Abstract
Statins have shown promise as anti-cancer agents in experimental and epidemiologic research. However, any benefit that they provide is likely context-dependent, for example applicable only to certain cancers or in combination with specific anti-cancer drugs. Here, we report that inhibition of HMG-CoA reductase (HMGCR) using statins enhances the pro-apoptotic activity of the B cell lymphoma-2 (BCL2) inhibitor venetoclax (ABT-199) in primary leukemia and lymphoma cells but not in normal human peripheral blood mononuclear cells. By blocking mevalonate production, HMGCR inhibition suppressed protein geranylgeranylation, resulting in up-regulation of pro-apoptotic protein p53 upregulated modulator of apoptosis (PUMA). In support of these findings, dynamic BH3 profiling confirmed that statins primed cells for apoptosis. Furthermore, in retrospective analyses of three clinical studies of chronic lymphocytic leukemia (CLL), background statin use was associated with enhanced response to venetoclax, as demonstrated by more frequent complete responses. Together, this work provides mechanistic justification and clinical evidence to warrant prospective clinical investigation of this combination in hematologic malignancies.
Introduction
As part of a growing effort to repurpose FDA-approved drugs to treat cancer (1), several groups have investigated whether HMGCR inhibitors (statins) elicit anti-cancer activity. Some researchers have reported promising experimental and epidemiological findings, but the overall body of evidence is mixed, even within individual cancers such as breast cancer (2–5). Therefore, any benefit statins exert on cancer outcomes is likely context-dependent, and factors such as tumor type and drug combinations must be accounted for when delineating rational applications for statins. Defining these applications would present the rare opportunity to integrate a well-tolerated and relatively inexpensive treatment option to enhance the efficacy of cancer therapeutics.
Statins promote apoptosis in acute myeloid leukemia (AML) (6, 7), acute lymphoblastic leukemia, chronic myeloid leukemia, and multiple myeloma cell lines (8), and epidemiologic studies suggest improved outcomes of statin users in some hematologic malignancies (9, 10). Mechanistically, statins lower plasma cholesterol concentrations by inhibiting the rate-limiting enzyme of the mevalonate pathway. Inhibition of mevalonate production also suppresses the synthesis of isoprenoids that are required for the normal function of key oncogenic proteins like the Ras superfamily (11). Furthermore, statins have been shown to modulate BCL2 family proteins (12), which promote survival and chemo-resistance in multiple cancers. Over-expression of BCL2 is frequently associated with poorer patient outcomes in CLL, AML, and diffuse large B cell lymphoma (DLBCL) (13).
We sought to determine whether statins can enhance the anti-cancer effects of BH3 (BCL2 homology domain-3) mimetics, a class of anticancer drugs that promote apoptosis in susceptible cancer cells. These agents work by mimicking the effects of the BH3-only subset of pro-apoptotic proteins (BIM, NOXA, PUMA, HRK), which antagonize their anti-apoptotic counterparts (BCL2, BCL-XL, MCL1) and thereby promote cell death (13, 14). These agents include venetoclax (ABT-199), a selective BCL2 inhibitor, and navitoclax (ABT-263), a dual BCL2 and BCL-XL inhibitor. Venetoclax was recently granted accelerated FDA approval for del(17p) CLL that has progressed after at least one prior therapy (15). Here, we present preclinical observations made at two independent laboratories, as well as retrospective analyses of patient-level data from clinical studies of venetoclax monotherapy in CLL patients.
Results
Statins selectively enhance the cytotoxicity of venetoclax against cancer cells in vitro
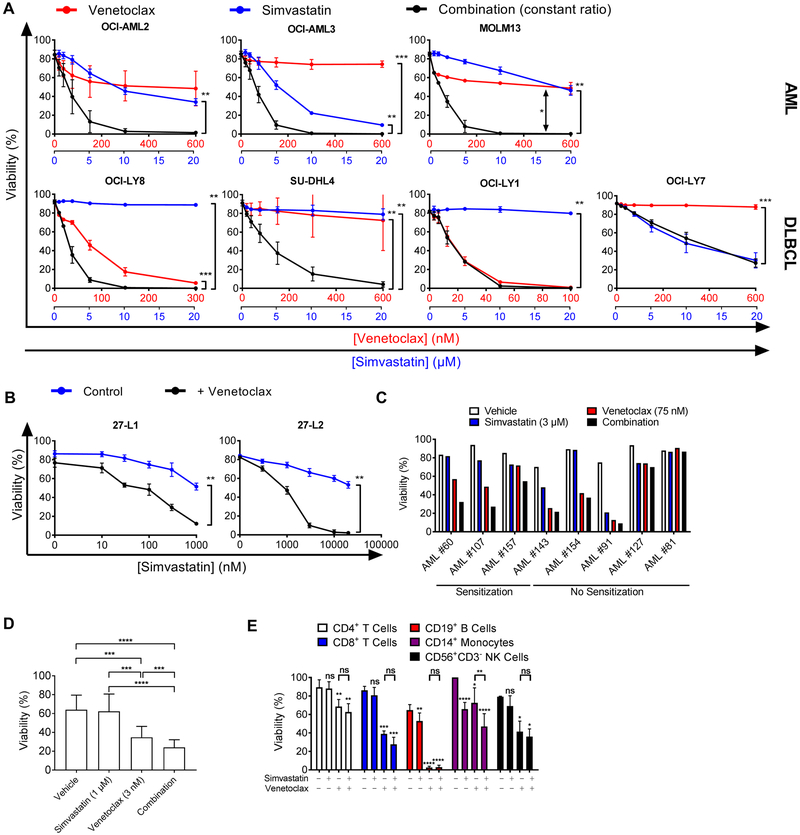
We first tested several human germinal center B cell-like (GCB) DLBCL and AML cell lines for sensitivity to venetoclax, simvastatin, or the combination. Strikingly, in all three AML cell lines (OCI-AML2, OCI-AML3, MOLM13) and in two out of four DLBCL cell lines (OCI-LY8, SU-DHL4), the combination of simvastatin and venetoclax induced more death than either treatment alone (Fig. 1A). In a separate laboratory, we confirmed the patterns seen in DLBCL cell lines and OCI-AML3 cells (fig. S1). We identified another AML cell line sensitive to this combination, HNT-34, and two additional AML cell lines with some sensitivity at higher statin concentrations (PL21 and UKE-1) (fig. S1). The simvastatin/venetoclax combination was synergistic, as assessed by the median-effect method (fig. S2A) (16). Statins were effective at sensitizing two independent clones (27-L1 and 27-L2) of primary murine lymphoma cells expressing human MYC and BCL2 oncogenes (17), representing human “double-hit” lymphoma (Fig. 1B and fig. S2B). The combination of simvastatin with navitoclax killed cells via the intrinsic apoptosis pathway (fig. S3A-C). In particular, a pan-caspase inhibitor rescued the viability of cells treated with the combination (fig. S3A). Rapid cleavage of caspase 3, caspase 9, and PARP was observed after combination treatment (fig. S3B-C). Over-expression of BCL2 or MCL1 increased the IC50 of navitoclax but did not prevent sensitization by simvastatin in DLBCL cell lines (fig. S3D).
Figure 1. Statins selectively enhance the efficacy of venetoclax against blood cancer cells.
