbilt University Medical Center for DBS device implantation between 2013 and 2017. Study imaging was acquired prior to surgery. A movement disorders neurologist diagnosed all participants with ET according the Movement Disorder Society consensus criteria5 or with PD according to the Queen Square Brain Bank criteria.6 Clinically definite ET was defined by the presence of bilateral postural and kinetic tremors without any additional neurologic abnormalities including no dystonia or signs of hypokinetic-rigid syndrome, absent history of exposure to tremorogenic drugs before the onset of symptoms, and without history or examination suggestive of psychogenic tremor or sudden onset of tremor with a stepwise deterioration.7 PD was diagnosed based on the presence of at least 3 of the 4 diagnostic criteria—rest tremor, rigidity, bradykinesia, and asymmetric onset—and the absence of features suggesting an alternative diagnosis, including the absence of dystonic tremor.8
Medical history was reviewed for documentation of medication use; disease duration was calculated from time of onset of motor symptoms to time of imaging study.
Motor severity was quantified in patients with PD using the Unified Parkinson's Disease Rating Scale part III (UPDRS-III) in the “on”- and “off”-medication state and in patients with ET using the Washington Heights–Inwood Genetic Study of ET (WHIGET; range 0–52)9 or Fahn-Tolosa-Marin (FTM; range 0–144) rating scale10 in the “on”-medication state. Global cognitive function was assessed using either the Dementia Rating Scale (DRS) or the Mini-Mental State Examination (MMSE).
MRI acquisition and processing
MRI data were acquired using a 3.0T Philips Achieva whole body scanner (Philips, Best, the Netherlands) with body coil radiofrequency transmission and sensitivity-encoded 8-channel head coil reception. Patients were placed under general anesthesia for high-resolution, motion-free MRI as part of their standard-of-care protocol for stereotactic planning for DBS surgery.11 General anesthesia was induced using propofol prior to scanning to reduce patient discomfort from placement of bone markers. Sedation was maintained during scanning by administration of sevoflurane through an MRI-compatible anesthesia machine. DTI was performed using a spin-echo echoplanar readout and 33 diffusion directions (repetition time/echo time = 10,000/60 ms; spatial resolution = 2 × 2 × 2 mm3; b = 0 and 1,000 s/mm2).
Preprocessing of DTI data included eddy current correction and brain extraction after which a diffusion tensor model was fit to extract FA, MD, AD, and RD measures using the FMRIB Diffusion Toolbox12,13 (FSL,14 FMRIB, Oxford, UK). Next, DTI data from each participant were registered to standard space (Montreal Neurological Institute, ICBM-152,15 spatial resolution = 1.0 × 1.0 × 1.0 mm3). To obtain a comprehensive white matter segmentation, the union of white matter tracts from 3 widely available atlases (Juelich atlas,16,17 Johns Hopkins University [JHU] tractography atlas,18,19 and JHU labels atlas20) was used. Finally, mean FA, MD, AD, and RD values were computed in each white matter region of interest (ROI) in standard space for each participant, and were logit transformed to ensure the range of support for a linear model. For cortical and cerebellar regions, data were averaged bilaterally.
Bootstrap analysis
The goal of this initial analysis was to identify candidate regions with differences in DTI measures between ET and PD groups using FA, MD, AD, or RD values. DTI measures in white matter ROIs were used as independent variables in least absolute shrinkage and selection operator (LASSO) regressions, where disease status (i.e., ET or PD) was the dependent variable. LASSO logistic regression involves variable selection to determine those that are particularly relevant for explaining the dependent variable21 and was performed as previously described22 using the glmnet package23 and R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). LASSO logistic regression was performed separately for each DTI metric (FA, MD, AD, and RD). In each regression, age and sex were included as a nonpenalized explanatory variable (i.e., controlled for). The LASSO logistic regression was performed on 500 bootstrapped samples with replacement for each DTI metric, and the frequency at which each white matter ROI was chosen was recorded. White matter ROIs were considered important for distinguishing ET and PD if they were chosen in ≥60% or more bootstraps. This cut off selected regions with a greater-than-chance likelihood of distinguishing the groups for further analysis.
Group-level differences
A linear regression analysis was used to quantify the magnitude and direction of differences between ET and PD groups in candidate regions identified by the bootstrap analysis. Nonlinear effects of age were controlled for using a restriction cubic spline with 3 knots placed at the 0.1, 0.5, and 0.9 quantiles. An ordinary least square model was then applied with the logit-transformed FA, MD, AD, and MD data as dependent variables and disease status (i.e., ET or PD) as an independent variable; de-meaned age was included as a nonlinear covariate, and sex was included as a binary covariate. After controlling the false discovery rate (FDR) at 0.1,24 statistical significance was established as p value less than the adjusted threshold for the group variable. Effect size was established as the magnitude of the coefficient for each ROI variable, and the sign of the coefficient indicated the direction of the difference for each variable. Additional regions with p < 0.10 but not less than the adjusted threshold were deemed marginally significant, deserving future study. Statistical analysis was conducted using R Statistical Software. Continuous variables are reported as mean ± SD.
