Table of Contents

| KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease | |
| 93 | Tables, figures, algorithms, and supplementary material |
| 95 | KDIGO executive committee |
| 96 | Reference keys |
| 97 | CKD nomenclature |
| 98 | Conversion factors |
| 99 | Abbreviations and acronyms |
| 100 | Notice |
| 101 | Foreword |
| 102 | Work Group membership |
| 103 | Abstract |
| 104 | Summary of recommendation statements |
| 108 | Chapter 1: Detection and evaluation of HCV in CKD |
| 114 | Chapter 2: Treatment of HCV infection in patients with CKD |
| 121 | Chapter 3: Preventing HCV transmission in hemodialysis units |
| 130 | Chapter 4: Management of HCV-infected patients before and after kidney transplantation |
| 137 | Chapter 5: Diagnosis and management of kidney diseases associated with HCV infection |
| 142 | Methods for guideline development |
| 151 | Biographic and disclosure information |
| 157 | Acknowledgments |
| 158 | References |
The development and publication of this guideline were supported by KDIGO. The opinions or views expressed in this professional education supplement are those of the authors and do not necessarily reflect the opinions or recommendations of the International Society of Nephrology or Elsevier. Dosages, indications, and methods of use for products that are referred to in the supplement by the authors may reflect their clinical experience or may be derived from the professional literature or other clinical sources. Because of the differences between in vitro and in vivo systems and between laboratory animal models and clinical data in humans, in vitro and animal data may not necessarily correlate with clinical results.
Tables
| 106 | Table 1. Infection control practices (“hygienic precautions”) particularly relevant in preventing HCV transmission |
| 122 | Table 2. Recent reported HCV prevalence in hemodialysis patients |
| 122 | Table 3. Factors and lapses in infection control practices associated with transmission of HCV infection in dialysis units |
| 125 | Table 4. Hygienic precautions for hemodialysis (dialysis machines) |
| 127 | Table 5. Steps to initiate concurrently and undertake following identification of a new HCV infection in a hemodialysis patient |
| 127 | Table 6. Strategies to support adherence to infection control recommendations in hemodialysis centers |
| 128 | Table 7. Key hygienic precautions for hemodialysis staff |
| 143 | Table 8. Systematic review topics and screening criteria |
| 144 | Table 9. Hierarchy of outcomes |
| 145 | Table 10. Work products for the guideline |
| 146 | Table 11. Classification of study quality |
| 146 | Table 12. GRADE system for grading quality of evidence |
| 146 | Table 13. Final grade for overall quality of evidence |
| 147 | Table 14. Balance of benefits and harms |
| 147 | Table 15. KDIGO nomenclature and description for grading recommendations |
| 147 | Table 16. Determinants of strength of recommendation |
| 148 | Table 17. The Conference on Guideline Standardization (COGS) checklist for reporting clinical practice guidelines |
Figures
| 105 | Figure 1. Recommended DAA treatment regimens for patients with CKD G4–G5D and kidney transplant recipients, by HCV genotype |
| 144 | Figure 2. Search yield |
Algorithms
| 118 | Algorithm 1. Treatment scheme for CKD G1–G5D |
| 119 | Algorithm 2. Treatment scheme for kidney transplant recipients |
| 132 | Algorithm 3. Proposed strategy in an HCV-infected kidney transplant candidate |
Supplementary Material
| Appendix A. Search strategies |
| Appendix B. Concurrence with Institute of Medicine standards for systematic reviews and for guidelines |
| Table S1. Summary table: diagnostic testing for liver fibrosis (by biopsy) |
| Table S2. Evidence profile: diagnostic testing for liver fibrosis (by biopsy) |
| Table S3. Summary table: HCV infection as independent predictor of CKD progression |
| Table S4. Evidence profile: HCV infection as independent predictor of CKD progression |
| Table S5. Summary table: treatment with direct-acting antiviral regimens in chronic HCV-infected CKD patients |
| Table S6. Evidence profile: treatment with direct-acting antiviral regimens in chronic HCV-infected CKD patients |
| Table S7. Summary table: treatment with direct-acting antiviral regimens in kidney transplant recipients with chronic HCV infection |
| Table S8. Evidence profile: treatment with direct-acting antiviral regimens in kidney transplant recipients with chronic HCV infection |
| Table S9. Summary table: isolation of HCV patients receiving hemodialysis |
| Table S10. Evidence profile: isolation of HCV patients receiving hemodialysis |
| Table S11. Summary table: transplantation versus waitlist among patients with HCV infection |
| Table S12. Evidence profile: transplantation versus waitlist among patients with HCV infection |
| Table S13. Summary table: HCV infection as predictor of death among kidney transplant recipients |
| Table S14. Evidence profile: HCV infection as predictor of death and graft loss among kidney transplant recipients |
| Table S15. Summary table: clinical outcomes of HCV-positive kidney transplant recipients from HCV-positive donors |
| Table S16. Summary table: induction and immunosuppression in kidney transplant recipients with HCV infection |
| Table S17. Summary table: HCV treatment of HCV-associated glomerular disease |
| Table S18. Evidence profile: HCV treatment of HCV-associated glomerular disease |
Supplementary material is linked to the online version of the article at www.kisupplements.org.
KDIGO Executive Committee
| Garabed Eknoyan, MD Norbert Lameire, MD, PhD Founding KDIGO Co-Chairs | |
| Bertram L. Kasiske, MD Immediate Past Co-Chair | |
| David C. Wheeler, MD, FRCP KDIGO Co-Chair |
Wolfgang C. Winkelmayer, MD, MPH, ScD KDIGO Co-Chair |
| Ali K. Abu-Alfa, MD Geoffrey A. Block, MD Jürgen Floege, MD John S. Gill, MD, MS Kunitoshi Iseki, MD Zhi-Hong Liu, MD, PhD Magdalena Madero, MD Ziad A. Massy, MD, PhD |
Ikechi G. Okpechi, MBBS, FWACP, PhD Brian J.G. Pereira, MBBS, MD, MBA Rukshana Shroff, MD, FRCPCH, PhD Paul E. Stevens, MB, FRCP Marcello A. Tonelli, MD, SM, FRCPC Suzanne Watnick, MD Angela C. Webster, MBBS, MM (Clin Epi), PhD Christina M. Wyatt, MD |
|
KDIGO Staff John Davis, Chief Executive Officer Danielle Green, Executive Director Michael Cheung, Chief Scientific Officer Tanya Green, Communications Director Melissa Thompson, Implementation Director | |
Reference keys
Nomenclature and Description for Rating Guideline Recommendations
Within each recommendation, the strength of recommendation is indicated as Level 1, Level 2, or not graded, and the quality of the supporting evidence is shown as A, B, C, or D.
| Gradea | Implications |
||
|---|---|---|---|
| Patients | Clinicians | Policy | |
|
Level 1 “We recommend” |
Most people in your situation would want the recommended course of action, and only a small proportion would not. | Most patients should receive the recommended course of action. | The recommendation can be evaluated as a candidate for developing a policy or a performance measure. |
|
Level 2 “We suggest” |
The majority of people in your situation would want the recommended course of action, but many would not. | Different choices will be appropriate for different patients. Each patient needs help to arrive at a management decision consistent with her or his values and preferences. | The recommendation is likely to require substantial debate and involvement of stakeholders before policy can be determined. |
The additional category “not graded” is used, typically, to provide guidance based on common sense or where the topic does not allow adequate application of evidence. The most common examples include recommendations regarding monitoring intervals, counseling, and referral to other clinical specialists. The ungraded recommendations are generally written as simple declarative statements. They should not be interpreted as being weaker recommendations than Level 1 or 2 recommendations.
| Grade | Quality of evidence | Meaning |
|---|---|---|
| A | High | We are confident that the true effect lies close to the estimate of the effect. |
| B | Moderate | The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| C | Low | The true effect may be substantially different from the estimate of the effect. |
| D | Very low | The estimate of effect is very uncertain, and often will be far from the truth. |
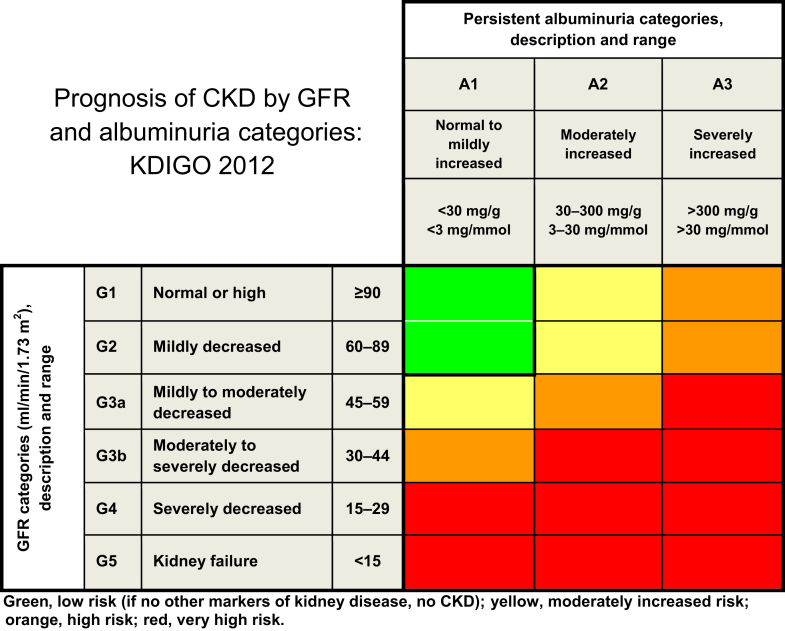
Current Chronic Kidney Disease (CKD) Nomenclature Used by KDIGO
CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health. CKD is classified based on cause, GFR category (G1–G5), and albuminuria category (A1–A3), abbreviated as CGA.
Prognosis of CKD by GFR and albuminuria category
Conversion Factors of Conventional Units to SI Units
| Conventional unit | Conversion factor | SI unit | |
|---|---|---|---|
| Creatinine | mg/dl | 88.4 | μmol/l |
Note: conventional unit × conversion factor = SI unit.
Albuminuria Categories in CKD
| Category | AER (mg/24 h) | ACR (approximate equivalent) |
Terms | |
|---|---|---|---|---|
| (mg/mmol) | (mg/g) | |||
| A1 | <30 | <3 | <30 | Normal to mildly increased |
| A2 | 30–300 | 3–30 | 30–300 | Moderately increaseda |
| A3 | >300 | >30 | >300 | Severely increasedb |
ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; CKD, chronic kidney disease.
Relative to young adult level.
Including nephrotic syndrome (albumin excretion usually > 2200 mg/24 h [ACR > 2200 mg/g; > 220 mg/mmol]).
Interpretation of HCV Assays
| Anti-HCV | HCV-NAT | Interpretation |
|---|---|---|
| Positive | Positive | Acute or chronic HCV infection depending on the clinical context |
| Positive | Negative | Resolution of HCV infection (i.e., successfully treated or spontaneously cleared) |
| Negative | Positive | Early acute HCV infection; chronic HCV in the setting of immunosuppressed state; false anti-HCV negative or false HCV-NAT positive |
| Negative | Negative | Absence of HCV infection |
Anti-HCV, HCV antibody; HCV, hepatitis C virus; NAT, nucleic acid testing.
Abbreviations and acronyms
| AASLD | American Association for the Study of Liver Diseases |
| ALT | alanine aminotransferase |
| Anti-HCV | HCV antibody |
| APRI | aspartate aminotransferase–platelet ratio index |
| ASN | American Society of Nephrology |
| AUC | area under the curve |
| BSI | bloodstream infection |
| CDC | Centers for Disease Control and Prevention |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CKD G4 CKD G5 | chronic kidney disease GFR category 4 chronic kidney disease GFR category 5 |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CNI | calcineurin inhibitor |
| CPG | clinical practice guideline |
| CrCl | creatinine clearance |
| DAA | direct-acting antiviral |
| DOPPS | Dialysis Outcomes and Practice Patterns Study |
| EASL | European Association for the Study of the Liver |
| eGFR | estimated glomerular filtration rate |
| ERT | evidence review team |
| ESKD | end-stage kidney disease |
| FDA | Food and Drug Administration |
| GFR | glomerular filtration rate |
| GN | glomerulonephritis |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| GT | genotype |
| HAV | hepatitis A virus |
| HBcAb | antibody to hepatitis B core antigen |
| HBsAb | antibody to hepatitis B surface antigen |
| HBsAg | hepatitis B surface antigen |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| HR | hazard ratio |
| IFN | interferon |
| IU | international unit |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| MMF | mycophenolate mofetil |
| MN | membranous nephropathy |
| MPGN | membranoproliferative glomerulonephritis |
| NAT | nucleic acid test(ing) |
| NS5A | nonstructural protein 5A |
| NS5B | nonstructural protein 5B |
| OR | odds ratio |
| PrOD (3D regimen) | paritaprevir/ritonavir/ombitasvir and dasabuvir |
| RBV | ribavirin |
| RCT | randomized controlled trial |
| RR | relative risk |
| SVR (weeks) | sustained virologic response (at stated weeks) |
| US | United States |
Notice
Section I: Use of the Clinical Practice Guideline
This Clinical Practice Guideline document is based upon literature searches last conducted in May 2017, supplemented with additional evidence through July 2018. It is designed to assist decision making. It is not intended to define a standard of care, and should not be interpreted as prescribing an exclusive course of management. Variations in practice will inevitably and appropriately occur when clinicians consider the needs of individual patients, available resources, and limitations unique to an institution or type of practice. Health care professionals using these recommendations should decide how to apply them to their own clinical practice.
Section II: Disclosure
Kidney Disease: Improving Global Outcomes (KDIGO) makes every effort to avoid any actual or reasonably perceived conflicts of interest that may arise from an outside relationship or a personal, professional, or business interest of a member of the Work Group. All members of the Work Group are required to complete, sign, and submit a disclosure and attestation form showing all such relationships that might be perceived as or are actual conflicts of interest. This document is updated annually and information is adjusted accordingly. All reported information is published in its entirety at the end of this document in the Work Group members’ Biographic and Disclosure section, and is kept on file at KDIGO.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Copyright © 2018, KDIGO. Published by Elsevier on behalf of the International Society of Nephrology. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Single copies may be made for personal use as allowed by national copyright laws. Special rates are available for educational institutions that wish to make photocopies for nonprofit educational use. No part of this publication may be reproduced, amended, or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without explicit permission in writing from KDIGO. Details on how to seek reprints, permission for reproduction or translation, and further information about KDIGO’s permissions policies can be obtained by contacting Danielle Green, Executive Director, at danielle.green@kdigo.org.
To the fullest extent of the law, neither KDIGO, Kidney International Supplements, nor the authors, contributors, or editors assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
Foreword
With the growing awareness that chronic kidney disease (CKD) is an international health problem, Kidney Disease: Improving Global Outcomes (KDIGO) was established in 2003 with its stated mission to “improve the care and outcomes of kidney disease patients worldwide through promoting coordination, collaboration, and integration of initiatives to develop and implement clinical practice guidelines.”
The high prevalence of hepatitis C virus (HCV) in the CKD population was recognized once diagnostic testing became available in the early 1990s, as was its transmission within dialysis units. A series of publications subsequently identified the adverse consequences of HCV infection in the CKD population as well as its detrimental effect on recipient and graft outcomes following kidney transplantation. Although screening of blood products for HCV reduced its acquisition by blood transfusion, the unique aspects of its epidemiology in the CKD population were apparent. Studies also established that transmission was frequent in dialysis patients and typically reflected insufficient attention to body fluid precautions. Also confounding the management of HCV in the CKD population was an absence of biochemical liver dysfunction in most HCV-infected hemodialysis patients, which contributed to the lack of recognition of its presence and clinical significance. An additional difficulty was the lack of effective and tolerable antiviral agents to treat HCV in patients with CKD because interferon, especially in combination with ribavirin, had considerable toxicity. Furthermore, interferon was implicated in graft dysfunction in kidney transplant recipients.
