Abstract
Traumatic brain injury (TBI) affects over 2.8 million people annually, and has been shown to increase motor impulsivity in both humans and animals. However, the root cause of this behavioral disinhibition is not fully understood. The goal of the current study was to evaluate whether timing behavior is disrupted after TBI, which could potentially explain increases in impulsive responding. Twenty-one male three-month old Long-Evans rats were trained on a fixed interval-18 s schedule. Following training, rats were placed on the Peak Interval Procedure, with intermittent peak trials. On peak trials, no behaviors were reinforced and response rates were recorded to determine timing ability. After reaching a stable baseline, rats received bilateral frontal TBI (n = 12) using controlled cortical impact or sham procedures (n = 9). After one week recovery, rats were re-assessed on the Peak Procedure for six weeks. An amphetamine challenge was carried out after behavior reached stable post-injury performance. TBI caused a chronic decrease/acceleration in peak time, increase in response variability, and reduction in response rate. The shifted peak time suggests that altered perception of time may contribute to impairments in response inhibition after TBI. Amphetamine significantly increased response variability, with TBI animals demonstrating greater sensitivity, but did not affect peak time in either group. These data suggest that timing may not be the sole factor explaining impulsive action after TBI given that amphetamine reduced motor impulsivity in prior studies. Further investigations will be needed to dissociate the effects of amphetamine on TBI with regard to timing behavior.
Keywords: Brain injury, Impulsivity, Amphetamine, Psychiatric dysfunction, Peak interval
1. Introduction
Traumatic brain injury (TBI) affects over 10 million people annually across the world and is a major concern for the medical field [1]. While mortality due to TBI has trended downward, there is a large population of patients living with enduring deficits. In addition to the well-known effects of TBI on risk for neurodegenerative disease [2], brain injury can also lead to a host of psychiatric disorders and subclinical symptoms [3]. In particular, psychiatric disease, as well as impulsivity-related syndromes such as aggression, and disinhibition are relatively common, even in cases of mild brain injury [4, 5]. In the absence of a full-fledged psychiatric disorder, these behavioral pathologies can still have severe consequences for individuals and their close relations, significantly affecting quality of life. Understanding why symptoms such as impulsivity emerge after TBI could significantly improve clinical efforts to treat brain injury, through both pharmacological and rehabilitative means.
Impulsivity is a complex construct, and can be measured in many different ways. At its core, impulsivity reflects action without forethought and largely results in suboptimal outcomes. Many researchers find it useful to classify impulsivity into two distinct subtypes: choice impulsivity, or decisions that result in short-term gain at the cost of long-term benefits, and motor impulsivity, or the inability to inhibit actions [6, 7]. Patients with TBI display chronic deficits across both forms of impulsivity, typically in a severity-dependent fashion [8–10]. Recently, we have employed animal models to investigate the trajectory of impulsivity symptoms after focal TBI [11, 12]. In particular, TBI severity was strongly predictive of the degree of motor impulsivity deficit, and mild-injured animals displayed modest increases relative to severely-injured animals. For all injured animals, deficits lasted for at least 14 weeks post-injury [11]. However, when testing choice impulsivity on the delay discounting task, severely-injured animals only displayed a transient increase, which returned to baseline levels within three weeks [12]. While there is clearly a relationship between TBI and impulsivity, it is difficult to pinpoint the root cause of this functional impairment, and why differences should occur between impulsivity subtypes. However, given the specific task requirements for testing motor and choice impulsivity in the previous studies, disruption in timing behavior, and specifically an acceleration of time perception may explain these observations. If the subjective rate of passage of time is increased after TBI, this would make motor impulsivity tasks much more difficult as they require waiting a prerequisite length of time before responding, when in contrast, on the delay discounting paradigm, the aversive longer delays to reinforcement may seem to pass faster.
Timing and impulsivity are intricately linked, as reflected by examinations of temporal control over behavior in conditions with impulsivity as a core facet, such as ADHD. Specifically, adolescents with ADHD display poorer estimation abilities for intervals as short as 5–17 s relative to their peers or immediate family [13, 14]. Adults with ADHD exhibit similar errors, and increased variability in their temporal judgments [15]. Moreover, psychostimulant treatment of ADHD reduces symptoms related to timing behaviors whereas nonstimulant treatments do not [16], suggesting a strong role for dopamine signaling in the control of interval timing. This may be specifically relevant to brain injury as dopamine release is severely attenuated after focal experimental injury [17], and dopamine transporters and receptor levels are altered in patients with TBI [18, 19]. While relatively few studies have examined timing in TBI patients, the collective data are suggestive of increased variability in timing abilities, and very much task dependent [20–22], particularly when considering injury severity and recovery [23]. To better understand changes in timing due to TBI, and its relation to impulsivity, animal models are needed to parse specific contributing variables.