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Participant data
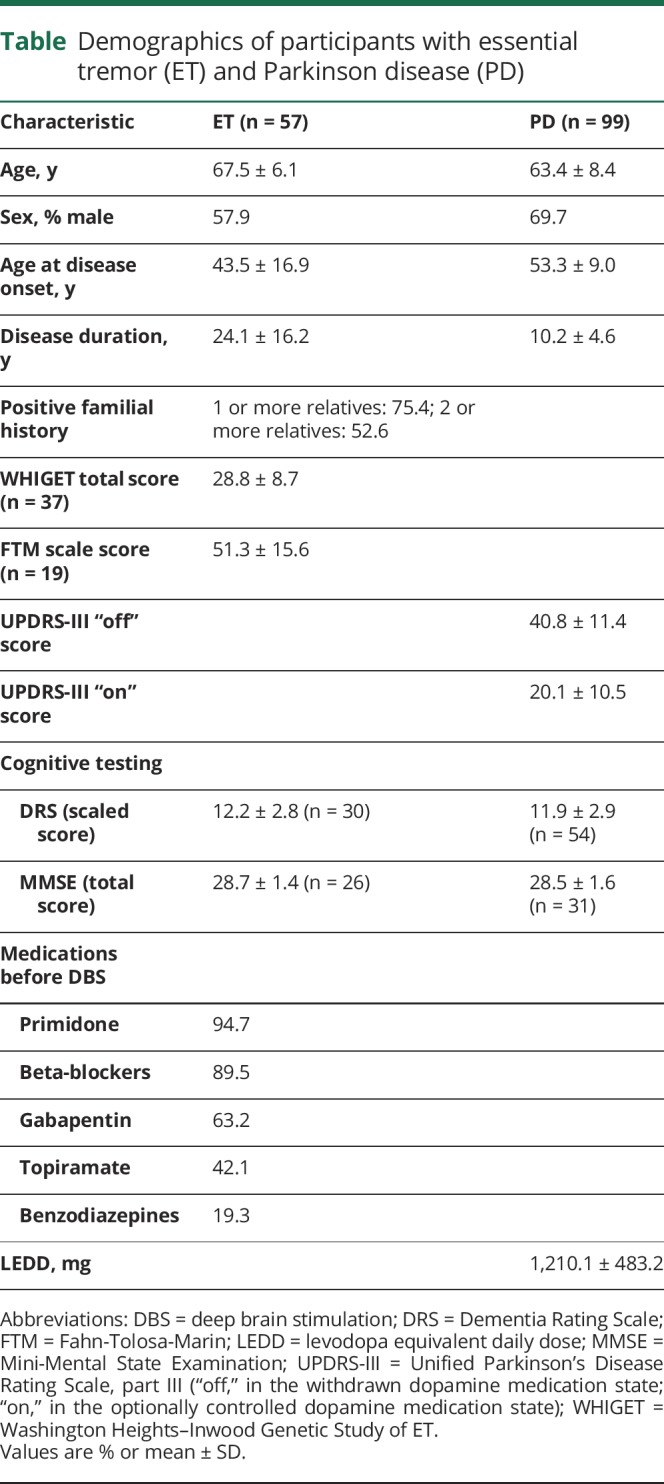
A total of 156 participants (57 ET and 99 PD) were included. Onset of tremor in ET participants was at 43.5 ± 16.9 years of age with a disease duration of 24.0 ± 16.2 years. Disease severity in the ET cohort assessed with WHIGET (n = 37; mean score = 28.84 ± 8.66) was comparable with severity in the ET cohort assessed with FTM (n = 19; mean score = 51.3 ± 15.6), consistent with moderate to severe clinical symptoms. Of note, for one ET participant, a formal tremor assessment using the WHIGET or FTM was not recorded, yet this patient was clinically determined to have moderately severe ET by the study neurologist. In ET participants, family history of tremor was noted in 75% of cases, where at least one first-degree relative had a medical history of ET. In PD compared to ET participants, age at motor onset was higher (53.3 ± 9.0 years) with a lower disease duration (10.2 ± 4.6 years). PD motor severity was assessed using the UPDRS-III, both “on” and “off” dopamine therapy. Most patients with ET included in this study were considered refractory to medical therapy, and DBS was recommended due to progressive symptoms that did not respond adequately to medical management; 84% of patients with ET used 3 or more different types of medications. The most common medication was primidone (93%), followed by beta-blockers (89%), gabapentin (62%), and topiramate (42%). The calculated levodopa equivalent daily dose for patients with PD was 1,210 mg.25 No differences in cognitive function were observed between the ET and PD groups assessed with DRS (n = 30 ET and n = 55 PD; p = 0.54) or with MMSE (n = 27 ET and n = 31 PD; p = 0.84). Clinical and demographic data are summarized in the table for both groups.