KDIGO convened a group of experts in this area to develop guideline recommendations for the prevention, diagnosis, and management of HCV in CKD a decade ago, which resulted in the publication of the very first KDIGO guideline in 2008. Since then there have been major advances in HCV management, particularly in antiviral therapy. As a result, much of the hesitancy in advising therapy for HCV-infected patients with CKD and following kidney transplant has now disappeared. In addition, diagnostic testing has evolved in chronic liver disease to the extent that fibrosis can now be assessed with noninvasive techniques such as transient elastography. Because of these advances in diagnostics and therapeutics, it was deemed appropriate to undertake a comprehensive review and update of the KDIGO HCV guideline in patients with kidney disease. It has been KDIGO’s philosophy to provide recommendations based on the best available clinical evidence without direct consideration of costs, as they vary widely across countries. The recent Lancet Commission on Essential Medicines articulated the importance and challenges of providing access to safe, effective, and affordable essential medicines, including treatments for combating HCV.1 In this vein, the World Health Organization has issued its first global report to offer practical steps to expand access for such treatments.2
We thank Michel Jadoul, MD, and Paul Martin, MD, for leading this important initiative, and we are especially grateful to the Work Group members who provided their time and expertise to this endeavor. In addition, this Work Group was ably assisted by colleagues from the independent evidence review team led by Ethan Balk, MD, MPH, Craig Gordon, MD, MS, Amy Earley, BS, and Mengyang Di, MD, PhD, who made this guideline possible.
In keeping with KDIGO’s policy for transparency and rigorous public review during the guideline development process, its scope and the draft guideline were both made available for open commenting. The feedback received was carefully considered by the Work Group members who critically reviewed the public input and revised the guideline as appropriate for the final publication.
David C. Wheeler, MD, FRCP
Wolfgang C. Winkelmayer, MD, ScD
KDIGO Co-Chairs
Work Group membership
| Work Group Co-chairs | |
| Michel Jadoul, MD Cliniques Universitaires Saint Luc Université Catholique de Louvain Brussels, Belgium |
Paul Martin, MD Miller School of Medicine University of Miami Miami, FL, USA |
| Work Group | |
| Marina C. Berenguer, MD La Fe University Hospital, IIS La Fe University of Valencia-CIBERehd Valencia, Spain |
Bertram L. Kasiske, MD, FACP Hennepin County Medical Center Minneapolis, MN, USA |
| Wahid Doss, MD National Hepatology and Tropical Medicine Research Institute Cairo, Egypt |
Ching-Lung Lai, MD, FRCP, FRACP, FHKAM (Med), FHKCP, FAASLD University of Hong Kong Hong Kong, China |
| Fabrizio Fabrizi, MD Maggiore Hospital and IRCCS Foundation Milan, Italy |
José M. Morales, MD, PhD Hospital Universitario 12 de Octubre Madrid, Spain |
| Jacques Izopet, PharmD, PhD Centre de Physiopathologie de Toulouse Purpan Toulouse, France |
Priti R. Patel, MD, MPH Centers for Disease Control and Prevention Atlanta, GA, USA |
| Vivekanand Jha, MBBS, MD, DM, FRCP, FRCP (Edin), FAMS The George Institute for Global Health New Delhi, India |
Stanislas Pol, MD, PhD Hôpital Cochin Paris, France |
| Nassim Kamar, MD, PhD CHU Rangueil Toulouse, France |
Marcelo O. Silva, MD Hospital Universitario Austral Pilar, Argentina |
|
Evidence Review Team Center for Evidence Synthesis in Health, Brown University School of Public Health Providence, RI, USA Ethan M. Balk, MD, MPH, Project Director, Evidence Review Team Director Craig E. Gordon, MD, MS, Assistant Project Director, Evidence Review Team Associate Director Amy Earley, BS, Research Associate Mengyang Di, MD, PhD, Physician Researcher | |
Abstract
The Kidney Disease: Improving Global Outcomes (KDIGO) 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease represents a complete update of the prior guideline published in 2008. This guideline is intended to assist the practitioner caring for patients with hepatitis C virus (HCV) and chronic kidney disease (CKD), including those who are on chronic dialysis therapy and individuals with a kidney transplant. Specifically, the topic areas for which new recommendations are issued include detection and evaluation of HCV in CKD; treatment of HCV infection in patients with CKD; management of HCV-infected patients before and after kidney transplantation; prevention of HCV transmission in hemodialysis units; and diagnosis and management of kidney diseases associated with HCV infection. Development of this guideline update followed an explicit process of evidence review and appraisal. Treatment approaches and guideline recommendations are based on systematic reviews of relevant studies, and appraisal of the quality of the evidence and the strength of recommendations followed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Limitations of the evidence are discussed, with areas of future research also presented.
Keywords: chronic kidney disease; cryoglobulinemia; dialysis; direct-acting antivirals; glomerular diseases; hemodialysis; hepatitis C virus; infection control; guideline; KDIGO; kidney transplantation; liver testing; nosocomial transmission; screening; systematic review
CITATION
In citing this document, the following format should be used: Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int Suppl. 2018;8:91–165.
Summary of recommendation statements
Chapter 1: Detection and evaluation of HCV in CKD
- 1.1 Screening patients with CKD for HCV infection
- 1.1.1: We recommend screening all patients for HCV infection at the time of initial evaluation of CKD (1C).
- 1.1.1.1: We recommend using an immunoassay followed by nucleic acid testing (NAT) if immunoassay is positive (1A).
- 1.1.2: We recommend screening all patients for HCV infection upon initiation of in-center hemodialysis or upon transfer from another dialysis facility or modality (1A).
- 1.1.2.1: We recommend using NAT alone or an immunoassay followed by NAT if immunoassay is positive (1A).
- 1.1.3: We suggest screening all patients for HCV infection upon initiation of peritoneal dialysis or home hemodialysis (2D).
- 1.1.4: We recommend screening all patients for HCV infection at the time of evaluation for kidney transplantation (1A).
1.2 Follow-up HCV screening of in-center hemodialysis patients
- 1.2.1: We recommend screening for HCV infection with immunoassay or NAT in in-center hemodialysis patients every 6 months (1B).
- 1.2.1.1: Report any new HCV infection identified in a hemodialysis patient to the appropriate public health authority (Not Graded).
- 1.2.1.2: In units with a new HCV infection, we recommend that all patients be tested for HCV infection and the frequency of subsequent HCV testing be increased (1A).
- 1.2.1.3: We recommend that hemodialysis patients with resolved HCV infection undergo repeat testing every 6 months using NAT to detect possible re-infection (1B).
- 1.2.2: We suggest that patients have serum alanine aminotransferase (ALT) level checked upon initiation of in-center hemodialysis or upon transfer from another facility (2B).
- 1.2.2.1: We suggest that hemodialysis patients have ALT level checked monthly (2B).
1.3 Liver testing in patients with CKD and HCV infection
1.3.1: We recommend assessing HCV-infected patients with CKD for liver fibrosis (1A).
1.3.2: We recommend an initial noninvasive evaluation of liver fibrosis (1B).
1.3.3: When the cause of liver disease is uncertain or noninvasive testing results are discordant, consider liver biopsy (Not Graded).
1.3.4: We recommend assessment for portal hypertension in CKD patients with suspected advanced fibrosis (F3–4) (1A).
1.4 Other testing of patients with HCV infection
- 1.4.1: We recommend assessing all patients for kidney disease at the time of HCV infection diagnosis (1A).
- 1.4.1.1: Screen for kidney disease with urinalysis and estimated glomerular filtration rate (eGFR) (Not Graded).
1.4.2: If there is no evidence of kidney disease at initial evaluation, patients who remain NAT-positive should undergo repeat screening for kidney disease (Not Graded).
1.4.3: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be followed up regularly to assess progression of kidney disease (1A).
1.4.4: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be screened and, if appropriate, vaccinated against hepatitis A virus (HAV) and hepatitis B virus (HBV), and screened for human immunodeficiency virus (HIV) (1A).
Chapter 2: Treatment of HCV infection in patients with CKD
- 2.1: We recommend that all CKD patients infected with HCV be evaluated for antiviral therapy (1A).
- 2.1.1: We recommend an interferon-free regimen (1A).
- 2.1.2: We recommend that the choice of specific regimen be based on HCV genotype (and subtype), viral load, prior treatment history, drug–drug interactions, glomerular filtration rate (GFR), stage of hepatic fibrosis, kidney and liver transplant candidacy, and comorbidities (1A).
- 2.1.3: Treat kidney transplant candidates in collaboration with the transplant center to optimize timing of therapy (Not Graded).
2.2: We recommend that patients with GFR ≥ 30 ml/min per 1.73 m2 (CKD G1–G3b) be treated with any licensed direct-acting antiviral (DAA)-based regimen (1A).
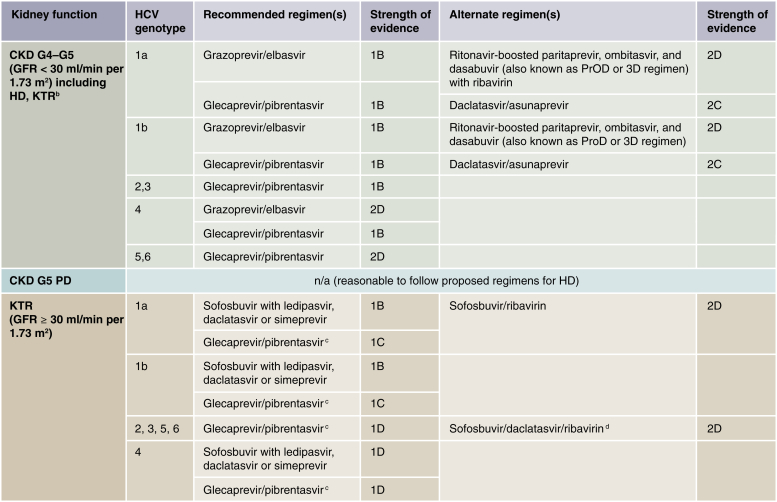
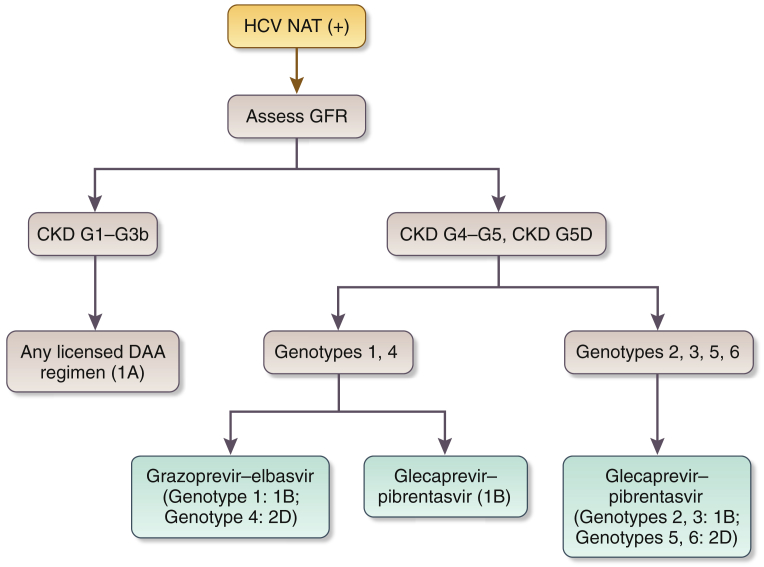
2.3: Patients with GFR < 30 ml/min per 1.73 m2 (CKD G4–G5D) should be treated with a ribavirin-free DAA-based regimen as outlined in Figure 1.
- 2.4: We recommend that all kidney transplant recipients infected with HCV be evaluated for treatment (1A).
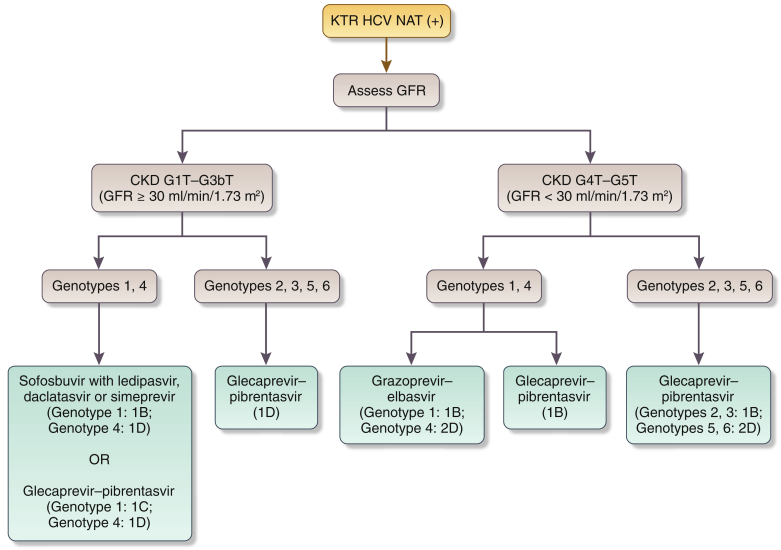
- 2.4.1: We recommend treatment with a DAA-based regimen as outlined in Figure 1 (1A).
- 2.4.2: We recommend that the choice of regimen be based on HCV genotype (and subtype), viral load, prior treatment history, drug–drug interactions, GFR, stage of hepatic fibrosis, liver transplant candidacy, and comorbidities (1A).
- 2.4.3: We recommend avoiding treatment with interferon (1A).
- 2.4.4: We recommend pre-treatment assessment for drug–drug interactions between the DAA-based regimen and other concomitant medications including immunosuppressive drugs in kidney transplant recipients (1A).
- 2.4.4.1: We recommend that calcineurin inhibitor levels be monitored during and after DAA treatment (1B).
- 2.5: All treatment candidates should undergo testing for HBV infection prior to therapy (Not Graded).
- 2.5.1: If hepatitis B surface antigen [HBsAg] is present, the patient should undergo assessment for HBV therapy (Not Graded).
- 2.5.2: If HBsAg is absent but markers of prior HBV infection (HBcAb-positive with or without HBsAb) are detected, monitor for HBV reactivation with serial HBV DNA and liver function tests during DAA therapy (Not Graded).
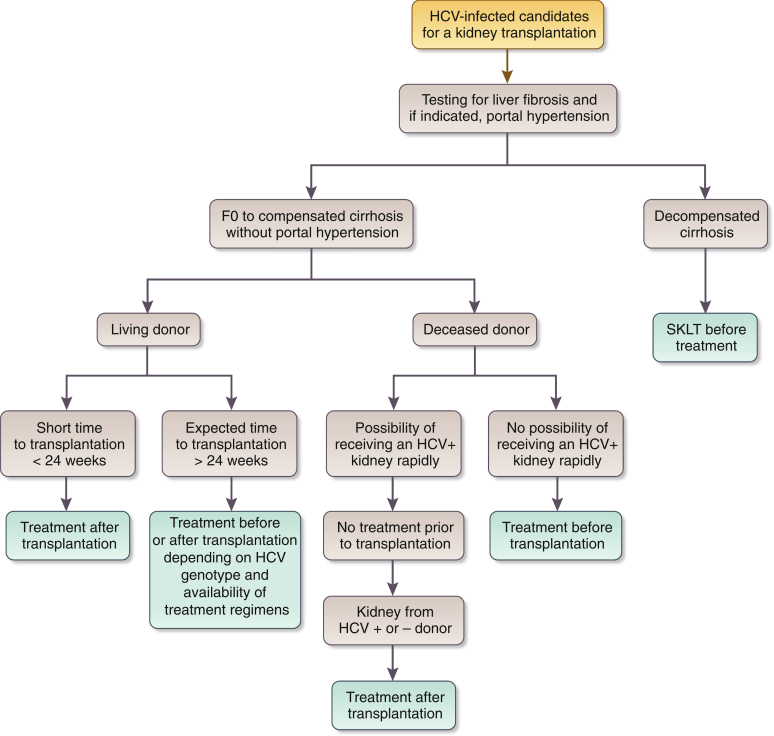
Figure 1.