There are multiple paradigms for assessing timing function in humans and other nonhuman animals [24]. Two of the most common are time discriminations (e.g. temporal bisection procedure) or schedules of reinforcement with explicit timing components. In particular, the fixed interval (FI) schedule is useful for examining timing sensitivity. Under an FI schedule, response rates typically accelerate as the interval progresses up to the point of reinforcer delivery. This produces what is conventionally known as a “scalloped” pattern of responding [25]. This characteristic pattern of responding can be utilized to test timing in a manipulation known as the Peak Interval (PI) procedure [26, 27]. The PI procedure encompasses a typical FI schedule with probe or “peak trials” in which reinforcement is withheld. During peak trials, responding increases until near the FI time, then descends when no reinforcer is given, resulting in a roughly Gaussian distribution of presses centered around the FI time. A fitted Gaussian function over the data produces several useful parameters which can be used to evaluate how strong temporal control is over behavior [28].
The current study evaluated whether timing behavior was disrupted due to TBI, which could explain dissociations between choice and motor impulsivity after frontal brain injury. We hypothesized that TBI would cause poorer temporal control, with an earlier peak time on the PI procedure. Finally, an amphetamine challenge was also conducted to investigate sensitivity to monoaminergic disruption between groups. We hypothesized that TBI animals would show improvements in timing similar to those previously reported in motor impulsivity under amphetamine [11].
2. Methods
2.1. Subjects
Twenty-four male three-month old Long-Evans rats were included in this study (three were excluded due to lack of baseline stability, resulting in 21 total subjects included). All elements of this study were reviewed by the West Virginia University Institutional Animal Care and Use Committee. The rats were pair-housed pre-injury in standard rat cages, and single-housed after surgery. Rats were kept on a 12-h light/dark cycle with access to water ad libitum and restricted to 14g of rodent chow daily during the experimental period, with weights monitored weekly. Shredded nesting paper was available to the rats as enrichment. Training was implemented between 11:30 a.m. and 1:30 p.m. on weekdays.
2.2. Apparatus
Testing was performed in a bank of 16 operant chambers, each enclosed in a sound-attenuated box. Each chamber was equipped with a grid floor consisting of stainless steel rods, a pellet hopper and a food trough with light in the center of the right wall with two retractable response levers on either side, a cue light above each lever, a 5-choice array on the opposite side, and a houselight and tone generator. Only the left lever, left cue light, food hopper, and hopper light were used in this experiment. Data were recorded using two PC computers running custom software programs written in MedPC-IV. Sessions were accompanied by a white noise machine to mask background noises.
2.3. Peak procedure training
Training was accomplished in five stages based on previous studies [29]. In stage 1, rats were acclimated to operant chambers in a 10 min habituation session with five sucrose pellets available. Stage 2 was an autoshaping procedure, where a variable-time 25 s schedule extended the lever and lit the cue light, then delivered a pellet after 10 s, and rats could earn up to 50 reinforcers. A concurrent continuous reinforcement schedule was available when the lever was extended such that a lever press resulted in immediate reinforcement. Stage 3 of training was a fixed interval (FI)-5 s schedule which was gradually leaned to an FI-10 and then an FI-18 schedule, which reinforced lever presses that occurred after the requisite time had elapsed. For the fourth stage, rats were assessed on an FI-18 s schedule with a limited hold of 18 s and a total time between trials of 72 s. At the start of a trial, the lever extended and cue light illuminated. Omissions and the pattern of responding were recorded. Once rats developed a characteristic scalloped pattern of responding [25] with low omissions, the Peak Procedure was implemented (stage 5). Peak trials were randomly interspersed such that rats received approximately 25% PI trials versus normal FI trials in a single session. During a peak trial, reinforcement was withheld and the trial continued out to 54 s before terminating. The total trials for each day was 72, and sessions lasted approximately 90 minutes. Rats were assessed daily until reaching a stable baseline (~20 sessions PI, ~50 total sessions). Each session averaged approximately 55 FI trials and approximately 17 PI trials. Baseline stability was determined by visual analysis of the data, with a requirement of no major change across a one-week (5 session) period, and a requirement that the subject displayed some level of peak responding.
2.4. Surgery
Once reaching a stable baseline, rats received a bilateral frontal TBI or a sham procedure. The 21 rats were matched based on peak interval, variance parameters, and omissions, then randomly assigned TBI (n = 12) or Sham (n = 9). Controlled cortical impact (CCI) procedures were carried out as previously described [11, 12]. In brief, rats were anesthetized with isoflurane anesthetic (5% induction, 2–4% maintenance) in 0.5 L/min oxygen. Local analgesic (bupivacaine, 0.25%) was given at the incision site and general analgesic (ketoprofen, 5 mg/kg) was given subcutaneously. Rats were placed in a stereotaxic frame, surgical site sterilized, and a midline incision performed. After retracting the periosteum, a 6 mm circular craniotomy was measured out, centered at +3.0, +0.0 mm from bregma and performed using a surgical drill. A bilateral, frontal CCI (−2.5 mm depth, 3 m/s velocity, 500 ms dwell) was then induced using a Leica Impact One device (Leica Biosystems, Buffalo Grove, IL). Bleeding was stopped, the incision site sutured closed, and triple-antibiotic lotion applied to the site. Sham animals received intact procedures, which did not include craniotomy or impact. Our prior studies have shown no impact of craniotomy on sensitive functional outcomes of decision-making and impulsivity [11, 30], and thus intact sham procedures were chosen.
