Abstract
Objective
To evaluate the prospective association of long-term intake of vegetables and fruits with late-life subjective cognitive function (SCF).
Methods
Among 27,842 men with a mean age of 51 years in 1986, we used multinomial logistic regression to examine the relation of vegetable and fruit consumption to future SCF. Average dietary intake was calculated from 5 repeated food frequency questionnaires collected every 4 years until 2002. SCF score was assessed twice (2008 and 2012) using a 6-item questionnaire; validity was supported by strong associations with APO ε4 genotype. We categorized the average of the 2 scores as good, moderate, and poor SCF.
Results
Higher intakes of total vegetables, total fruits, and fruit juice were each significantly associated with lower odds of moderate or poor SCF after controlling for major nondietary factors and total energy intake. The association with total fruit intake was weaker after further adjusting for major dietary factors. In this model, the multivariate odds ratios (95% confidence intervals) for vegetable intake (top vs bottom quintile) were 0.83 (0.76–0.92), p trend <0.001 for moderate SCF and 0.66 (0.55–0.80), p trend <0.001 for poor SCF. For orange juice, compared to <1 serving/mo of intake, daily consumption was associated with a substantially lower odds of poor SCF (0.53 [0.43–0.67], p trend <0.001). Higher consumption of vegetables and fruits 18 to 22 years before SCF assessment was associated with lower odds of poor SCF independent of more proximal intake.
Conclusion
Our findings support a long-term beneficial role of vegetable, fruit, and orange juice consumption on SCF.
The role of diet in cognitive function is a subject of strong and growing research interest.1,2 Intakes of fruits, vegetables, and juices, which are rich sources of antioxidant nutrients and bioactive substances, have been studied extensively because of biological plausibility.3,4 However, inconsistent findings have been seen among observational studies and clinical trials. Many of these had relatively small sample sizes and limited periods of follow-up, and thus may have failed to capture the most relevant time window of exposure.5,6
Neuropsychological testing of cognitive function has been the primary outcome measurement in most studies.7 Although providing an objective and comprehensive assessment, those tests are likely to be insensitive to small changes in cognitive performance among healthy individuals, including older individuals in early stages of predementia.8 To capture the earliest sign of cognitive decline, a growing body of research has identified subjective cognitive function (SCF), a self-reported and validated meta-cognition measure, as a precursor to mild cognitive impairment.9–12
We therefore used the many repeated assessments of diet over 20 years and questionnaires on SCF in the Health Professionals Follow-up Study (HPFS), a prospective cohort of US men, to investigate consumption of overall and specific fruits, vegetables, and juices in relation to future SCF.
Methods
Study design
The HPFS began in 1986 when 51,529 male US health professionals (dentists, optometrists, pharmacists, podiatrists, and veterinarians) aged 40 to 75 years answered a detailed questionnaire that included a comprehensive dietary survey, and items on lifestyle practice and medical history.13 Potential participants were selected using the name and address lists obtained from professional organizations; invitations were sent to those who were eligible by age. Of those contacted, 33% responded after up to 3 invitations. Participants have been followed through biennial mailed questionnaires thereafter. The follow-up rate has been approximately 94% at each biennial questionnaire. Study details have been described previously.14 A total of 28,133 men responded to the baseline dietary questionnaires starting in 1986 (excluding individuals with >70 food items blank, and with energy intake of <800 or >4,200 kcal/d), were still living in 2008, and completed the SCF questions on the 2008 and/or 2012 questionnaire. We further excluded 291 participants who developed Parkinson disease before 2012. The final study included 27,842 men with a mean age of 51 years at enrollment in 1986.15,16
Standard protocol approvals, registrations, and patient consents
The study was approved by the Human Subjects Committees of the Harvard T.H. Chan School of Public Health and Brigham and Women's Hospital. As approved by our Human Subjects Committee, the return of a completed questionnaire by our participants is interpreted to imply informed consent.
Dietary assessment
Dietary data have been updated every 4 years in the HPFS since 1986 with a validated semiquantitative food frequency questionnaire (SFFQ) with approximately 130 items about usual intake of foods and beverages over the preceding year (available at channing.harvard.edu/). Validity of the questionnaire to assess long-term foods and nutrients has been documented in HPFS.17–22 Participants reported how often, on average, they consumed each food with a specified portion size. The frequency of consumption of each food was reported in 9 categories ranging from “never or less than once per month” to “6 or more times per day.” Twenty-four vegetable items, 13 fruit items, and 5 fruit juice items listed on the SFFQ are included in this study. We stopped updating dietary data in 2002 to minimize possible effects of altered cognitive function on diet. More than 97% of participants completed at least 2 dietary assessments. Average dietary intake was calculated from the 5 repeated SFFQs to reduce within-subject variation and best represent long-term diet.23 Average total energy intake and alcohol consumption, and major food groups from these dietary questionnaires, were also calculated. Subgroups of vegetables, fruits, and fruit juices included green leafy vegetables (spinach, kale, lettuce), cruciferous vegetables (broccoli, cauliflower, cabbage, sauerkraut, brussels sprouts), carotenoid-rich vegetables (tomatoes, tomato juice, tomato sauce, carrots, yams/sweet potatoes, squash, kale, spinach), starchy vegetables (corn), other nonstarchy vegetables (mixed vegetables, squash, eggplant/zucchini, green pepper, garlic, celery, mushrooms, alfalfa sprouts, beets, onion), citrus fruits (oranges, grapefruit), berry fruits (strawberries, blueberries), other noncitrus fruits (raisins/grapes, prunes, avocado, bananas, cantaloupe, watermelon, apples/pears, applesauce, peaches/apricots/plums and orange juice, grapefruit juice, and other juices (apple juice, prune juice, other juice).
