Abstract
Background
Children with sickle cell disease experience vaso-occlusive crises (VOC) that requires opioid pharmacotherapy. Multimodal analgesic therapy may reduce pain and opioid-induced adverse effects.
Objective
The primary objective was to examine the effectiveness of intravenous (IV) acetaminophen in children presenting with pain from VOC. Secondary objectives were to document the safety and opioid-sparing effects of IV acetaminophen during VOC in pediatric patients.
Setting
Children’s Medical Center, NYU-Winthrop Hospital
Method
This retrospective study had two groups of patients, those who received opioids alone (group O) and those who received acetaminophen with opioids (group OA). Children two to 19 years of age who were admitted to the children’s medical center for VOC were eligible for inclusion.
Main outcome measure
A reduction in pain by at least 1 out of 10. With every analgesic dose, we documented pain scales and pain scores before and after each dose, the number of doses administered per day, and mg/kg/day. Data were analyzed using the mixed effect model. All opioids administered to patients were converted to morphine equivalents. We documented length of stay and adverse events.
Results
We had a total of 46 children: 28 in group O and 18 in group OA. Acetaminophen reduced the pain from VOC by 2.3/10. There were trends in different assessments of opioid-sparing effects, in reducing opioid dosage (−0.5 mg/kg morphine equivalent; P = 0.45), reducing overall morphine equivalent doses (−18.5 mg; P = 0.066), and opioid-related adverse effects.
Conclusion
This is the first study to demonstrate the effectiveness of IV acetaminophen in treating VOC pain in children, supporting multimodal analgesic therapy in this setting. Opioid-sparing effects were also encouraging.
Keywords: pain, sickle cell disease, opioid, acetaminophen IV, children
INTRODUCTION
Children with sickle cell disease (SCD) frequently experience VOC that may lead to acute, severe pain. This pain is a result of hypoxia, ischemia, and inflammation, and typically lasts from 5 to 7 days and requires hospitalization.1,2 During a crisis, pain can vary widely in intensity among patients, therefore the experience of pain is subjective and must be treated accordingly. Opioid analgesics remain central to the pharmacotherapy of moderate to severe pain from VOC. While these analgesics are effective, they are associated with adverse effects, including sedation, constipation, and respiratory depression.1 A multimodal analgesic approach, using adjuvant analgesics with different mechanisms of action, maximizes pain-receptor targeting and reduces adverse effects. This approach aims to improve pain control while reducing the need for opioid medications, and thus their related adverse effects.3,4
In 2010, an IV form of acetaminophen was approved by the U.S. Food and Drug Administration (FDA) in children two years of age and older for the management of mild to moderate pain, and severe pain when combined with opioids.5 Acetaminophen is widely distributed in the body and readily penetrates the cerebrospinal fluid.6 It has been successfully used in postoperative pain in children and is well tolerated when used at therapeutic doses.7 The IV form provides higher serum levels with a more rapid and predictable analgesia over other routes of administration, including oral or rectal routes.8 Analgesia begins in five to 10 minutes, peaks at approximately one hour, and lasts four to six hours.8
Following FDA approval, our institution adopted IV acetaminophen for pain management, including pain from VOC in SCD, in pediatric patients older than two years of age. While studies have been conducted on the use of IV acetaminophen as an adjunct in surgery, we were unable to find published data on its use in the treatment of VOC from SCD in children.3,7,8
AIM OF STUDY
The primary objective of this retrospective study was to evaluate the efficacy of IV acetaminophen in reducing pain in children during an SCD pain crisis. Secondary objectives were to evaluate its efficacy in reducing opioid requirements, length of stay, and/or the adverse effects associated with opioids. This study was determined to be exempt from full Institutional Review Board approval at our institution.
Methods
This retrospective chart review included: 1) children 2 to 19 years of age with a history of sickle cell anemia and who were admitted to the hospital for pain management from VOC; 2) treatment with an opioid alone, IV acetaminophen, and/or both; and 3) a length of stay of 48 hours or more. Excluded were children who did not receive any analgesic, and those with missing data. The study period was from January 1, 2011, through May 31, 2015. This four-year period was divided into two segments: before IV acetaminophen use at our institution (January 1, 2011, through June 30, 2013) and after IV acetaminophen use (July 1, 2013 through May 31, 2015). Hence, there were two groups of patients: those who received opioid agents alone (Group O) and those who received IV acetaminophen with opioid analgesics (Group OA). If a subject received IV acetaminophen alone without opioids, they were placed with Group OA, because opioid use may have been spared. Data were collected on patients’ age, gender, weight, length of stay, pain scales used, all analgesic agents and doses administered, and adverse effects, when documented. To determine efficacy, pain scores were recorded when available, prior to and within four hours of analgesic administration. All acetaminophen and opioid doses were recorded and documented in mg/kg/day and by the number of doses per day.