2.5. Behavior assessment
After 7-days of recovery rats were re-assessed on the peak procedure for TBI-related deficits. Testing was conducted for 6 weeks (week 7 post-injury), until behavior stabilized. An amphetamine challenge was given in post-injury weeks 8 and 9.
2.6. Pharmacological challenge
An amphetamine challenge was implemented after the post-injury assessment. Animals were randomly assigned to amphetamine or saline conditions for week one, and then conditions reversed in week two. Ten minutes before the start of a session, d-amphetamine hemisulfate (Sigma, St. Louis, MO), dissolved in 0.9% phosphate buffered saline (PBS), was injected (1.0 mg/kg, i.p.).
2.7. Histology and lesion analysis
After behavioral testing concluded (10-weeks post-injury), rats were euthanized with sodium pentobarbital. Brains were removed and post-fixed in 3.7% formaldehyde for 72 hours, then placed in a 30% sucrose cyroprotectant solution. Brains were embedded in a gel matrix (15% gelatin) with five brains per gel block, and sliced, frozen, on a sliding microtome at 40 μm. Sections were mounted to slides and stained for cresyl violet to visualize the extent of the lesion. Images were captured on a Konica Minolta copier at 600 DPI, and remaining brain size estimated in ImageJ (NIH, Bethesda, MD) by measuring 4 sections transversing the lesion cavity (+4.5, +3.5, +2.5, +1.5 from bregma), averaging their area, and multiplying by the thickness [31].
2.8. Data recording and analysis
During each session, total omissions were tracked. For the PI trials, every press, the interresponse time (IRT) and lever hold duration were recorded. Presses during PI trials were averaged, and binned into 1 s increments. A Gaussian function was then fit similar to previously described [28], using one week’s worth of data (5 sessions). The function, a * e^{−0.5*[(t-t0)/b]2}, contains parameters of t0 corresponding to peak time, b/t0 corresponding to variance, and a corresponding to peak response rate. This simplified formula (no parameters accounting for the tail of the function) was adopted due to low response rates in the TBI group impacting ability to fit additional parameters.
For each week of testing, the function was fit to estimate the parameters of the equation for each subject as well as for the group to generate figures and unbiased estimates of group parameters. The estimates of peak time, variability, and peak response rate as well as directly recorded parameters of omissions, IRT, and lever hold duration were analyzed in a linear mixed effects regression with fixed-effect predictors of Injury, Week, and the Injury X Week interaction and random-effect predictors of individual subject. Analyses of amphetamine data used fixed-effect predictors of Injury, Drug, and Injury X Drug interaction. One-way ANOVA was used to compare pre-injury baseline performance, and remaining brain volume between the two groups. Transformations were applied to the data as appropriate to normalize distributions, with the log transformation for data bounded on the lower end (omissions, IRTs, lever holds). All data were analyzed using R statistical software (http://www.r-project.org/) with the lme4, lmerTest, and stats libraries. A p-value equal to or less than 0.05 was considered significant.
