Abstract
Development and planning of health care services requires robust health information systems to define the burden of disease, inform policy development, and identify opportunities to improve service provision. The global coverage of kidney disease health information systems has not been well reported, despite their potential to enhance care. As part of the Global Kidney Health Atlas, a cross-sectional survey conducted by the International Society of Nephrology, data were collected from 117 United Nations member states on the coverage and scope of kidney disease health information systems and surveillance practices. Dialysis and transplant registries were more common in high-income countries. Few countries reported having nondialysis chronic kidney disease and acute kidney injury registries. Although 62% of countries overall could estimate their prevalence of chronic kidney disease, less than 24% of low-income countries had access to the same data. Almost all countries offered chronic kidney disease testing to patients with diabetes and hypertension, but few to high-risk ethnic groups. Two-thirds of countries were unable to determine their burden of acute kidney injury. Given the substantial heterogeneity in the availability of health information systems, especially in low-income countries and across nondialysis chronic kidney disease and acute kidney injury, a global framework for prioritizing development of these systems in areas of greatest need is warranted.
Keywords: acute kidney injury, chronic kidney disease, end-stage kidney disease, health information systems, registries, screening
Health information systems are the cornerstone of health service surveillance and monitoring, governance and regulation, and planning and development.1 Encompassing registries, electronic health records, and disease surveillance systems, health information systems provide an overview of disease incidence, prevalence and patient outcomes,2, 3 allow the objective assessment of the quality and safety of care,4, 5 and facilitate comparisons between and within health services.3, 6, 7, 8, 9 Health information systems have become essential for informing health service growth and expansion,10, 11 enabling policy development,11, 12 stimulating research and hypothesis generation,13, 14, 15 and guiding allocation of resources and funding.16, 17
All countries can benefit from comprehensive health information systems. For high-income countries, such systems can provide a cost-effective means by which to identify, implement, and share best practices,2, 11 and potentially to reduce health care costs by identifying areas with unduly high or unnecessary expenditures.2 Additional benefits for low-income countries include identification of areas of need to enable prioritization, and appropriate channeling of health care resources.18 Despite these benefits, there is significant heterogeneity in the global availability of health information systems,19, 20 which is unsurprising given the financial and organizational resources required to establish such systems.
The global coverage of health information systems across the spectrum of kidney disease has not been well described. Liu et al. reported global variability in the availability and scope of dialysis-specific renal registries.21 In their review, they found 48 dialysis registries, almost half of which were based in Europe. Most did not record clinical outcomes other than mortality, and public access to information was uncommon. Similar issues undoubtedly exist in transplantation, nondialysis chronic kidney disease (CKD), and acute kidney injury (AKI) registries. Identification of deficiencies in the global coverage of kidney disease health information systems will facilitate targeted improvement in areas of need. In turn, an accurate estimation of the global burden of kidney disease will be achievable and regional, national, and global resources can be focused appropriately.
Results
Of the 130 United Nations member states that received an invitation to participate, 125 states completed 227 surveys. In total, 117 states provided information pertaining to their kidney disease health information systems, with representation across all International Society of Nephrology (ISN) regions and 2014 World Bank country classifications (i.e., low-, lower-middle–, upper-middle–, and high-income).22, 23 Most of the 227 survey respondents were nephrologists (n = 202, 89%), followed by health care administrators or policy makers (n = 9, 4%), non-nephrology physicians (n = 7, 3%), and other individuals (n = 9, 4%).