A, viability of AML and DLBCL cell lines treated with increasing doses of venetoclax, simvastatin, or the combination for 48 hours. Concentrations for each drug are shown on the x-axes for venetoclax (red) and simvastatin (blue). Significance testing was done using one-tailed paired Student’s t-test comparing IC50 values of indicated treatment groups (n = 3). B, viability of primary murine lymphoma cells co-cultured with irradiated 3T3 stroma and treated for 48 hours with indicated inhibitors ([venetoclax] = 10 nM for 27-L1 and 1000 nM for 27-L2). Significance testing was done using one-tailed paired t-test on IC50 values (n = 3). C, viability of primary AML cells treated with simvastatin for 16 hours before addition of venetoclax for an additional 8 hours. Cell lines were classified as sensitized if their response to the combination exceeded the response to both single agent treatments by greater than 10%; n = 1 for each AML sample. D, Viability of primary CLL cells grown on NK.tert stroma and treated with simvastatin for 16 hours before addition of venetoclax for an additional 8 hours. One-way ANOVA with Tukey’s multiple comparisons test, n = 12. E, viability of PBMC subsets treated with simvastatin (3 µM), venetoclax (100 nM), or the combination for 48 hours before staining. Significance testing was done by two-tailed paired Student’s t-test; n ≥ 3. (A-E) In this and other figures, * p < 0.05, ** p < 0.01, *** p < 0.001. ns = not significant.
We next tested whether the combination was equally effective in primary human patient cells. The combination showed an increased effect relative to single-agent treatments in three of eight primary human AML samples (Fig. 1C). In agreement with previous reports (14, 18, 19), primary CLL cells (table S1) in short-term culture were sensitive to venetoclax, with 3 nM significantly reducing viability (Fig. 1D, p < 0.001). 1 µM simvastatin augmented venetoclax killing of primary CLL samples, including some with 17p(del) (Fig. 1D, fig. S4A, table S1). Furthermore, simvastatin partially reversed venetoclax resistance conferred by CD40L, which mimics T cell-derived survival signals present in the CLL microenvironment (fig. S4B, (20)).
Given that the combination was effective in three blood cancers, we next investigated whether the combination would exacerbate any toxicities in normal human peripheral blood mononuclear cell (PBMC) subsets. Venetoclax had modest toxicity in PBMCs except CD19+ B cells (consistent with B cells being exquisitely sensitive to BCL2i), whereas simvastatin was only slightly toxic to CD14+ monocytes. When simvastatin was combined with venetoclax, there was no enhanced toxicity in any subset except CD14+ monocytes (Fig. 1E).
Dynamic BH3 profiling predicts sensitization of DLBCL cell lines to venetoclax by simvastatin
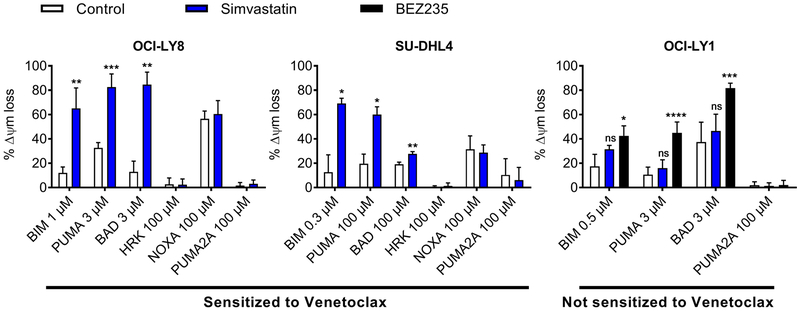
The development of functional diagnostics for patient selection is a growing area of emphasis in precision medicine (21). One such approach is dynamic BH3 profiling (DBP), which measures how readily cells undergo mitochondrial outer membrane permeabilization before and after treatment (22, 23). DBP can rapidly predict whether a treatment is likely to enhance an apoptotic response, enabling patients to be matched with drugs to which their cancers are likely to be sensitive (24). We previously reported that DBP could be used to predict enhanced sensitivity of CLL and DLBCL to combinations involving BCL2 antagonists (25, 26). Similarly, in the two DLBCL cell lines where the combination of simvastatin with venetoclax was synergistic (fig. S2A), DBP identified increased mitochondrial priming by simvastatin (Fig. 2). This can be seen by the increased percentage of mitochondrial depolarization when peptides from BIM or PUMA are added to permeabilized cells after simvastatin treatment. Among BH3-only proteins, BAD binds both BCL2 and BCL-XL, whereas HRK binds selectively to BCL-XL (22). Therefore, the increased response to BAD peptide but not HRK suggests that simvastatin increases cellular dependence on BCL2 for survival. Both the priming effect and the sensitization to venetoclax were dose-dependent, with significant priming observed using 1 µM simvastatin (fig. S5A, p < 0.05). In OCI-LY1 cells, where simvastatin did not synergize with venetoclax (Fig. 1A), there was no measurable increase in priming (assessed using BIM, PUMA, or BAD peptides) under conditions where the phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor BEZ235 did increase priming (Fig. 2). The priming effect sensitized selectively to BCL2 antagonists, and simvastatin did not significantly increase sensitivity to either doxorubicin or vincristine in OCI-LY8 or SU-DHL4 cells (fig. S5B).
Figure 2. Dynamic BH3 profiling predicts sensitization to venetoclax by simvastatin.
Dynamic BH3 profiles for all cells treated with 10 µM simvastatin or 50 nM BEZ235 for 16 hours. Significance testing was performed by two-tailed paired Student’s t-test relative to vehicle-treated control samples; n = 4 (OCI-LY8) or 3 (SU-DHL4, OCI-LY1).
Combination of statin and venetoclax is effective in a mouse model of lymphoma
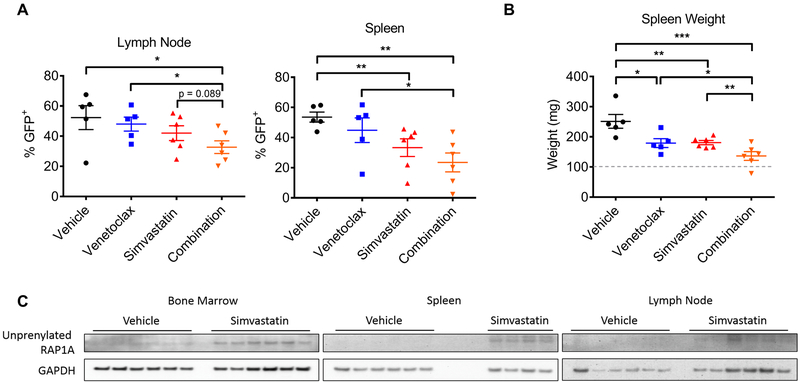
We next sought to determine whether statins could synergize with venetoclax in vivo using a syngeneic mouse model of lymphoma. The murine lymphoma cell line 27-L1, which was sensitive to the combination in vitro (Fig. 1B), was injected into C57BL6/N mice (30) before treating with venetoclax, simvastatin, or both agents together. After only five days of dosing, the combination significantly reduced lymphoma burden (%GFP+) in both lymph nodes and spleens compared to venetoclax treatment alone (Fig. 3A, p < 0.05). Additionally, the degree of splenomegaly was significantly reduced in mice receiving both simvastatin and venetoclax relative to venetoclax alone (p < 0.05), simvastatin alone (p < 0.01), or vehicle (p < 0.001) (Fig. 3B). We confirmed the activity of simvastatin at this dose by measuring unprenylated RAP1A (Fig. 3C), a marker of suppressed mevalonate production (27). In a separate in vivo cohort, the combination of simvastatin and venetoclax significantly extended survival of mice transplanted with lymphoma cells, whereas single agent treatment had no effect (fig. S6, p < 0.05).
Figure 3. Combination of simvastatin and venetoclax is effective in a syngeneic mouse model of lymphoma.