3. Results
All pre-injury variables were analyzed with a one-way ANOVA to determine if there were any differences between the groups. There were no significant differences on any of the variables at baseline (F’s < 0.62, p’s > 0.441).
3.1. Effects of TBI on timing behavior
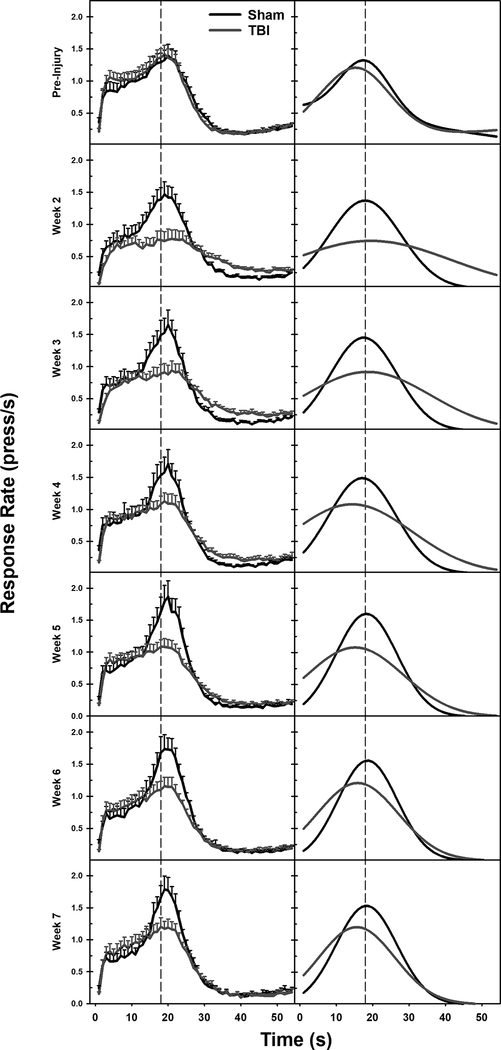
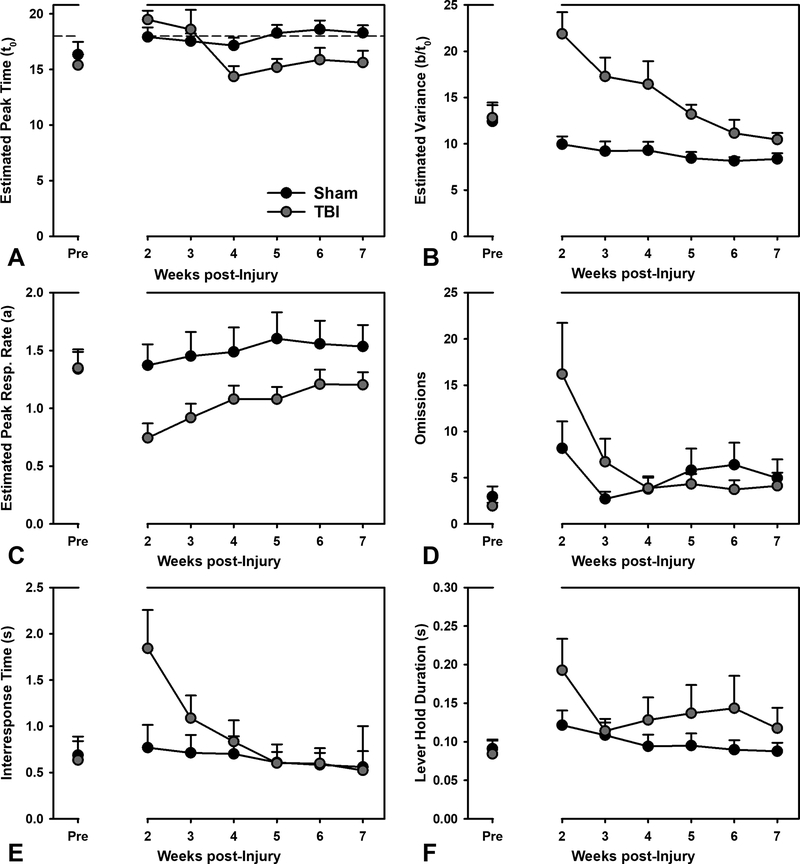
To assess timing behavior, the parameters from the model (peak time: t0, variance: b/t0, peak rate: a) were compared across the groups and time. The TBI group had a reduction in peak time from week 4 post-injury until the end of testing (Injury X Week interaction: β = −0.26, t = 3.34, p < 0.001). Variance was increased in the TBI group, although it declined over time, (Injury X Week interaction: β = −0.31, t = 5.48, p < 0.001). Peak response rate was similarly affected in TBI animals, but in an opposite fashion: starting low, and increasing across time, (Injury X Week interaction: β = 0.10, t = 2.85, p = 0.005). None of these parameters had reached sham levels by 7-weeks post-injury (see Figure 1 and Figure 2).
Figure 1.
Averaged raw response rate data (left) and fitted Gaussian functions of that data (right). TBI caused a marked reduction in overall response rate, and a leftward shift in peak time off of the 18 s FI point (marked by a dashed line). Parameters extracted from the Gaussian function were analyzed to understand between-group differences.
Figure 2.
Performance on the Peak Interval task. Panels A-C are estimated from the Gaussian function in Figure 1. A) TBI caused a significant and persistent decrease in peak time across the testing period (p < 0.001). B) Variance in peak was significantly elevated in the TBI group, but reduced across time (p < 0.001), never quite returning to sham level. C) Peak response rates were significantly reduced due to TBI, but improved to some degree over time (p = 0.005). D) TBI initially increased omissions, which recovered over time (p < 0.001). E) Interresponse times were transiently increased due to TBI (p = 0.047). F) Lever hold durations were not significantly affected by TBI (p = 0.353).
3.2. Effects of TBI on response-related variables
To determine if actions other than timing behavior were affected by injury, omissions (motivation/task engagement), lever hold durations (subtle motoric effects), and IRTs (response patterns) were analyzed. Omitted responses were initially higher in TBI animals, but declined rapidly and reached sham levels relatively quickly (Injury X Week interaction: β = −0.21, t = 3.62, p < 0.001). An examination of lever hold duration, revealed no significant injury-related deficits, but a weak trend for of change across time (Injury X Week interaction: β = −0.02, t = −0.35, p = 0.727; Injury: β = 0.36, t = 0.95, p = 0.353; Week: β = −0.09, t = 1.97, p = 0.051). However, IRTs declined across time from high initial values in the TBI group (Injury X Week: β = −0.09, t = 2.01, p = 0.047; see Figure 2).