Table.
Demographics of participants with essential tremor (ET) and Parkinson disease (PD)
Bootstrap analysis
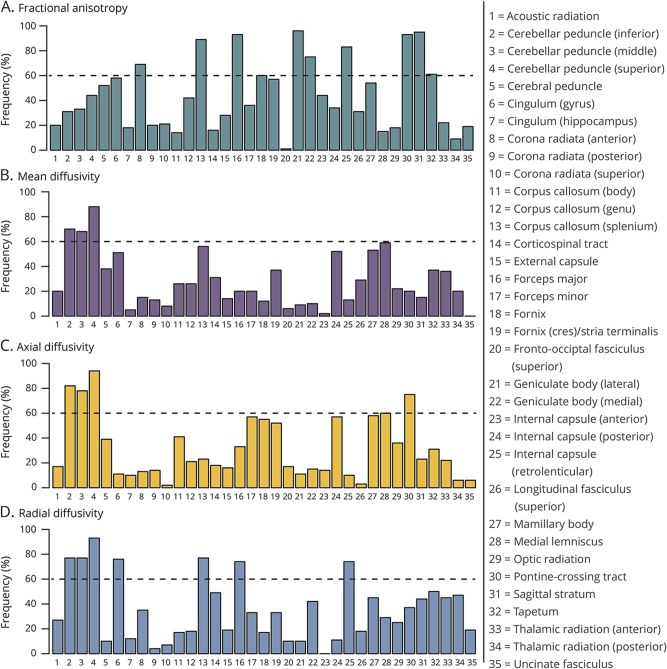
The LASSO regression analysis resulted in 15 distinct regions that were selected with a frequency of ≥60% for distinguishing ET and PD based on FA, MD, AD, or RD (figure 1). For FA, 10 regions were identified: lateral geniculate body (96%), sagittal stratum (95%), forceps major (93%), pontine crossing tract (93%), splenium of corpus callosum (89%), retrolenticular internal capsule (83%), medial geniculate body (75%), fornix (70%), anterior corona radiata (69%), and tapetum (61%) (figure 1A). For MD, 3 regions were identified: superior cerebellar peduncle (SCP; 88%), inferior cerebellar peduncle (ICP; 70%), and middle cerebellar peduncle (MCP; 68%) (figure 1B). For AD, 5 regions were identified: SCP (94%), ICP (82%), MCP (78%), pontine crossing tract (75%), and medial lemniscus (60%) (figure 1C). For RD, 7 regions were identified: SCP (93%), ICP (77%), splenium of corpus callosum (77%), cingulum gyrus (76%), MCP (77%), retrolenticular internal capsule (74%), and forceps major (74%) (figure 1D). These results indicate that the white matter microstructure in cerebellum, brainstem, and occipital lobe differs in ET compared with PD. To determine the direction and size of group differences, these white matter tracts were included in a linear regression analysis.
Figure 1. Bootstrap analysis.
Bootstrapping technique was applied with the least absolute shrinkage and selection operator, and each white matter tract was ranked according to the frequency at which it was chosen as important for distinguishing patients with essential tremor and Parkinson disease for each of fractional anisotropy (A), mean diffusivity (B), axial diffusivity (C), and radial diffusivity (D). Threshold of 60% was utilized to determine the most important tracts for further analysis with a region of interest approach.
Group differences
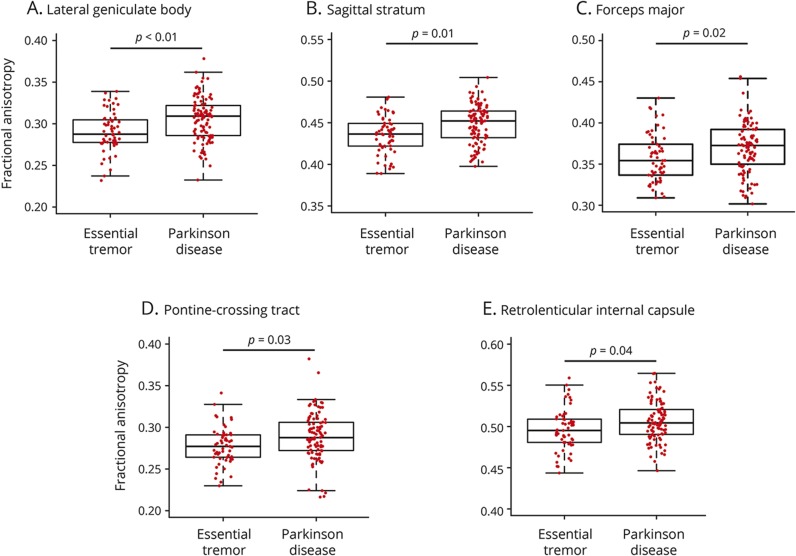
Positive coefficients for a region indicate lower values in ET compared to PD. Lower FA values were observed in ET compared to PD in 5 white matter regions: the lateral geniculate body (p < 0.01; coefficient = 0.05), sagittal stratum (p = 0.01; coefficient = 0.04), forceps major (p = 0.02; coefficient = 0.05), pontine crossing tract (p = 0.03; coefficient = 0.04), and retrolenticular internal capsule (p = 0.04; coefficient = 0.04) (figure 2).