A, percent lymphoma burden in indicated organs of mice treated with venetoclax (75 mg/kg/day, n = 5), simvastatin (50 mg/kg/day, n = 6), or the combination (n = 6) for 5 days. B, spleen weights for mice treated as in A. Significance testing was done using unpaired one-tailed t-tests. C, western blots for pharmacodynamic effect of simvastatin (accumulation of unprenylated RAP1A) in indicated organs of mice treated as in A. Note that two spleens from the simvastatin-treated group were lost due to errors in processing.
Effect of statins is due to on-target inhibition of HMGCR
Several chemically distinct statins similarly enhanced the efficacy of venetoclax and navitoclax in lymphoma cell lines (fig. S1C and S7A-B), suggesting that the effects were due to a shared on-target effect. Supplementing cells with mevalonate (the product of HMGCR activity) completely negated the enhanced killing effect conferred by simvastatin in DLBCL and AML cells (fig. S7C and S7D). Notably, mevalonate specifically counteracted the effects of simvastatin, but not the dual PI3K/mTOR inhibitor BEZ235, which also synergizes with venetoclax in DLBCL (26). Collectively, these data show that the ability of statins to enhance killing by BCL2 inhibitors stems from on-target inhibition of HMGCR.
Sensitization to venetoclax requires inhibition of protein geranylgeranylation.
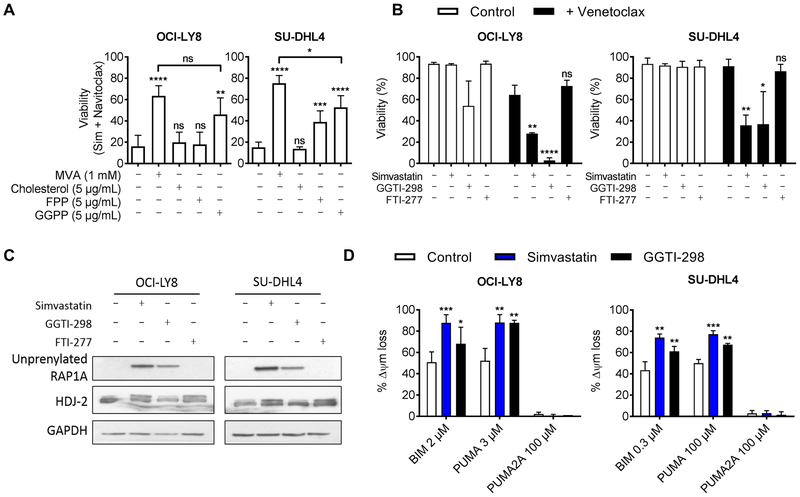
Mevalonate is used in several key cellular processes, including cholesterol biosynthesis, protein synthesis, and signal transduction (12). To determine which pathways downstream of HMGCR were critical for the sensitizing effect of statins, we investigated whether the addition of mevalonate metabolites required for these processes could also rescue cells from simvastatin. We found that only addition of geranylgeranyl pyrophosphate (GGPP) could consistently rescue viability (Fig. 4A and fig. S8). Although a related metabolite, farnesyl pyrophosphate (FPP), moderately rescued some cells, cholesterol had no effect on the viability of cells treated with a combination of simvastatin and venetoclax or navitoclax. Both FPP and GGPP are required for protein prenylation, a post-translational modification that mediates membrane localization (28). Therefore, to confirm reduced protein prenylation, we assessed unprenylated RAP1A (fig. S9A), a marker of reduced geranylgeranylation (27). In all DLBCL cell lines tested, treatment with simvastatin resulted in an accumulation of unprenylated RAP1A in a dose-dependent manner that correlated with single agent cytotoxicity (in OCI-LY7 cells) or with the degree of sensitization to venetoclax (fig. S9B).
Figure 4. Sensitization to venetoclax requires inhibition of protein geranylgeranylation.
A, viability of cells treated with the combination of 300 nM navitoclax and 10 µM simvastatin supplemented with indicated metabolites for 48 hours. Abbreviations are as follows; MVA: mevalonate, FPP: farnesyl pyrophosphate, GGPP: geranylgeranyl pyrophosphate. n = 4 (OCI-LY8 control and GGPP); n = 3 (OCI-LY8 MVA/cholesterol, and all SU-DHL4 groups). B, viability of cells treated as indicated with 10 µM simvastatin, GGTI-298, or FTI-277 with (black bars) or without (white bars) venetoclax (30 nM for OCI-LY8, 300 nM for SU-DHL4) for 48 hours. Viability was assessed by flow cytometry using Annexin-V and PI double-negativity; n = 3 (Sim and FTI), n = 5 or 6 (GGTI and DMSO). C, western blot of cells treated with 10 µM of indicated inhibitors for 16 hours. Statin and FTI-277 but not GGTI-498 caused a mobility shift of HDJ-2 (slower migrating, unprenylated form). Statin and GGTI-498 but not FTI-277 induced the appearance of unprenylated RAP1A. D, dynamic BH3 profile of cells treated with 10 µM simvastatin (blue bars) or GGTI-298 (black bars) for 16 hours; n = 5 (OCI-LY8, DMSO, and Sim), n = 2 (OCI-LY8, GGTI), n = 4 (SU-DHL4, all conditions). Significance testing was performed by two-tailed paired Student’s t-test relative to vehicle-treated control samples in panels A and B, one-tailed in panel D.
We next tested whether selective inhibition of either geranylgeranyl transferase (GGT) or farnesyl transferase (FT) was sufficient to recapitulate the effects of simvastatin. In most cell lines tested, the FT inhibitor (FTI-277) did not enhance the killing by venetoclax, whereas the GGT-1 inhibitor (GGTI-298) consistently sensitized cells to venetoclax (Fig. 4B and fig. S10A). We confirmed that each inhibitor adequately suppressed its specific target pathway by western blotting for mobility shifts in HDJ-2 (a marker of FT inhibition) or the appearance of an unprenylated RAP1A band (Fig. 4C and fig. S10B). Collectively, these data suggest that inhibition of protein geranylgeranylation is both required and sufficient to sensitize cells to BCL2 antagonism in both DLBCL and AML cells.
We also tested whether GGTI-298 could prime DLBCL cells for apoptosis. In both OCI-LY8 and SU-DHL4 cell lines, where GGTI-298 sensitized cells to venetoclax, DBP detected increases in mitochondrial priming (Fig. 4D).