Availability of registries
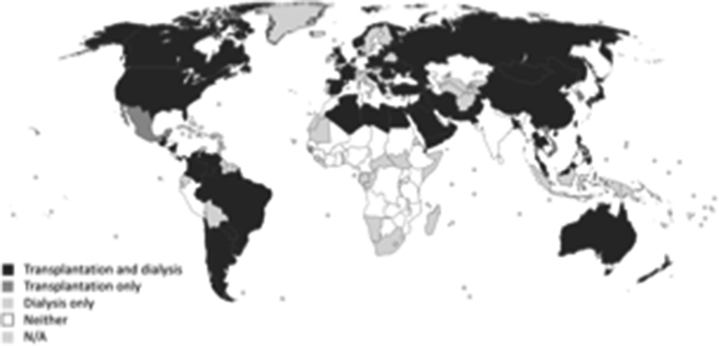
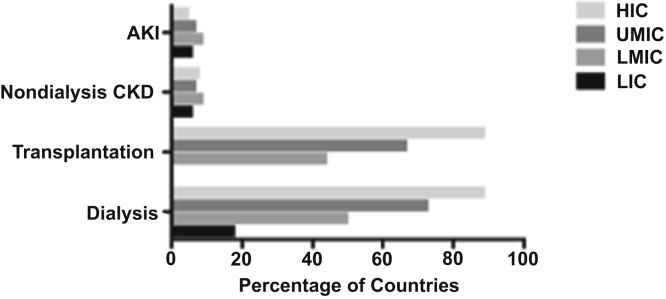
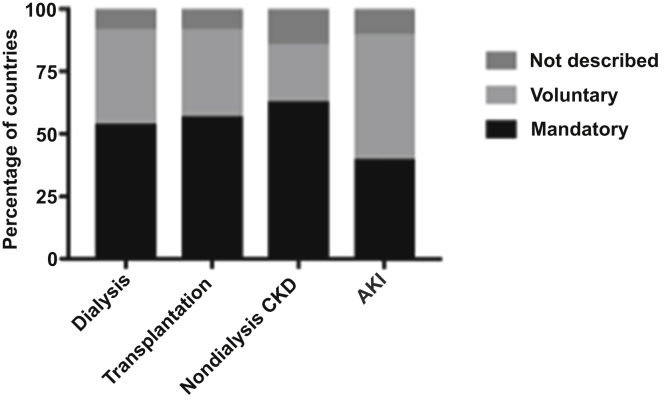
The majority of countries (n = 75, 64%) reported the existence of a national or regional registry for dialysis, and over half (n = 68, 58%) had a registry for transplantation. There was wide variation between ISN regions and World Bank income groups in terms of dialysis and transplant registry availability, with the lowest representation in low-income countries in Africa, the Middle East, and South Asia (Table 1, Figures 1 and 2). Most high-income countries had a dialysis (n = 34, 89%) or transplant registry (n = 34, 89%). Few low-income countries had a dialysis registry (n = 3, 18%), and none had a transplant registry. Only 9 (8%) countries had a nondialysis CKD registry and 8 (7%) an AKI registry, with little variation across ISN regions and World Bank income groups (Figure 2). The majority of nondialysis CKD registries (n = 5, 56%) covered all stages of CKD and were based nationally (n = 8, 89%). Mandatory provider participation was required for most dialysis (n = 40, 54%), transplant (n = 39, 57%) and nondialysis CKD (n = 5, 63%) registries; however, AKI registries tended to be voluntary (n = 4, 50%) (Figure 3).
Table 1.
Availability of renal registries by International Society of Nephrology region and World Bank income group (n=117)
| Region/group | Countries in region/group, n | Countries with dialysis registries, n (%) | Countries with transplant registries, n (%) | Countries with nondialysis CKD registries, n (%) | Countries with AKI registries, n (%) |
|---|---|---|---|---|---|
| Overall | 117 | 75 (64) | 68 (58) | 9 (8) | 8 (7) |
| ISN region | |||||
| Africa | 31 | 11 (35) | 6 (19) | 1 (3) | 1 (3) |
| Eastern and Central Europe | 16 | 15 (94) | 14 (88) | 2 (13) | 4 (25) |
| Latin America and the Caribbean | 16 | 11 (69) | 11 (69) | 2 (13) | 0 (0) |
| Middle East | 13 | 10 (77) | 8 (62) | 1 (8) | 1 (8) |
| NIS and Russia | 6 | 4 (67) | 4 (67) | 1 (17) | 1 (17) |
| North America | 2 | 2 (100) | 2 (100) | 0 (0) | 0 (0) |
| North and East Asia | 6 | 5 (83) | 6 (100) | 0 (0) | 0 (0) |
| Oceania and Southeast Asia | 13 | 7 (54) | 7 (54) | 0 (0) | 0 (0) |
| South Asia | 5 | 2 (40) | 2 (40) | 0 (0) | 1 (20) |
| Western Europe | 9 | 8 (89) | 8 (89) | 2 (22) | 0 (0) |
| World Bank income group | |||||
| Low-income | 17 | 3 (18) | 0 (0) | 1 (6) | 1 (6) |
| Lower-middle–income | 32 | 16 (50) | 14 (44) | 3 (9) | 3 (9) |
| Upper-middle–income | 30 | 22 (73) | 20 (67) | 2 (7) | 2 (7) |
| High-income | 38 | 34 (89) | 34 (89) | 3 (8) | 2 (5) |
AKI, acute kidney injury; CKD, chronic kidney disease; ISN, International Society of Nephrology; NIS, newly independent states.