Assessment of SCF
SCF was self-reported on the paper questionnaires that were mailed to the participants. We assessed SCF twice (2008 and 2012) by 6 questions on changes in memory and cognition24: Do you have more trouble than usual remembering recent events? Do you have more trouble than usual remembering a short list of items, such as a shopping list? Do you have trouble remembering things from one second to the next? Do you have any difficulty in understanding things or following spoken instructions? Do you have more trouble than usual following a group conversation or a plot in a TV program due to your memory? Do you have trouble finding your way around familiar streets?25 We then assigned equal value to each question, giving 1 point for every “yes” and summed the points for each questionnaire. If an individual completed only 1 of the 2 SCF assessments, we computed their SCF score using only one questionnaire. Otherwise, the average of the 2 questionnaires was used; 73% of individuals completed both questionnaires. We categorized the scores as good (0 points), moderate (0.5–2.5 points), and poor (3–6 points).
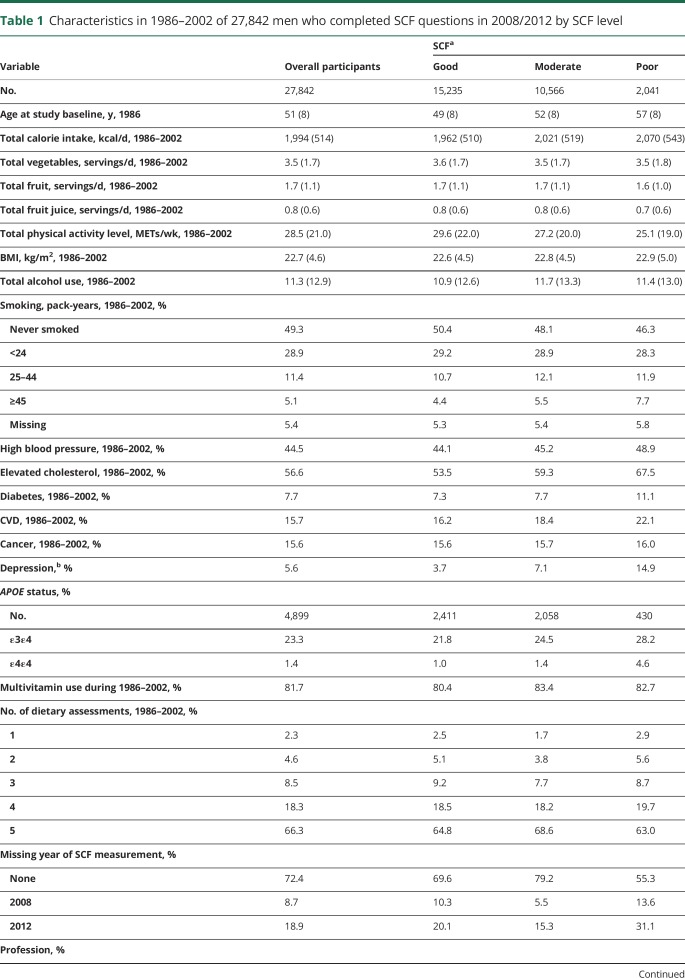
The validity of the SCF score is supported by strong associations with APO ε4 genotype; the age-standardized prevalence of the homozygous APOE ε4 allele was 1.0% in the good SCF group, 1.4% in the moderate SCF group, and 4.6% in the poor SCF group (p trend <0.001). In addition, the average baseline age was 57 years in the poor SCF group compared to 49 years in the good SCF group. Known risk factors for dementia, including depression, heavy smoking, elevated blood cholesterol, high blood pressure, type 2 diabetes, and cardiovascular disease, were all associated prospectively with low SCF (table 1).
Table 1.
Characteristics in 1986–2002 of 27,842 men who completed SCF questions in 2008/2012 by SCF level
Assessment of other covariates
Information on covariates of interest, including lifestyle factors and medical history, was prospectively collected at HPFS baseline and from follow-up questionnaires. Information from baseline at 1986 until 2002 was utilized to be consistent with the time frame of dietary assessment. Baseline age and profession, averaged body mass index (weight in kilograms divided by height in meters squared), and physical activity (metabolic equivalents, MET-h/wk) from 1986 to 2002, and other self-reported covariates, each updated to 2002 (except where indicated differently), included multivitamin use, smoking status in pack-years, diabetes, high blood pressure, elevated cholesterol, cardiovascular disease (stroke, myocardial infarction, angina, or coronary artery surgery), cancer (prostate, colon/rectum, melanoma, lymphoma, leukemia, or other cancer), and depression (defined as use of antidepressants in 1990 or self-reported depression for the last 2 years in 2008).