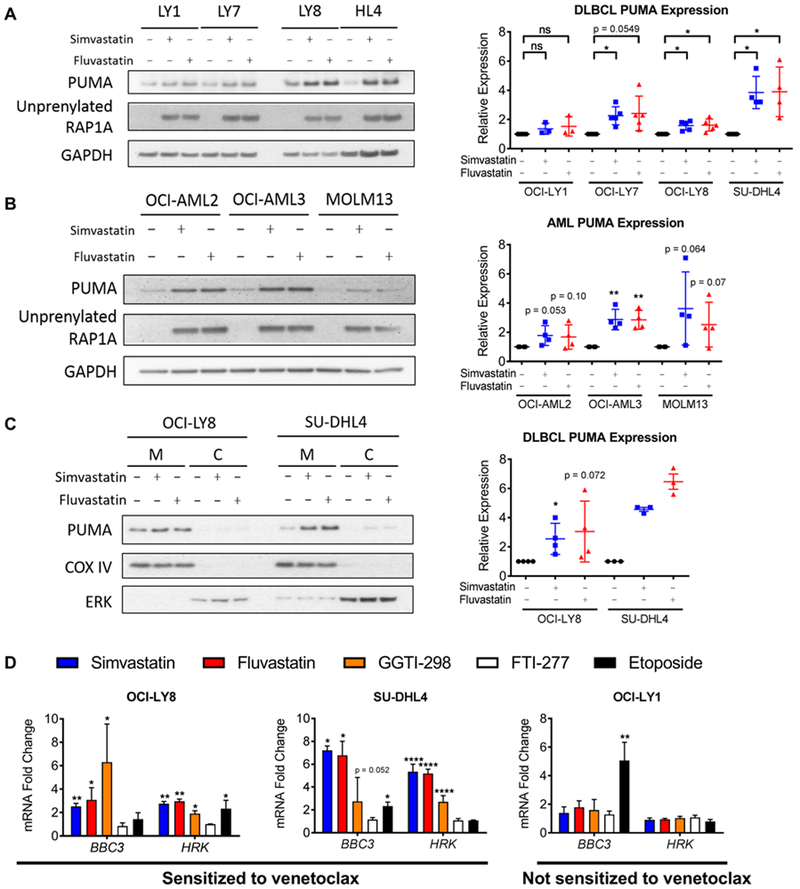
Statins induce expression of PUMA and HRK
We next performed immunoblotting to investigate whether statins could affect the abundance of BCL2 family members. Although most BCL2 family proteins were unaltered by treatment with simvastatin in the DLBCL cells analyzed (fig. S11A, S11B), we observed consistent induction of the pro-apoptotic BH3-only protein PUMA in DLBCL and AML cell lines that were sensitive to the combination of statins and venetoclax (Fig. 5A-B), as well as one primary mouse lymphoma line (fig. S11C). Two chemically distinct statins (simvastatin, fluvastatin) increased PUMA, indicating an on-target effect. In 11 out of 12 primary CLL samples, treatment with simvastatin (3 µM) for 16 hours increased BBC3 mRNA (encoding PUMA) from 2- to 5-fold (fig. S11D). Simvastatin treatment resulted in accumulation of PUMA at the mitochondria (Fig. 5C, S11B), where it can neutralize anti-apoptotic BCL2 family proteins. In support, PUMA association with BCL2 increased after simvastatin treatment (fig. S12).
Figure 5. Statins induce transcription of PUMA and HRK in DLBCL and AML cell lines.
A-B, western blot (left) and quantification (right) of whole cell lysates from DLBCL cells (A) or AML cells (B) treated with 10 µM of indicated inhibitors for 16 hours. C, western blot (left) and quantification (right) of mitochondria-enriched fractions from DLBCL cells treated with 10 µM of indicated inhibitors for 16 hours, M = mitochondrial and C = cytoplasmic fractions. COX IV is a mitochondrial marker, and ERK is a cytoplasmic marker. D, qPCR of DLBCL cell lines treated with 10 µM of indicated inhibitors for 16 hours. (A-C) Paired one-tailed t-tests, n = 3–5. (D) One-way ANOVA with Dunnett’s post-test (all samples compared to control).
To investigate the mechanism of increased PUMA expression, we tested whether statins affected the amounts of PUMA transcript. Notably, both simvastatin and fluvastatin increased the abundance of BBC3 mRNA in OCI-LY8 and SU-DHL4 cell lines (Fig. 5D), consistent with these cell lines being sensitive to the priming effect of statins (Fig. 1A and2A). Statins did not affect the amount of BBC3 transcript in OCI-LY1 cells (Fig. 5D), which were insensitive to the combination (Figs. 1A and2A) and did not upregulate PUMA protein in response to statins (Fig. 5A). The amount of HRK transcript increased with a similar pattern as BBC3 in DLBCL cells (Fig. 5D); however, HRK did not increase in CLL cells treated with simvastatin (fig. S11D). We observed similar patterns of BBC3 induction with simvastatin and GGTI-298 in DLBCL cells (Fig. 5D), supporting the hypothesis that statins and GGTIs affect priming through a similar mechanism. Simvastatin did not enhance venetoclax killing of OCI-AML3 cells in which PUMA was stably knocked down (fig. S13), supporting the functional importance of PUMA upregulation.
Transcriptional induction of BBC3 encoding PUMA can be driven by p53 or Forkhead Box Subgroup O (FOXO) transcription factors (29). However, statins did not affect FOXO phosphorylation, p53 phosphorylation, or total p53 expression in OCI-LY8 or SU-DHL4 cells (fig. S14A), consistent with these lines having mutations in p53 (30, 31). In OCI-AML3 cells, which have functional p53, expression of a p53 dominant-negative mutant (GSE56) blocked PUMA induction and venetoclax sensitization by DNA-damaging agent etoposide but not by simvastatin (fig. S14B, C). Thus, our data suggest that inhibition of the mevalonate pathway primes hematologic cancer cells by increasing expression of PUMA in a manner independent of p53 or FOXO.
Complete Remission Rates Are Increased Among Statin Users in Venetoclax CLL Clinical Trials
To evaluate the clinical relevance of our findings, post-hoc analyses of clinical trial data were conducted using patient-level data pooled from three single-arm, open-label Phase 1 or 2 trials of venetoclax monotherapy in CLL patients. Study 1 (NCT01889186, (32)) enrolled patients with relapsed/refractory (R/R) del(17p) CLL; Study 2 (NCT01328626, (15)) was a first-in-human dose-escalation study in hematologic malignancies that included a cohort of patients with R/R CLL or small lymphocytic lymphoma (SLL); Study 3 (NCT02141282, (33)) enrolled patients with CLL previously treated with the B-cell receptor inhibitors (BCRi) ibrutinib or idelalisib. The target dose in each study was 400 mg daily (QD), the approved dose for del(17p) CLL, except in the dose-escalation period of Study 2, where doses ranged from 150–1200 mg QD. This analysis compared investigator-assessed response between patients with and without background statin use for the following endpoints: overall response rate (ORR), which reflects the proportion of subjects with any response (partial or complete); the composite complete remission rate (CR), which includes subjects who achieved complete remission or complete remission with incomplete bone marrow recovery (CRi); progression free survival (PFS); and overall survival (OS).
Across studies, the majority of patients (289/338, 85.5%) were assigned to receive 400 mg of venetoclax. The other 15% participated in the dose-escalation portion of Study 2. Twenty-two percent of patients (75/338) were receiving a statin as background therapy; simvastatin and atorvastatin each represented approximately 35% of statin use. Mean duration of treatment for both subgroups was approximately 18 months. More than 90% of subjects in both groups were white; statin users were more frequently male (79% vs. 67%) and more often older than 65 years of age (73% vs. 54%). More statin users had previously received a BCRi and were enrolled at US sites, whereas fewer statin users had a baseline absolute lymphocyte count ≥25×109/L. The demographics of each subgroup are provided in table S2.