Results shown include total number of countries in each region or income group and numbers (%) of countries with registries in that category.
Figure 1.
Global distribution of registries for dialysis and transplantation across 117 United Nations member states.
Figure 2.
Availability of registries for acute kidney injury (AKI), nondialysis chronic kidney disease (CKD), transplantation and dialysis by World Bank income group (HIC, high-income country; LIC, low-income country; LMIC, lower-middle–income country; UMIC, upper-middle–income country) in 117 United Nations member states.
Figure 3.
Nature of provider participation in registries for dialysis (n = 75), transplantation (n = 68), chronic kidney disease (CKD, n = 9), and acute kidney injury (AKI, n = 8).
CKD incidence and prevalence
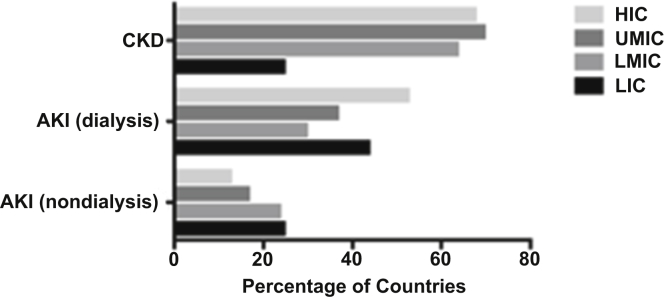
According to respondents from about two-thirds of countries, data were available on national prevalence of CKD (n = 72, 62%) (Table 2, Figure 4). Although CKD prevalence data were available in most lower-middle– (n = 21, 64%), upper-middle– (n = 21, 70%), and high-income (n = 26, 68%) countries, only a small number of low-income countries (n = 4, 25%) reported access to the same information. One-quarter (n = 32, 27%) of countries identified ethnic groups at high-risk of CKD in their countries, of which very few were low-income countries (n = 3, 19%).
Table 2.
Availability of data on prevalence of kidney disease by International Society of Nephrology region and World Bank income group (n=117)
| Region/group | Countries in region/group, n | Countries with CKD prevalence data, n (%) | Countries with AKI (nondialysis) prevalence data, n (%) | Countries with AKI (dialysis) prevalence data, n (%) |
|---|---|---|---|---|
| Overall | 117 | 72 (62) | 22 (19) | 48 (41) |
| ISN region | ||||
| Africa | 31 | 12 (39) | 8 (26) | 13 (42) |
| Eastern and Central Europe | 16 | 9 (56) | 4 (25) | 10 (63) |
| Latin America and the Caribbean | 16 | 13 (81) | 2 (13) | 4 (25) |
| Middle East | 13 | 9 (69) | 2 (15) | 3 (23) |
| South Asia | 6 | 4 (67) | 1 (17) | 3 (50) |
| North America | 2 | 2 (100) | 2 (100) | 2 (100) |
| North and East Asia | 6 | 5 (83) | 0 (0) | 3 (50) |
| Oceania and Southeast Asia | 13 | 10 (77) | 2 (15) | 5 (38) |
| NIS and Russia | 5 | 3 (60) | 0 (0) | 0 (0) |
| Western Europe | 9 | 5 (56) | 1 (11) | 5 (56) |
| World Bank income group | ||||
| Low-income | 16 | 4 (25) | 4 (25) | 7 (41) |
| Lower-middle–income | 32 | 21 (66) | 8 (54) | 10 (31) |
| Upper-middle–income | 30 | 21 (70) | 5 (17) | 11 (37) |
| High-income | 38 | 26 (68) | 5 (13) | 20 (53) |
AKI, acute kidney injury; CKD, chronic kidney disease; ISN, International Society of Nephrology; NIS, newly independent states.
Results shown include total number of countries in each region or income group and numbers (%) of countries with registries in that category.
Figure 4.
Availability of data on the prevalence of chronic kidney disease (CKD) and acute kidney injury (AKI) by World Bank income group (HIC, high-income country; LIC, low-income country; LMIC, lower-middle–income country; UMIC, upper-middle–income country) in 117 United Nations member states.
Identification of CKD
A total of 28 (24%) countries had an established population-based CKD screening program for individuals without specific CKD risk factors; most were in high-income countries (n = 12, 32%). The only low-income country with a CKD screening program implemented it actively through both routine health encounters and specific screening processes. Lower-middle–income countries tended to use active screening more often than reactive screening (50% vs. 25%), while upper-middle– and high-income countries used both active and reactive approaches equally.