IV acetaminophen was determined to be effective if there was a minimum reduction of 1/10 in pain scores according to documentation in the patient’s chart. We assessed all sources of acetaminophen exposure (oral [PO], IV, rectal, and acetaminophen-containing, opioid-combination agents) to ensure that the recommended daily dose was not exceeded. All opioid dosages (IV or PO) were recorded and converted to PO morphine equivalents (ME), in mg/day and ME mg/kg/day to quantify overall opioid exposure, using an equianalgesic opioid dosing chart.9 We documented the number of analgesic doses administered each day, for days 1 through 5 of admission, given the nature of sickle-cell crisis duration. We compared ME requirements between the groups to assess acetaminophen’s opioid-sparing effects, and we conducted a parallel comparison of ketorolac and other non-steroidal anti-inflammatory drug (NSAID) use between the groups. We documented all adverse effects and compared them between groups.
Statistical Analysis
Data were analyzed and compared using the two-sample t-test, Wilcoxon rank sum test, Fisher’s exact test, and a linear mixed-effect model. Data were summarized using mean ± standard deviation, median (interquartile range), and count (percent). All analyses were done using SAS 9.4®.
RESULTS
The study population consisted of 46 children: 28 in group O and 18 in group OA. All subjects satisfied the study entry criteria. The mean age was 9.6 ± 5.8 years and there was no significant difference in gender between groups. The length of stay was similar between groups (P = 0.81), with 50% of patients in both groups admitted for ≥ 5 days (Table 1). Although demographics were not statistically different between groups, subjects in OA tended to be older and to weigh more, therefore weight-based dosage calculations were used to ensure uniformity in comparing opioid requirements between groups. Pain scales varied according to age and included Face, Legs, Activity, Cry, Consolability (FLACC); Wong-Baker FACES; and numeric pain scales. In practice, to assess pain in non-verbal children, we use various scales according to each sub-patient population: neonates, infants and toddlers, and older children. All the scales used and mentioned in this article have been validated and are reproducible in the pediatric literature. The three different pain scales mentioned above, including FLACC and Wong-Baker, are graded to provide the correlate of the numerical pain scale of 1 to 10 that is used in older children and adults. This allows us to have one numerical number to assess and address a child’s pain. Two subjects who were treated with acetaminophen alone (without opioids) were included in group OA to examine acetaminophen’s efficacy.
Table 1.
Demographic Data for Patients in Group OA and Group O
| Group OA N = 18 |
Group O N = 28 |
Mean Difference | P–Value | |
|---|---|---|---|---|
| Age in Years | 11.1 ± 5.8 | 8.7 ± 5.8 | −2.4 | 0.17 |
| Female, N (%) | 5 (27.8%) | 9 (32.1%) | 4 | 1 |
| Weight, Kg | 48.8 ± 34.1 | 33.6 ± 20.44 | −15.2 | 0.17 |
| Length of Stay, Days (IQ Range) | 4.5 (4–7) | 4.5 (4–6) | 0 | 0.81 |
Morphine IV was most commonly prescribed to treat pain in both groups, followed by hydromorphone IV. The most commonly prescribed oral opioid was oxycodone; however, oral opioid prescribing varied widely, encompassing morphine, acetaminophen with codeine, tramadol, or methadone in both groups. Mean ± sd opioid doses for O and OA were 3.2 ± 1.4 doses and 2.5 ± 1.3 doses per day, respectively.
A total of 124 IV acetaminophen doses were administered in group OA, with a mean (± sd) number of acetaminophen doses of 7.18 ± 5.8 per subject and a duration of therapy of 2.76 ± 1.89 days. Doses ranged from 12.5 mg/kg/dose IV every 4 hours to 15 mg/kg/dose IV every 6 hours. Adult doses were prescribed for patients whose weight exceeded 40 kg. Acetaminophen was prescribed around-the-clock for 24-hour periods of time, then the order was renewed if deemed necessary by the medical team. Excluding combination analgesics that had acetaminophen as a component, the minimum time between the administration of opioid and IV acetaminophen was 45 minutes.