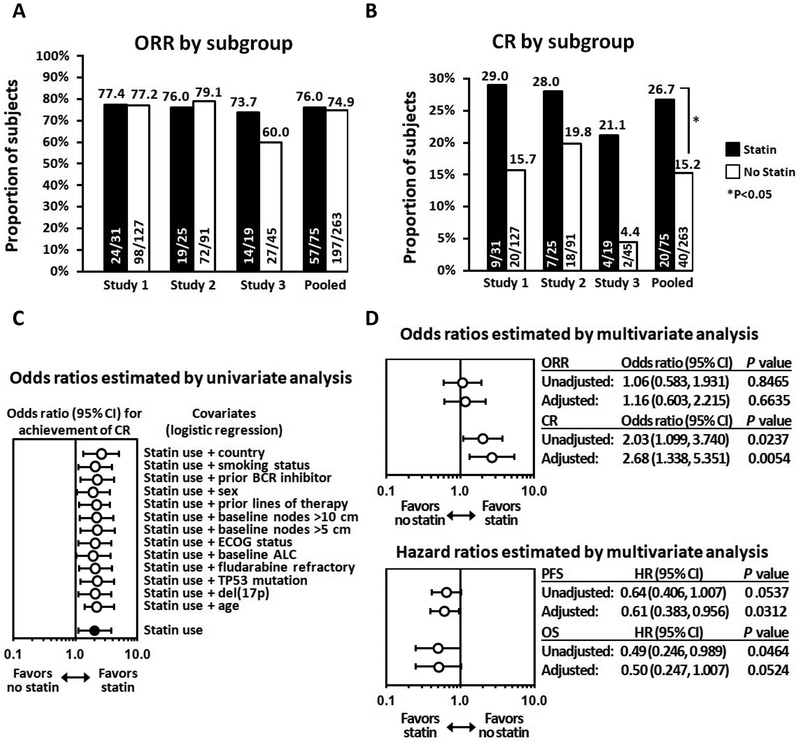
In the pooled analysis, ORR was similar for patients with and without background statin use (76% vs 75%, respectively; Fig. 6A). However, more statin users achieved CR both within the individual studies and overall, with cumulative CR rates of 26.7% vs. 15.2% in the pooled analysis, and an unadjusted odds ratio (95% confidence interval (CI)) of 2.03 (1.099, 3.740) in favor of statin users; results were consistent when adjusted for baseline covariates (Fig. 6B, C). The corresponding multivariable-adjusted odds ratio for CR was 2.676 (1.338, 5.351; P=0.0054) in favor of statin users. PFS and OS results were consistent with the CR results, with respective multivariable-adjusted hazard ratios of 0.61 (0.383, 0.956; P=0.0312) and 0.50 (0.247, 1.007; P=0.0524) (Fig. 6D and fig. S15), which was statistically significant for PFS. Results were similar when the analyses were repeated by including only the subset of patients (n=289) whose target dose was 400 mg (fig. S16). Table S3 includes a summary of the safety findings across studies. Overall, there were no unexpected toxicity differences between the two subgroups.
Figure 6. Response to venetoclax was enhanced among statin users in CLL clinical trials.
A, Proportion of CLL subjects in clinical studies of venetoclax monotherapy who achieved any response, depicted as overall response rate (ORR) by investigator assessment; results are shown by presence or absence of statin use in individual studies and when pooled. B, Proportion of subjects in these studies who achieved complete remission (CR) by investigator assessment; this result reflects the composite of subjects who achieved CR and those with CR with incomplete bone marrow recovery (CRi). C, Odds ratio for achievement of CR across the pool of studies when adjusted for individual covariates. D, Adjusted and unadjusted odds ratios for achievement of ORR and CR and hazard ratios for PFS and OS estimated by multivariate analyses across the pool of studies.
Pharmacokinetic analysis demonstrated no significant effect of background statin use on venetoclax exposures. Dose-normalized peak (Cmax) and area under the curve (AUC) exposures of venetoclax were comparable between patients who reported taking statins as compared to those who did not (fig. S17).
Discussion
Although there is abundant research on potential uses for statins as anti-cancer agents, no clear indications for their use have been confirmed to date. Here we report a series of experiments and analyses that highlight a priority area for further exploration, namely, the use of statins in combination with BH3 mimetics as a treatment for hematologic malignancies. Preclinical work from two different laboratories independently demonstrated that multiple statins profoundly enhance the ability of venetoclax and navitoclax to kill cancer cells. The combination of statins with venetoclax was cytotoxic to DLBCL, CLL, and AML cells, and was effective at reducing lymphoma burden in a syngeneic mouse model of BCL2/MYC-driven “double-hit” lymphoma. In contrast, synergism was not observed when statins were combined with other agents (doxorubicin or vincristine). From a mechanistic standpoint, statins appeared to enhance pro-apoptotic activity of BCL2 protein inhibition by suppressing protein geranygeranylation, which resulted in up-regulation of the pro-apoptotic BH3-only protein PUMA in DLBCL and AML cell lines and in primary CLL cells. Notably, inhibition of protein geranylgeranylation was both necessary and sufficient to recapitulate the sensitizing effect of statins and to increase expression of PUMA. Knockdown experiments supported the concept that PUMA upregulation is important for statin sensitization to venetoclax. Retrospective analyses of venetoclax clinical trials in relapsed CLL demonstrated that patients with background statin use were more likely to achieve complete remission, providing strong support for the clinical relevance of our findings.
It is important to note that preclinical experiments reported here and elsewhere examining pleiotropic effects of statins are typically conducted with low to mid-micromolar concentrations, whereas the concentrations observed clinically are generally in the low nanomolar range (34). However, our retrospective analysis of clinical trial data supports the clinical relevance of the preclinical findings, particularly given that statin-treated patients in these studies were receiving standard doses for concurrent cardiovascular indications. It is also important to acknowledge that there are inherent limitations associated with the retrospective, non-randomized comparisons such as the post-hoc subgroup analyses presented here; nonetheless, the nature of the endpoints evaluated in the current work increases confidence in the findings. Specifically, in each clinical study included in the analysis, investigators were required to follow International Working Group on CLL guidelines to assess response. Additionally, achievement of CR required documented resolution in objective measures of disease burden, including improvement in hematologic laboratory parameters, resolution of lymphadenopathy as evidenced by CT scan, and clearance of CLL bone marrow infiltrate as determined by biopsy. As such, the objective nature of these parameters and the use of multivariable analyses to account for baseline differences in patient demographics provide additional confidence in the results. Nevertheless, the deeper responses observed among statin users in these analyses require confirmation in a prospective manner before widespread implementation can be considered. It would also be interesting to test whether statins enhance clinical responses to emerging CLL combination treatments such as venetoclax with rituximab (35).
Exposure-response analyses on venetoclax in subjects with CLL showed that higher venetoclax exposures are associated with higher probability of achieving complete responses and longer PFS (36, 37). Therefore, we explored the possibility that statins improve response rates simply by increasing venetoclax exposures. The analysis showed no difference in venetoclax exposures between patients who reported taking statin drugs as compared to those who did not. These results were consistent with the current knowledge about factors affecting venetoclax pharmacokinetics (38–41).
Although the observation of improved response among patients receiving a standard statin dose is appealing, given the relatively well-described and well-tolerated safety profile of these agents, higher statin dosing may further augment the efficacy of this combination. In support, several studies have reported achievable systemic exposures reaching the micromolar range (42, 43). A remaining concern is whether the combination will exacerbate any single-agent toxicities. Whereas the dose-limiting toxicity of venetoclax is tumor lysis syndrome, statins are associated with myotoxicity (particularly myositis (44)). Previous work on smooth muscle cells suggests that statins can induce apoptosis via downregulation of BCL2 (45), cautioning that venetoclax may exacerbate this toxicity through shared targeting of BCL2. However, the venetoclax studies included in this analysis did not reveal any new safety concerns, and there were no major differences with respect to adverse events of interest such as myalgia or tumor lysis syndrome. However, it must be recognized that patients included in these trials generally had long-standing statin use based on medical history, and presumably were tolerating these agents well before initiation of treatment with venetoclax, and the tolerability profile of the combination may differ in patients who are naïve to both treatments. In addition, in preclinical studies, simvastatin did not enhance venetoclax toxicity among most PBMC subpopulations. Collectively, these results suggest that the combination may retain an acceptable safety profile and achieve reasonable cancer cell selectivity.