3.3. Effects of amphetamine on timing behavior & response-related variables
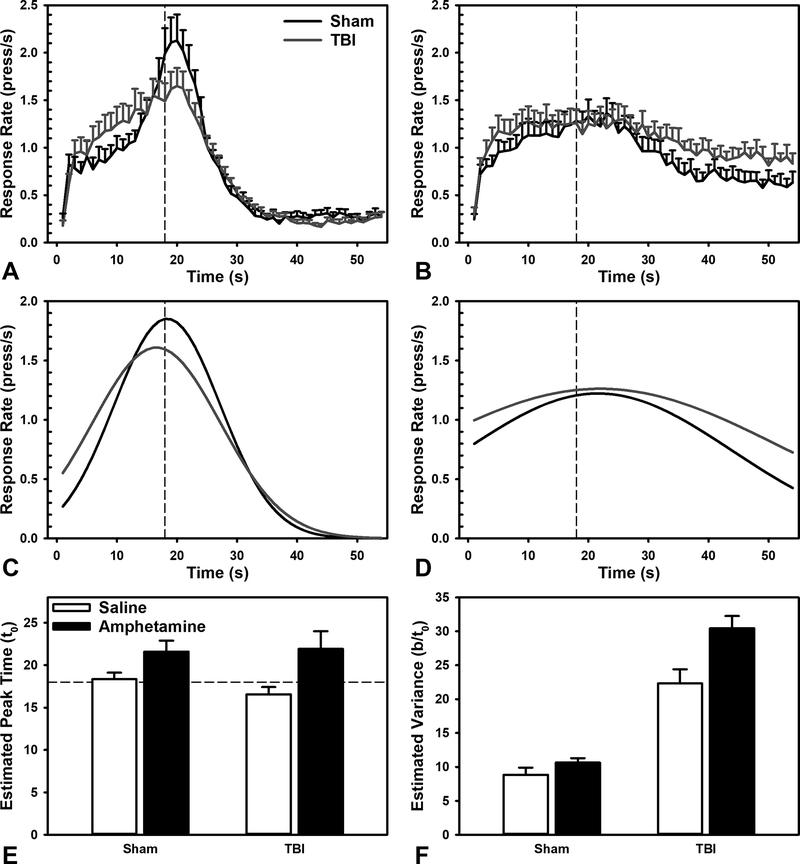
To evaluate the effects of a general monoaminergic challenge to timing behavior, and any injury-related sensitivity, data from the peak interval task were compared in amphetamine and saline conditions. Amphetamine caused distinct effects in behaviors related to timing relative to response-related parameters. Interestingly, amphetamine did not significantly alter peak time or interact with Injury, (Injury X Drug: β = 0.45, t = 0.82, p = 0.421; Drug: β = 0.63, t = 1.49, p = 0.152). However, amphetamine led to a significant increase in variance, and interacted with Injury, such that TBI animals had even larger increases, (Injury X Drug: β = 0.62, t = 2.07, p = 0.046; Drug: β = 1.31, t = 5.7, p < 0.001). Amphetamine also decreased peak rates of responding, across both groups (Injury X Drug: β = 0.55, t = 1.02, p = 0.322; Drug: β = −1.14, t = 2.72, p = 0.015; see Figure 3). Amphetamine had no effects on omissions, lever hold duration, or IRTs (β’s < 0.61, t’s < 1.56, p’s > 0.139). Likewise, there were no Drug X Injury interactions (β’s < 0.59, t’s < 1.02, p’s > 0.321; data not shown).
Figure 3.
Effects of amphetamine on the Peak Interval task. A) Averaged raw response rates after saline injection. B) Averaged raw response rates after 1.0 mg/kg amphetamine injection. C) Gaussian fit of response rates after saline injection. D) Gaussian fit of response rates after 1.0 mg/kg amphetamine injection. E) Amphetamine did not significantly change estimated peak time (p = 0.152). F) Amphetamine significantly increased variance in responding (p < 0.001), and had a larger effect in TBI animals (p = 0.046).
3.4. Lesion analysis
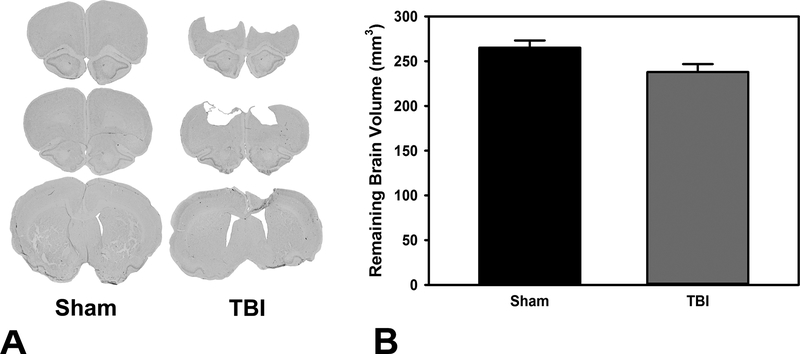
Damage was confirmed in all TBI animals, and total brain volume was significantly reduced relative to Sham, F(1,17) = 5.12, p = 0.037 (see Figure 4).
Figure 4.
Remaining brain volume after brain injury. A) Histoplate demonstrating extent of damage in a representative brain. B) Brain volume was significantly reduced after TBI (p = 0.037).