Figure 2. Fractional anisotropy.
Box-and-whisker plots are shown for all participants with essential tremor and Parkinson disease, and the middle line, box, and whiskers represent the median, 25th and 75th percentiles, and 1.5 times the interquartile range, respectively. Fractional anisotropy values are displayed for the lateral geniculate body (A), sagittal stratum (B), forceps major (C), pontine-crossing tract (D), and retrolenticular internal capsule (E).
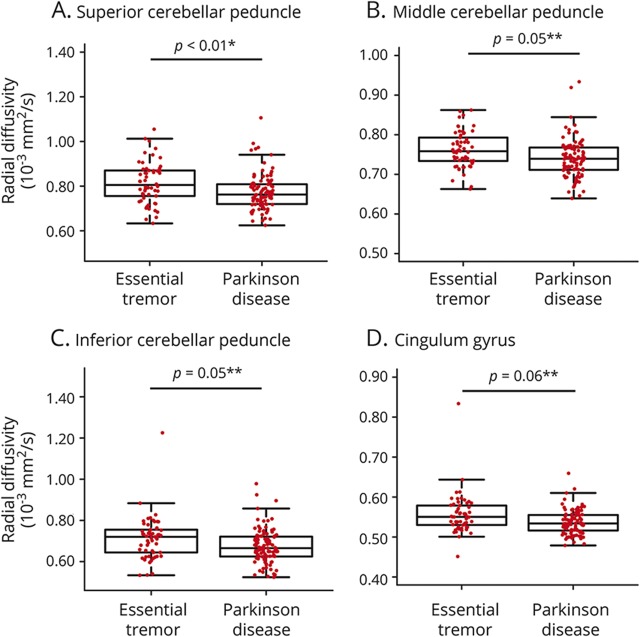
Elevated MD values were observed in ET compared to PD in 3 white matter regions: SCP (p < 0.01; coefficient = −3.87 × 10−3), MCP (p = 0.02; coefficient = −1.58 × 10−3), and ICP (p = 0.07; coefficient = −2.61 × 10−3). Likewise, higher AD values in ET were observed in the same 3 regions: SCP (p < 0.01; coefficient = −4.70 × 10−3), MCP (p = 0.01; coefficient = −1.76 × 10−3), and ICP (p = 0.04; coefficient = −2.79 × 10−3).
Elevated RD values were observed in ET compared to PD in one region: SCP (p < 0.01; coefficient = −3.45 × 10−3). RD values in the MCP (p = 0.05; coefficient = −1.39 × 10−3), ICP (p = 0.05; coefficient = −2.88 × 10−3), and cingulum gyrus (p = 0.06; coefficient = −1.13 × 10−3) were marginally higher in ET than PD, but did not survive FDR correction (figure 3). A graphical summary of white matter tracts involved is presented in figure 4.
Figure 3. Radial diffusivity.
Box-and-whisker plots are shown here for all participants with essential tremor and Parkinson disease with the median, 25th and 75th percentiles, and 1.5 times the interquartile range indicated by the middle line, box, and whiskers, respectively. Radial diffusivity values are displayed for the superior cerebellar peduncle (A), middle cerebellar peduncle (B), inferior cerebellar peduncle (C), and cingulum (D). *Significance after false discovery rate (FDR) correction; **marginal significance not surviving FDR correction.
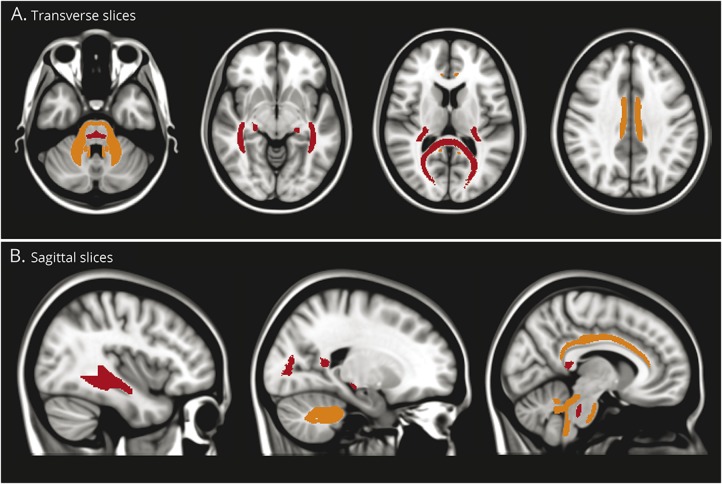
Figure 4. White matter tracts affected in essential tremor (ET) relative to Parkinson disease (PD).