A goal of precision medicine is to develop biomarkers and/or diagnostics that can match patients with efficacious drugs. Although variability in response to combined BCL2 and HMGCR inhibition was observed across cell lines, statin-induced mitochondrial priming (measured by dynamic BH3 profiling) correlated with increased sensitivity to the combination. Further studies are needed to determine whether these observations translate to primary patient cells, but these data illustrate the potential of DBP as a functional diagnostic. In addition to predictive diagnostics, we also identified markers of pharmacodynamic (PD) response that correlate with enhanced sensitivity to venetoclax. In particular, accumulation of unprenylated RAP1A can determine the extent of statin-induced suppression of protein prenylation, and increased PUMA may be used to predict cells that respond to the combination. PUMA induction cooperates with BH3 mimetics to antagonize anti-apoptotic BCL2 proteins and promote apoptosis, and therefore represents a functionally relevant PD readout. Indeed, across DLBCL and AML cell lines and primary CLL cells, only those cells that upregulated PUMA expression after statin treatment were more sensitive to combination with venetoclax.
One limitation of our study is that the geranylgeranylated proteins and downstream pathways that drive PUMA upregulation remain to be defined. PUMA is the product of the p53 target gene BBC3, suggesting that statins may induce mitochondrial priming through activation of p53. However, the combination of statins and venetoclax was equally effective across primary CLL samples irrespective of p53 status and occurred in p53-deficient DLBCL cells (SU-DHL4) as well as an AML cell line expressing dominant-negative p53. Furthermore, clinical data demonstrating enhanced responses to venetoclax with concomitant statin use in 17p-del CLL patients strongly support a p53-independent mechanism (study 1 was the largest individual clinical study and enrolled exclusively 17p-del patients). In support, early work using prenyltransferase inhibitors identified p53-independent mechanisms of apoptosis (46). Statin effects may be broader in cells with mutant p53, based on their ability to destabilize mutant p53 to enable the function of wild-type p53 (47). Overall, the finding that statins have activity regardless of p53 status broadens the potential for statins as apoptotic sensitizers and provides a strong rationale for testing statins prospectively.
Our results support a growing body of evidence regarding the importance of the mevalonate pathway in cancer (11). More specifically, this work provides a preclinical foundation and clinical evidence warranting prospective clinical evaluation of combining statins and BH3 mimetics to treat blood cancers.
Materials and methods
Study Design
Initial experiments were designed to test the hypothesis that combining statins and venetoclax could synergize to kill blood cancer cell lines. These experiments were performed in controlled laboratory settings, repeated three times (unless otherwise noted), and were consistently observed by two independent laboratories. Based off these results, our initial hypothesis expanded to interrogate the generalizability (across cell lines, cancer subtypes, and species), in vivo reproducibility (using a syngeneic mouse model), and clinical relevance (by retrospective analyses of human clinical trial data) of these findings. In parallel, we also sought to elucidate the mechanism of synergy with the goal of identifying markers of pharmacodynamics response in controlled laboratory experiments using primary human cells as well as human and mouse cell lines. Unless otherwise noted, no data or outliers were excluded from any analyses. For in vivo studies, mice were randomized upon initiation of dosing, and animal caretakers and investigators were blinded to the treatment groups. Sample size was pre-determined by power analysis to reliably detect 20% differences between treatment groups. Details of the clinical analyses are included below. Sample numbers and numbers of replicates performed for each experiment are included in the figure legends. Original data are provided in table S4.
Animal studies
All animal studies were conducted in accordance with guidelines of the University of California Institutional Animal Care and Use Committee. 10-week-old female C57BL/6N mice were purchased from Charles River and sub-lethally irradiated (4G) 24 hours before injection of lymphoma cells by tail vein. Lymphoma burden was measured by FACS using peripheral blood collected via Goldenrod animal lancets (Braintree Scientific, Inc.). Drug administration was performed by oral gavage with vehicle formulations as follows: venetoclax in 10% ethanol, 30% polyethylene glycol 400, and 60% Phosal 50 PG, and simvastatin in 0.5% methylcellulose and 0.1% Tween-80.
Chemicals
Simvastatin, atorvastatin calcium salt, rosuvastatin calcium salt, and fluvastatin sodium salt were obtained from Cayman Chemical Company. Simvastatin was activated as reported elsewhere (48). Venetoclax and navitoclax were obtained from Active Biochem. InSolution Q-VD-OPh was obtained from EMD Millipore. NVP-BEZ235 was obtained from LC Laboratories. Doxorubicin, vincristine, mevalonate, squalene, cholesterol, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate were all obtained from Sigma-Aldrich.
BH3 profiling
BH3 profiling of DLBCL cells was performed as described previously (26). Briefly, cells were pre-treated for 16 hours with either DMSO or indicated compounds. Cells were then harvested and washed with PBS, then incubated in T-EB buffer (300 mM trehalose, 10 mM HEPES, 80 mM potassium chloride, 1 mM EGTA, 1 mM EDTA, 0.1% BSA, and 5 mM succinic acid) with 200 nM JC-1 (Life Technologies), 0.001–0.005% digitonin (Sigma-Aldrich), and 10 μg/ml oligomycin (Sigma-Aldrich) with either DMSO or BH3-only peptides for 60 minutes before analysis using a FACScalibur (Becton-Dickinson). The sequences and method of synthesis of BH3-only peptides were described previously (49). The PUMA2a peptide contains alanine substitutions that abolish its binding interactions with BCL2 family proteins and therefore served as a negative control. Percent depolarization caused by each BH3-only peptide was calculated as the percent difference in the JC-1 red fluorescence (590 nm) relative to DMSO-treated control cells. Doses of peptides for each cell line were empirically chosen based on minimal effects on mitochondrial depolarization (akin to an IC10 concentration).
Pharmacokinetic analysis
Venetoclax samples were collected at steady state (Weeks 3, 6, or 7) of Study 2 enrolling patients with CLL/SLL or NHL. Pharmacokinetic parameters Cmax and AUC were calculated using non-compartmental methods in Statistical Analysis System
(SAS) version 9.2. These parameters were normalized to the administered dose to allow comparisons across all doses assuming pharmacokinetic linearity (50). For all analyses, the actual dose received by the patient, rather than the assigned dose was used in calculations. If the 24-hour post-dose concentration value at steady state (such as week 6 day 1, week 7 day 1) was not available, the pre-dose (0 hour) concentration on that same day was used to calculate AUC24. Venetoclax steady-state pharmacokinetic data available from patients who were reported to have been taking statins concomitantly were used to assess if statins affect venetoclax exposures. Although food has a marked effect on venetoclax pharmacokinetics (51), all steady-state pharmacokinetic data were collected under low-fat conditions, which allowed an integrated analysis.
Statistical Analysis
Statistical analyses of preclinical data were performed in the GraphPad Prism software version 5c (GraphPad Software). Unless otherwise indicated, results indicate means±S.D. of three independent experiments. P < 0.05 was considered statistically significant and was annotated throughout as: * P < 0.05, ** P < 0.005, *** P < 0.001. For drug synergy calculations, combination index versus fraction affected curves were generated using CalcuSyn software (Biosoft).
Statistical analyses included clinical data collected through 10 June 2016. Statin therapy was identified from investigator reports of concurrent medication use, and subjects with any statin use while receiving venetoclax were included in the statin subgroup. Response criteria followed the IWCLL NCI-WG 2008 guidelines and were based on investigator assessment. For the univariate analyses, odds ratios and 95% confidence intervals (CIs) were calculated for ORR and CR and compared via Fisher exact test; CIs were determined by the Wald method. PFS and OS were analyzed by Kaplan-Meier methodology. Multivariate analyses were conducted by using Cox proportional hazard model (for OS and PFS) and logistic regression model (ORR and CR) with implementation of a stepwise model selection procedure. No adjustments were made for multiple comparisons. Analyses were conducted using SAS 9.4.
Supplementary Material
Supplemental Figure S1. Simvastatin enhances the effects of venetoclax on a subset of AML and DLBCL cell lines.