Recommended direct-acting antiviral (DAA) treatment regimens for patients with chronic kidney disease (CKD) G4–G5D and kidney transplant recipients (KTRs), by hepatitis C virus (HCV) genotypea. Duration of therapy for all above regimens is usually 12 weeks but readers should consult Association for the Study of Liver Diseases (AASLD) or European Association for the Study of the Liver guidelines for latest guidance. aWe recommend that CKD patients with glomerular filtration rates (GFRs) ≥ 30 ml/min per 1.73 m2 (CKD G1T–G3bT) be treated with any licensed DAA regimen. bThere is little published evidence to guide treatment regimens in KTRs with GFR < 30 ml/min per 1.73 m2 (CKD G4T–G5T). Regimens in KTRs should be selected to avoid drug–drug interactions, particularly with calcineurin inhibitors. cBased on Reau et al.3dAs suggested in AASLD guidelines (https://www.hcvguidelines.org/). CKD G, chronic kidney disease (GFR category); HD, hemodialysis; n/a, no data or evidence available; PD, peritoneal dialysis.
Chapter 3: Preventing HCV transmission in hemodialysis units
- 3.1: We recommend that hemodialysis facilities adhere to standard infection control procedures including hygienic precautions that effectively prevent transfer of blood and blood-contaminated fluids between patients to prevent transmission of blood-borne pathogens (see Table 1) (1A).
- 3.1.1: We recommend regular observational audits of infection control procedures in hemodialysis units (1C).
- 3.1.2: We recommend not using dedicated dialysis machines for HCV-infected patients (1D).
- 3.1.3: We suggest not isolating HCV-infected hemodialysis patients (2C).
- 3.1.4: We suggest that the dialyzers of HCV-infected patients can be reused if there is adherence to standard infection control procedures (2D).
- 3.2: We recommend that hemodialysis centers examine and track all HCV test results to identify new cases of HCV infections in their patients (1B).
- 3.2.1: We recommend that aggressive measures be taken to improve hand hygiene (and proper glove use), injection safety, and environmental cleaning and disinfection when a new case of HCV is identified that is likely to be dialysis-related (1A).
3.3: Strategies to prevent HCV transmission within hemodialysis units should prioritize adherence to standard infection control practices and should not primarily rely upon the treatment of HCV-infected patients (Not Graded).
Table 1.
Infection control practices (“hygienic precautions”) particularly relevant for preventing HCV transmission
|
|
|
|
Chapter 4: Management of HCV-infected patients before and after kidney transplantation
4.1 Evaluation and management of kidney transplant candidates regarding HCV infection
4.1.1: We recommend kidney transplantation as the best therapeutic option for patients with CKD G5 irrespective of presence of HCV infection (1A).
- 4.1.2: We suggest that all HCV-infected kidney transplant candidates be evaluated for severity of liver disease and presence of portal hypertension (if indicated) prior to acceptance for kidney transplantation (2D).
- 4.1.2.1: We recommend that HCV-infected patients with compensated cirrhosis (without portal hypertension) undergo isolated kidney transplantation (1B).
- 4.1.2.2: We recommend referring HCV-infected patients with decompensated cirrhosis for combined liver-kidney transplantation (1B) and deferring HCV treatment until after transplantation (1D).
- 4.1.3: Timing of HCV treatment in relation to kidney transplantation (before vs. after) should be based on donor type (living vs. deceased donor), wait-list times by donor type, center-specific policies governing the use of kidneys from HCV-infected deceased donors, HCV genotype, and severity of liver fibrosis (Not Graded).
- 4.1.3.1: We recommend that all HCV-infected patients who are candidates for kidney transplantation be considered for DAA therapy, either before or after transplantation (1A).
- 4.1.3.2: We suggest that HCV-infected kidney transplant candidates with a living kidney donor can be considered for treatment before or after transplantation according to HCV genotype and anticipated timing of transplantation (2B).
- 4.1.3.3: We suggest that if receiving a kidney from an HCV-positive donor improves the chances for transplantation, the HCV NAT–positive patient can undergo transplantation with an HCV-positive kidney and be treated for HCV infection after transplantation (2B).
4.2 Use of kidneys from HCV-infected donors
4.2.1: We recommend that all kidney donors be screened for HCV infection with both immunoassay and NAT (if NAT is available) (1A).
4.2.2: We recommend that transplantation of kidneys from HCV NAT-positive donors be directed to recipients with positive NAT (1A).
4.2.3: After the assessment of liver fibrosis, HCV-positive potential living kidney donors who do not have cirrhosis should undergo HCV treatment before donation; they can be accepted for donation if they achieve sustained virologic response (SVR) and remain otherwise eligible to be a donor (Not Graded).
4.3 Use of maintenance immunosuppressive regimens
4.3.1: We suggest that all conventional current induction and maintenance immunosuppressive regimens can be used in HCV-infected kidney transplant recipients (2C).
4.4 Management of HCV-related complications in kidney transplant recipients
4.4.1: We recommend that patients previously infected with HCV who achieved SVR before transplantation be tested by NAT 3 months after transplantation or if liver dysfunction occurs (1D).
4.4.2: Untreated HCV-positive kidney transplant recipients should have the same liver disease follow-up as HCV-positive non-transplant patients, as outlined in the American Association for the Study of Liver Diseases (AASLD) guidelines (Not Graded).
- 4.4.3: HCV-infected kidney transplant recipients should be tested at least every 6 months for proteinuria (Not Graded).
- 4.4.3.1: We suggest that patients who develop new-onset proteinuria (either urine protein-to-creatinine ratio > 1 g/g or 24-hour urine protein > 1 g on 2 or more occasions) have an allograft biopsy with immunofluorescence and electron microscopy included in the analysis (2D).
4.4.4: We recommend treatment with a DAA regimen in patients with post-transplant HCV-associated glomerulonephritis (1D).
Chapter 5: Diagnosis and management of kidney diseases associated with HCV infection
5.1: We recommend that a kidney biopsy be performed in HCV-infected patients with clinical evidence of glomerular disease (Not Graded).
- 5.2: We recommend that patients with HCV-associated glomerular disease be treated for HCV (1A).
- 5.2.1: We recommend that patients with HCV-related glomerular disease showing stable kidney function and/or non-nephrotic proteinuria be treated initially with DAA (1C).
- 5.2.2: We recommend that patients with cryoglobulinemic flare, nephrotic syndrome, or rapidly progressive kidney failure be treated, in addition to DAA treatment, with immunosuppressive agents with or without plasma exchange (1C).
- 5.2.3: We recommend immunosuppressive therapy in patients with histologically active HCV-associated glomerular disease who do not respond to antiviral therapy, particularly those with cryoglobulinemic kidney disease (1B).
- 5.2.3.1: We recommend rituximab as the first-line immunosuppressive treatment (1C).
Chapter 1: Detection and evaluation of HCV in CKD
1.1 Screening patients with CKD for HCV infection
Patients receiving maintenance hemodialysis and subgroups of CKD patients not yet on dialysis are known to have a high prevalence of HCV infection. The reasons for testing CKD patients for HCV infection include early detection and treatment of HCV infection, diagnostic evaluation of the cause of CKD, identification of infection control lapses in hemodialysis centers, and guidance on decisions surrounding kidney transplantation care.
- 1.1.1: We recommend screening all patients for HCV infection at the time of initial evaluation of CKD (1C).
- 1.1.1.1: We recommend using an immunoassay followed by nucleic acid testing (NAT) if immunoassay is positive (1A).
- 1.1.2: We recommend screening all patients for HCV infection upon initiation of in-center hemodialysis or upon transfer from another dialysis facility or modality (1A).
- 1.1.2.1: We recommend using NAT alone or an immunoassay followed by NAT if immunoassay is positive (1A).
1.1.3: We suggest screening all patients for HCV infection upon initiation of peritoneal dialysis or home hemodialysis (2D).
1.1.4: We recommend screening all patients for HCV infection at the time of evaluation for kidney transplantation (1A).
Rationale
- 1.1.1: We recommend screening all patients for HCV infection at the time of initial evaluation of CKD (1C).
- 1.1.1.1: We recommend using an immunoassay followed by nucleic acid testing (NAT) if immunoassay is positive (1A).
Any CKD patient who has a risk factor for HCV infection should be tested.4 Additionally, HCV testing is warranted for the evaluation of CKD because: (i) the prevalence of HCV infection may be higher in patients with CKD not yet on dialysis than in the general population;5, 6 (ii) HCV infection increases the risk of developing CKD;7 and (iii) HCV infection can accelerate progression of CKD.8, 9, 10
Diagnosis of HCV infection relies on various assays.11, 12 Serological assays that detect HCV antibody (anti-HCV) are based on enzyme immunoassays or chemoluminescence immunoassays. Anti-HCV tests are unable to distinguish between resolved HCV infection and current HCV infection. Detection of HCV viremia relies on NAT technologies. Qualitative and quantitative HCV RNA methods are available and have similar limits of detection (10–20 international units [IU]/ml). HCV antigen tests that detect core antigen alone or in combination with other HCV proteins have the potential to be less costly than NAT, but their limit of detection is higher (equivalent to about 150–3000 IU/ml).11, 13, 14, 15
The most usual strategy for diagnosis of HCV infection consists of initial screening with an inexpensive serological assay and, if the assay is positive, subsequent NAT. However, in high prevalence settings or very high risk groups, immediate NAT is an appropriate alternative.
- 1.1.2: We recommend screening all patients for HCV infection upon initiation of in-center hemodialysis or upon transfer from another dialysis facility or modality (1A).
- 1.1.2.1: We recommend using NAT alone or an immunoassay followed by NAT if immunoassay is positive (1A).
The prevalence of HCV infection in patients undergoing hemodialysis (CKD G5 on dialysis) is higher than in the general population16, 17 and has been associated with the number of years one has been on hemodialysis. Patient-to-patient transmission of HCV infection in outpatient hemodialysis centers has occurred repeatedly despite widespread knowledge of this risk and published guidelines for prevention. Identification of HCV transmission within a dialysis facility should prompt immediate reevaluation of infection control practices and determination of appropriate corrective action (see Chapter 3).18, 19, 20, 21, 22 The majority of persons with HCV infection are asymptomatic, making screening necessary to detect infection in high-risk populations, particularly in hemodialysis patients in whom signs or symptoms of acute HCV infection are rarely recognized. Screening of maintenance hemodialysis patients for HCV infection is recommended by the United States (US) Centers for Disease Control and Prevention (CDC) and also the US Preventive Services Task Force.23, 24 Goals of screening in this patient population include early detection of HCV infection, treatment of infection, and detection of dialysis-related transmission. HCV screening is indicated in patients starting in-center maintenance hemodialysis and also in patients who transfer from another dialysis facility or modality. In dialysis units with a high prevalence of HCV, initial testing with NAT should be considered. An anti-HCV–negative, HCV RNA–positive (i.e., NAT-positive) profile strongly suggests acute HCV infection.
Samples collected to test for HCV by NAT should be drawn before dialysis, because hemodialysis sessions reduce viremia level, although the mechanism remains unclear.25
1.1.3: We suggest screening all patients for HCV infection upon initiation of peritoneal dialysis or home hemodialysis (2D).
HCV transmission has typically been described in the context of in-center hemodialysis. In this setting, blood contamination on the hands of staff members or of medications, supplies, and equipment can contribute to HCV transmission. The current risk of health care–related HCV infection among patients who receive peritoneal dialysis or home hemodialysis has not been quantified. Many of these patients will require in-center hemodialysis at some point during their care, and may be at risk of acquiring HCV infection during that time. Screening of peritoneal dialysis and home hemodialysis patients should be considered upon initiation of dialysis to document baseline HCV infection status. If these patients transiently receive in-center hemodialysis, they should undergo HCV infection screening as per the recommendations for in-center hemodialysis patients, with consideration of continued screening until 6 months after the completion of in-center hemodialysis (and transition to a different modality).
1.1.4: We recommend screening all patients for HCV infection at the time of evaluation for kidney transplantation (1A).
Kidney transplantation candidates should be tested for HCV infection during evaluation for transplantation. Determination of HCV status in recipients is essential for optimal management and potentially for acceptance of kidneys from HCV-infected donors (see Chapter 4).
1.2 Follow-up HCV screening of in-center hemodialysis patients
- 1.2.1: We recommend screening for HCV infection with immunoassay or NAT in in-center hemodialysis patients every 6 months (1B).
- 1.2.1.1: Report any new HCV infection identified in a hemodialysis patient to the appropriate public health authority (Not Graded).
- 1.2.1.2: In units with a new HCV infection, we recommend that all patients be tested for HCV infection and the frequency of subsequent HCV testing be increased (1A).
- 1.2.1.3: We recommend that hemodialysis patients with resolved HCV infection undergo repeat testing every 6 months using NAT to detect possible re-infection (1B).
- 1.2.2: We suggest that patients have serum alanine aminotransferase (ALT) level checked upon initiation of in-center hemodialysis or upon transfer from another facility (2B).
- 1.2.2.1: We suggest that hemodialysis patients have ALT level checked monthly (2B).
Rationale
- 1.2.1: We recommend screening for HCV infection with immunoassay or NAT in in-center hemodialysis patients every 6 months (1B).
- 1.2.1.1: Report any new HCV infection identified in a hemodialysis patient to the appropriate public health authority (Not Graded).
- 1.2.1.2: In units with a new HCV infection, we recommend that all patients be tested for HCV infection and the frequency of subsequent HCV testing be increased (1A).
- 1.2.1.3: We recommend that hemodialysis patients with resolved HCV infection undergo repeat testing every 6 months using NAT to detect possible re-infection (1B).
Patients who are not infected with HCV should be screened for presence of new infection every 6 months.23 This recommendation includes anti-HCV–negative patients and anti-HCV–positive, HCV RNA–negative patients screened initially by immunoassay, as well as HCV RNA–negative patients screened initially by NAT. Patients who are anti-HCV–positive and HCV RNA–negative (i.e., NAT-negative) have resolved infection but remain at risk for re-infection if exposed.26 Therefore, these patients should also undergo repeat screening. For dialysis patients who are anti-HCV–positive and HCV NAT–negative, screening for HCV reinfection should be conducted every 6 months using NAT.
The purpose of the repeat screening is to identify new infections (i.e., newly acquired infections) that could represent transmission within the dialysis center. The baseline HCV testing results should be reviewed for any patient who has a positive HCV screening test result to determine whether there was a change in infection status indicating a new infection, and results must be communicated to the patient. Any patient with a current infection, whether new or pre-existing, should be linked to HCV care and considered for antiviral therapy.