4. Discussion
Understanding the core variables which cause impulsivity after TBI could lead to novel therapeutic targets or more effective behavioral interventions for individuals suffering from these debilitating symptoms. Notably, the ability to accurately discriminate the passage of time may be a significant contributing factor. In the current study we observed that frontal brain injury caused a persistent decrease in peak time, a clear deficit in timing. This was accompanied by increased variability in responses, and reduced rates of responding, neither of which reached baseline by 7-weeks post-injury (Figure 1 and Figure 2). Despite these functional impairments, no enduring deficits were demonstrated on the non-timing related behavioral measures (Figure 2). An amphetamine challenge had no overall effect on peak time, but this was largely due to the increase in response variability across both groups, with the greatest drug sensitivity in the TBI group (Figure 3). In previous studies, animals with TBI have shown a differential sensitivity to amphetamine, however theses effects were beneficial in the direction of reduced impulsivity [11].
Multiple research groups have reported deficits in motor impulsivity after experimental TBI [11, 32–35], however some of these involve directly inhibiting an action (e.g. go/no-go, stop-signal task) based upon signals versus merely waiting for a prescribed passage of time (e.g. differential reinforcement of low-rate responding, five-choice signal reaction time task). Notably, these impulsivity deficits occur in both focal injuries, such as the data shown here, and in more diffuse models [33]. Shifts in the ability to time events, and specifically an increase in perceived rate of time passage, could explain at least the major deficits in waiting impulsivity present following TBI. In a prior study, we have observed increases in impulsive responding after injury [11]. Given that this behavioral assessment required animals to wait a specific period of time (i.e. 5 s) before initiating a response, perceptions of time may play a large role in dictating response patterns. While, we cannot conclusively say how animals experience time, in the current study, we observed an almost 3 s accelerated shift in the TBI animals relative to sham, which would equate to as much as a 120% difference. Even more interesting is that this developed over time (by 4-weeks post-injury), which maps on to a very similar progression of increased impulsive responding over time in prior studies [11, 36]. Finally, these changes in time discrimination may explain discrepancies observed when TBI animals were previously tested on a delay-discounting task. Specifically, animals with the same injury as those reported here, only had a transient increase in impulsive choice, which normalized by week 4 post-injury [12]. The major difference in this task is that increased perception of the passage of time could lend to a ‘less impulsive’ phenotype because delays may be perceived as shorter. Interestingly, in that study, animals with a milder injury (~10% of force) had a more enduring impulsive phenotype, highlighting the complexities of TBI, particularly with regard to cognitive function.
Given the complicated nature of brain injury, studies investigating the neural basis of timing in intact animals may yield explanations for post-injury deficits. The neural underpinnings of timing have been explored extensively to determine the circuits responsible for representing the passage of time, particularly with regard to environmentally-relevant stimuli (e.g. obtaining food). Multiple regions have been implicated, however timing at intervals on the order of multiple seconds, is strongly linked to the frontal cortex and its motor output via the basal ganglia [37, 38]. Given the ongoing and progressive nature of brain damage, these circuits and their white matter connections may continue to degrade over time [39]. While this was not addressed in the current study, investigation of these pathological changes and associated behavioral aberrations at later time points would be of great benefit. Another target of interest is dopamine, given its role in supporting timing behavior. Specific disruptions to D1-mediated signaling in the frontal cortex distinctly alter high-frequency burst firing and subsequently impair response patterns for timed stimuli [40]. Thus, it is not particularly surprising that strong dopaminergic drugs such as amphetamine have been reported to alter timing. In the current study, amphetamine merely disrupted response patterns without any discernible specific effect on timing behavior (Figure 3). This stands in contrast to previous work using the same 1.0 mg/kg dose, which observed a small, but significant acceleration of timing, and a large increase in response rates [29]. Further research into the interaction of pharmacological interventions may yield a better understanding of these subtle dissociations between studies and the use of timing paradigms to study disease states, including TBI, may lead to a better understanding of the nature of timing, and provide critical information upon which therapies may be developed.
In particular, pharmacotherapies may be beneficial in the brain-injured population to treat chronic deficits. Given similar impulsive phenotypes reported in TBI, drugs that benefit individuals with ADHD may also yield improvements after brain injury [41], although more study is needed in the area, particularly with regard to timing. Recent research has suggested that psychostimulants may indeed reduce issues with impulse control in patients with TBI [42], however based upon animal studies there may be some concern about whether conventional doses are appropriate after TBI [11]. While medication may be desirable due to the ease of administration, more involved behavioral training paradigms may also yield desirable results. For example, interventions directly designed to improve timing abilities have been demonstrated to have carry-over benefits into other areas such as impulsive decision-making [43, 44] and may be augmented by co-administration of therapeutic compounds [45]. More research will be needed to determine whether either behavioral training or pharmacotherapies may yield improvements in timing ability after brain injury.
The current study provides evidence that timing is altered after TBI, and yields a potential mechanism explaining increases in motor impulsivity. Moreover, these deficits appeared to be chronic in nature, similar to unrelenting symptoms reported in human patients [4]. However, more work is needed to determine why amphetamine may reduce impulsivity after TBI [11], yet impairs timing function in a similar fashion to sham animals. These conflicting data suggest the existence of additional contributors to behavioral disinhibition, particularly in the case of more severe injuries. Going forward, future studies should focus on the evaluation of pharmacotherapeutic and behavioral interventions that may be successful in remediating timing-related deficits. By doing this, effective therapeutics may be developed to help the millions of people suffering from the chronic consequences of TBI.