Statistical analysis
We calculated age-standardized characteristics for all participants and according to quintiles (Q1 and Q5) of total vegetable, fruits, and fruit juice intakes. To evaluate associations between intakes of fruits, vegetables, and fruit juice from 1986 to 2002 and odds of SCF status at 2008/2012, we used multinomial logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Intakes of vegetables, fruits, and total fruit juice were divided into quintiles. Intakes of subgroups of fruit juice were divided into the following categories: never or <1 serving/mo, ≤1 serving/wk, 2–4 servings/wk, 5–7 servings/wk, and >1 serving/d. Comparisons were made between the moderate and poor SCF vs good SCF. We calculated age-adjusted and multivariable-adjusted estimates of associations, adjusting for baseline age (continuous), profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), averaged total energy intake (kcal/d), alcohol intake (g/d), body mass index (<23, 23–24.9, 25–29.9, ≥30 kg/m2), and physical activity (quintiles, MET-h/wk) in 1986–2002, smoking status (never, 1–24 pack-years, 25–44 pack-years, 45+ pack-years, missing) and multivitamin use (never, ever use, missing) in 2002, self-reported diagnosis of hypercholesterolemia, hypertension, diabetes, cancer (prostate, colon/rectum, melanoma, lymphoma, leukemia, or other cancer), and cardiovascular disease (stroke, myocardial infarction, angina, or coronary artery bypass graft surgery) updated until 2002, depression defined as use of antidepressants in 1990 or self-reported depression for the last 2 years in 2008, missing indicator for SCF measurement at 2008 or 2012, and number of dietary assessments during 1986–2002. To examine whether the observed associations were independent of other major food groups, we further conducted analyses mutually adjusting for vegetable, fruit, fruit juice, coffee, potatoes, legumes, refined grains, and dairy products. The specific food groups included in the final model were selected among 14 food groups using the stepwise methods (entry criterion = 0.10, retention criterion = 0.05). Missing indicators were created for variables with missing values. In addition, to increase power in analyses within subgroups and for specific fruits and vegetables, we treated intakes of vegetables, fruits, and fruit juice as continuous variables and estimated multivariable-adjusted ORs for each 3 servings/d of total vegetable and fruit intake, for each 1 serving/d of total fruit juice intake, and for each 3 servings/wk of subgroups and individual food. The p values for linear trend were computed by modeling each dietary variable as a continuous variable.
We further examined whether the associations between diet and SCF varied by baseline age (<53 years, ≥53 years), disease status (self-reported cancer, stroke, and depression), and APOE ε4 allele carrier status (yes/no) in a subgroup of 4,899 men who had APOE ε4 directly genotyped or imputed from a genome-wide association analysis as part of case-control studies for various endpoints (APOE ε4 allele carrier status was determined using 2 imputed single-nucleotide polymorphisms: rs429358 and rs741226). To address the possibility of residual confounding, we conducted sensitivity analyses by further adjusting for marital status (married, divorced/separated, widowed, never married), working status (full-time, part-time, retired), and living arrangement (alone, with wife, with other relative, or other). To evaluate the potential influence of personality traits on the reporting of SCF, we conducted additional analyses after further adjusting for personality traits measured at the same time with SCF assessment, including life satisfaction (“Are you basically satisfied with your life?”), happiness (“Do you feel happy most of the time?”), and optimism (measured by 6 questions using the Life Orientation Test–Revised).
To evaluate the temporal relationships of consuming vegetables, fruits, and fruit juice with SCF, we examined diet at individual years (1986, 1990, 1994, 1998, and 2002) in relation to SCF. We also mutually adjusted for the mean of dietary intakes for 1986–1990 and for 1998–2002 in a multivariable model to evaluate the independent association of remote and recent dietary intakes with SCF. In these analyses, the covariates closest in time with the dietary assessment were used. All analyses were conducted using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Data availability
Any data not published within the article will be shared at the request of other qualified investigators for purposes of replicating procedures and results. Our HPFS website (sites.sph.harvard.edu/hpfs/) includes a description of the cohort, links to all questionnaires, and guidelines for external users.
Results
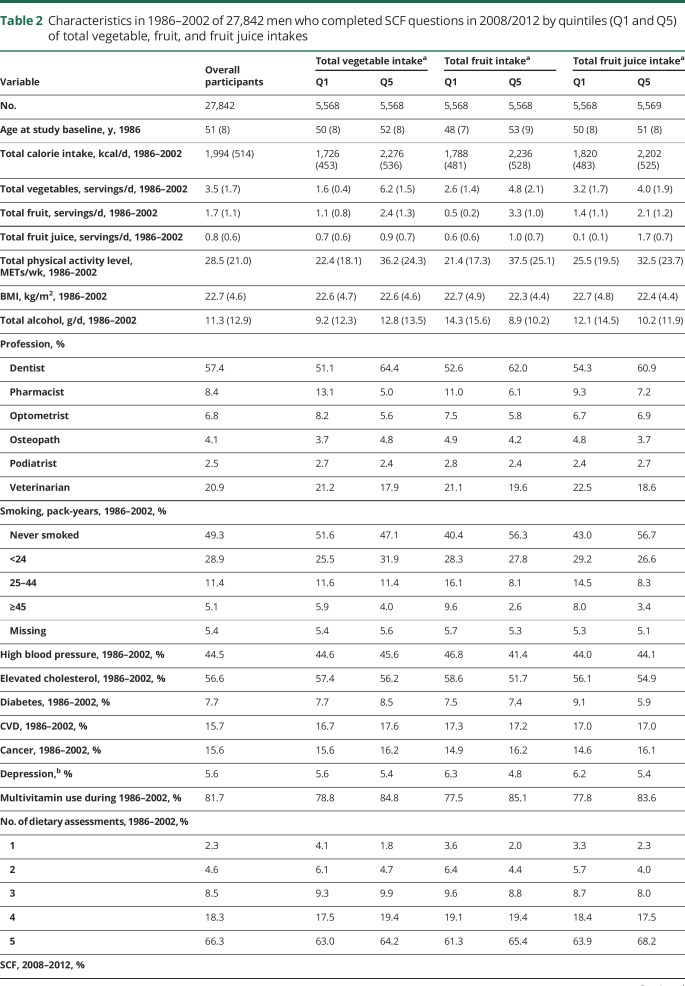
Among the 27,842 men with an average age of 73 years at the time of first SCF measurement, 54.7% had good cognitive function in 2008–2012, 38% had moderate function, and 7.3% had poor function. The age-standardized characteristics of study participants by quintiles (Q1 and Q5) of vegetable, fruit, and fruit juice intake are presented in table 2. The average intakes of total vegetables, total fruit, and total fruit juice were 3.5 servings/d, 1.7 servings/d, and 0.8 serving/d, respectively. Participants with higher vegetable and fruit intake tended to be older, dentists, and more engaged in physical activities, and to have higher multivitamin use, fruit juice intake, and total energy intake. Individuals with higher intakes of fruits and fruit juice were more likely to be dentists, nonsmokers, and tended to have lower alcohol intake and higher multivitamin use, vegetable intake, and physical activity level.