Acute HCV infection in a hemodialysis patient should be reported to the appropriate public health authority. Reporting may be mandated by law, as in the US, where a documented negative HCV antibody or NAT laboratory test result followed within 12 months by a positive HCV test result (test conversion) must be reported to public health authorities.27 Acute HCV infection in a hemodialysis patient should be investigated and considered health care–related until proven otherwise.28 Behavioral risk factors, along with dialysis and nondialysis health care exposures, should be evaluated by public health authorities. Molecular sequencing of HCV RNA from other patients in the facility may help to identify a source.22, 29, 30, 31
Acute HCV infection should also prompt immediate evaluation of all other patients in the same facility to identify additional cases. The status of all patients should be reviewed at the time a new infection is identified, and all patients previously known to be uninfected should be retested for HCV infection. The frequency of repeat screening should also be increased for a limited time: for example, monthly testing for 3 months, followed by testing again in 3 months, and then resumption of screening every 6 months if no additional infections are identified.20, 23 This strategy can help to identify delayed seroconversions (from the same exposure period as the index case) or other cases resulting from recurrent breaches. Use of this strategy has led to the detection of additional new cases in several reported outbreaks.22, 32
For anti-HCV–positive patients with chronic HCV infection who become HCV NAT–negative with a sustained virologic response (SVR) to HCV therapy, initiate NAT screening 6 months after documentation of SVR. SVR is determined based on results of NAT testing ≥ 12 weeks after the conclusion of therapy.
For patients with spontaneous resolution of acute HCV infection as documented by a negative test for HCV RNA at ≥ 6 months after the onset of acute infection, NAT screening should begin 6 months after documented resolution of infection.
- 1.2.2: We suggest that patients have serum alanine aminotransferase (ALT) level checked upon initiation of in-center hemodialysis or upon transfer from another facility (2B).
- 1.2.2.1: We suggest that hemodialysis patients have ALT level checked monthly (2B).
A baseline serum ALT test, followed by monthly testing, in susceptible patients has been recommended to enable early detection of new HCV infection in hemodialysis patients.23 Newly infected patients may have an increase in ALT levels prior to antibody conversion, which should prompt additional evaluation. If an unexplained elevation (i.e., to greater than upper-limit normal) of ALT occurs, the patient should be tested for HCV infection. The exact predictive value of ALT screening for detection of HCV infection has been assessed in a single study and found to be moderate.33 However, ALT monitoring is an inexpensive way to ensure that hemodialysis patients are assessed for possible acquisition of infection between regular antibody or NAT screenings. Because few hemodialysis patients with a new HCV infection report symptoms or have symptoms documented in their dialysis medical records, ALT levels are also often used retrospectively to define the likely exposure period for patients who acquired infection. Thus, monthly ALT levels are valuable to help narrow the focus of an HCV case investigation to the most likely exposure and source. The value of monthly ALT testing in patients who have resolved HCV infection has not been studied.
1.3 Liver testing in patients with CKD and HCV infection
1.3.1: We recommend assessing HCV-infected patients with CKD for liver fibrosis (1A).
1.3.2: We recommend an initial noninvasive evaluation of liver fibrosis (1B).
1.3.3: When the cause of liver disease is uncertain or noninvasive testing results are discordant, consider liver biopsy (Not Graded).
1.3.4: We recommend assessment for portal hypertension in CKD patients with suspected advanced fibrosis (F3–4) (1A).
Rationale
Evaluation of liver fibrosis in HCV-infected patients with CKD
In the prior Kidney Disease: Improving Global Outcomes (KDIGO) HCV guideline published in 2008,34 liver biopsy had been considered the gold standard to assess liver fibrosis in patients with CKD, including candidates for transplantation and transplant recipients. The primary objective of liver biopsy in patients with advanced CKD had been to diagnose cirrhosis. Because of the risk of liver-related mortality after kidney transplantation, cirrhosis had been considered a contraindication to kidney transplantation alone and led to consideration of combined liver-kidney transplantation.
Current evidence suggests that biochemical noninvasive markers (FibroTest/FibroMeter, aspartate aminotransferase–platelet ratio index [APRI], Forns, or FIB-4 index) and morphological evaluation (liver stiffness by elastography) may have comparable accuracy in evaluating liver fibrosis in patients with CKD G4–5 as in the general population.35 Noninvasive methods, especially elastography, are sufficiently reliable to detect extensive fibrosis and/or cirrhosis (F3–F4)36, 37 though noninvasive tests other than elastography may be less accurate (Supplementary Tables S1 and S2). Furthermore, although serious complications of liver biopsy are uncommon, patients are often reluctant to consider it, and its validity may be diminished by sampling as well as interpretation errors. Liver biopsy use in HCV-infected patients generally has declined.
Because SVR can now be anticipated in the vast majority of patients treated for HCV, the management of the HCV-infected kidney transplant candidate, even with cirrhosis, has evolved. SVR is associated with sustained and long-lasting suppression of necroinflammation and may even result in regression of cirrhosis, potentially resulting in decreased disease-related morbidity and improved survival.38 Even in the absence of regression of cirrhosis, kidney transplantation alone is feasible in the absence of major complications of portal hypertension, just like in patients with hepatitis B virus (HBV)–related cirrhosis.39
Thus, the role of liver biopsy in evaluation of liver fibrosis in HCV-infected patients with CKD G4–5 will evolve given the high SVR rates obtained with current DAA regimens. Defining the severity of cirrhosis involves assessment for clinically significant portal hypertension (hepatic-vein wedge-pressure gradient of ≥ 10 mm Hg).40 Methods include upper endoscopy, noninvasive radiological evaluation, or direct portal pressure measurement. Based on the Baveno VI consensus,41 portal hypertension is very unlikely (and hence an upper endoscopy can be avoided with > 90% reliability) in patients with compensated cirrhosis but elastography < 20 kPa and platelet count > 150,000/mm3. Whether this approach is also valid for patients on hemodialysis remains unknown.
In summary, all HCV-infected patients with kidney failure should undergo a noninvasive biochemical and/or morphological evaluation to stage fibrosis and determine the role of antiviral therapies (see Chapter 2) and to facilitate the choice of kidney or combined liver-kidney transplantation in cirrhotic patients. When results between biochemical and morphological evaluation are discordant or when liver comorbidities are suspected, liver biopsy is suggested.42
1.4 Other testing of patients with HCV infection
Although HCV infection predominantly causes liver disease, it is also associated with extrahepatic manifestations including kidney disease.43 HCV has been shown to infect both hepatocytes and lymphocytes; thus, lymphoproliferative disorders such as lymphoma and mixed cryoglobulinemia are linked to HCV infection.44 HCV has also been implicated in derangements of multiple organ systems including cardiovascular, endocrine, muscular, nervous, ocular, respiratory, skeletal, cutaneous, and urinary systems. In addition, HCV can have a deleterious impact on psychosocial status.45
The relationship between HCV infection and CKD is complex. HCV infection and CKD are prevalent in the general population and associated in various ways: patients on chronic hemodialysis are at increased risk of acquiring HCV, and some types of kidney disease are precipitated by HCV infection. Conventional risk factors for CKD such as aging, diabetes, hypertension, and metabolic syndrome do not fully explain the current frequency of CKD in the adult general population of developed countries. In addition to these conventional risk factors, accumulating evidence in the last decade has implicated HCV infection as a cause of kidney disease. HCV co-infection has also been implicated as a risk factor for CKD in HIV-infected patients.46 A meta-analysis7 of observational studies47, 48, 49, 50, 51, 52, 53, 54, 55 demonstrated a relationship between anti-HCV–positive serologic status and an increased incidence of CKD in the adult general population, with an adjusted hazard ratio (HR) of 1.43 (95% confidence interval [CI]: 1.23–1.63). Based on current information, patients with HCV infection should be regarded as being at increased risk of CKD, regardless of the presence of conventional risk factors for kidney disease.
- 1.4.1: We recommend assessing all patients for kidney disease at the time of HCV infection diagnosis (1A).
- 1.4.1.1: Screen for kidney disease with urinalysis and estimated glomerular filtration rate (eGFR) (Not Graded).
1.4.2: If there is no evidence of kidney disease at initial evaluation, patients who remain NAT-positive should undergo repeat screening for kidney disease (Not Graded).
1.4.3: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be followed up regularly to assess progression of kidney disease (1A).
1.4.4: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be screened and, if appropriate, vaccinated against hepatitis A virus (HAV) and hepatitis B virus (HBV), and screened for human immunodeficiency virus (HIV) (1A).
Rationale
- 1.4.1: We recommend assessing all patients for kidney disease at the time of HCV infection diagnosis (1A).
- 1.4.1.1: Screen for kidney disease with urinalysis and estimated glomerular filtration rate (eGFR) (Not Graded).
The prevalence of CKD, defined by a reduction in eGFR and/or increased urinary albumin excretion,56 exceeds 10% in the adult general population, according to numerous population-based studies. The prevalence of low GFR alone is around 5% to 6% but increases sharply with older age. Testing for CKD appears logical in HCV-infected individuals, as many authors have suggested a potential role of HCV infection as a cause of CKD. However, epidemiologic supporting data regarding the prevalence of CKD in HCV-infected patients were until recently limited and used variable criteria for the definition of CKD; the demographic/clinical characteristics of the representative patient population were variable as well. According to 3 studies performed in the US,47, 52, 55 the unadjusted prevalence of low GFR (<60 ml/min per 1.73 m2) ranged at baseline between 5.1% and 8.0% among middle-aged anti-HCV–seropositive individuals. The unadjusted prevalence of renal insufficiency (serum creatinine >1.5 mg/dl [>133 μmol/l]) in one large study of anti-HCV-seropositive veterans from the US was 4.8%.57 In another large cohort of HCV-positive, HIV-positive patients from North America, the unadjusted frequency of low GFR (<60 ml/min per 1.73 m2) at baseline ranged between 3.7% and 4.0%.58
Kidney involvement in HCV infection was first recognized more than 2 decades ago; however, the association between HCV and CKD (low GFR or presence of proteinuria) in the adult general population was controversial until a few years ago. An increasing body of evidence has recently highlighted the detrimental impact of HCV infection on the risk of CKD (Supplementary Tables S3 and S4). One meta-analysis7 reported an HR of 1.43 (95% CI: 1.23–1.63) between positive HCV serologic status and increased incidence for CKD, while another recent study59 demonstrated that patients with HCV had a 27% increased risk of CKD compared with patients without HCV. This study also revealed that HCV-positive patients experienced a 2-fold higher risk of membranoproliferative glomerulonephritis (MPGN) and a nearly 17-fold higher risk of cryoglobulinemia. Effective antiviral treatments have been shown to reduce risk for development of CKD by 30%. Cohort studies performed in patients with HIV and HCV co-infection,10 patients with diabetes,8, 60 and patients with biopsy-proven chronic glomerulonephritis (GN)9 have confirmed a significant relationship between anti-HCV–positive serologic status and accelerated progression of CKD. The prevalence of anti-HCV in serum was significantly greater in patients with CKD before reaching end-stage kidney disease (ESKD) than in a healthy population.5, 6 Among liver transplant recipients infected with HCV who were treated with antiviral therapy, SVR led to improved eGFR in those with CKD G2 (GFR 60–89 ml/min per 1.73 m2) before treatment.61 HCV co-infection is a risk factor for increased health care resource utilization in HIV-infected individuals in the US; a multivariate Poisson model showed that HCV co-infection was associated with higher frequency of emergency department visits: adjusted relative risk (RR) 2.07 (95% CI: 1.49–2.89). In particular, emergency department visits related to kidney disease were much more common among co-infected patients (37%) than among those with HIV infection alone (10%).62 Another meta-analysis of observational studies63 reported a relationship between positive anti-HCV serologic status and an increased risk of reduced GFR among HIV-infected individuals, with an adjusted HR of 1.64 (95% CI: 1.28–2.0), compared with those having HIV infection alone.
1.4.2: If there is no evidence of kidney disease at initial evaluation, patients who remain NAT-positive should undergo repeat screening for kidney disease (Not Graded).
The recommendation to repeat testing for proteinuria or GFR in anti-HCV–positive, HCV NAT–positive patients comes from epidemiologic data. In one study, serial measurements of eGFR and proteinuria were obtained in a large cohort of US metropolitan residents. The prevalence of CKD was greater among anti-HCV–positive, HCV NAT–positive patients compared with matched anti-HCV–negative controls (9.1% vs. 5.1%, P = 0.04).64 In addition, using data from the Third National Health and Nutrition Examination Survey, at least 2 studies have observed an increased risk of albuminuria in patients with HCV.65, 66 Classically, HCV infection predisposes to cryoglobulinemic MPGN; however, HCV-positive individuals may also be at risk for kidney injury related to decompensated cirrhosis, injection drug use, and HIV or HBV co-infection. Overall, multiple studies have now shown that HCV infection is associated with an increased risk of developing CKD, as summarized in a recent meta-analysis.7 It is possible that accelerated atherosclerosis also contributes to the increased risk of developing kidney disease among HCV-infected individuals.67
1.4.3: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be followed up regularly to assess for progression of kidney disease (1A).
Although studies are heterogeneous and some controversy persists,68 overall, HCV-infected patients appear to be at greater risk for incidence and progression of kidney disease and require monitoring as outlined in the KDIGO CKD guideline.56 In the Women’s Interagency HIV study, anti-HCV–positive serologic status was independently associated with a net decrease in eGFR of approximately 5% per year (95% CI: 3.2–7.2) compared with women who were seronegative.69
Of note, antiviral therapy for HCV significantly improves hepatic and extrahepatic outcomes in the general population70, 71 and among patients co-infected with HIV and HCV.72 Six studies have addressed the impact of interferon (IFN)-based regimens on the progression of CKD.64, 73, 74, 75, 76, 77 Five multivariate analyses64, 73, 74, 75, 76 suggested that treatment of HCV infection may improve renal survival per se. In a nationwide cohort study from Taiwan, patients who had received antiviral treatment (pegylated IFN plus ribavirin [RBV]) had a calculated 8-year cumulative incidence of ESKD of 0.15% versus 1.32% in untreated patients (P < 0.001).75 Multivariate-adjusted Cox regression revealed that antiviral treatment was associated with lower risks of ESKD (HR: 0.15; 95% CI: 0.07–0.31). Antiviral treatment was also associated with an adjusted HR of 0.77 (95% CI: 0.62–0.97) for acute coronary syndrome, and 0.62 (95% CI: 0.46–0.83) for ischemic stroke.75 These favorable associations were not observed in patients treated for less than 16 weeks, suggesting that shorter-duration therapy was inadequate.
In a study on 650 Japanese patients with liver cirrhosis,73 multivariate Cox proportional hazards analysis showed that failure to achieve SVR was a predictor of development of CKD, with an adjusted HR of 2.67 (95% CI:1.34–5.32). In a hospital-based study from the US, 552 HCV-infected patients were evaluated, and 159 received IFN therapy during a 7-year follow-up. Multivariate logistic regression indicated that a history of IFN treatment was a significant independent negative predictor for CKD (odds ratio [OR]: 0.18; 95% CI: 0.06–0.56).64 Finally, a recent meta-analysis of controlled and uncontrolled studies (11 studies; n = 225 patients) that evaluated efficacy and safety of antiviral treatment for HCV-related glomerular disease found that the summary estimate of the mean decrease in serum creatinine levels was 0.23 mg/dl (20 μmol/l) (95% CI: 0.02–0.44) after IFNα-based therapy.78
1.4.4: We recommend that all CKD patients with a history of HCV infection, whether NAT-positive or not, be screened and, if appropriate, vaccinated against HAV and HBV, and screened for human immunodeficiency virus (HIV) (1A).
HCV is a blood-borne pathogen and shares routes of transmission with HBV and HIV. Although hepatitis A virus (HAV) infection is frequently mild in healthy individuals, superinfection with HAV and HBV in patients with liver disease (including chronic HCV) may result in significant morbidity and mortality.79 Thus, as HAV80 and HBV81 are vaccine-preventable infections, appropriate vaccination should be encouraged, although response rates to vaccination are diminished in patients with advanced CKD.