Acknowledgements
We would like to thank Trinity Shaver, Robelle Dalida, Caitlyn Cabral, Chris O’Hearn, and other members of the Vonder Haar lab for their assistance with behavioral testing and tissue processing. Funding for this project was provided by a grant from the National Institute of General Medical Sciences (NIGMS; 5P20GM109098–04) and West Virginia University.
Footnotes
Conflict of Interest
The authors have no financial or commercial relationships based on the research reported in this paper.
References
- [1].Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC, The impact of traumatic brain injuries: A global perspective, NeuroRehabilitation 22(5) (2007) 341–353. [PubMed] [Google Scholar]
- [2].Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JCS, Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias, Neurology 55(8) (2000) 1158–1166. [DOI] [PubMed] [Google Scholar]
- [3].Stocchetti N, Zanier ER, Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review, Critical Care 20(1) (2016) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vaishnavi S, Rao V, Fann JR, Neuropsychiatric problems after traumatic brain injury: Unraveling the silent epidemic, Psychosomatics 50(3) (2009) 198–205. [DOI] [PubMed] [Google Scholar]
- [5].Rao V, Lyketsos C, Neuropsychiatric sequelae of traumatic brain injury, Psychosomatics 41(2) (2000) 95–103. [DOI] [PubMed] [Google Scholar]
- [6].Winstanley CA, Eagle DM, Robbins TW, Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies, Clin. Psychol. Rev 26(4) (2006) 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Evenden JL, Varieties of impulsivity, Psychopharmacology 146(4) (1999) 348–361. [DOI] [PubMed] [Google Scholar]
- [8].Dixon MR, Jacobs EA, Sanders S, Guercio JM, Soldner J, Parker-Singler S, Robinson A, Small S, Dillen JE, Impulsivity, self-control, and delay discounting in persons with acquired brain injury, Behavioral Interventions 20(1) (2005) 101–120. [Google Scholar]
- [9].Ord JS, Boettcher AC, Greve KW, Bianchini KJ, Detection of malingering in mild traumatic brain injury with the Conners’ Continuous Performance Test–II, J. Clin. Exp. Neuropsychol 32(4) (2010) 380–387. [DOI] [PubMed] [Google Scholar]
- [10].McHugh L, Wood RL, Using a temporal discounting paradigm to measure decision-making and impulsivity following traumatic brain injury: A pilot study, Brain Inj. 22(9) (2008) 715–721. [DOI] [PubMed] [Google Scholar]
- [11].Vonder Haar C, Lam FCW, Adams WA, Riparip L-K, Kaur S, Muthukrishna M, Rosi S, Winstanley CA, Frontal traumatic brain injury in rats causes long-lasting impairments in impulse control that are differentially sensitive to pharmacotherapeutics and associated with chronic neuroinflammation., ACS Chem. Neurosci 7(11) (2016) 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vonder Haar C, Martens KM, Riparip L-K, Rosi S, Wellington CL, Winstanley CA, Frontal traumatic brain injury increases impulsive decision making in rats: A potential role for the inflammatory cytokine interleukin-12, J. Neurotrauma 34(19) (2017) 2790–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hwang SL, Gau SSF, Hsu WY, Wu YY, Deficits in interval timing measured by the dual‐task paradigm among children and adolescents with attention‐deficit/hyperactivity disorder, Journal of Child Psychology and Psychiatry 51(3) (2010) 223–232. [DOI] [PubMed] [Google Scholar]
- [14].Hwang-Gu S-L, Gau SS-F, Interval timing deficits assessed by time reproduction dual tasks as cognitive endophenotypes for attention-deficit/hyperactivity disorder, PLoS One 10(5) (2015) e0127157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suarez I, Lopera F, Pineda D, Casini L, The cognitive structure of time estimation impairments in adults with attention deficit hyperactivity disorder, Cogn. Neuropsychol 30(4) (2013) 195–207. [DOI] [PubMed] [Google Scholar]
- [16].Smith A, Cubillo A, Barrett N, Giampietro V, Simmons A, Brammer M, Rubia K, Neurofunctional effects of methylphenidate and atomoxetine in boys with attention-deficit/hyperactivity disorder during time discrimination, Biol. Psychiatry 74(8) (2013) 615–622. [DOI] [PubMed] [Google Scholar]
- [17].Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE, Controlled cortical impact injury affects dopaminergic transmission in the rat striatum, J. Neurochem 95(2) (2005) 457–465. [DOI] [PubMed] [Google Scholar]
- [18].Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK, Impaired dopaminergic neurotransmission in patients with traumatic brain injury: A SPET study using 123 I-β-CIT and 123 I-IBZM, Eur. J. Nucl. Med 27(9) (2000) 1410–1414. [DOI] [PubMed] [Google Scholar]
- [19].Wagner AK, Scanion JM, Becker CR, Ritter AC, Niyonkuru C, Dixon CE, Conley YP, Price JC, The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI, J. Cereb. Blood Flow Metab 34(8) (2014) 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nichelli P, Clark K, Hollnagel C, Grafman J, Duration processing after frontal lobe lesions, Ann. N. Y. Acad. Sci 769(1) (1995) 183–190. [DOI] [PubMed] [Google Scholar]
- [21].Mioni G, Mattalia G, Stablum F, Time perception in severe traumatic brain injury patients: a study comparing different methodologies, Brain Cogn. 81(3) (2013) 305–312. [DOI] [PubMed] [Google Scholar]
- [22].Perbal S, Couillet J, Azouvi P, Pouthas V, Relationships between time estimation, memory, attention, and processing speed in patients with severe traumatic brain injury, Neuropsychologia 41(12) (2003) 1599–1610. [DOI] [PubMed] [Google Scholar]
- [23].Anderson JW, Schmitter-Edgecombe M, Recovery of time estimation following moderate to severe traumatic brain injury, Neuropsychology 25(1) (2011) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lejeune H, Richelle M, Wearden J, About Skinner and time: Behavior‐analytic contributions To research on animal timing, J. Exp. Anal. Behav 85(1) (2006) 125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferster CB, Skinner BF, Schedules of Reinforcement, Appleton-Century-Crofts, East Norwalk, CT, 1957.