Table 2.
Characteristics in 1986–2002 of 27,842 men who completed SCF questions in 2008/2012 by quintiles (Q1 and Q5) of total vegetable, fruit, and fruit juice intakes
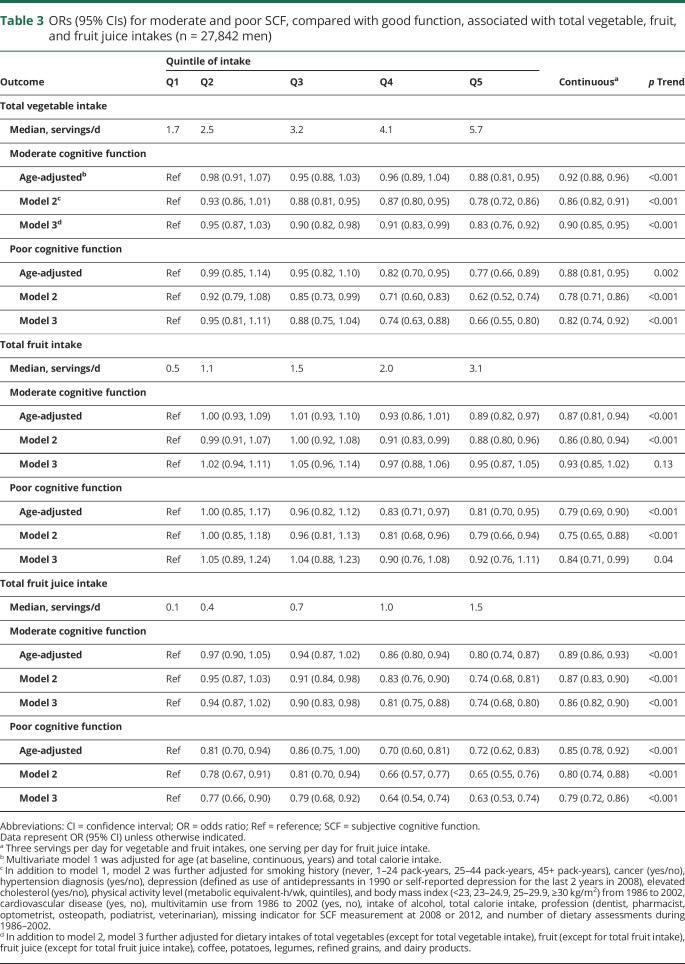
In the primary analyses, total vegetables, total fruits, and fruit juice were each significantly associated with lower odds of moderate and poor SCF after controlling for age, and these associations became stronger with further adjustment for other major nondietary factors and total energy intake (table 3). The associations with total fruit intake were weaker and marginally significant only for poor SCF after further adjusting for intakes of total vegetables, fruit juice, coffee, potatoes, legumes, refined grains, and dairy products. In this model, the multivariate ORs (95% CIs) for vegetable intake (top vs bottom quintile) were 0.83 (0.76–0.92), p trend <0.001 for moderate SCF and 0.66 (0.55–0.80), p trend <0.001 for poor SCF. The multivariable-adjusted OR (95% CI) for poor SCF was 0.82 with each 3 servings/d increase in vegetable intake, 0.84 (0.71, 0.99) with each 3 servings/d increase in fruit intake, and 0.79 (0.72, 0.86) for each 1 serving/d increase in total fruit juice intake. The associations between vegetable, fruit, and fruit juice intake and SCF were similar across strata of age, disease status, and APOE ε4 allele carrier status. When we also included marital status, working status, living arrangement, happiness, life satisfaction, and optimism in the model, the results were unchanged. Specifically, the OR for poor SCF was 0.81 (0.70, 0.94) for an increase of 3 servings/d of total vegetables intake, which is similar to the OR without adjustment of personality traits (0.82 [0.74, 0.92]).
Table 3.