Graphical representation of the location of the white matter tracts from the region of interest analysis where fractional anisotropy (FA) is lower (red) or radial diffusivity (RD) is higher (orange) in ET compared to PD. Lower FA could indicate demyelination or axonal degeneration while higher RD is more specific to demyelination. The white matter tracts with prominent differences appear to localize to cerebello-thalamo-cortical trajectories associated with the visual pathway.
Discussion
Results suggest that there are microstructural white matter distinctions that differentiate ET from PD. Interestingly, these regions appear to converge on a cerebello-thalamo (posterior) cortical network and not on a cerebello-thalamo-motor cortical network as was hypothesized. Conventional interpretation of DTI results indicates that decreased FA coupled with elevated RD is attributed to a demyelinating process, while reduced AD values indicate axonal damage, and concomitant increases in AD and RD (and thus MD) are indicative of inflammation or tissue loss.26 Reduced FA values alone are nonspecific, and can reflect either demyelination or axonal damage.26 Findings generate the hypothesis that there may be a vulnerable white matter pathway in ET, where reduced FA and elevated RD values suggest a demyelinating-type process that may account for microstructural group differences. Here, we discuss the previous evidence linking white matter changes to ET and discuss the putative ET-related network linking the cerebellum to thalamus and visual-related pathways.
The role of white matter dysfunction in ET is supported by recent studies of the LINGO127–30 and the TENM431 genes, which are thought to associate with axonal regeneration and central myelination,32 respectively. We found that white matter tracts with differential disruption in ET include the cerebellar peduncles (superior, middle, and inferior), pontine crossing tract, lateral geniculate body, sagittal stratum, forceps major, and retrolenticular internal capsule. Specifically, reduced FA values were observed in the lateral geniculate body of the thalamus, sagittal stratum, forceps major, pontine crossing tract, and retrolenticular internal capsule of patients with ET, suggesting that an underlying demyelination-related or axonal degeneration-related process may occur in these regions. Meanwhile, elevated AD and MD in the SCP, MCP, and ICP of patients with ET suggest inflammation or tissue loss in these regions. Given that these changes localize to a white matter network extending from the cerebellum to brainstem, thalamus, and occipital lobe, further study on visual–motor integration in ET is warranted.
Some previous studies utilizing diffusion MRI have indicated isolated areas of increased MD or reduced FA in the cerebellum, brainstem, and cortex.2,3,33,34 However, other studies fail to observe differences between patients with ET and controls.3 These discrepancies may be due to small sample sizes and clinical heterogeneity of study cohorts35; in this study, we sought to address these limitations by utilizing a relatively large cohort of clinically well-characterized patients with ET (n = 57) or PD (n = 99). Our results suggest that white matter changes in ET follow a cerebello-thalamo-cortical pattern and are in good agreement with data presented in a recent review36 that synthesized findings from multimodal neuroimaging studies in ET, including structural MRI, diffusion MRI, and PET.
The cerebellum integrates bidirectional pathways linking the peripheral nervous system and cortex, allowing simultaneous and ongoing regulation of motor control. While DTI studies emphasize the cerebellum as the most consistently affected region in ET, the spatial distribution of other white matter changes is poorly localized. Experimental animal models of tremor often use the neurotoxin harmaline to excite the inferior olivary nucleus, inducing cerebellar Purkinje cell loss.37,38 Also, pacemaker properties of the inferior olivary nucleus directly alter synchronization of Purkinje cell firing.39
In our study, the greatest distinctions between PD and ET localize to all 3 cerebellar peduncles, which are densely packed with afferent and efferent cerebellar projections, mediating the dentato-rubro-thalamic and cortico-ponto-cerebellar tracts. Afferents from the inferior olivary nucleus and spinocerebellar tract pass through the ICP to inhibitory Purkinje neurons. These in turn project to the dentate nucleus, through the SCP, to the brainstem, thalamus, and motor and visual cortex. Given that we see increased RD in the SCP and marginally increased RD in the ICP in patients with ET, we hypothesize that a myelin-related process disrupts this cerebello-thalamic-cortical and reciprocal network, altering neuronal conduction between these regions, and ultimately manifesting as an action or intention tremor. This finding is in agreement with other studies localizing ROI-based diffusion differences in ET to the SCP3,33 and ICP.2,3
We have also identified white matter differences (elevated AD and MD and marginally elevated RD) in the MCP of patients with ET compared to PD. Given the role of the MCP as a main afferent pathway from the cortex to cerebellum, and additional findings of diffusion changes in brainstem and pons,34 these data emphasize the role of the cortico-ponto-cerebellar loops in tremor control and ET pathology. Interestingly, demyelination of the MCP is noted in fragile X–associated tremor–ataxia syndrome, a tremor disorder that can resemble ET and responds to similar therapies.40
Together, these data suggest that pathologic white matter changes to both the afferent and efferent pathways of the cerebellum occur in patients with ET compared to PD. Given that these patients have presented for treatment of a medical refractory tremor, we speculate that this cohort represents a population with more severe disease, limiting our confidence to broadly attribute this pathophysiologic process to all patients with ET. There is clear clinical evidence over the last 5 years that targeting the tracts specifically involved in the dentato-rubro-thalamic tract results in a therapeutic advantage for patients receiving DBS of the thalamus in the treatment of ET41 or PD.42 However, in light of evidence from this study, the mechanism of improvement for DBS therapy in tremor may be reflected in the pathology of the pathway, and not just targeting the pathway itself.