Research recommendations
-
•
Studies are needed to examine HCV antigen testing as an alternative to NAT to diagnose HCV viremic infection.
-
•
The clinical utility of HCV antigen immunoassays and antigen and antibody combination assays should be determined.
-
•
The predictive value of different levels of ALT for identifying HCV infection and the additive value of ALT screening to the current generation of immunoassays or NAT testing should be investigated. Data should already exist to address this question among dialysis providers that perform routine screening of their patients. The utility of ALT testing after resolved HCV infection should be studied.
-
•
With the availability of effective treatments for HCV, the role of DAAs in preventing and slowing the progression of CKD in HCV-infected population should be assessed.
Chapter 2: Treatment of HCV infection in patients with CKD
The recommendations are presented below by GFR category. GFR can be measured GFR or estimated GFR. If eGFR is used, we suggest using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula or the creatinine and cystatin C-based CKD-EPI formula.82
Because multiple studies from the general population have found a strong correlation between mortality and SVR,83 regulatory agencies such as the US Food and Drug Administration (FDA) have generally accepted SVR response as a surrogate endpoint for trials used in their drug approval process.84 The FDA recently replaced SVR at 24 weeks after cessation of therapy (SVR24) with SVR at 12 weeks (SVR12). Although there are no data demonstrating that SVR12 reduces mortality in CKD, a meta-analysis showed that SVR24 predicted mortality not only in the general population, but also in patients with cirrhosis and patients with HIV co-infection.85 Currently, duration of therapy for DAA regimens is usually 12 weeks but may change in the future.
For most CKD patients, as in the general population, the potential benefits of antiviral treatment outweigh potential harms.86 However, some patients may not be expected to live long enough to benefit from therapy (e.g., those with metastatic cancer). The Work Group was hesitant to specify a minimum life expectancy that would justify treatment, given the inaccuracy of predictions and the need to individualize this decision. However, as noted in the American Association for the Study of Liver Diseases/Infectious Diseases Society of America (AASLD/IDSA) guidance, little evidence exists to support initiation of HCV treatment in patients with a limited life expectancy (<12 months).87
IFN is often poorly tolerated in advanced CKD (CKD G4–G5) patients who have prolonged IFN exposure due to decreased renal clearance. RBV is also associated with adverse events. Hemolytic anemia induced by RBV is especially common in patients with CKD G3b–G5 and can be severe. The RBV dose needs to be reduced in patients with advanced CKD, but dose reductions can only be approximated. An initial starting dose of 200 mg daily is typical but does not preclude development of anemia, despite initiation or increased dosing of erythropoiesis stimulating agents (ESAs). Because DAAs are effective, well-tolerated, and often do not require dose reductions in those with CKD, it is clearly desirable to avoid IFN completely in all patients and to minimize use of RBV in patients with advanced CKD.
- 2.1: We recommend that all CKD patients infected with HCV be evaluated for antiviral therapy (1A).
- 2.1.1: We recommend an interferon-free regimen (1A).
- 2.1.2: We recommend that the choice of specific regimen be based on HCV genotype (and subtype), viral load, prior treatment history, drug–drug interactions, glomerular filtration rate (GFR), stage of hepatic fibrosis, kidney and liver transplant candidacy, and comorbidities (1A).
- 2.1.3: Treat kidney transplant candidates in collaboration with the transplant center to optimize timing of therapy (Not Graded).
2.2: We recommend that patients with GFR ≥ 30 ml/min per 1.73 m2 (CKD G1–G3b) be treated with any licensed direct-acting antiviral (DAA)-based regimen (1A).
2.3: Patients with GFR < 30 ml/min per 1.73 m2 (CKD G4–G5D) should be treated with a ribavirin-free DAA-based regimen as outlined in Figure 1.
- 2.4: We recommend that all kidney transplant recipients infected with HCV be evaluated for treatment (1A).
- 2.4.1: We recommend treatment with a DAA-based regimen as outlined in Figure 1 (1A).
- 2.4.2: We recommend that the choice of regimen be based on HCV genotype (and subtype), viral load, prior treatment history, drug–drug interactions, GFR, stage of hepatic fibrosis, liver transplant candidacy, and comorbidities (1A).
- 2.4.3: We recommend avoiding treatment with interferon (1A).
- 2.4.4: We recommend pre-treatment assessment for drug–drug interactions between the DAA-based regimen and other concomitant medications including immunosuppressive drugs in kidney transplant recipients (1A).
- 2.4.4.1: We recommend that calcineurin inhibitor levels be monitored during and after DAA treatment (1B).
- 2.5: All treatment candidates should undergo testing for HBV infection prior to therapy (Not Graded).
- 2.5.1: If hepatitis B surface antigen [HBsAg] is present, the patient should undergo assessment for HBV therapy (Not Graded).
- 2.5.2: If HBsAg is absent but markers of prior HBV infection (HBcAb-positive with or without HBsAb) are detected, monitor for HBV reactivation with serial HBV DNA and liver function tests during DAA therapy (Not Graded).
Rationale
CKD G1–G3b (GFR ≥ 30 ml/min per 1.73 m2)
For mild to moderate decreases in kidney function, patients with CKD can generally be treated as per evidence-based guidelines for the general population. Currently in the US, the AASLD/IDSA guidelines recommend few dosage modifications for people with mild to moderate reductions in GFR. For CKD G1–G3b (GFR ≥ 30 ml/min per 1.73 m2), no dosage adjustment is required when using daclatasvir (60 mg); daily fixed-dose combination of elbasvir (50 mg) and grazoprevir (100 mg); daily fixed dose combination of glecaprevir (300 mg) and pibrentasvir (120 mg); fixed dose combination of sofosbuvir (400 mg) with either ledipasvir (90 mg) or velpatasvir (100 mg); simprevir (150 mg); fixed-dose combination of sofosbuvir (400 mg), velpatasvir (100 mg), and voxilaprevir (100 mg); or sofosbuvir (400 mg). At the time of publication, regimens including velpatasvir have not been formally approved for use in patients with CKD G1–G3 in some jurisdictions, however.
The 2018 European Association for the Study of the Liver (EASL) guideline42 also recommends no dosage modifications of DAAs for CKD G1–G3 patients, but recommends that these patients should be carefully monitored.
In summary, for patients with CKD G1–G3 the choice of DAA is not restricted. However, it must be stressed that recommended drugs and dosage are constantly evolving, and clinicians should consult the latest guidelines from AASLD (https://www.hcvguidelines.org/unique-populations/renal-impairment) or EASL (http://www.easl.eu/research/our-contributions/clinical-practice-guidelines) for the most up-to-date treatment information.
CKD G4–G5 and G5D (Advanced CKD: GFR < 30 ml/min per 1.73 m2 and those on hemodialysis)
DAAs have variable renal elimination; thus, advanced CKD, if present, is an important determinant in the choice of agent. Until recently, patients with advanced CKD had limited options for HCV therapy. Importantly sofosbuvir, which had been the cornerstone of most DAA regimens, is predominantly renally cleared (80%) and is licensed for use only in individuals with GFR ≥ 30 ml/min per 1.73 m2 (CKD G1–G3b).
A regimen combining a nonstructural protein 5A (NS5A) replication complex inhibitor (elbasvir) and a new-generation nonstructural protein NS3/4A protease inhibitor (grazoprevir) has been licensed for patients infected with HCV genotypes (GTs) 1 and 4, with safety and efficacy data available in patients with advanced CKD. Both agents are metabolized by CYP3A and primarily (>90%) excreted in feces with minimal renal clearance (<1%). Although pharmacokinetic analyses show that area under the curves (AUCs) are higher in individuals with advanced CKD requiring hemodialysis (up to 46% higher compared with individuals with normal kidney function), these changes in exposure to the drugs are not considered clinically relevant.88 Of note, Reddy et al.89 identified 32 patients with CKD G3a/G3b included in trials with grazoprevir and elbasvir and found no evidence of deterioration of kidney function as a result of treatment with these agents.
Grazoprevir is a substrate of OATP1B1/3, and co-administration with drugs that inhibit OATP1B1/3 (such as enalapril, statins, digoxin, some angiotensin-receptor blockers) may result in increased levels of grazoprevir that may lead to clinically significant hyperbilirubinemia. Elbasvir and grazoprevir are substrates of CYP3A, and co-administration with strong CYP3A inducers (such as rifampin, phenytoin, and St John’s wort) is contraindicated, as it may result in decreased plasma concentrations and potentially reduced antiviral activity of both agents. The Hepatitis Drug Interactions website from the University of Liverpool (http://www.hep-druginteractions.org) or another reliable expert source should be accessed to determine the risk and management recommendations for drug–drug interactions.
In contrast to sofosbuvir, agents such as grazoprevir-elbasvir, paritaprevir-ritonavir-ombitasvir with or without dasabuvir, simeprevir, daclatasvir as well as glecaprevir/pibrentasvir can be safely used in CKD G4 and G5 patients (Supplementary Tables S5 and S6). Data on several regimens have been published in patients with advanced CKD (CKD G4–G5D). In the C-SURFER trial, a phase 3 placebo-controlled, randomized, multicenter trial, 12-week treatment with grazoprevir and elbasvir was evaluated in HCV GT1–infected patients with advanced CKD (81% with eGFR < 15 ml/min per 1.73 m2 [CKD G5] and 76% on hemodialysis [CKD G5D]), including 6% of patients with cirrhosis).90 The majority of them were infected with GT1a (52%), and 80% were treatment-naïve. SVR12 was 99% (95% CI: 95.3–100.0; 115 of 116), with 1 relapse 12 weeks after end of treatment with no significant difference between GTs 1a and 1b, nor between those undergoing hemodialysis and those with advanced CKD not on dialysis therapy. Tolerability was excellent. The most common adverse events (≥10% frequency) were headache, nausea, and fatigue, and were comparable in the treatment versus control arms. The frequencies of hemoglobin levels < 8.5 g/dl (< 85 g/l) were also comparable between treated and untreated groups (4.5% and 4.4%, respectively), and similar proportions of patients in both groups required treatment with ESAs. Renal events such as a rise in serum creatinine and/or blood urea nitrogen, change in eGFR, and need to start hemodialysis were comparable between both groups.90, 91 These RCT results have recently been confirmed in a real-world French cohort study.92 The combination of ritonavir-boosted paritaprevir with ombitasvir and dasabuvir (“PrOD” or 3D regimen) has been evaluated in a small single-arm study as well as in observational cohorts demonstrating excellent efficacy in patients infected with HCV GT1 and CKD G4 and G5.93 RBV may be required when using the PrOD regimen in patients infected with HCV GT1a. However, even with a reduced dose of 200 mg RBV daily, further dosing reduction was required in half of the treated patients despite the use of ESAs.94
Virological factors that may impact response to HCV therapy especially in GT1a-infected patients include the presence of resistance-associated variants.95 Resistance testing may not be available in some centers, and if use of RBV is not feasible due to baseline anemia, extension of therapy with grazoprevir/elbasvir to 16 weeks for patients infected with HCV GT1a should be considered. In HCV GT1a patients with high viral load (>800,000 IU/ml), prolonging duration of therapy to 16 weeks and the use of RBV, if possible, to avoid a reduction in SVR12 (from 99% with RBV to 88% without in 1 study) is suggested.96
In the RUBY II trial presented at the 2016 AASLD Annual Meeting, dialysis patients with HCV GT1a were treated with ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir, and those infected with GT4 were treated with the first 2 agents without dasabuvir. RBV was not included in the regimen. Of the 13 treated subjects, 12 achieved SVR (92%). The remaining patient who discontinued antiviral therapy elected to undergo kidney transplantation.97 All components of the combination regimen containing ombitasvir, paritaprevir, ritonavir, and dasabuvir (used in GT1 and without dasabuvir in GT4) are predominantly excreted in the feces, with <11% renal clearance; thus, pharmacokinetics are not significantly altered in advanced CKD (CKD G4–G5), and no dose adjustment is recommended. In a single-arm, multicenter study of treatment-naïve adults with HCV GT1 infection without cirrhosis and with CKD G4 or G5, 20 patients were treated with this regimen for 12 weeks. Patients with HCV GT1a infection also received RBV (n = 13), whereas those with GT1b infection did not (n = 7). Eighteen of the 20 patients achieved SVR12 (90%; 95% CI: 69.9–97.2), but 1 treatment failure was nonvirological (death after the end of the treatment unrelated to the treatment). The only patient who relapsed was a GT1-infected patient with advanced liver fibrosis on hemodialysis. Adverse events were primarily mild or moderate, and no patient discontinued treatment due to an adverse event. RBV therapy was interrupted in nine patients due to anemia; 4 received ESAs. No blood transfusions were required.94
Similar to other protease inhibitors (simeprevir and paritaprevir), grazoprevir is contraindicated in decompensated patients with Child-Turcotte-Pugh class B or C due to diminished hepatic metabolism and risk of adverse event, particularly hepatic toxicity.
In practice, no dose adjustment for kidney function is needed with NS5A inhibitors such as daclatasvir and protease inhibitors such as simeprevir.
Prior to the recent introduction of glecaprevir-pibrentasvir, a sofosbuvir-based regimen had been the only option for patients with CKD G4 and G5 infected with HCV GTs 2, 3, 5, and 6, particularly those with cirrhosis and those with a history of prior nonresponse to IFN-based therapies. However, the glecaprevir-pibrentasvir regimen is pan-genotypic, with no dose reduction necessary for diminished GFR. In the EXPEDITION-4 trial, which included 104 patients with CKD G4–G5 and HCV GTs 1–6 of whom 82% were receiving hemodialysis therapy,98 subjects received the combination of glecaprevir, a protease inhibitor, and pibrentasvir, an NS5A inhibitor, for 12 weeks. Forty-two percent of subjects had been treated previously, including 2 who had received sofosbuvir-based therapy; 19% of patients had compensated cirrhosis. SVR12 was 98%; of the 2 patients who did not achieve SVR, 1 received only 4 weeks of therapy and the other died of an unrelated cause shortly after completion of therapy. Detection of resistance-associated variants, present in 29% of subjects, did not impact SVR, although HCV GT 3 patients with prior therapy failure had been excluded from inclusion.
We recognize that preferred regimens such as grazoprevir-elbasvir and glecaprevir-pibrentasvir for CKD G4–G5D patients may not be available in some countries or regions, and sofosbuvir-based regimens may be all that is available despite the fact that they are not licensed for use in CKD G4–G5D patients. Sofosbuvir undergoes extensive hepatic metabolism and is biotransformed to the pharmacologically active nucleotide analog uridine-triphosphate (SOF-007TP) which, once dephosphorylated, results in the formation of the predominant sofosbuvir inactive metabolite GS-331007 (SOF-007). SOF-007 is mainly eliminated through the renal route, and the 4-hour hemodialysis extraction ratio is about 53%.99 For creatinine clearance (CrCl) < 30 ml/min, pharmacokinetics data showed marked plasma overexposure of sofosbuvir (AUC0-IFN 171% higher), and particularly SOF-007 (AUC0-IFN 451% higher) after a single dose of 400 mg, as compared with subjects with normal kidney function.100
Despite these pharmacokinetics studies, there are preliminary data with sofosbuvir-based regimen in CKD patients suggesting that sofosbuvir with a daily or 3-times weekly regimen is safe and well tolerated in HCV-infected patients, most with cirrhosis, who require hemodialysis.100, 101, 102, 103, 104, 105, 106, 107 In a recent prospective study, 2 dosing regimens, sofosbuvir full dose (400 mg daily, n = 7) and 3 times a week (n = 5) after hemodialysis with simeprevir, daclatasvir, ledipasvir, or RBV, were compared in hemodialysis patients.105 While both groups showed higher SOF-007 plasma concentrations than those previously reported in patients with normal kidney function, plasma concentrations of sofosbuvir or its inactive metabolite SOF-007 did not accumulate with either regimen between hemodialysis sessions or throughout the treatment course.