ORs (95% CIs) for moderate and poor SCF, compared with good function, associated with total vegetable, fruit, and fruit juice intakes (n = 27,842 men)
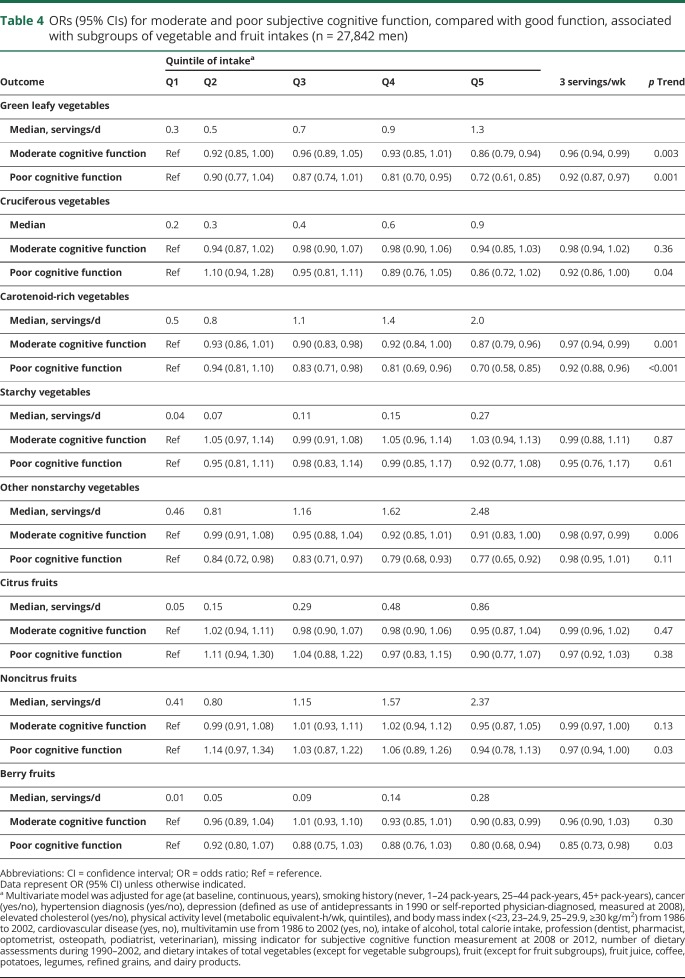
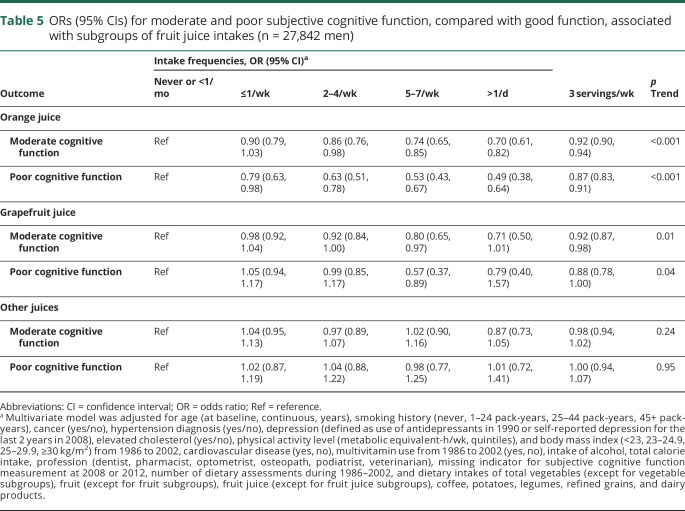
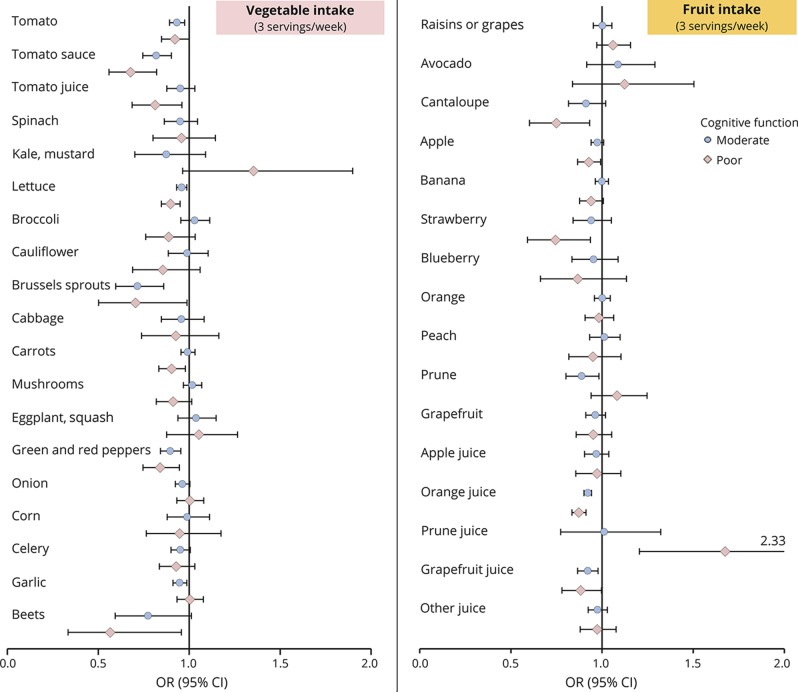
In the vegetable and fruit subgroups analyses, we found that higher consumption of green leafy vegetables, carotenoid-rich vegetables, and berry fruits were significantly associated with reduced odds of both moderate and poor SCF (table 4). No significant associations were observed for cruciferous vegetables, starchy vegetables, citrus fruits, and other noncitrus fruits subgroups. The associations for total juice was mainly observed for orange juice intake, whereby participants with daily consumption had a substantially lower odds of poor SCF (0.53 [0.43–0.67], p trend <0.001) compared to those with no or <1 serving/mo intake (table 5). When evaluating the associations with intakes of individual vegetables, fruits, and fruit juices, we observed that tomatoes, lettuce, brussels sprouts, peppers, cantaloupe, and strawberries were associated with significantly lower odds of moderate and poor SCF, and especially strong associations were seen for tomato sauce and orange juice (figure).