We analyzed the analgesic effects of 49 doses of IV acetaminophen when it was administered without other analgesics, in instances where pain scores were adequately documented. Pain scores were reduced in 34 (69%) of the total doses administered, with a mean reduction in pain of 2.4 ± 1.9 on a scale of 1 to 10. Individual pain scores while patients were on acetaminophen therapy ranged from zero change in pain reduction to a drop of 7 points on the pain scale. There was no instance in which total acetaminophen exposure (IV, PO, or combination) exceeded the maximum that is recommended per day.
Mean overall pain scores were lower in group OA than in group O: 4.6 out of 10 versus 5.4 out of 10, respectively. The addition of acetaminophen further reduced pain by 0.8 out of 10, a 14.8% relative reduction.
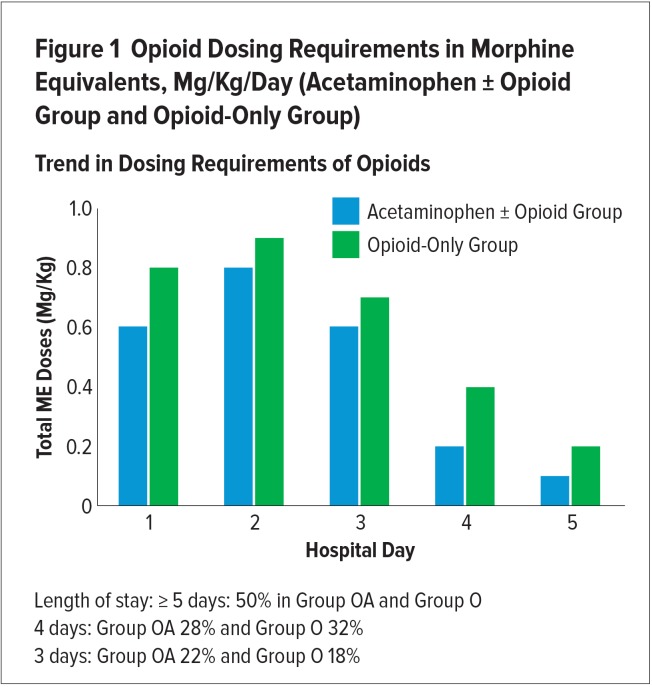
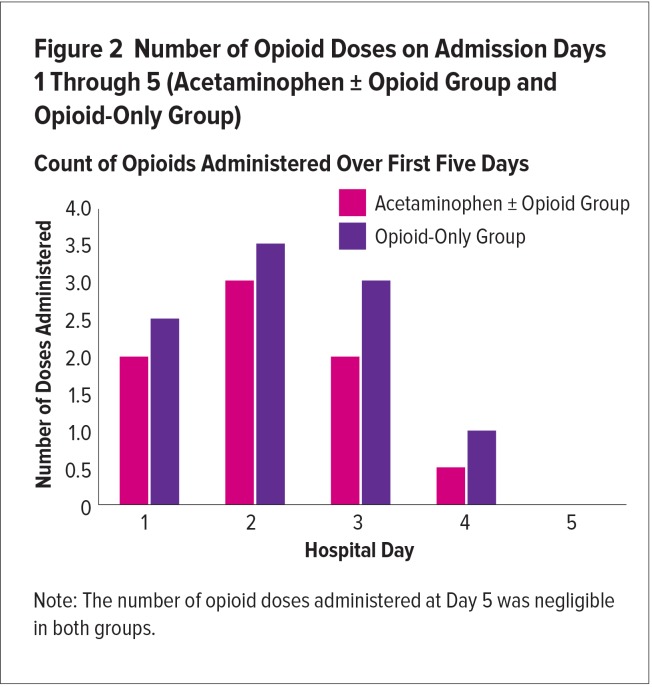
There was a trend for reduced overall and daily opioid exposure in group OA, by three different analyses: 1) ME, mg/kg was reduced over the five days by 0.5 mg/kg, a 16% relative reduction (group OA, 2.6 ± 2.4 vs. group O, 3.1 ± 2.2; P = 0.45); 2) ME doses decreased overall (−18.5 mg; P = 0.066); and 3) a mean overall reduction in the number of opioid doses administered (−2.5 ME mg/kg doses; group OA, 7.5 vs. group O, 10), a relative reduction of 25% (Figures 1 and 2).