Countries varied in their approach to CKD testing in high-risk individuals (Table 3). All countries performed CKD testing through routine health encounters in patients with diabetes mellitus, and almost all (n = 113, 97%) did so in those with hypertension. Approximately 80% of countries tested patients with a history of cardiovascular disease, autoimmune or multisystem disease, or urological disorder. Patients with a family history of CKD, those aged ≥65 years, or those with chronic use of nephrotoxic medications were offered CKD testing in 68%, 62%, and 60% of countries, respectively. Only 20 countries (17%) performed CKD testing in high-risk ethnic groups, most of which were high-income (n = 10).
Table 3.
Availability of chronic kidney disease and subgroup screening by World Bank income group (n=117)
| Detection strategy | All (N = 117), n (%) | LIC (N = 17), n (%) | LMIC (N = 32), n (%) | UMIC (N = 30), n (%) | HIC (N = 38), n (%) |
|---|---|---|---|---|---|
| Active CKD detection | 28 (24) | 1 (6) | 7 (22) | 8 (27) | 12 (32) |
| Subgroup screening | |||||
| DM | 117 (100) | 17 (100) | 32 (100) | 30 (100) | 38 (100) |
| HTN | 113 (97) | 17 (100) | 30 (94) | 29 (97) | 37 (97) |
| CVD | 95 (81) | 13 (76) | 24 (75) | 25 (83) | 33 (87) |
| Autoimmune | 93 (79) | 9 (53) | 26 (81) | 25 (83) | 33 (87) |
| Older age (≥65 years) | 73 (62) | 7 (41) | 15 (47) | 22 (73) | 29 (76) |
| Urological disorders | 91 (78) | 16 (94) | 25 (78) | 23 (77) | 27 (71) |
| Nephrotoxic drugs | 70 (60) | 10 (59) | 19 (59) | 15 (50) | 26 (68) |
| Family history of CKD | 79 (68) | 12 (71) | 16 (50) | 22 (73) | 29 (76) |
| High-risk ethnic groups | 20 (17) | 1 (6) | 5 (16) | 4 (13) | 10 (26) |
CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HIC, high-income countries; HTN, hypertension; LIC, low-income countries; LMIC, lower-middle–income countries; UMIC, upper-middle–income countries.
AKI incidence and prevalence
Most countries (n = 73, 62%) were unable to determine the prevalence of AKI not requiring dialysis (19% able and 19% unsure), and 57% were unable to estimate the incidence (20% able and 23% unsure). A higher proportion of countries were able to determine their incidence and prevalence of AKI requiring dialysis (44% and 41%, respectively).
Identification of AKI
Compared to the 27% of countries who identified specific groups at high risk for CKD, a higher proportion of countries identified specific groups at risk for AKI (n = 67, 57%). These groups included patients who were elderly, diabetic, hypertensive, pregnant, or had a history of HIV or malaria. There was no difference in the proportion of countries able to identify high-risk groups between ISN regions and World Bank income groups.
Discussion
This report demonstrates that there is significant heterogeneity within and between ISN regions and World Bank income groups in the coverage and availability of kidney disease health information systems. Dialysis and transplant registries were more likely to be established in high- and upper-middle–income countries, compared with low-income countries. There were significant gaps in the global coverage of nondialysis CKD and AKI registries. The majority of dialysis and transplant registries required mandatory provider participation, while AKI registries tended to be voluntary. Although almost three-quarters of countries could estimate their prevalence of CKD, less than 24% of low-income countries had access to the same data. Most countries were unable to estimate their incidence or prevalence of AKI. A small proportion of countries had an established CKD detection program based on national policy; these programs were usually implemented through routine health encounters. Almost all countries offered CKD testing to diabetic and hypertensive patients, but few offered testing to high-risk ethnic groups, especially in low-income countries.