Table 4.
ORs (95% CIs) for moderate and poor subjective cognitive function, compared with good function, associated with subgroups of vegetable and fruit intakes (n = 27,842 men)
Table 5.
ORs (95% CIs) for moderate and poor subjective cognitive function, compared with good function, associated with subgroups of fruit juice intakes (n = 27,842 men)
Figure. ORs (95% CIs) of moderate and poor SCF, compared with good function, associated with individual vegetables, fruits, and juices among 27,842 men (for each 3 servings/wk as continuous variables).
Multivariate model was adjusted for age (at baseline, continuous, years), smoking history (never, 1–24 pack-years, 25–44 pack-years, 45+ pack-years), cancer (yes/no), hypertension diagnosis (yes/no), depression (defined as use of antidepressants in 1990 or self-reported depression for the last 2 years in 2008), elevated cholesterol (yes/no), physical activity level (metabolic equivalent-h/wk, quintiles), and body mass index (<23, 23–24.9, 25–29.9, ≥30 kg/m2) from 1986 to 2002, cardiovascular disease (yes, no), multivitamin use from 1986 to 2002 (yes, no), intake of alcohol, total calorie intake, profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), missing indicator for SCF measurement at 2008 or 2012, number of dietary assessments during 1986–2002, and dietary intakes of total vegetables (except for individual vegetables), fruit (except for individual fruits), fruit juice (except for individual fruit juices), coffee, potatoes, legumes, refined grains, and dairy products. CI = confidence interval; OR = odds ratio; SCF = subjective cognitive function.
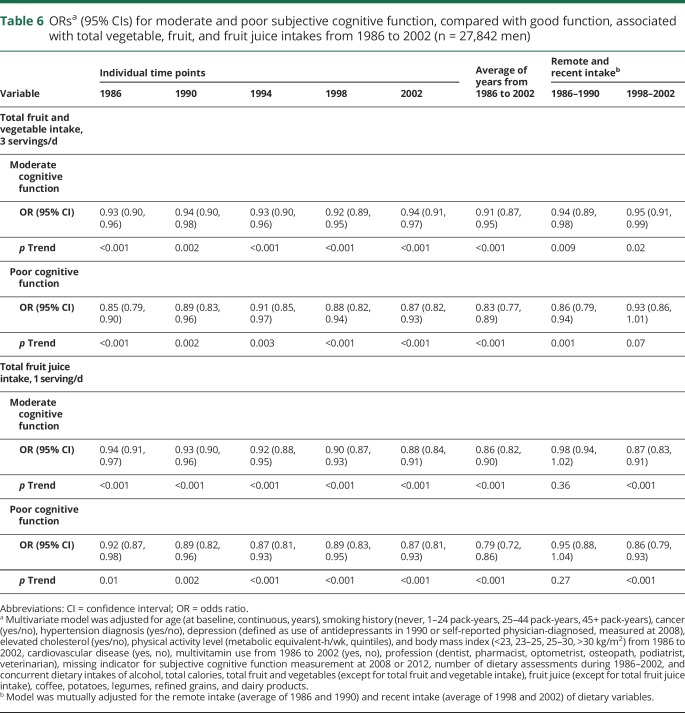
In the evaluation of the temporal relationships, we found that higher intake of total vegetables and fruits, and fruit juice at each of the 5 time points during follow-up was strongly associated with lower odds of moderate and poor SCF (table 6). The strongest associations were with the average of all dietary assessments. For total vegetable and fruit intake, the association was slightly pronounced for more remote years. However, when both remote (18–22 years before SCF assessment) and recent (6–10 years before SCF assessment) intakes were included in the model, the association between vegetable and fruit intakes during proximal periods and poor SCF became marginally significant. The findings were similar for vegetables alone (data not shown). For total fruit juice intake, the association with SCF was slightly stronger in more proximal years, and including both remote and proximal intakes in the same model, the associations were only seen for proximal fruit juice intake.
Table 6.
ORsa (95% CIs) for moderate and poor subjective cognitive function, compared with good function, associated with total vegetable, fruit, and fruit juice intakes from 1986 to 2002 (n = 27,842 men)
Discussion
In this large cohort of US male health professionals, we found that greater intakes of vegetables, fruit, and fruit juice across middle to late adulthood were associated with lower odds of both moderate and poor SCF in later life. Of note, consumption of vegetables and fruits 18 to 22 years before assessment of SCF was associated with poor SCF independent of more proximal intake. In addition, our study adds to the literature that regular consumption of orange juice at elderly age may have a protective role in later-life SCF.