Supplemental Figure S2. Statins synerg ize with venetoclax in blood cancer cells.
Supplemental Figure S3. Simvastatin plus venetoclax induce apoptosis in DLBCL and AML cell lines.
Supplemental Figure S4. Simvastatin enhances killing of primary CLL samples cultured with stimuli from the microenvironment.
Supplemental Figure S5. Statins induce dose-dependent increase in mitochondrial priming, but do not sensitize to chemotherapy.
Supplementary Figure S6. The combination of statin with venetoclax extends survival of mice with syngeneic B cell lymphoma.
Supplemental Figure S7. The effect of statins is due to on-target HMGCR inhibition.
Supplemental Figure S8. Mevalonate and geranylgeranyl pyrophosphate are sufficient to rescue from the effects of simvastatin.
Supplemental Figure S9. Simvastatin inhibits protein geranylgeranylation in a dose-dependent manner in DLBCL.
Supplemental Figure S10. Inhibition of GGT is sufficient to recapitulate the effects of simvastatin in AML cell lines.
Supplemental Figure S11. Simvastatin does not affect expression of many major BCL-2 family proteins but does increase PUMA.
Supplemental Figure S12. Simvastatin increases association of BCL2 with PUMA in sensitive OCI-AML3 cells but not resistant OCI-LY1 cells.
Supplemental Figure S13. PUMA knockdown in OCI-AML3 cells rescues them from sensitization to venetoclax by simvastatin.
Supplemental Figure S14. Statins increase PUMA upregulation through a mechanism independent of p53 in DLBCL and AML cells.
Supplemental Figure S15. Statin use is associated with longer progression-free survival in CLL patients treated in venetoclax clinical trials.
Supplemental Figure S16. Response to venetoclax was enhanced in CLL clinical trials among patients who received the 400 mg statin dose.
Supplemental Figure S17. Statin drugs do not show a PK interaction with venetoclax.
Supplemental Table S1. Characteristics of CLL patient samples.
Supplemental Table S2. Demographics: Characteristics of CLL patients enrolled across three clinical trials of venetoclax monotherapy shown by background statin use.
Supplemental Table S3. Summary of adverse events in CLL patients enrolled across three clinical trials of venetoclax monotherapy shown by background statin use.
Table S4. Raw data (in a separate Excel file)
Acknowledgments:
We thank Laura Pasqualucci and Alexandru Almasan for providing DLBCL cell lines, and Lindsey Mayo for providing dominant-negative p53. AR was an employee of AbbVie while this work was conducted and is an employee of PRA Health Sciences.
Funding: This work was supported by NIH grants R21-CA209341 and UL1 TR001414 (to DAF), by Cancer Center Support Grant P30-CA062203 (to UC Irvine), and by startup funds from the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine (to JS). JS was supported by NIH TL1TR001415 grant awarded to the University of California Irvine Institute for Clinical and Translational Science. TTV and DJ were supported by NIH Training Grant T32CA009054. DJ also received support from T32-GM08620.
Footnotes
Competing Interests: RJB, SKA, AHS, TX, JJ, LTL, and JDL are AbbVie employees and may own stock options. AR was an employee of AbbVie while this work was conducted. AL has consulted for and his laboratory has received research funding from AbbVie. MSD and SO have consulted for AbbVie. MSD has consulted for and his laboratory has received research funding from Genentech. AL has consulted for and his laboratory has received research funding from Novartis and AstraZeneca. DAF has consulted for and his laboratory has received research funding from Revolution Medicines.
Data and Materials Availability: Materials are available from the corresponding authors upon request.
References and notes
- 1.Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB, Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol. Sci 34, 508–517 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Nickels S, Vrieling A, Seibold P, Heinz J, Obi N, Flesch-Janys D, Chang-Claude J, Mortality and Recurrence Risk in Relation to the Use of Lipid-Lowering Drugs in a Prospective Breast Cancer Patient Cohort. PLOS ONE 8, e75088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardwell CR, Hicks BM, Hughes C, Murray LJ, Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology 26, 68–78 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Smith A, Murphy L, Sharp L, O’Connor D, Gallagher WM, Bennett K, Barron TI, De novo post-diagnosis statin use, breast cancer-specific and overall mortality in women with stage I-III breast cancer. Br. J. Cancer 115, 592–598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manthravadi S, Shrestha A, Madhusudhana S, Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer 139, 1281–1288 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Minden MD, Dimitroulakos J, Nohynek D, Penn LZ, Lovastatin Induced Control of Blast Cell Growth in an Elderly Patient with Acute Myeloblastic Leukemia. Leuk. Lymphoma 40, 659–662 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Dimitroulakos J, Nohynek D, Backway KL, Hedley DW, Yeger H, Freedman MH, Minden MD, Penn LZ, Increased Sensitivity of Acute Myeloid Leukemias to Lovastatin-Induced Apoptosis: A Potential Therapeutic Approach. Blood 93, 1308 (1999). [PubMed] [Google Scholar]
- 8.Bockorny B, Dasanu CA, HMG-CoA reductase inhibitors as adjuvant treatment for hematologic malignancies: what is the current evidence? Ann. Hematol 94, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi X, Jia W, Jin Y, Zhen S, Statin Use Is Associated with Reduced Risk of Haematological Malignancies: Evidence from a Meta-Analysis. PLOS ONE 9, e87019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanfilippo KM, Keller J, Gage BF, Luo S, Wang T-F, Moskowitz G, Gumbel J, Blue B, O’Brian K, Carson KR, Statins Are Associated With Reduced Mortality in Multiple Myeloma. J. Clin. Oncol 34, 4008–4014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ, The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 16, 718–731 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Wong WW, Dimitroulakos J, Minden MD, Penn LZ, HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16, 508–519 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Leverson JD, Sampath D, Souers AJ, Rosenberg SH, Fairbrother WJ, Amiot M, Konopleva M, Letai A, Found in Translation: How Preclinical Research Is Guiding the Clinical Development of the BCL2-Selective Inhibitor Venetoclax. Cancer Discovery 7, 1376–1393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW, ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med 19, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF, Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. New Engl. J. Med 374, 311–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou T-C, Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res 70, 440 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Li L, Pongtornpipat P, Tiutan T, Kendrick SL, Park S, Persky DO, Rimsza LM, Puvvada SD, Schatz JH, Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia 29, 1702–1712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ, Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia 10, 456–459 (1996). [PubMed] [Google Scholar]