- [26].Catania AC, Reinforcement schedules and psychophysical judgments, 1970.
- [27].Roberts S, Isolation of an internal clock, J. Exp. Psychol.: Anim. Behav. Processes 7(3) (1981) 242. [PubMed] [Google Scholar]
- [28].Buhusi CV, Meck WH, Timing for the absence of a stimulus: the gap paradigm reversed, J. Exp. Psychol.: Anim. Behav. Processes 26(3) (2000) 305. [DOI] [PubMed] [Google Scholar]
- [29].Taylor KM, Horvitz JC, Balsam PD, Amphetamine affects the start of responding in the peak interval timing task, Behav. Processes 74(2) (2007) 168–175. [DOI] [PubMed] [Google Scholar]
- [30].Martens KM, Vonder Haar C, Hutsell BA, Hoane MR, A discrimination task used as a novel method of testing decision-making behavior following traumatic brain injury, J. Neurotrauma 29 (2012) 2505–2512. [DOI] [PubMed] [Google Scholar]
- [31].Coggeshall RE, A consideration of neural counting methods, Trends Neurosci. 15(1) (1992) 9–13. [DOI] [PubMed] [Google Scholar]
- [32].Crane AT, Fink KD, Smith JS, The effects of acute voluntary wheel running on recovery of function following medial frontal cortical contusions in rats, Restor. Neurol. Neurosci 30(4) (2012) 325–333. [DOI] [PubMed] [Google Scholar]
- [33].Mychasiuk R, Hehar H, Esser MJ, A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats, Behav. Brain Res 286 (2015) 285–292. [DOI] [PubMed] [Google Scholar]
- [34].Hehar H, Yeates K, Kolb B, Esser MJ, Mychasiuk R, Impulsivity and concussion in juvenile rats: Examining molecular and structural aspects of the frontostriatal pathway, PLoS One 10(10) (2015) e0139842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lindner MD, Plone MA, Cain CK, Frydel B, Francis JM, Emerich DF, Sutton RL, Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions, J. Neurotrauma 15(3) (1998) 199–216. [DOI] [PubMed] [Google Scholar]
- [36].Shaver TK, Ozga JE, Zhu B, Anderson KG, Martens KM, Vonder Haar C, Traumatic brain injury disrupts probabilistic decision making in rats, Brain Res. (under review). [Google Scholar]
- [37].Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK, Contextual encoding by ensembles of medial prefrontal cortex neurons, Proceedings of the National Academy of Sciences 109(13) (2012) 5086–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu M, Zhang S.-y., Dan Y, Poo M.-m., Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex, Proceedings of the National Academy of Sciences 111(1) (2014) 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pischiutta F, Micotti E, Hay JR, Marongiu I, Sammali E, Tolomeo D, Vegliante G, Stocchetti N, Forloni G, De Simoni M-G, Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury, Exp. Neurol 300 (2018) 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS, D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing, J. Neurosci 34(50) (2014) 16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Writer BW, Schillerstrom JE, Psychopharmacological treatment for cognitive impairment in survivors of traumatic brain injury: A critical review, The Journal of Neuropsychiatry and Clinical Neurosciences 21(4) (2009) 362–370. [DOI] [PubMed] [Google Scholar]
- [42].Moreno-López L, Manktelow AE, Sahakian BJ, Menon DK, Stamatakis EA, Anything goes? Regulation of the neural processes underlying response inhibition in TBI patients, Eur. Neuropsychopharmacol 27(2) (2016) 159–169. [DOI] [PubMed] [Google Scholar]
- [43].Bailey C, Peterson JR, Schnegelsiepen A, Stuebing SL, Kirkpatrick K, Durability and generalizability of time-based intervention effects on impulsive choice in rats, Behav. Processes (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peterson JR, Kirkpatrick K, The effects of a time-based intervention on experienced middle-aged rats, Behav. Processes 133 (2016) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Margulies S, Anderson G, Atif F, Badaut J, Clark R, Empey P, Guseva M, Hoane M, Huh J, Pauly J, Combination therapies for traumatic brain injury: Retrospective considerations, J. Neurotrauma 33(1) (2016) 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]