Large studies evaluating long-term intakes of vegetables, fruit, and fruit juice in relation to SCF are rare. Previous studies on these associations have mainly focused on objective measures of cognitive function and dementia with shorter follow-up periods and limited dietary data. A recent review of 9 prospective cohort studies with a follow-up of 6 months or longer found that 5 studies observed a decreased risk of dementia27,28 or cognitive decline29–31 with high consumptions of vegetables, but not fruit.32 Three other studies found significant associations for vegetables and fruit analytically combined.33–35 In particular, our group has previously reported similar findings for total and specific vegetables in the Nurses' Health Study using a large sample, and long-term repeated measures of diet and repeated objective telephone-administered cognitive tests.30 Another review that also included studies of 100% juices found supportive evidence of benefits for cognition or memory function based on limited data from prospective cohort studies36 and acute interventions37–39 in older adults who were experiencing cognitive decline.4
Our findings extend and refine evidence in this area. Utilizing repeated dietary assessment every 4 years over 20 years of follow-up, we found a 34% lower odds of poor SCF among vegetable consumers in the top quintile (median intake: 5.7 servings/d) vs bottom quintile (median intake: 1.7 servings/d), which is consistent with some previous findings of a 26% to 40% reduced risk of dementia or cognitive decline in similar quintile comparisons, although the quintile-specific median serving size was not identical across studies.34,35,40 In addition, our finding was seen even with a more than 20-year lag between assessment of diet and SCF, which is much longer than most existing studies. We found only a weak relation between fruit consumption and poor SCF when mutually adjusting for other dietary factors. Many of the previous studies did not observe an association between fruit intake and risk of cognitive decline or dementia.29–31 Our study was one of the few that examined the prospective association between fruit juice intake and SCF. We found a strong dose-response association of fruit juice, with both moderate and poor SCF. In particular, orange juice, the major source of the carotenoid β-cryptoxanthin, was the main contributor to this association. Moreover, the association was mainly pronounced for more recent years, suggesting a potential beneficial role of orange juice consumed at older ages. Future research is warranted to confirm this finding and to understand the possible mechanisms underlying this relationship.
Many antioxidant nutrients and bioactive substances (including vitamins A, B, C, and E, carotenoids, flavonoids, and polyphenols) that are found naturally in vegetables, fruits, and juices, are hypothesized to reduce the brain oxidative stress, thereby preventing age-related neurologic dysfunction.41–43 Although not consistently replicated in human studies, several animal studies have reported that antioxidants (mainly vitamin E) improve cognitive performance and prevent neuronal damage.44–47
This current study has multiple strengths. The prospective study design, large sample size, careful control of various potential confounders, and more than 20 years of follow-up allowed us to examine the relation of long-term dietary intake with late-life SCF and to provide statistically precise estimates of associations. Moreover, the average dietary intakes calculated from multiple dietary assessments over time reduce within-subject variation and best represent long-term diet. Reverse causation is of less concern in our prospective analyses, and the fact that we stopped updating dietary data 6 years before SCF measurement minimized possible effects of altered cognitive function on diet. A limitation of our study is the lack of baseline cognitive assessment to derive cognitive decline over time. However, because all participants completed professional training, they can be assumed to have started with relatively high cognitive function in early adult life. Therefore, poor SCF at the time of our assessment can be interpreted as indicating decline during adult life. Moreover, the questions on SCF themselves are framed changes compared to earlier function. An additional limitation is the lack of objective cognitive measurement and that SCF assessment used in our study may be subject to errors. However, considerable evidence has demonstrated the validity of SCF measurement as it is clearly related to concurrent level of cognitive function measured by neuropsychological testing,25 faster rates of subsequent cognitive decline,48 risk of future dementia, and the underlying brain pathology of dementia. The strong associations with APO ε4 genotype, age, depression, heavy smoking, elevated blood cholesterol, high blood pressure, type 2 diabetes, and cardiovascular disease in this study also indirectly support its validity. In particular, adjustment of depression in the multivariate model partially accounted for the potential influence of personality traits on the self-report of SCF. In addition, our results remained the same with further adjustment of personality traits including happiness, life satisfaction, and optimism. Moreover, the highly consistent findings with the previous study in a large-scale long-term cohort of female nurses using objective measurement of cognition indirectly support the validity of the subjective measurement used in this study. Another limitation is that individuals who did not complete the 2012 follow-up questionnaire are more likely to have cognitive difficulty. However, if this is the case, this may bias the results toward null. Finally, the homogeneous population of male health professionals may limit the generalizability of the findings to women and other groups.
Our prospective findings relating diet over 2 decades to SCF in later life support the hypothesis that higher long-term intake of vegetables and fruits can have an important role in maintaining cognitive function. The relation with vegetable and fruit intake was seen independently for intake 18–22 years and 6–10 years before assessment of SCF. Subgroups of vegetables, fruits, and fruit juices that appeared particularly important included green leafy vegetables, carotenoid-rich vegetables, berry fruits, and orange juice.
Glossary
- CI
confidence interval
- HPFS
Health Professionals Follow-up Study
- MET
metabolic equivalent
- OR
odds ratio
- SCF
subjective cognitive function
- SFFQ
semiquantitative food frequency questionnaire
Footnotes
CME Course: NPub.org/cmelist
Author contributions
C. Yuan contributed to data analysis and interpretation, and to drafting and revising the manuscript. E. Fondell contributed to data analysis and review. A. Bhushan contributed to the interpretation of the results and revision of the manuscript for important intellectual content. A. Ascherio contributed to data analysis, the interpretation of the results, and revision of the manuscript for important intellectual content. O. Okereke contributed to data acquisition, the interpretation of the results, and revision of the manuscript for important intellectual content. F. Grodstein contributed to the interpretation of the results and revision of the manuscript for important intellectual content. W. Willett contributed to study concept and design, data analysis, and interpretation and revision of the manuscript for important intellectual content.
Study funding
This work was supported by a grant from the NIH (UM1CA167552) and an anonymous gift to the Harvard T.H. Chan School of Public Health.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology February 5, 2018. Accepted in final form September 6, 2018.