In addition to differences in cerebellar white matter, we also noted changes in cerebral white matter pathways, particularly in regions related to the occipital visual cortex including the lateral geniculate body, sagittal stratum, forceps major, and retrolenticular internal capsule. Vision plays a major role in the guidance of movements, and the role for the cerebellum in visually guided movement, and its disturbance in tremor, has been well-described.43 The lateral geniculate body, sagittal stratum, and retrolenticular internal capsule, in which we found reduced FA in ET, relay information from the retina to the primary visual cortex for processing. The sagittal stratum and retrolenticular internal capsule also connect the occipital cortex to the thalamus or brainstem. The forceps major, in which reduced FA was also observed in ET, connects the 2 hemispheres of the occipital lobe. Together, these data show pathologic structural changes to the white matter pathways from the thalamus to the primary visual cortex and from the cortex to thalamic and brainstem structures. Interestingly, marginally elevated RD values in ET were also observed in the cingulum, which connects the cingulate gyrus and the entorhinal cortex, indicating limbic-based networks may also be affected in ET.
As we did not have an a priori hypothesis regarding the potential involvement of the visual pathway when conducting this study, we did not acquire functional imaging data sequentially with eyes open and closed. However, a recent study by Archer et al.44 utilized fMRI to describe a functional network involving the visual pathway that is associated with the severity of tremor in ET. Their results emphasize that patients with ET displayed abnormal fMRI signal with visual feedback in regions beyond the traditionally studied cerebellum and thalamus, and this network involves the primary visual cortex and the extrastriate visual areas. However, Archer et al.44 did not observe microstructural changes in these regions, which could be attributed to the lower severity (mean FTM = 39) of disease in their sample of patients with ET compared with the patients with ET in our study. Taking these results together with our findings, we speculate that abnormalities in functional networks in the visual and cerebellar pathways could be indicative of changes to underlying tissue microstructure in a disease severity–dependent manner. However, further work is required to study this possibility.
While our study focused on the microstructure differences between patients with ET and PD, these findings may be generalizable to other conditions, including spinocerebellar ataxias, in which postural or action tremors are present. However, further work is required before the generalizability of these findings can be known. In addition, the white matter abnormalities observed may also arise from changes in gray matter structures through deafferentation, though we cannot speculate on this topic based on the diffusion data acquired for this current study.