Figure 1.
Opioid Dosing Requirements in Morphine Equivalents, Mg/Kg/Day (Acetaminophen ± Opioid Group and Opioid-Only Group)
Trend in Dosing Requirements of Opioids
Figure 2.
Number of Opioid Doses on Admission Days 1 Through 5 (Acetaminophen ± Opioid Group and Opioid-Only Group)
Count of Opioids Administered Over First Five Days
The trends in the opioid-sparing effects of acetaminophen were consistent during the days of admission; however, statistical analysis calculation on each day was not possible because of the mixed effect model. Duration of opioid therapy was not significantly different between the groups (3.4 days for group OA and 3.8 days for group O (P = > 0.5).
Adjuvant analgesics, which included ketorolac IV, ibuprofen PO/IV, naproxen PO, and acetaminophen PO, were used less frequently in group OA than in group O, with 8.9 doses and 10.1 doses per patient, respectively (Table 2). Gastrointestinal and respiratory adverse effects were reported less frequently in OA patients than in O patients (50% vs. 61%, 18% relative reduction). No patient developed hepatotoxicity. Our study limitations are those that are inherent in a retrospective study design, including a lack of pain-score documentation after administration of every analgesic dose, use of different pain scales, and inconsistencies in the timing of the assessment. If a patient was resting, sleeping, or not complaining of pain, the health care provider may not have recorded pain scores as therapy was successful. Another limitation was that our sample size was small and uneven because of the recent addition of acetaminophen to our institution’s medication formulary.
Table 2.
Adjuvant Analgesic Use
| Analgesic | N (%) | Mean Number of Doses | Median Number of Doses | Maximum Number of Doses | Total Number of Doses (Dose Per Patient) | |
|---|---|---|---|---|---|---|
| Group OA, N = 18 | Ketorolac IV | 14/18 (78%) | 9.3 | 8.5 | 20 | 130 (7.2) |
| Ibuprofen PO | 4/18 (22%) | 1.75 | 1 | 4 | 7 (0.4) | |
| Acetaminophen PO | 8/18 (44%) | 2.9 | 2.5 | 18 | 23 (1.3) | |
| Group OA: Subtotal (total number of doses per patient): 160 (8.9) | ||||||
| Group O, N = 28 | Ketorolac IV | 22 (79%) | 8.2 | 7 | 24 | 181 (6.5) |
| Ibuprofen PO | 12 (43%) | 4.3 | 3 | 11 | 52 (1.9) | |
| Naproxen PO | 1 (0.04%) | 0 | 0 | 7 | 7 (0.3) | |
| Acetaminophen PO | 13 (46%) | 3.1 | 3 | 7 | 40 (1.4) | |
| Group O: Subtotal (total number of doses per patient): 280 (10.1) | ||||||
DISCUSSION
To our knowledge, this is the first study to examine the efficacy, safety, and opioid-sparing effects of IV acetaminophen in the management of VOC pain in children. Our main objective was achieved: we established the efficacy of IV acetaminophen alone in reducing VOC pain by 2.4 out of 10 (a 24% reduction). Our results are consistent with a review of IV acetaminophen in adults and a limited number of pediatric patients having pain after surgery, which showed a 20% to 36% reduction in pain.10,11 In our study, when IV acetaminophen was combined with opioid analgesic agents, pain scores were further reduced by 14.6%.
Using different measures, we observed a trend for the opioid-sparing effects of IV acetaminophen in our study. There was an overall relative reduction in opioid dosing requirements by 16%, in the number of opioid doses by 25%, and in the duration of opioid therapy by 10.5%. At our institution, some prescribers tend to use opioids as needed rather than around-the-clock, while ensuring that adjuvant analgesics are prescribed aroundthe-clock. This explains the lower number of daily opioid doses in this patient series; however, this treatment approach was applied equally to both groups of patients. Given the variety of IV and PO opioids prescribed, we used conversions to oral ME for the purposes of a unified quantification and comparison between groups. Because of patient weight differences between groups, we compared opioid exposure using weight-based opioid dosing requirements, ME mg/kg/day.