- 19.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A, Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest 117, 112–121 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thijssen R, Slinger E, Weller K, Geest CR, Beaumont T, van Oers MH, Kater AP, Eldering E, Resistance to ABT-199 induced by microenvironmental signals in chronic lymphocytic leukemia can be counteracted by CD20 antibodies or kinase inhibitors. Haematologica 100, e302–306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman AA, Letai A, Fisher DE, Flaherty KT, Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Gaizo Moore V, Letai A, BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 332, 202–205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini MG, Letai A, Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, Piao H, Horowitz NS, Berkowitz RS, Matulonis U, Janne PA, Amrein PC, Cichowski K, Drapkin R, Letai A, Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS, Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 31, 2075–2084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JS, Tang SS, Ortiz V, Vo TT, Fruman DA, MCL-1-independent mechanisms of synergy between dual PI3K/mTOR and BCL-2 inhibition in diffuse large B cell lymphoma. Oncotarget 6, 35202–35217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berndt N, Sebti SM, Measurement of protein farnesylation and geranylgeranylation in vitro, in cultured cells and in biopsies, and the effects of prenyl transferase inhibitors. Nat. Protocols 6, 1775–1791 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Casey PJ, Protein prenylation: unique fats make their mark on biology. Nature reviews. Mol. Cell Biol 17, 110–122 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Lomonosova E, Chinnadurai G, BH3-only proteins in apoptosis and beyond: an overview. Oncogene 27 Suppl 1, S2–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang H, Blondal JA, Benchimol S, Minden MD, Messner HA, p53 mutations, c-myc and bcl-2 rearrangements in human non-Hodgkin’s lymphoma cell lines. Leuk. Lymphoma 19, 165–171 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Allman R, Errington RJ, Smith PJ, Delayed expression of apoptosis in human lymphoma cells undergoing low-dose taxol-induced mitotic stress. Br. J. Cancer 88, 1649–1658 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, Puvvada SD, Wendtner CM, Roberts AW, Jurczak W, Mulligan SP, Bottcher S, Mobasher M, Zhu M, Desai M, Chyla B, Verdugo M, Enschede SH, Cerri E, Humerickhouse R, Gordon G, Hallek M, Wierda WG, Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 17, 768–778 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Jones J, Choi MY, Mato AR, Furman RR, Davids MS, Heffner LT, Cheson BD, Lamanna N, Barr PM, Eradat H, Halwani A, Chyla B, Zhu M, Verdugo M, Humerickhouse RA, Potluri J, Wierda WG, Coutre SE, Venetoclax (VEN) Monotherapy for Patients with Chronic Lymphocytic Leukemia (CLL) Who Relapsed after or Were Refractory to Ibrutinib or Idelalisib. Blood 128, 637 (2016). [Google Scholar]
- 34.Kovarik JM, Hartmann S, Hubert M, Berthier S, Schneider W, Rosenkranz B, Rordorf C, Pharmacokinetic and pharmacodynamic assessments of HMG-CoA reductase inhibitors when coadministered with everolimus. J. Clin. Pharmacol 42, 222–228 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, Anderson MA, Beaven AW, Rosen ST, Tam CS, Prine B, Agarwal SK, Munasinghe W, Zhu M, Lash LL, Desai M, Cerri E, Verdugo M, Kim SY, Humerickhouse RA, Gordon GB, Kipps TJ, Roberts AW, Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 18, 230–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freise KJ, Jones AK, Menon RM, Verdugo ME, Humerickhouse RA, Awni WM, Salem AH, Relationship between venetoclax exposure, rituximab coadministration, and progression-free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol. Oncol 35, 679–684 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Freise KJ, Jones AK, Eckert D, Mensing S, Wong SL, Humerickhouse RA, Awni WM, Salem AH, Impact of Venetoclax Exposure on Clinical Efficacy and Safety in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Clin. Pharmacokin 56, 515–523 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Agarwal SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA, Wong SL, Menon RM, Konopleva MY, Salem AH, Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments. Clin. Therap 39, 359–367 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Agarwal SK, Hu B, Chien D, Wong SL, Salem AH, Evaluation of Rifampin’s Transporter Inhibitory and CYP3A Inductive Effects on the Pharmacokinetics of Venetoclax, a BCL-2 Inhibitor: Results of a Single- and Multiple-Dose Study. J. Clin. Pharmacol 56, 1335–1343 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M, Chien D, Lewis LD, Wong SL, Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br. J. Clin. Pharmacol 83, 846–854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH, Clinical Predictors of Venetoclax Pharmacokinetics in Chronic Lymphocytic Leukemia and Non-Hodgkin’s Lymphoma Patients: a Pooled Population Pharmacokinetic Analysis. AAPS J. 18, 1192–1202 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ, Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother. Pharmacol 57, 155–164 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Siekmeier R, Lattke P, Mix C, Park JW, Jaross W, Dose Dependency of Fluvastatin Pharmacokinetics in Serum Determined by Reversed Phase HPLC. J. Card. Pharmacol. Therap 6, 137–145 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Ucar M, Mjorndal T, Dahlqvist R, HMG-CoA reductase inhibitors and myotoxicity. Drug Safety 22, 441–457 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Blanco-Colio LM, Villa A, Ortego M, Hernandez-Presa MA, Pascual A, Plaza JJ, Egido J, 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, atorvastatin and simvastatin, induce apoptosis of vascular smooth muscle cells by downregulation of Bcl-2 expression and Rho A prenylation. Atherosclerosis 161, 17–26 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Martirosyan A, Clendening JW, Goard CA, Penn LZ, Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer 10, 103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrales A, Ranjan A, Iyer Swathi V., Padhye S, Weir Scott J., Roy A, Iwakuma T, DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat. Cell Biol 18, 1233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong W, Vuletic S, Albers JJ, Differential effects of simvastatin and pravastatin on expression of Alzheimer’s disease-related genes in human astrocytes and neuronal cells. J. Lipid Res 50, 2095–2102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A, Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Salem AH, Agarwal SK, Dunbar M, Enschede SL, Humerickhouse RA, Wong SL, Pharmacokinetics of Venetoclax, a Novel BCL-2 Inhibitor, in Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia or Non-Hodgkin Lymphoma. J. Clin. Pharmacol 57, 484–492 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Salem AH, Agarwal SK, Dunbar M, Nuthalapati S, Chien D, Freise KJ, Wong SL, Effect of Low- and High-Fat Meals on the Pharmacokinetics of Venetoclax, a Selective First-in-Class BCL-2 Inhibitor. J. Clin. Pharmacol 56, 1355–1361 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Simvastatin enhances the effects of venetoclax on a subset of AML and DLBCL cell lines.
Supplemental Figure S2. Statins synerg ize with venetoclax in blood cancer cells.
Supplemental Figure S3. Simvastatin plus venetoclax induce apoptosis in DLBCL and AML cell lines.
Supplemental Figure S4. Simvastatin enhances killing of primary CLL samples cultured with stimuli from the microenvironment.
Supplemental Figure S5. Statins induce dose-dependent increase in mitochondrial priming, but do not sensitize to chemotherapy.
Supplementary Figure S6. The combination of statin with venetoclax extends survival of mice with syngeneic B cell lymphoma.
Supplemental Figure S7. The effect of statins is due to on-target HMGCR inhibition.
Supplemental Figure S8. Mevalonate and geranylgeranyl pyrophosphate are sufficient to rescue from the effects of simvastatin.
Supplemental Figure S9. Simvastatin inhibits protein geranylgeranylation in a dose-dependent manner in DLBCL.
Supplemental Figure S10. Inhibition of GGT is sufficient to recapitulate the effects of simvastatin in AML cell lines.
Supplemental Figure S11. Simvastatin does not affect expression of many major BCL-2 family proteins but does increase PUMA.
Supplemental Figure S12. Simvastatin increases association of BCL2 with PUMA in sensitive OCI-AML3 cells but not resistant OCI-LY1 cells.
Supplemental Figure S13. PUMA knockdown in OCI-AML3 cells rescues them from sensitization to venetoclax by simvastatin.
Supplemental Figure S14. Statins increase PUMA upregulation through a mechanism independent of p53 in DLBCL and AML cells.
Supplemental Figure S15. Statin use is associated with longer progression-free survival in CLL patients treated in venetoclax clinical trials.
Supplemental Figure S16. Response to venetoclax was enhanced in CLL clinical trials among patients who received the 400 mg statin dose.
Supplemental Figure S17. Statin drugs do not show a PK interaction with venetoclax.
Supplemental Table S1. Characteristics of CLL patient samples.
Supplemental Table S2. Demographics: Characteristics of CLL patients enrolled across three clinical trials of venetoclax monotherapy shown by background statin use.
Supplemental Table S3. Summary of adverse events in CLL patients enrolled across three clinical trials of venetoclax monotherapy shown by background statin use.
Table S4. Raw data (in a separate Excel file)