The findings presented in this study should be considered in the context of the following limitations. First, ethical issues and other challenges associated with administering sedation in healthy adults preclude acquisition of normal comparator data for inclusion in this study. Given that the goal was to determine whether ET effects on white matter are distinct from the effects of PD, we have refrained from making conclusions about pathologic implications of the findings beyond differences between patients with ET and PD. However, a cross-sectional lifespan study across 435 participants ranging from 8 to 85 years of age has shown that maturation of white matter microstructure occurs at approximately 30 years of age, followed by a steady deterioration of these metrics with increasing age.45 Given this, it is reasonable to expect comparisons with patients with PD should indicate regions that are uniquely affected in ET. Patients with PD may also exhibit disease process–specific degenerative changes in the white matter, even though their contribution to cardinal motor symptoms remains controversial.46 One recent study47 has reported findings of increased FA in the motor tracts of patients with PD compared to healthy controls, and the authors suggest that this could represent neuroplasticity in PD. However, these observed differences were primarily localized to motor tracts, and no such differences were found in the nonmotor tracts studied. Second, the number of participants with ET (n = 57) is lower than the number of participants with PD (n = 99), and the mean age of participants with ET is slightly older than the mean age of participants with PD. However, to our knowledge, this cohort is the largest to date of any imaging study of white matter in ET, and we have conducted all analyses while controlling for participant age and sex in order to mitigate differences between the participant groups. Third, tremor severity data were not available for one participant with ET in this study. However, as this participant was selected for DBS, the tremor severity in this participant is similar to that seen in remaining participants and so this participant was not excluded. Fourth, we did not study differences between different subtypes of PD, including those with and without tremor and those with resting as well as postural or kinetic tremor. However, the categorical definition of patients with PD who have improvements in motor scores in response to dopamine therapy, regardless of the type of tremor, is a suitable comparator to determine differences as compared to an ET cohort. Similarly, we did not stratify patients with ET into different subtypes, and dopamine transporter scans were not conducted to identify those who may have subclinical PD as the diagnosis was clinically based. However, this is expected to affect only a small fraction of patients with ET, and our sample size (n = 57) helps to reduce the effects of this potential confound. Fifth, while family history of ET in our sample is higher than usual, we did not systematically assess genetic contributions to white matter pathology. Finally, while MRI data were acquired under sedation, previous work has revealed the effects of anesthesia on diffusivity metrics to be relatively small,48 and anesthesia was similarly utilized for all participants in both ET and PD groups, mitigating any potential confounds of anesthesia on between-group differences in diffusion.
We have utilized a cohort of clinically well-characterized patients to demonstrate that patients with clinically severe ET have an altered white matter microstructure compared to patients with PD, and that these differences are localized primarily to a cerebello-thalamic-cortical pathway involving the visual network. Future work will investigate the association of white matter degeneration in ET with disease severity, potential functional differences that may contribute to these microstructural changes, and the potential to differentiate ET pathophysiology from that of PD using noninvasive MRI methods.
Glossary
- AD
axial diffusivity
- DBS
deep brain stimulation
- DRS
Dementia Rating Scale
- DTI
diffusion tensor imaging
- ET
essential tremor
- FA
fractional anisotropy
- FDR
false discovery rate
- FTM
Fahn-Tolosa-Marin
- ICP
inferior cerebellar peduncle
- JHU
Johns Hopkins University
- LASSO
least absolute shrinkage and selection operator
- MD
mean diffusivity
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- RD
radial diffusivity
- ROI
region of interest
- SCP
superior cerebellar peduncle
- UPDRS-III
Unified Parkinson's Disease Rating Scale part III
- WHIGET
Washington Heights–Inwood Genetic Study of ET
Author contributions
M.R. Juttukonda: analysis and interpretation of data, drafting and critical revision of manuscript. G. Franco: analysis and interpretation of data, critical revision of manuscript. D.J. Englot: analysis and interpretation of data, critical revision of manuscript. Y.-C. Lin: analysis and interpretation of data, critical revision of manuscript. K.J. Petersen: analysis and interpretation of data, critical revision of manuscript. P. Trujillo: analysis and interpretation of data, critical revision of manuscript. P. Hedera: critical revision of manuscript. B.A. Landman: analysis and interpretation of data, critical revision of manuscript. H. Kang: analysis and interpretation of data, critical revision of manuscript. M.J. Donahue: critical revision of manuscript. P.E. Konrad: study concept and design, critical revision of manuscript. B.M. Dawant: study concept and design, critical revision of manuscript. D.O. Claassen: study concept and design, analysis and interpretation of data, critical revision of manuscript, study supervision.
Study funding
NIH: R01NS095291 to B.D.; 5R01NS078828 to M.D.; R01 NS097783 to D.C.
Disclosure
M. Juttukonda, G. Franco, D. Englot, Y.-C. Lin, K. Petersen, P. Trujillo, P. Hedera, B. Landman, and H. Kang report no disclosures relevant to the manuscript. M. Donahue reports research-related support from Philips Healthcare. P. Konrad and B. Dawant report no disclosures relevant to the manuscript. D. Claassen reports grant support from NIH/NINDS, Lundbeck Foundation, Michael J Fox Foundation, AbbVie, Acadia, Biogen, BMS, CHDI, Teva Neuroscience, Wave Life Sciences, and Vaccinex and personal fees from Adamas, Lundbeck, Neurocrine, and Teva Neuroscience. Go to Neurology.org/N for full disclosures.