These findings highlight several gaps in the existing health information system infrastructure. First, there was a clear disparity in the distribution of dialysis and transplant registries across ISN regions and World Bank income groups, with particularly low representation in low-income countries in Africa, the Middle East, and South Asia. Dialysis and transplant registries provide useful information regarding the epidemiology and outcomes of renal replacement therapy, as well as facilitating benchmarking across regions and tailoring of existing health care services to areas of need.17 Extrapolation of registry data from high-income countries to low-income countries is not possible due to well-recognized differences in the etiology of kidney disease, the accessibility of renal replacement therapy, and long-term clinical outcomes between income groups.24 In particular, the epidemiology of end-stage kidney disease in low-income countries is particularly complex, with the dual burden of communicable and noncommunicable diseases, so it is critical that this is accurately reported.25
Second, this report highlighted that there was a dearth of nondialysis CKD and AKI registries across all ISN regions and World Bank income groups. This indicates that much of our current understanding of the prevalence, natural history, and clinical outcomes of nondialysis CKD and AKI has been informed by anecdotal clinical practice, observational studies, and small registries run by regional health services or networks.26 However, efforts to create new registries in these areas are currently under way. For example, the International Acute Kidney Injury Registry, endorsed by the ISN, has been established with the aim of determining the prevalence, natural history, and outcome of AKI among critically ill patients. Although the registry has recruited internationally, it currently only includes patients from 13 centers, and because of its critical care focus it does not capture AKI in the community, outpatient, or ward setting, all of which contribute substantially to the overall incidence and prevalence of disease.
Third, this study identified that several heavily populated countries reported having no registry across any of the 4 domains of kidney disease. Collectively, these countries represented over 20% of the world population and included large countries such as India, Nigeria, Ethiopia, Germany, and the Democratic Republic of the Congo. The lack of data from such populous countries has broad implications for the accurate estimation of the global prevalence of kidney disease. Facilitating the development of health information systems in these countries should be a global priority. In the first instance, consideration should be given to incorporation of these countries into existing registries. For example, North African countries have occasionally contributed data to the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) Registry,27 and other countries to the United States Renal Data System (USRDS) registry.28
Barriers to successful development of comprehensive health information systems in low-income countries must be addressed.18, 29, 30 Infrastructure for the diagnosis and screening of kidney disease is unavailable in many countries, or may be concentrated in major towns, despite a primarily rural population. Recruitment for nondialysis CKD and AKI registries will need to encompass patients managed in primary health care, general medical, and critical care settings. Estimation of disease prevalence and the collection of longitudinal data concerning patient outcomes are challenging due to the migratory nature of some populations, and the lack of a national census affects the adequacy of the reference population. Also, health information systems rely on the systematic identification of individuals; however, the feasibility of using identifiers will vary by local custom. Establishing a health information system requires adequate numbers of trained personnel, including data collectors, coders, and analysts, as well as suitable data processing facilities for data storage and security.10 Complex and lengthy questionnaires may limit participation. Ensuring sustainability of a health information system is challenged by resource limitations.27
Improving the global status of health information systems requires a framework for prioritizing system development that ensures coverage of areas of greatest need.31 Establishing dialysis and transplant registries is important, because these areas are associated with high health care costs and significant variation in clinical processes or outcomes, and registry data may inform more efficient use of resources. This is already under way in Africa.27 Registries prioritized for development or expansion must be underpinned by robust governance structures and mechanisms to ensure transparency in terms of managerial decision making and oversight, data collection and analysis methodologies, and systems for reporting and disseminating results.19 These systems will need to be continuously mentored, monitored, and evaluated. In low- and middle-income countries, existing regional health information systems could expand to the national level and increase in scope. Resources should be focused toward guiding the sustainable design and implementation of these systems, ensuring their security and privacy, enhancing the analysis and interpretation of their data, and evaluating their quality and scope. Capacity should be developed to provide health information system support and guide emerging systems to maximize their likelihood of success. The ISN plans to support such collaborations.
At the same time, expansion of nondialysis CKD and AKI registries should also be prioritized, given the need to accurately estimate the global incidence and prevalence of these conditions and their outcomes. Currently, the burden of nondialysis CKD and AKI is difficult to estimate, which inhibits optimal allocation of finances and health resources to manage these highly prevalent conditions. Because de novo creation of registries requires substantial investment of resources, consideration should be given to the inclusion of patients with nondialysis CKD and AKI into existing registries with infrastructure already in place. Because it is impractical to include all individuals in such registries, focus should initially be on inclusion of those with advanced disease. The ability of nondialysis CKD and AKI health information systems to accurately estimate disease burden and outcome will be reliant on the availability of appropriate population-based screening procedures.