References
- 1.Tucker KL. Nutrient intake, nutritional status, and cognitive function with aging. Ann NY Acad Sci 2016;1367:38–49. [DOI] [PubMed] [Google Scholar]
- 2.Smith PJ, Blumenthal JA. Dietary factors and cognitive decline. J Prev Alzheimers Dis 2016;3:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canevelli M, Lucchini F, Quarata F, Bruno G, Cesari M. Nutrition and dementia: evidence for preventive approaches? Nutrients 2016;8:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamport DJ, Saunders C, Butler LT, Spencer JP. Fruits, vegetables, 100% juices, and cognitive function. Nutr Rev 2014;72:774–789. [DOI] [PubMed] [Google Scholar]
- 5.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann NY Acad Sci 2016;1367:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamroziewicz MK, Barbey AK. Nutritional cognitive neuroscience: innovations for healthy brain aging. Front Neurosci 2016;10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jager CA, Dye L, de Bruin EA, et al. Criteria for validation and selection of cognitive tests for investigating the effects of foods and nutrients. Nutr Rev 2014;72:162–179. [DOI] [PubMed] [Google Scholar]
- 8.Gardner RC, Langa KM, Yaffe K. Subjective and objective cognitive function among older adults with a history of traumatic brain injury: a population-based cohort study. PLoS Med 2017;14:e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology 2015;85:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;69:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 2015;48:S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 2004;251:671–675. [DOI] [PubMed] [Google Scholar]
- 13.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 14.Grobbee DE, Rimm EB, Giovannucci E, Colditz G, Stampfer M, Willett W. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med 1990;323:1026–1032. [DOI] [PubMed] [Google Scholar]
- 15.Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol 2018;33:223–234. [DOI] [PubMed] [Google Scholar]
- 16.Fondell E, Townsend MK, Unger LD, et al. Physical activity across adulthood and subjective cognitive function in older men. Eur J Epidemiol 2018;33:79–87. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126. [DOI] [PubMed] [Google Scholar]
- 18.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–249. [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Giovannucci EL, Ascherio A, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev 1998;7:283–290. [PubMed] [Google Scholar]
- 21.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992;135:418–427. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991;133:810–817. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol 2011;26:877–886. [DOI] [PubMed] [Google Scholar]
- 24.Go RC, Duke LW, Harrell LE, et al. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol 1997;10:161–167. [DOI] [PubMed] [Google Scholar]
- 25.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc 2011;59:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radmanesh F, Devan WJ, Anderson CD, Rosand J, Falcone GJ. Alzheimer's Disease Neuroimaging I. Accuracy of imputation to infer unobserved APOE epsilon alleles in genome-wide genotyping data. Eur J Hum Genet 2014;22:1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts RO, Geda YE, Cerhan JR, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord 2010;29:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287:3223–3229. [DOI] [PubMed] [Google Scholar]
- 29.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006;67:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol 2005;57:713–720. [DOI] [PubMed] [Google Scholar]
- 31.Nooyens AC, Bueno-de-Mesquita HB, van Boxtel MP, van Gelder BM, Verhagen H, Verschuren WM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br J Nutr 2011;106:752–761. [DOI] [PubMed] [Google Scholar]
- 32.Loef M, Walach H. Fruit, vegetables and prevention of cognitive decline or dementia: a systematic review of cohort studies. J Nutr Health Aging 2012;16:626–630. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie K, Carrière I, Ritchie CW, Berr C, Artero S, Ancelin ML. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ 2010;341:c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the three-city cohort study. Neurology 2007;69:1921–1930. [DOI] [PubMed] [Google Scholar]
- 35.Hughes TF, Andel R, Small BJ, et al. Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish twins. Am J Geriatr Psychiatry 2010;18:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame project. Am J Med 2006;119:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krikorian R, Boespflug EL, Fleck DE, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem 2012;60:5736–5742. [DOI] [PubMed] [Google Scholar]
- 38.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr 2010;103:730–734. [DOI] [PubMed] [Google Scholar]
- 39.Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem 2010;58:3996–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Sun D, Tan Y. Intake of fruit and vegetables and the incident risk of cognitive disorders: a systematic review and meta-analysis of cohort studies. J Nutr Health Aging 2017;21:1284–1290. [DOI] [PubMed] [Google Scholar]
- 41.Johnson EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am J Clin Nutr 2012;96:1161S–1165S. [DOI] [PubMed] [Google Scholar]
- 42.Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey AN, Joseph JA. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br J Nutr 2015;114:1542–1549. [DOI] [PubMed] [Google Scholar]
- 43.Lamport DJ, Dye L, Wightman JLD, Lawton C. The effects of flavonoid and other polyphenol consumption on cognitive performance: a systematic research review of human experimental and epidemiological studies. Nutr Aging 2012;1:5–25. [Google Scholar]
- 44.Yokota T, Igarashi K, Uchihara T, et al. Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci USA 2001;98:15185–15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging 2002;23:809–818. [DOI] [PubMed] [Google Scholar]
- 46.Maneesub Y, Sanvarinda Y, Govitrapong P. Partial restoration of choline acetyltransferase activities in aging and AF64A-lesioned rat brains by vitamin E. Neurochem Int 1993;22:487–491. [DOI] [PubMed] [Google Scholar]
- 47.Socci DJ, Crandall BM, Arendash GW. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Res 1995;693:88–94. [DOI] [PubMed] [Google Scholar]
- 48.Samieri C, Proust-Lima C, Glymour MM, et al. Subjective cognitive concerns, episodic memory, and the APOE ε4 allele. Alzheimers Dement 2014;10:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article will be shared at the request of other qualified investigators for purposes of replicating procedures and results. Our HPFS website (sites.sph.harvard.edu/hpfs/) includes a description of the cohort, links to all questionnaires, and guidelines for external users.