References
- 1.Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson's tremor. Curr Neurol Neurosci Rep 2013;13:378. [DOI] [PubMed] [Google Scholar]
- 2.Klein JC, Lorenz B, Kang JS, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp 2011;32:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saini J, Bagepally BS, Bhatt MD, et al. Diffusion tensor imaging: tract-based spatial statistics study in essential tremor. Parkinsonism Relat Disord 2012;18:477–482. [DOI] [PubMed] [Google Scholar]
- 4.Martinelli P, Rizzo G, Manners D, et al. Diffusion-weighted imaging study of patients with essential tremor. Mov Disord 2007;22:1182–1185. [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor: Ad Hoc Scientific Committee. Mov Disord 1998;13(suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 6.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elble RJ; Tremor Research Group. Report from a U.S. conference on essential tremor. Mov Disord 2006;21:2052–2061. [DOI] [PubMed] [Google Scholar]
- 8.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 9.Louis ED, Ottman R, Ford B, et al. The Washington Heights–Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology 1997;16:124–133. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovik J, Tolosa E, eds. Parkinson's Disease and Movement Disorders. Baltimore: Urban & Schwarzenberg; 1988:225–234. [Google Scholar]
- 11.Konrad PE, Neimat JS, Yu H, et al. Customized, miniature rapid-prototype stereotactic frames for use in deep brain stimulator surgery: initial clinical methodology and experience from 263 patients from 2002 to 2008. Stereotact Funct Neurosurg 2011;89:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003;50:1077–1088. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 15.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Assist Interv 2006;9:58–66. [DOI] [PubMed] [Google Scholar]
- 16.Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 2006;29:1092–1105. [DOI] [PubMed] [Google Scholar]
- 17.Bürgel U, Schormann T, Schleicher A, Zilles K. Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. Neuroimage 1999;10:489–499. [DOI] [PubMed] [Google Scholar]
- 18.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- 21.Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc B Met 1996;58:267–288. [Google Scholar]
- 22.Petersen K, Van Wouwe N, Stark A, et al. Ventral striatal network connectivity reflects reward learning and behavior in patients with Parkinson's disease. Hum Brain Mapp 2018;39:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–878. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 26.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delay C, Tremblay C, Brochu E, et al. Increased LINGO1 in the cerebellum of essential tremor patients. Mov Disord 2014;29:1637–1647. [DOI] [PubMed] [Google Scholar]
- 28.Stefansson H, Steinberg S, Petursson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet 2009;41:277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Jimenez FJ, Garcia-Martin E, Lorenzo-Betancor O, Pastor P, Alonso-Navarro H, Agundez JA. LINGO1 and risk for essential tremor: results of a meta-analysis of rs9652490 and rs11856808. J Neurol Sci 2012;317:52–57. [DOI] [PubMed] [Google Scholar]
- 30.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med 2007;13:1228–1233. [DOI] [PubMed] [Google Scholar]
- 31.Hor H, Francescatto L, Bartesaghi L, et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum Mol Genet 2015;24:5677–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki N, Fukushi M, Kosaki K, et al. Teneurin-4 is a novel regulator of oligodendrocyte differentiation and myelination of small-diameter axons in the CNS. J Neurosci 2012;32:11586–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicoletti G, Manners D, Novellino F, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology 2010;74:988–994. [DOI] [PubMed] [Google Scholar]
- 34.Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol 2008;29:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharifi S, Nederveen AJ, Booij J, van Rootselaar AF. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin 2014;5:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaming R, Annese J. Functional anatomy of essential tremor: lessons from neuroimaging. AJNR Am J Neuroradiol 2014;35:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res 1973;18:69–87. [DOI] [PubMed] [Google Scholar]
- 38.O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience 1993;55:303–310. [DOI] [PubMed] [Google Scholar]
- 39.Hansel C. Reading the clock: how Purkinje cells decode the phase of olivary oscillations. Neuron 2009;62:308–309. [DOI] [PubMed] [Google Scholar]
- 40.Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical phenotype, diagnosis, and treatment. J Invest Med 2009;57:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson JM. Connectomic surgery: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Front Integr Neurosci 2012;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Halloran RL, Chartrain AG, Rasouli JJ, Ramdhani RA, Kopell BH. Case study of image-guided deep brain stimulation: magnetic resonance imaging-based white matter tractography shows differences in responders and nonresponders. World Neurosurg 2016;96:613.e9–613.e16. [DOI] [PubMed] [Google Scholar]
- 43.Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev 1992;72:967–1017. [DOI] [PubMed] [Google Scholar]
- 44.Archer DB, Coombes SA, Chu WT, et al. A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain 2018;141:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 2010;20:2055–2068. [DOI] [PubMed] [Google Scholar]
- 46.Hall JM, Ehgoetz Martens KA, Walton CC, et al. Diffusion alterations associated with Parkinson's disease symptomatology: a review of the literature. Parkinsonism Relat Disord 2016;33:12–26. [DOI] [PubMed] [Google Scholar]
- 47.Mole JP, Subramanian L, Bracht T, Morris H, Metzler-Baddeley C, Linden DE. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur Radiol 2016;26:3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe Y, Tsurugizawa T, Le Bihan D. Water diffusion closely reveals neural activity status in rat brain loci affected by anesthesia. PLoS Biol 2017;15:e2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.