It is likely that in the future it will be possible to monitor a broad range of medical conditions using registries that collect data inexpensively from electronic medical records and administrative datasets.32 For example, both nondialysis CKD and AKI are ideal computable phenotypes.33 To reduce the burden and cost of registry data collection, registry development will need to evolve to ensure that data items are reliably captured within, and easily extracted from, these routinely collected electronic datasets. Incorporation of patient-reported outcome measures to existing health information systems in order to enhance understanding of patient experiences should be considered.34 System design that enables easy comparisons between and within regions warrants consideration.35 To this end, the NephroQUEST project was launched by the ERA-EDTA Registry with the aim of developing a consensus on the selection of quality of care indicators recorded in participating European national registries and to standardize data collection and dissemination.36 At the same time, international comparison of registry data between diverse nations may not be appropriate because of differences in patient selection and health service provision.37 Novel use of health information system data is occurring in the field of clinical research and study design. In addition to use in cohort studies, registry data may be utilized for cluster-randomized studies nested within an ongoing registry, and for studies that link the registry database with other data sources.
This is the most comprehensive report of the global coverage of health information systems for kidney disease. Its key strengths lie in its use of a validated survey, based on the WHO health system building blocks, as well as its inclusion of respondents from 117 countries, with representation from all ISN regions and World Bank income groups. These strengths must be balanced against the limitations and potential bias of population surveys, including subjective responses, and dependence on the knowledge and experience of the respondent. Furthermore, the content, quality, and inclusiveness of kidney disease health information systems were not within the scope of this study. However, these factors are critical determinants of the value, comparability, validity, and usability of their data.
In summary, there are significant gaps in the global coverage of health information systems, particularly across low-income countries and in the domains of nondialysis CKD and AKI. Moving forward, the global objective should be to develop robust and reliable health information systems that include countries from all income groups and cover all domains of kidney disease. Establishment of a dedicated platform to guide and support the development of new kidney disease registries is under consideration by the ISN. An approach of this type has several key advantages, including enhanced feasibility, likelihood of success, and cost-effectiveness. International collaboration between registries should be encouraged because an integrated, centralized repository of kidney disease health information systems could illustrate global trends, lead to improved quality and consistency in care, and ultimately facilitate allocation of resources to areas of need.
Methods
The GKHA Project was a cross-sectional survey conducted by the ISN. All United Nations member states were invited to participate, with a specific focus on 130 countries with ISN-affiliated societies. The online questionnaire was distributed through the ISN’s 10 regional boards (Africa, East and Central Europe, Latin America, Middle East, North America, North and East Asia, Oceania and Southeast Asia, newly independent states [NIS] and Russia, South Asia, and Western Europe). For the purpose of analysis, countries were grouped by World Bank country classification of 2014 and ISN region (Supplementary Tables S1 and S2). A comprehensive description of the sampling approach, development and validation of the survey, data handling, and statistical analysis is available elsewhere.38, 39
As part of the survey, countries were asked to provide data on the local availability of health information systems for dialysis, transplant, nondialysis CKD and AKI. Details of the nature of provider participation (mandatory or voluntary) were requested. For countries with a nondialysis CKD registry, the scope of the registry (all CKD vs. stages 4-5 and national vs. regional) was identified.
Each country’s ability to estimate their prevalence of CKD and AKI (dialysis- and nondialysis-dependent) was covered. The availability of screening and surveillance systems for CKD and AKI was also explored. Countries were asked about the process of identification of cases. A “reactive” approach was defined as the identification of cases through routine practice; “active, routine” surveillance involved identification of cases through active screening of high-risk populations at routine health encounters; and “active, specific” surveillance involved the active screening of high-risk populations through specific screening processes.
Disclosure
Publication of this article was supported by the International Society of Nephrology.
EB-F declared seeing private patients on a part-time basis. MBG declared receiving lecture fees from AMGEN, B Braun, Leo Pharma, Novartis, Novo-Nordisk, Promopharm, Roche, Sanofi, Servier, Sophadial, and Sothema. BB declared receiving consulting fees from Otsuka and receives current grant support from Amgen. DCH declared receiving lecture fees from Roche Myanmar and Otsuka. VJ declared receiving consulting fees from Baxter and Medtronic; and current grant support from the Department of Biotechnology, Government of India, Baxter, and GlaxoSmithKline. KK-Z declared receiving past and future consulting and lecture fees from Abbott, Abbvie, Alexion, Amgen, AstraZeneca, Aveo, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, and UpToDate; future consulting and lecture fees from ZS-Pharma; current grant support from National Institutes of Health; and expert witness engagement for GranuFlo. RK declared receiving lecture fees from Baxter Healthcare. JP declared receiving consulting fees from Fresenius Medical Care, Baxter Healthcare, Otsuka, and Boehringer Ingelheim; lecture fees from Baxter Healthcare; and current grant support from Canadian Institutes of Health Research and Baxter Healthcare. DWJ declared receiving consulting fees from AstraZeneca; lecture fees from Baxter Healthcare and Fresenius Medical Care; and support from Baxter Extramural and Clinical Evidence Council grants. All the other authors declared no competing interests.