The literature contains conflicting reports on the opioid-sparing effects of acetaminophen. Ceelie et al.12 found postoperative IV acetaminophen reduced morphine requirements by 66% in 71 children younger than one year of age who had undergone various surgical procedures. Hong et al.13 showed a reduction in fentanyl requirements and adverse effects by 50% in a group of 63 infants and children following ureteroneocystostomy. Hiller et al.14 documented the efficacy of IV acetaminophen in reducing pain exceeding 6/10 in 36 children and adolescents, following spine surgery; however, they did not find oxycodone-sparing effects. In their review of adult and pediatric patients who received IV acetaminophen, Yeh et al.10 found a reduction in opioid consumption by 20%, which is similar to our findings. There were no other studies of IV acetaminophen in pediatric patients during VOC with which to compare our results.
Adverse effects in Group OA tended to occur less frequently than in Group O. Other studies did not find a significant reduction in opioid-related adverse effects from the addition of IV acetaminophen.7,11,12 This is intriguing in light of observances by other researchers of opioid-sparing effects with the addition of this medication.
A prospective trial of IV acetaminophen in children during VOC may further support its opioid-sparing effects. At our institution, these findings were sufficient to encourage the use of IV acetaminophen as an analgesic during VOC.
CONCLUSION
This study is the first to confirm the effectiveness of IV acetaminophen in reducing pain from a VOC in children with sickle cell disease. Clinically, acetaminophen reduced opioid requirements and opioid-related adverse effects. Future trials with a prospective, randomized, crossover design and a larger sample size may further confirm our findings.
Footnotes
Disclosure: The authors report no financial or commercial interests in regard to this article.
Ethics approval: All procedures performed in this study that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent: This study was considered to be exempt from Institutional Review Board approval. As this was a retrospective study, it was not possible to obtain informed consent from all individual participants included in the study.
REFERENCES
- 1.National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services. Evidence-based management of sickle cell disease: expert panel report. Sep, 2014. [Accessed January 9, 2019]. Available at: https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease.
- 2.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal [published online August 24, 2012] Blood. 2012;120(18):3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 3.Groudine S, Fossum S. Use of intravenous acetaminophen in the treatment of postoperative pain. J Perianesth Nurs. 2011;26(2):74–80. doi: 10.1016/j.jopan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Stinson J, Naser B. Pain management in children with sickle cell disease. Pediatr Drugs. 2003;5(4):229–241. doi: 10.2165/00128072-200305040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Shastri N. Intravenous acetaminophen use in pediatrics. Pediatr Emerg Care. 2015;31(6):444–450. doi: 10.1097/PEC.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 6.Jahr JS, Lee VK. Intravenous acetaminophen. Anesthesiol Clin. 2010;28(4):619–645. doi: 10.1016/j.anclin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials [published online January 28, 2005] Br J Anaesth. 2005;94(4):505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 8.Chidambaran V, Sadhasivam S. Pediatric acute and surgical pain management: recent advances and future perspectives. Int Anesthesiol Clin. 2012;50(4):66–82. doi: 10.1097/AIA.0b013e31826f3284. [DOI] [PubMed] [Google Scholar]
- 9.Baumann TJ, Herndon CM, Strickland JM. Pain management. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014. [Accessed December 17, 2018]. pp. 925–942. Available at: https://accesspharmacy.mhmedical.com/book.aspx?bookid=689. [Google Scholar]
- 10.Yeh YC, Reddy P. Clinical and economic evidence for intravenous acetaminophen [published online May 8, 2012] Pharmacotherapy. 2012;32(6):559–579. doi: 10.1002/j.1875-9114.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 11.McNicol ED, Ferguson MKC, Haroutounian S, et al. Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev. 2016;(5):CD007126. doi: 10.1002/14651858.CD007126.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceelie I, de Wildt SN, van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309(2):149–154. doi: 10.1001/jama.2012.148050. [DOI] [PubMed] [Google Scholar]
- 13.Hong JY, Kim WO, Koo BN, et al. Fentanyl-sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent-/nurse-controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology. 2010;113(3):672–677. doi: 10.1097/ALN.0b013e3181e2c34b. [DOI] [PubMed] [Google Scholar]
- 14.Hiller A, Helenius I, Nurmi E, et al. Acetaminophen improves analgesia but does not reduce opioid requirement after major spine surgery in children and adolescents. Spine. 2012;37(20):e1225–e1231. doi: 10.1097/BRS.0b013e318263165c. [DOI] [PubMed] [Google Scholar]