Additional experience with reduced sofosbuvir doses, such as 200 mg daily or 400 mg 3 times weekly, suggests that while very well tolerated, these suboptimal doses may lead to inferior SVR rates. In one study, Gane et al. presented results for 10 patients with advanced CKD (9 infected with HCV GT1 and 1 with HCV GT3, all with CrCl < 30 ml/min) receiving sofosbuvir, 200 mg daily, combined with RBV, 200 mg daily.100 This schedule resulted in 6 relapses in HCV GT1-infected patients. In 2 case reports, Perumpail et al. reported the successful treatment of 2 liver transplant patients on hemodialysis therapy who received sofosbuvir, 200 mg and 400 mg daily, respectively, with simeprevir at standard dose.103, 104 Bhamidimarri et al.106 evaluated 2 different schedules in 15 patients with advanced CKD (n = 3) or requiring hemodialysis (n = 12). Eleven patients received sofosbuvir, 200 mg daily, and 4 patients received sofosbuvir, 400 mg 3 times weekly, all with simeprevir at a standard dose. Two relapses occurred, one in each group. Finally, preliminary results from another case series in 11 patients requiring hemodialysis receiving sofosbuvir, 400 mg daily, and simeprevir reported no relapse.102 Very recently, a larger study (n = 50) also suggested that sofosbuvir-based antiviral therapy, with a reduced dose of sofosbuvir, is reasonably safe and effective for the treatment of HCV patients with ESKD, including hemodialysis patients.108
Use of full-dose off-label use of sofosbuvir daily has been reported in HCV patients on dialysis and in those at high risk of treatment failure such as those with cirrhosis, previously pretreated or nonresponders and those infected with GT3. Such patients should be closely monitored, with clinical, biological, and cardiac assessment.109
A related and unresolved issue is whether use of sofosbuvir in patients with advanced CKD may accelerate its progression. Most of the studies that examined this issue were conducted in patients with moderate CKD. Gonzalez-Parra and colleagues110 observed a significant mean decrease in GFR of 9 ml/min per 1.73 m2 in 35 patients treated with a sofosbuvir-based regimen with a baseline GFR of 30 to 60 ml/min per 1.73 m2, whereas no significant decline in GFR occurred in 8 patients treated with the PrOD regimen. Rosenblatt et al.111 also reported that in a series of 90 patients, a baseline CrCl < 60 ml/min predicted a decline in kidney function with sofosbuvir therapy. Saxena et al. also observed a decline in kidney function in 73 patients with a baseline eGFR ≤ 45 ml/min per 1.73 m2 treated with sofosbuvir.107 Mallet et al.,112 in a retrospective study of 814 HCV patients mostly with baseline eGFR ≥ 60 ml/min per 1.73 m2, reported a mean eGFR decrease of 2.6 and 1.7 ml/min per 1.73 m2 over a maximum of 37 months in patients treated with sofosbuvir-based and non–sofosbuvir-based regimens, respectively. In contrast, Sise et al.113 recently reported that in patients with CKD G3a–G3b who received sofosbuvir-based regimens, HCV cure was associated with a 9.3 ml/min per 1.73 m2 improvement in eGFR during the 6-month post-treatment follow-up period. Despite these conflicting findings, if a sofosbuvir-based regimen is selected, monitoring of kidney function should be performed with serial serum creatinine measurements during therapy, although it is unclear whether dose reduction or withdrawal is indicated if GFR declines further.
Algorithm 1 summarizes the recommended choice of DAAs according to the level of kidney function and HCV GT. The Work Group recognizes that not all preferred regimens are available in all jurisdictions, and as such we have also recommended alternate regimens to provide further potential treatment options. There is no evidence to support specific DAA regimens in patients on peritoneal dialysis, but it is reasonable to follow guidance for patients on hemodialysis.114
Algorithm 1.
Treatment scheme for chronic kidney disease (CKD) G1–G5D. Recommendation grading is provided for each specific treatment regimen and hepatitis C virus (HCV) genotype. CKD G, chronic kidney disease, GFR category; DAA, direct-acting antiviral; GFR, glomerular filtration rate; NAT, nucleic acid testing.
In summary, we recommend that patients with CKD G4–G5 and G5D be treated with a RBV-free DAA-based regimen. Glecaprevir-pibrentasvir has pan-genotypic efficacy including in patients with prior sofosbuvir treatment and cirrhosis. Grazoprevir-elbasvir and the PrOD regimen are also approved for use in CKD G4–G5 and G5D patients with GTs 1 and 4. Although there are studies reporting the use of sofosbuvir in patients with CKD G4–G5D, in jurisdictions where there is availability of well-tolerated regimens (i.e., grazoprevir-elbasvir and glecaprevir-pibrentasvir), its use is not recommended given the limited information about its safety in this population. Our guidance is in general concordance with those provided by AASLD (https://www.hcvguidelines.org/unique-populations/renal-impairment) and EASL (http://www.easl.eu/research/our-contributions/clinical-practice-guidelines), but given that recommended drugs and dosage are constantly evolving, clinicians should consult these resources for the most up-to-date treatment information.
Kidney transplant recipients: CKD G1T–G5T (see also Chapter 4)
Although published data on DAAs in kidney transplant recipients are less abundant,115 the study results seem as satisfactory as those observed in liver transplant recipients (Supplementary Tables S7 and S8). In a recent trial comparing 12 and 24 weeks of sofosbuvir and ledipasvir in 114 kidney transplant recipients infected with HCV GTs 1 and 4 (96% GT1) with an eGFR of 40 ml/min per 1.73 m2 or greater (median eGFR 56 ml/min per 1.73 m2), the therapy was very well tolerated, and SVR rates were close to 100% without differences between arms, suggesting that a 12-week regimen is also indicated in kidney transplant recipients.116 Smaller cohort studies recently also reported excellent results in kidney transplant recipients with sofosbuvir-based regimens.117, 118, 119 Sofosbuvir/velpatasvir has also been shown to be highly effective and well tolerated in liver transplant recipients with GTs 1–4 and may be considered for kidney transplant recipients in the future, although at the present, efficacy and safety data for the latter group are lacking.120 Reau et al.3 have recently described the use of glecapravir/pibrentasvir in 100 organ transplant recipients, 20 of whom had received a kidney transplant with high SVR and excellent tolerability.
In transplant recipients, drug–drug interactions with immunosuppressive agents may result in increased or diminished plasma levels of immunosuppressive agents, with consequent risk of toxicity or graft rejection, respectively. For instance, concurrent use of elbasvir-grazoprevir and cyclosporine is not recommended, as it results in a 15-fold increase in grazoprevir AUC and 2-fold increase in elbasvir AUC. Elbasvir-grazoprevir increases levels of tacrolimus by 43%; thus, close monitoring of levels is indicated, and dose reductions of tacrolimus may be needed. Other protease inhibitors such as simeprevir and paritaprevir have similar drug–drug interactions with cyclosporine, tacrolimus, and everolimus. There are no significant drug–drug interactions with these protease inhibitors and mycophenolate mofetil (MMF). No significant interactions between NS5A and polymerase inhibitors such as sofosbuvir and calcineurin inhibitors (CNIs) have been described, but close monitoring of immunosuppressive drugs is mandatory because changes in liver metabolism concurrent with HCV eradication may require modification of immunosuppressive drug doses.
Overall, drug–drug interactions are an important factor in the choice of a DAA regimen. Protease inhibitors are associated with significant risk for drug–drug interactions, particularly in patients who are treated with immunosuppressive agents such as CNIs and mTOR inhibitors.93, 121 Nonstructural protein 5B (NS5B) inhibitors such as sofosbuvir or NS5A inhibitors such as ledipasvir and daclatasvir are associated with a low risk of drug–drug interaction with CNIs and mTOR inhibitors, but may have interactions with other concomitant medications. The Hepatitis Drug Interactions website from the University of Liverpool (http://www.hep-druginteractions.org) or another reliable expert source should be accessed to determine the risk and management recommendations for drug–drug interactions.
Waiting times for deceased donor kidney transplantation are very long in many parts of the world, and many transplant candidates die while waiting for a deceased donor transplant. (see Chapter 4). Survival after transplantation is generally better than survival on dialysis including for HCV-infected patients. With access to DAA, it may be better to receive a kidney transplant from an HCV-positive donor than to face a long wait for an HCV-negative kidney. It has been suggested that an HCV-positive transplant candidate should forego treatment of HCV until after kidney transplantation, to allow receipt of a kidney transplant from an HCV-positive deceased donor. Adoption of this strategy would expand the deceased donor organ pool as well as diminish wait times as suggested by Kucirka et al.122
If an HCV-negative transplant candidate has a potential living donor who is HCV NAT–positive, then it seems reasonable for the donor to be treated for HCV, and donate the kidney after SVR has been achieved. Because the probability of SVR is very high, and the time it takes to achieve SVR is only 12 weeks, this strategy makes intuitive sense even if there are no supporting data. The potential donor also requires careful evaluation of severity of liver disease. Another consideration is the use of a kidney from an HCV NAT–positive donor in an HCV-negative recipient with prompt DAA treatment after transplant, as recently reported by Goldberg et al.123 and Durand et al.124 in 2 encouraging small case series. This approach requires further study before it can be endorsed.
In summary, kidney transplant recipients with GFR ≥ 30 ml/min per 1.73 m2 (CKD G1T–G3bT) and HCV GTs 1 or 4 can utilize sofosbuvir-based regimens and glecaprevir-pibrentasvir. For those with HCV GTs 2, 3, 5, and 6, we recommend glecaprevir-pibrentasvir. For kidney transplant recipients with GFR < 30 ml/min per 1.73 m2 (CKD G4T–G5T), the same regimens proposed for patients with CKD G4–G5D apply (i.e., grazoprevir-elbasvir for GTs 1 and 4 and glecaprevir-pibrentasvir for all GTs). Our guidance is in general concordance with those provided by AASLD (https://www.hcvguidelines.org/unique-populations/kidney-transplant) and EASL (http://www.easl.eu/research/our-contributions/clinical-practice-guidelines), but given that recommended drugs and dosage are constantly evolving, clinicians should consult these resources for the most up-to-date treatment information. Algorithm 2 summarizes the recommended choice of DAAs for kidney transplant recipients according to the level of kidney function and HCV GT.
Algorithm 2.
Treatment scheme for kidney transplant recipients (KTRs). Recommendation grading is provided for each specific treatment regimen and hepatitis C virus (HCV) genotype. Chronic kidney disease (CKD) G, CKD glomerular filtration rate (GFR) category (suffix T denotes transplant recipient); NAT, nucleic acid testing.
Reactivation of HBV infection after DAA therapy
A number of reports have recently described apparent reactivation of HBV infection in individuals following successful therapy of HCV infection with DAA-based therapy.125, 126 This has prompted an FDA warning.127 As part of routine evaluation of patients with HCV and CKD, serum markers of HBV infection (i.e., hepatitis B surface antigen [HBsAg) and HBV DNA) should be obtained prior to antiviral therapy. Initiation of therapy with an oral HBV suppressive agent is recommended if criteria for HBV therapy are met, based on initial testing prior to HCV therapy or during follow-up after HCV. If HBsAg is initially absent but markers of prior HBV infection (positive antibody to hepatitis B core antigen [HBcAb-positive] with or without antibody to hepatitis B surface antigen [HBsAb]) are detected, patients should be monitored for HBV reactivation with serial HBV DNA and liver function tests during DAA therapy (see also https://www.hcvguidelines.org/evaluate/monitoring).
Research recommendations
-
•
Further studies should be conducted on whether RBV is required after kidney transplantation in some specific groups such as prior nonresponders infected with HCV GT1a. Treatment of NS5A-resistant variants after kidney transplantation should also be evaluated.
-
•
Optimal timing of antiviral therapy before or after transplantation in candidates for kidney transplantation should be clarified. Because the time to transplantation with kidneys from deceased donors is unpredictable, delaying treatment carries higher vascular, metabolic, and malignancy risks as well as the risk of drug–drug interactions with CNIs after transplantation. As such, treatment before transplantation may be more appropriate. However, in regions where the prevalence of anti-HCV–positive donors is high, post-kidney transplant therapy should be considered.
-
•
Use of organs from HCV-positive donors for HCV-negative recipients with DAA therapy needs to be further explored.
-
•
The impact of treating HCV infection on CKD progression should be further investigated.
Chapter 3: Preventing HCV transmission in hemodialysis units
- 3.1: We recommend that hemodialysis facilities adhere to standard infection control procedures including hygienic precautions that effectively prevent transfer of blood and blood-contaminated fluids between patients to prevent transmission of blood-borne pathogens (see Table 1) (1A).
- 3.1.1: We recommend regular observational audits of infection control procedures in hemodialysis units (1C).
- 3.1.2: We recommend not using dedicated dialysis machines for HCV-infected patients (1D).
- 3.1.3: We suggest not isolating HCV-infected hemodialysis patients (2C).
- 3.1.4: We suggest that the dialyzers of HCV-infected patients can be reused if there is adherence to standard infection control procedures (2D).
- 3.2: We recommend that hemodialysis centers examine and track all HCV test results to identify new cases of HCV infections in their patients (1B).
- 3.2.1: We recommend that aggressive measures be taken to improve hand hygiene (and proper glove use), injection safety, and environmental cleaning and disinfection when a new case of HCV is identified that is likely to be dialysis-related (1A).
3.3: Strategies to prevent HCV transmission within hemodialysis units should prioritize adherence to standard infection control practices and should not primarily rely upon the treatment of HCV-infected patients (Not Graded).
Rationale
The prevalence of HCV infection in hemodialysis patients is usually higher than in the general population.128 HCV prevalence rates range from about 4%–9% in most high-income countries, but is significantly higher in other countries, particularly those in the Middle East, North and Sub-Sahara Africa, Asia, and Eastern Europe16, 129, 130, 131 (Table 2). Rates also vary during times of social crisis, war, or economic downturn.132, 133, 134 According to a recent systematic review of studies in hemodialysis patients based on data up to 2006, the overall global incidence rate of HCV infection was 1.47 per 100 patient-years: 4.44 per 100 patient-years in low- to middle-income countries, and 0.99 per 100 patient-years in high-income countries.135
Table 2.