Acknowledgments
We thank Drs. Marcello Tonelli and Valerie Luyckx for their contributions to the project and manuscript, Sandrine Damster, Research Project Manager at the International Society of Nephrology (ISN), and Alberta Kidney Disease Network (AKDN) staff (Ghennete Houston, Sue Szigety, and Sophanny Tiv) for their support with the organization and conduct of the survey and project management. We thank the ISN staff (Louise Fox and Luca Segantini) for their support. We thank the executive committee of the ISN, the ISN regional leadership, and the leaders of the ISN Affiliate Societies at regional and country levels for their support toward the success of this initiative.
Footnotes
Table S1. Categorization of the 117 United Nations member states included in this study by International Society of Nephrology region.
Table S2. Categorization of the 117 United Nations member states included in this study by 2014 World Bank income group.
Supplementary material is linked to the online version of the paper at www.kisupplements.org.
Supplementary Material
Categorization of the 117 United Nations member states included in this study by International Society of Nephrology region.
Categorization of the 117 United Nations member states included in this study by 2014 World Bank income group.
References
- 1.World Health Organization . WHO Press; Geneva, Switzerland: 2010. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. [Google Scholar]
- 2.Larsson S., Lawyer P., Garellick G. Use of 13 disease registries in 5 countries demonstrates the potential to use outcome data to improve health care’s value. Health Aff. 2012;31:220–227. doi: 10.1377/hlthaff.2011.0762. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D., Bray F., Ferlay J. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 4.Han W., Sharman R., Heider A. Impact of electronic diabetes registry “Meaningful Use” on quality of care and hospital utilization. J Am Med Informatics Assoc. 2015;23:1–7. doi: 10.1093/jamia/ocv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoque D.M.E., Kumari V., Ruseckaite R. Impact of clinical registries on quality of patient care and health outcomes: protocol for a systematic review. BMJ Open. 2016;6:e010654. doi: 10.1136/bmjopen-2015-010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins A.J., Foley R.N., Gilbertson D.T. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl. 2015;5:2–7. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoccali C., Kramer A., Jager K. The databases: renal replacement therapy since 1989-the European Renal Association and European Dialysis and Transplant Association (ERA-EDTA) Clin J Am Soc Nephrol. 2009;4:S18–S22. doi: 10.2215/CJN.05210709. [DOI] [PubMed] [Google Scholar]
- 8.Jin D.-C. Dialysis registries in the world: Korean Dialysis Registry. Kidney Int Suppl. 2015;5:8–11. doi: 10.1038/kisup.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung C.B., Cheung W.L., Li P.K.T. Renal registry in Hong Kong-the first 20 years. Kidney Int Suppl. 2015;5:33–38. doi: 10.1038/kisup.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morsy A., Lim T.O., Varatharajan S. 2010 5th Cairo International Biomedical Engineering Conference, CIBEC 2010. IEEE; 2010. National registries in developing countries: understanding construction challenges and implementation steps; pp. 75–78. [Google Scholar]
- 11.Mcdonald S.P. Australia and New Zealand Dialysis and Transplant Registry. Kidney Int Suppl. 2015;5:39–44. doi: 10.1038/kisup.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox N., McNeil J.J. Clinical quality registries have the potential to drive improvements in the appropriateness of care. Med J Aust. 2016;205:S27–S29. doi: 10.5694/mja15.00921. [DOI] [PubMed] [Google Scholar]
- 13.Lim T.O., Goh A., Lim Y.N. Use of renal registry data for research, health-care planning and quality improvement: what can we learn from registry data in the Asia-Pacific region? Nephrology. 2008;13:745–752. doi: 10.1111/j.1440-1797.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanafusa N., Nakai S., Iseki K. Japanese society for dialysis therapy renal data registry - a window through which we can view the details of Japanese dialysis population. Kidney Int Suppl. 2015;5:15–22. doi: 10.1038/kisup.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager K.J., Wanner C. Fifty years of ERA-EDTA Registry—a registry in transition. Kidney Int Suppl. 2015;5:12–14. doi: 10.1038/kisup.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nwomeh B.C., Lowell W., Kable R. History and development of trauma registry: lessons from developed to developing countries. World J Emerg Surg. 2006;1:32. doi: 10.1186/1749-7922-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counil É., Cherni N., Kharrat M. Trends of incident dialysis patients in Tunisia between 1992 and 2001. Am J Kidney Dis. 2008;51:463–470. doi: 10.1053/j.ajkd.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Jedy-Agba E.E., Oga E.A., Odutola M. Developing national cancer registration in developing countries – case study of the Nigerian national system of cancer registries. Front Public Heal. 2015;3:1–10. doi: 10.3389/fpubh.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black N., Barker M., Payne M. Cross sectional survey of multicentre clinical databases in the United Kingdom. BMJ. 2004;328:1478. doi: 10.1136/bmj.328.7454.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna T.P., Kangolle A.C.T. Cancer control in developing countries: using health data and health services research to measure and improve access, quality and efficiency. BMC Int Heal Hum Rights. 2010;10:24. doi: 10.1186/1472-698X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F.X., Rutherford P., Smoyer-Tomic K. A global overview of renal registries: a systematic review. BMC Nephrol. 2015;16:31. doi: 10.1186/s12882-015-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Society of Nephrology (ISN). ISN Regions. Available at: https://www.theisn.org/about-isn/regions. Accessed August 22, 2017.