Recent reported HCV prevalence in hemodialysis patients
| Country | N | Year of testing | HCV prevalence (%) | Source |
|---|---|---|---|---|
| Australia-New Zealand | 393 | 2012 | 3.8 | DOPPS 5147 |
| Belgium | 485 | 2012 | 4.0 | DOPPS 5147 |
| Brazil | 798 | 2011 | 8.4 | Rodrigues de Freitas148 |
| Canada | 457 | 2012 | 4.1 | DOPPS 5147 |
| China | 1189 | 2012 | 9.9 | DOPPS 5147 |
| Cuba | 274 | 2009 | 76 | Santana149 |
| Egypt | – | 2007–2016 | 50 | Ashkani-Esfahan149a |
| France | 501 | 2012 | 6.9 | DOPPS 4147 |
| Germany | 584 | 2012 | 4.5 | DOPPS 5147 |
| Gulf Cooperation Council | 910 | 2012 | 19.3 | DOPPS 5147 |
| India | 216 | 2012 | 16 | NephroPlus |
| 1050 | 2013 | 11 | ||
| 3068 | 2014 | 8 | ||
| Iran | – | 2006–2015 | 12 | Ashkani-Esfahan149a |
| Iraq | – | 2008–2015 | 20 | Ashkani-Esfahan149a |
| 7122 | 2015 | 10 | ||
| 7673 | 2016 | 9 | ||
| Italy | 485 | 2012 | 11.5 | DOPPS 5147 |
| Japan | 1609 | 2012 | 11.0 | DOPPS 5147 |
| Jordan | – | 2007–2015 | 35 | Ashkani-Esfahan149a |
| Lebanon | 3769 | 2010–2012 | 4.7 | Abou Rached150 |
| Libya | 2382 | 2009–2010 | 31.1 | Alashek151 |
| Nigeria | 100 | 2014 | 15 | Ummate152 |
| Palestine | – | 2010–2016 | 18 | Ashkani-Esfahan149a |
| Romania | 600 | 2010 | 27.3 | Schiller153 |
| Russia | 486 | 2012 | 14.0 | DOPPS 5147 |
| Saudi Arabia | – | 2007 | 19 | Ashkani-Esfahan149a |
| Senegal | 106 | 2011 | 5.6 | Seck154 |
| Syria | – | 2009 | 54 | Ashkani-Esfahan149a |
| Spain | 613 | 2012 | 8.9 | DOPPS 5147 |
| Sweden | 426 | 2012 | 6.0 | DOPPS 5147 |
| Turkey | 383 | 2012 | 7.0 | DOPPS 5147 |
| United Kingdom | 397 | 2012 | 4.6 | DOPPS 5147 |
| United States | 2977 | 2012 | 7.3 | DOPPS 5147 |
DOPPS, Dialysis Outcomes and Practice Patterns Study; HCV, hepatitis C virus.
HCV is easily transmitted parenterally, primarily through percutaneous exposure to blood. Dramatic reductions were noted in the incidence following introduction of screening for HCV in blood donors and reduction in blood transfusion requirements following introduction of ESAs,136 leaving nosocomial transmission as the main method of spread of HCV in dialysis units. Several studies have confirmed nosocomial transmission in dialysis units using epidemiologic and phylogenetic data obtained by viral sequencing.21, 34, 137, 138, 139, 140 These data are further supported by the observation of decline in infection rates following routine implementation of infection control practices and virological follow-up to detect anti-HCV using sensitive, specific new-generation serological tests.17, 141 A multicenter survey revealed that prevalence of anti-HCV positivity for a Belgian cohort of hemodialysis patients (n = 1710) dropped steadily from 13.5% in 1991 to 6.8% in 2000, and the same survey revealed significant drops in other European countries including France (42% to 30%), Italy (28% to 16%), and Sweden (16% to 9%).141 Table 2 provides an overview of HCV prevalence in hemodialysis patients as summarized from some recent studies.
Nevertheless, more than 50% of all health care–associated HCV outbreaks from 2008 to 2015 reported to the CDC occurred in hemodialysis settings.142 As a result, the CDC recently provided guidance on improving infection control practices to stop HCV transmission in dialysis units.143
Infection control
Infection control lapses responsible for HCV transmission contribute to transmission of other pathogens; hence implementation of improvement efforts will have broader salutary effects. Most importantly, HCV transmission can be prevented effectively through adherence to currently recommended infection control practices. There are no reports of transmission of HCV in dialysis units that had all infection control practices in place. Publication bias is unlikely to explain this observation. Additionally, in the experience of the authors, centers that have had HCV transmission identified and that subsequently responded with increased attention to appropriate infection control practices have not had continued transmission. This observation applies to unpublished outbreaks and transmission events.
Three systematic reviews have examined the reasons behind transmission of HCV in hemodialysis units.34, 140, 144 Root cause analysis of confirmed nosocomial outbreaks22, 29, 31, 145, 146 has revealed lapses in infection control to be associated with transmission of HCV infection between patients in dialysis units. For several reasons, including the long latency period of HCV infection, the number of dialysis treatments occurring during a patient’s likely exposure period (based on multiple treatments per week), and sparse documentation of details in the dialysis treatment record, retrospective investigation to determine an exact cause of dialysis-related HCV acquisition is challenging. Rarely, the exact cause can be surmised using epidemiologic and molecular virology data. More often, transmission is documented among patients in the same clinic, who lack other common exposures and/or risk factors, and lapses in infection control are identified in the clinic that could logically lead to transmission (Table 3). Other causes of infection such as undergoing dialysis during travel to developing countries, and nondialysis health care exposures (e.g., procedures performed in a common vascular access surgical center) can occur and are considered before concluding that transmission occurred in the dialysis unit.
Table 3.
Factors and lapses in infection control practices associated with transmission of HCV infection in dialysis units
|
|
|
|
|
|
|
|
HCV, hepatitis C virus.
Mishandling of parenteral medications has been implicated frequently in transmission. Medication vials can become contaminated with HCV when accessed with used needles or syringes, or through environmental or touch contamination of the vial diaphragm by health care personnel hands. The US CDC’s One & Only Campaign on safe injection practices (http://www.oneandonlycampaign.org/) should help address the former issue by promoting single use of syringes. The latter issue concerning contamination is more likely to occur when medications are stored or prepared in contaminated areas and blood-contaminated items are handled in close proximity. Sharing of multidose heparin or other medication vials or spring-triggered devices for glucose monitoring can lead to transmission. Inadequate cleaning and disinfection of shared environmental surfaces also increases risk of transmission. This may include failure to adequately clean and disinfect external surfaces of hemodialysis machines, treatment chairs, and other surfaces in the treatment station, and failure to clean blood spills.
It should be emphasized that blood contamination of environmental surfaces and equipment both at the patient treatment station and outside the immediate treatment area can be present, even in the absence of visible blood. HCV RNA has been detected on external surfaces of dialysis machines, a dialysate connector, on a shared waste cart, and in hand washings of dialysis personnel.155, 156, 157, 158, 159, 160, 161 Blood that is visible or not visible to the naked eye, as evidenced by chemical tests, has also been detected on dialysis treatment station surfaces that underwent routine cleaning procedures following an outbreak of HCV.21 HCV can persist in an infectious state for at least 16 hours, and potentially much longer, on surfaces at room temperature.160, 162 Hand hygiene also plays an important role in prevention of nosocomial transmission.163 Lack of adherence to standard practices, such as hand-washing and glove use and removal practices, has been documented in several audits. In most HCV outbreaks in US hemodialysis centers reported to the CDC, multiple lapses in infection control were identified, involving practices such as hand hygiene and glove use, injectable medication handling, and environmental surface disinfection.142
Petrosillo et al.164 conducted a multicenter study in 58 Italian hemodialysis centers and found that the adjusted risk of transmission was correlated with dialysis in units with a high prevalence of HCV-infected patients at baseline and those with a low personnel-patient ratio. A study of 87 US hemodialysis centers similarly found that baseline HCV prevalence of greater than 10%, low staff-to-patient ratio, and ≥2-year duration of treatment in the facility were independently associated with frequency of HCV infections that were likely to be acquired in the facility.165
Implementation of infection control practices can be advanced by establishing a list of evidence-based interventions, such as those recommended by the CDC, and regularly assessing and reinforcing adherence to practice through observational audits. Infection control practices that may be most critical to improve (based upon observation of breaches in outbreak situations that are likely to transmit HCV) are shown in Table 1. The CDC has checklists and audit tools to assist facilities in implementing and assessing many of these practices.166
Isolation
Isolating HCV-infected patients (or patients awaiting HCV screening results) during hemodialysis is defined as physical segregation from others for the express purpose of limiting direct or indirect transmission of HCV. The traditional definition of contact isolation is that used for HBV infections in hemodialysis centers (i.e., dedicated room, machine, equipment, gowns, and personnel). However, “isolation” as considered for HCV control has involved multiple varied approaches and policies, including the use of a dedicated dialysis machine, personnel, room, or shift, and/or other barrier precautions (e.g., aprons, gowns, or gloves) by health care professionals attending these patients.
Whereas the complete isolation of HBV-infected patients (by room, thus including machine, equipment, and staff) has proven invaluable in halting the nosocomial transmission of HBV within hemodialysis units,167 there are multiple reasons that argue against recommending isolation of HCV-positive patients:168
-
(i)
Isolation purely for HCV will have no impact on transmission of other infections. Segregation of patients can create a false sense of reassurance around practices that could easily result in bloodstream infections (BSIs) or transmission of multi-drug resistant organisms or other blood-borne pathogens.
-
(ii)
Segregating patients on the basis of HBV and HCV would create four separate cohorts, which creates a significant logistic challenge. The treatment of HCV infection in dialysis patients raises an additional logistical difficulty of how to cohort patients undergoing therapy.
-
(iii)
Isolating only on HCV infection status may expose the isolated patient to infection with a second HCV GT.
-
(iv)
HCV seroconversion may be delayed for several months in newly infected hemodialysis patients and serological testing cannot be relied on to exclude recent infection.169
-
(v)
Starting and maintaining isolation is likely to impose large costs on already expensive dialysis programs.
The evidence for the use of isolation of HCV-infected patients during hemodialysis is weak, based on very low-quality evidence (Supplementary Tables S9 and S10). The KDIGO 2008 HCV guideline34 stated that hemodialysis units should ensure implementation of and adherence to strict infection control procedures designed to prevent transmission of blood-borne pathogens, including HCV, but isolation of HCV-infected patients was not recommended as an alternative to strict infection control procedures (unless in cases of continued health care–acquired transmission, where a local isolation policy may be deemed necessary).
A recent Cochrane review170 examined the impact of isolation as a strategy for controlling transmission of HCV infection in hemodialysis units. Of the 123 full-text articles identified, the authors could find only 1 randomized controlled trial (RCT).171 This cluster RCT included a total of 12 hemodialysis centers (593 patients) assigned to either dedicated hemodialysis machines for HCV-infected patients or no dedicated machines. Two follow-up periods were included in the study, and each was 9 months long. Staff was educated on standard infection control practices. Although the original article reported a significant reduction in the proportion of new infections in the second follow-up period among the facilities using dedicated versus nondedicated machines (calculated using chi-square test), based on a more standard risk ratio analysis, the Cochrane review concluded that the use of dialysis machines dedicated for HCV-infected individuals, as compared with the use of nondedicated machines made no difference in terms of reducing the incidence of HCV infection during the follow-up period. In addition, the quality of evidence was rated as “very low” due to several methodological issues.
Other studies examining isolation as a means of reducing HCV transmission reported a reduction of transmission, but they were observational and had very poor-quality evidence with methodological challenges.172, 173, 174 The isolation policies studied included implementing the isolation or cohorting of infected patients in a separate room; using exclusive machines; or employing dedicated machines, room, and staff. Most studies have adopted a “before-and-after” design, and compared their results with their own historical controls.175, 176, 177, 178 Thus, it is unclear whether the reported improvement resulted from the isolation policy or rather from the simultaneous raising of awareness and reinforcement of the application of hygienic precautions. Furthermore, in some studies, there might be other contributing factors such as changes in baseline prevalence and injection safety and hygienic practices over time.
In contrast to these studies, a DOPPS (Dialysis Outcomes and Practice Patterns Study) multicenter study and an Italian multicenter study both concluded that isolation did not protect against transmission of HCV in hemodialysis patients,16, 164 and some prospective observational studies have shown reduction of transmission after adoption of universal precautions.179 A prospective observational study showed a reduction in the annual incidence of HCV seroconversion from 1.4% to 0% after the reinforcement of basic hygienic precautions, without any isolation measures.180
The CDC does not recommend the isolation of HCV-infected patients in its infection-prevention guidelines.23 The UK Renal Association also states that patients with HCV do not need to be dialyzed in a segregated area; however, more experienced staff should be assigned. They further recommend that if nosocomial transmission continues to occur despite reinforcement and audit of the precautions, a local segregation policy may be deemed necessary.181 The European Best Practice Work Group considers implementation of universal hygienic measures to be the standard of care.182
Finally, several experts and guidelines acknowledge that because transmission can be effectively prevented by adherence to currently recommended practices, considering isolation of seropositive patients indicates a failure of adherence to the current standard and would have a negative impact on the implementation and reinforcement of basic hygienic measures in the unit as a whole.
Dedicated dialysis machines
Evidence of HCV transmission through internal pathways of the modern single-pass dialysis machine has not been demonstrated.34 Transmission would require the virion to cross the intact dialyzer membrane, migrate from the drain tubing to the fresh dialysate circuit, and pass again through the dialyzer membrane of a second patient. However, the virus does not cross the intact membrane, and even in the event of a blood leak, transmission would require HCV to reach fresh dialysate used for a subsequent patient and enter the blood compartment for that patient through back-filtration across the dialyzer membrane, a highly unlikely scenario. Almost all the studies included in the various systematic reviews have conclusively excluded transmission via the internal dialysis pathway. In a few cases, a role for the dialysis circuit could not be excluded, but the environmental surfaces are more likely to have contributed to transmission.21
Receiving dialysis next to, rather than sharing the same dialysis machine with, an HCV-infected patient has been found to be a risk factor for HCV acquisition.183 In outbreak investigations with phylogenetic viral sequencing analysis, transmission is sometimes documented from an infected patient to a subsequent patient treated at the same station on the next shift, and also from an infected patient to patients treated in nearby stations during the same or subsequent shifts, which indicates transmission independent of the machine. Hurried and incomplete disinfection of external machine surfaces and other surfaces at the station (e.g., side table, dialysis chair, blood pressure cuff, or prime waste container) are lapses commonly identified in these outbreaks. In some investigations, transmission involving the dialysis machine was essentially ruled out.137 In several studies included in the systematic reviews of HCV transmission, nosocomial spread was documented despite the existence of a policy of dedicated machines. Taken together, this information confirms that contamination of dialysis machine components cannot be the sole contributor to transmission, and may have little to no role in HCV spread. While contaminated external surfaces of dialysis machines might facilitate HCV spread, other surfaces in the dialysis treatment station are likely to have the same impact, diminishing the purported value of using dedicated machines. Similar to the concern about the risks of isolating dialysis patients with HCV, it should be stressed that using dedicated machines may trigger the perception that there is no longer a risk of nosocomial HCV transmission and thus reduce the attention devoted by hemodialysis staff members to body fluid precautions.
Reuse
During the reuse procedure, patient-to-patient transmission can take place if the dialyzers or blood port caps are switched between patients and not sterilized effectively or if there is spillage of contaminated blood or mixing of reused dialyzers during transport. These situations can be eliminated by adherence to standard hygienic precautions and appropriate labeling. Two large studies have not identified reuse as a risk factor for HCV transmission,180, 184 whereas a weak association was shown in 1 study, likely due to unmeasured confounders.185
Management of a dialyzer membrane defect leading to blood leak
As HCV is transmitted by percutaneous exposure to blood from an infected person, effective implementation of the dialysis precautions recommended in the 2008 KDIGO HCV guideline34 and by the CDC should prevent nosocomial transmission. The risk that the virus leaving the dialyzer could be trapped in the Hansen connector and transferred to the fresh dialysate side through accidental misconnection is vanishingly low, hence the CDC does not recommend disinfection of “single-pass” machines between treatments on the same day, even when a blood leak has occurred.23 The 2008 KDIGO HCV guideline, however, recommends disinfection of both the internal fluid pathways and the Hansen connectors before the next patient if a leak has occurred as a matter of abundant caution, and justified it based on the rarity of such events34 (Table 4). We reaffirm our previous recommendation.
Table 4.