- 23.World Bank Country and Lending Groups - World Bank Data Help Desk. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed August 22, 2017.
- 24.Barsoum R.S. Overview: end-stage renal disease in the developing world. Artif Organs. 2002;26:737–746. doi: 10.1046/j.1525-1594.2002.07061.x. [DOI] [PubMed] [Google Scholar]
- 25.Boutayeb A., Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4:2. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani L., Stengel B., Combe C. The CKD Outcomes and Practice Patterns Study (CKDopps) Am J Kidney Dis. 2016;68:402–413. doi: 10.1053/j.ajkd.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 27.Razeen Davids M., Eastwood J.B., Selwood N.H. A renal registry for Africa: first steps. Clin Kidney J. 2016;9:162–167. doi: 10.1093/ckj/sfv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United States Renal Data System . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. 2014 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health. [Google Scholar]
- 29.Valsecchi M.G., Steliarova-Foucher E. Cancer registration in developing countries: luxury or necessity? Lancet Oncol. 2008;9:159–167. doi: 10.1016/S1470-2045(08)70028-7. [DOI] [PubMed] [Google Scholar]
- 30.Rastogi T., Hildesheim A., Sinha R. Opportunities for cancer epidemiology in developing countries. Nat Rev Cancer. 2004;4:909–917. doi: 10.1038/nrc1475. [DOI] [PubMed] [Google Scholar]
- 31.Evans S.M., Scott I.A., Johnson N.P. Development of clinical-quality registries in Australia: the way forward. Med J Aust. 2011;194:360–363. doi: 10.5694/j.1326-5377.2011.tb03007.x. [DOI] [PubMed] [Google Scholar]
- 32.Lucas H. Information and communications technology for future health systems in developing countries. Soc Sci Med. 2008;66:2122–2132. doi: 10.1016/j.socscimed.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Drawz P.E., Archdeacon P., McDonald C.J. CKD as a Model for Improving Chronic Disease Care through Electronic Health Records. Clin J Am Soc Nephrol. 2015;10:1488–1499. doi: 10.2215/CJN.00940115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breckenridge K., Bekker H.L., Gibbons E. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30:1605–1614. doi: 10.1093/ndt/gfv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schena F.P. Epidemiology of end-stage renal disease: international comparisons of renal replacement therapy. Kidney Int. 2000;57:S39–S45. [Google Scholar]
- 36.Jager K.J., Zoccali C. Quality of care in end-stage renal disease: the importance of comparing “apples with apples”. Nephrol Dial Transplant. 2008;23:1116. doi: 10.1093/ndt/gfm876. [DOI] [PubMed] [Google Scholar]
- 37.Kjellstrand C.M. Death on Hemodialysis: Preventable or Inevitable? Springer Netherlands; Dordrecht, Netherlands: 1994. International comparisons of dialysis survival are meaningless to evaluate differences in dialysis procedures; pp. 55–68. [Google Scholar]
- 38.Bello A.K., Levin A., Tonelli M. Assessment of Global Kidney Health Care Status. JAMA. 2017;317:1864. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bello A.K., Johnson D.W., Feehally J. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl. 2017;7:145–153. doi: 10.1016/j.kisu.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Categorization of the 117 United Nations member states included in this study by International Society of Nephrology region.
Categorization of the 117 United Nations member states included in this study by 2014 World Bank income group.