Hygienic precautions for hemodialysis (dialysis machines)
Definitions
|
| Transducer protectors |
|
External cleaning
|
| Disinfection of the internal fluid pathways |
|
Audits
Audits and use of surveillance data to implement prevention steps are critical to any infection control program. Routine observational audits of various infection control practices, combined with feedback of results to clinical staff, allows for regular assessment of actual practices and identification of gaps. Data from audits can facilitate immediate interventions to correct practice and should also inform broader quality improvement efforts, including unit-wide staff education and retraining. In the US, most dialysis centers use infection control audit tools (including tools developed by the CDC or the dialysis company) as part of their continuous quality improvement process.
Although there are no RCTs that examined the impact of audits on transmission of HCV infection in dialysis units, observational studies as part of quality improvement programs have shown reduction in the rates of BSIs following implementation of regular audits and an evidence-based intervention package. In a study from the US, 17 centers reported monthly event and denominator data to the National Healthcare Safety Network and received guidance from the CDC. The feedback included advice on chlorhexidine use for catheter exit site care, staff training and competency assessments focused on catheter care and aseptic technique, hand hygiene and vascular access care audits, and feedback of infection and adherence rates to staff. Modeled rates decreased 32% (P < 0.01) for BSIs and 54% (P < 0.001) for access-related BSIs.186 In a follow-up study, the reduction in access-related BSI rates was sustained for 4 years after the initial intervention implementation.187 The over-representation of hospital-based centers and lack of a control group limit generalization of these data. However, the ongoing simplification of audit tools for ease of reporting with the use of information technology—as used in this study—precludes the need of infection control professionals on site, and leaves little justification to not recommend implementation of audits. Moreover, the scope of such audits goes beyond measuring 1 particular outcome, such as HCV transmission, and permits wider implementation of infection control measures.
Audits done in other dialysis center studies routinely show suboptimal adherence to hygienic practices. A Spanish study showed that gloves were used on 93% of occasions, and hands were washed only 36% of the time after patient contact and only 14% of the time before patient contact.188 In a 2002 US survey, only 53% of US outpatient ESKD facilities reported preparing injected medications in a dedicated room or area separated from the treatment area; 25% prepared these medications at a medication cart or other location in the treatment area, and 4% prepared medications at the dialysis station.184 A survey of 420 dialysis personnel from 45 facilities reported on hand hygiene practices and knowledge regarding HCV infection risk.189 At these facilities, percentages of dialysis staff reported to always wash their hands and change gloves during the following activities were: 47% when going from one patient treatment station to another, 55% between administering intravenous medications to different patients, and 57% immediately before starting patients on dialysis. Other studies have shown similar findings.
Observational audits of hygienic precautions that were carried out in outbreak investigations have identified a range of problems, including lack of basic hand hygiene, failure to change gloves when touching the machine interface, or when urgently required to deal with bleeding from a fistula; carrying contaminated blood circuits through the ward unbagged; lack of routine decontamination of the exterior of machines and other surfaces even when blood spillages had occurred; and failure to change the internal transducer protector when potentially contaminated. On the other hand, when hygienic practice was reviewed through interviewing staff after an outbreak rather than by observation, no obvious breaches in procedure could be identified.
The frequency at which routine audits of infection control procedures should be carried out will depend on audit type, staff turnover and training, and on the results of previous audits. When setting up a new program, audits should be at intervals of no greater than 6 months to enable staff to gain experience with the process and ensure that any remedial actions taken have been effective. The CDC recommends that audits be performed as often as monthly to establish and constantly reinforce recommended practices. Observational audits should be conducted on various days of the week and different shifts to capture all staff, and should include particularly busy times of day such as shift changes. These factors and the number of opportunities (e.g., for hand hygiene) and procedures (e.g., injectable medication administration) observed will determine the representativeness of the results.
The CDC website (http://www.cdc.gov/dialysis/prevention-tools/audit-tools.html) has a number of audit tools and checklists intended to promote CDC-recommended practices for infection prevention in hemodialysis facilities. The audit tools and checklists can be used by individuals when assessing staff practices. They can also be used by facility staff themselves to help guide their practices. In some centers, audit tools have been shared with patients, who are asked to assess staff practice as a means of engaging patients in the infection control efforts of the facility and improving the culture of safety in units.190 Patients should be educated on correct practices and should feel empowered to speak up when they observe a breach in hand hygiene or other staff practice.
It is known that hand hygiene practices improve when study participants are aware they are under observation. In one study, video monitoring of hand hygiene (performed via review of video surveillance footage) was shown to be a more accurate method than direct observation.191 Video surveillance for hand hygiene adherence should be considered, and other innovative approaches to monitoring staff adherence to recommended infection control practices should be explored.
Screening
Screening for HCV infection is essential to identifying transmission in hemodialysis units. The CDC recommends that all maintenance hemodialysis patients be screened for anti-HCV and ALT level upon admission and that anti-HCV testing be repeated semiannually and ALT testing be repeated monthly for susceptible patients.192 This is discussed in Chapter 1. Detection of seroconversions should prompt an aggressive evaluation of infection control practices to correct lapses and prevent additional cases from occurring (Table 5).28 Importantly, HCV screening should not be used as a substitute for regular infection control audits.
Table 5.
Steps to initiate concurrently and undertake following identification of a new HCV infection in a hemodialysis patient (adapted from CDC Health Alert28)
|
CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus.
Infrastructure requirements
Audit data show that despite the existence of guidelines to prevent transmission of infections in hemodialysis units, their implementation remains suboptimal, leading to a large preventable burden of infections that not only adversely impacts clinical outcomes, but imposes large costs on the health care system. Experience from public health interventions shows that interventions that depend on behavior change require large effort, which can undermine their impact. In contrast, making systemwide changes, such as imposition of regulations and creating an environment that discourages unhealthy behavior, is likely to have greater impact. This impact has been shown in many fields such as smoking cessation and containing HIV infection.193 Examples in the dialysis field include endorsement of dialysis event BSI measure by the US National Quality Forum, and implementation of the Medicare Quality Initiative. Recommendation of uniform validated measures such as those used by the National Healthcare Safety Network are critical for comparisons and to facilitate interventions. Other systemwide changes that are likely to have a beneficial impact on infection prevention and control practices include increasing staff-to-patient ratios and instituting staff training and education requirements. Physical infrastructure changes to facilities might also be beneficial—for example, establishing minimum space requirements between treatment stations, creating walls around individual treatment stations to establish separate rooms instead of large open spaces, and using walls to separate clean and dirty processes (e.g., separate room for medication preparation). Such possibilities should be explored, along with strategies to improve work flow and reduce unnecessary staff maneuvers that add to the already substantial number of occasions during dialysis care when glove change and hand hygiene are warranted. As such, regulatory and accrediting agencies should issue and/or incorporate recommendations to favor compliance with basic infection control practices in dialysis units, and efforts to make the desired infection control behavior the simplest or most logical approach to care processes should be pursued (Table 6). Table 7 provides a summary of important hygienic precautions for hemodialysis center staff to follow.
Table 6.
Strategies to support adherence to infection control recommendations in hemodialysis centers
|
The following are useful CDC and WHO informational resources to improve hand hygiene, environmental cleaning and disinfection, and injection safety:
|
CDC, Centers for Disease Control and Prevention; US, United States; WHO, World Health Organization.
Table 7.
Key hygienic precautions for hemodialysis staffa
Definitions
|
| Education |
|
Hand hygiene
|
| Injection safety |
|
Equipment management(for management of the dialysis machine, seeTable 4)
|
Waste and specimen management
|
In addition to standard precautions.
Treatment of HCV infection as a means for prevention of transmission
With the availability of DAAs, there is a possibility that dialysis units might take recourse to starting HCV-infected patients on these agents with the hope that this will cure the infection and prevent transmission to uninfected patients. Several studies have shown that facility prevalence of HCV infection is associated with incidence of infection. Thus, it stands to reason that successful treatment of patients could reduce the likelihood of HCV spread in dialysis centers. However, it should be noted that transmission can occur even in centers with very low HCV prevalence.139 A study that modeled HCV transmission in hemodialysis centers found that HCV prevalence influenced incidence (as did staff-to-patient ratio), but the compliance rate with hand hygiene and glove change between patients was a much stronger determinant of transmission.163 Thus, even in the setting of low HCV prevalence, rigorous adherence to infection control practices is necessary. HCV prevention programs that focus solely on treatment of patients are likely to have a deleterious effect on observance of routine infection control practices, leading to paradoxically increased risk of transmission. Furthermore, reliance on HCV treatment to prevent transmission goes against the principle of treating patients primarily for their individual benefit. Use of treatment alone as an infection control measure might place patients at increased risk of HCV and other blood-borne infections from other sources.
Implementation issues
Despite such strong data, adherence to recommended practices remains suboptimal, often due to misconceptions of the dialysis staff. A survey of 420 dialysis personnel from 45 hemodialysis facilities showed that only 35% of dialysis personnel strongly believed that patients were at risk of acquiring HCV infection in the hemodialysis facility. In contrast, 46% strongly perceived themselves to be at risk of acquiring HCV infection through occupational exposure.189 Personnel also were much more likely to report knowing how to protect themselves from acquiring a blood-borne pathogen infection than knowing how to protect their patients. On the basis of their observational results, which included high compliance with glove use (93%) in contrast to poor hand hygiene compliance (36%), Arenas et al.188 similarly concluded that dialysis personnel had greater concern for patient-to-staff transmission and lacked awareness of their role in facilitating pathogen transmission to patients. These data support the need for improved training and education to address knowledge gaps, as well as other initiatives focused on optimizing adherence to recommended infection control practices (Table 7). As mentioned above, implementation is more likely when guidelines are accompanied by changes in regulations.
Research recommendations
-
•
Further observation studies should be conducted to ascertain features of facilities that do not have incident cases (e.g., staffing, physical layout, policies and practices, and baseline prevalence).
-
•
Large, multicenter long-term RCTs of good quality are required to answer the questions concerning the benefits and harms of isolating HCV-positive patients during hemodialysis. These studies should ideally evaluate costs, patient perceptions, and complications associated with isolation. These studies should ensure the physical separation of either the center or room, or separation by treatment shift; these programs should have strict isolation strategies in place that include staff. Studies should randomize centers to either the standard of care (i.e., efforts to adhere to recommended infection control practices) or the standard of care plus isolation; they should describe the infection control efforts and compliance rates in both sets of centers, and should ensure data assessors are blinded to the interventions. The above-suggested trials remain of interest because HCV therapies may not be universally available, affordable, or prioritized for all hemodialysis patient populations. In particular, we need innovative, effective strategies to improve infection control, and it is still important to overcome barriers to identification and treatment of all infected patients (e.g., costs and reimbursement for screening and treatment regimens) in hemodialysis centers; this has implications for improved infection control practices for other endemic and emerging infections even if HCV is eradicated from hemodialysis patient populations.
-
•
Studies should determine whether isolation of HCV-positive patients influences rates of transmission of HCV or other infections.
-
•
The costs and impact of improved facility staffing strategies, including higher staff-to-patient ratios, on HCV transmission should be further evaluated.
-
•
Future research should examine standard measures for detecting dialysis-associated HCV infection that do not require viral sequencing and phylogenetic analysis.
-
•
Future research should devise innovative approaches that accurately measure infection control processes at a reasonable cost.
Chapter 4: Management of HCV-infected patients before and after kidney transplantation
HCV infection remains more prevalent in CKD G5 patients compared with the general population. Although HCV infection can cause HCV-associated glomerular disease resulting in CKD G5D (ESKD),128, 194 kidney transplant candidates may also have acquired HCV infection within a dialysis unit195 or may have been infected when they had received a previous transplant or were transfused in the era before systematic screening for HCV.194, 196, 197 Because of the deleterious effects of HCV infection in dialysis and kidney transplant patients, evaluation of disease severity and need for antiviral therapy is crucial.198, 199, 200, 201, 202, 203, 204 Screening for HCV in kidney transplant candidates has been addressed in Chapter 1.
4.1 Evaluation and management of kidney transplant candidates regarding HCV infection
4.1.1: We recommend kidney transplantation as the best therapeutic option for patients with CKD G5 irrespective of presence of HCV infection (1A).
- 4.1.2: We suggest that all HCV-infected kidney transplant candidates be evaluated for severity of liver disease and presence of portal hypertension (if indicated) prior to acceptance for kidney transplantation (2D).
- 4.1.2.1: We recommend that HCV-infected patients with compensated cirrhosis (without portal hypertension) undergo isolated kidney transplantation (1B).
- 4.1.2.2: We recommend referring HCV-infected patients with decompensated cirrhosis for combined liver-kidney transplantation (1B) and deferring HCV treatment until after transplantation (1D).
- 4.1.3: Timing of HCV treatment in relation to kidney transplantation (before vs. after) should be based on donor type (living vs. deceased donor), wait-list times by donor type, center-specific policies governing the use of kidneys from HCV-infected deceased donors, HCV genotype, and severity of liver fibrosis (Not Graded).
- 4.1.3.1: We recommend that all HCV-infected patients who are candidates for kidney transplantation be considered for DAA therapy, either before or after transplantation (1A).
- 4.1.3.2: We suggest that HCV-infected kidney transplant candidates with a living kidney donor can be considered for treatment before or after transplantation according to HCV genotype and anticipated timing of transplantation (2B).
- 4.1.3.3: We suggest that if receiving a kidney from an HCV-positive donor improves the chances for transplantation, the HCV NAT–positive patient can undergo transplantation with an HCV-positive kidney and be treated for HCV infection after transplantation (2B).
Rationale
4.1.1: We recommend kidney transplantation as the best therapeutic option for patients with CKD G5 irrespective of presence of HCV infection (1A).
Several studies have shown that kidney transplantation is the best therapeutic option for patients with ESKD (Supplementary Tables S11 and S12). Survival is significantly greater in CKD G5 patients who have undergone kidney transplantation compared with those who have remained on the waiting list irrespective of recipient age and/or comorbidities.205, 206 As in the uninfected population, in patients with HCV it has also been clearly shown that survival is significantly lower in dialysis patients than in kidney transplant recipients.198, 207, 208 Thus, eligible patients should be considered for kidney transplantation regardless of their HCV status. In addition, the DAAs for HCV treatment in dialysis and kidney transplant patients (see Chapter 2) allow successful HCV clearance in nearly all patients before or after transplantation. Patients who achieve SVR before transplantation do not relapse after transplantation, despite the use of potent immunosuppressive drugs.209, 210
Although the survival of patients with persistent HCV replication after kidney transplantation is inferior compared with HCV-negative kidney transplant patients,200, 201, 204 it remains higher than if they had remained on dialysis.198, 207, 208 Graft survival is also significantly decreased in HCV-positive kidney transplant patients compared with HCV-negative patients (Supplementary Tables S13 and S14).200, 201, 202, 204, 211, 212 Although liver fibrosis progression in HCV-infected kidney transplant patients is variable, development of cirrhosis and hepatocellular carcinoma (HCC) has been reported.213, 214, 215, 216 As HCC typically develops only in HCV-infected patients with stage 3 or 4 fibrosis, surveillance for HCC should be offered if extensive fibrosis is present.
- 4.1.2: We suggest that all HCV-infected kidney transplant candidates be evaluated for severity of liver disease and presence of portal hypertension (if indicated) prior to acceptance for kidney transplantation (2D).
- 4.1.2.1: We recommend that HCV-infected patients with compensated cirrhosis (without portal hypertension) undergo isolated kidney transplantation (1B).
- 4.1.2.2: We recommend referring HCV-infected patients with decompensated cirrhosis for combined liver-kidney transplantation (1B) and deferring HCV treatment until after transplantation (1D).
HCV-positive patients who are candidates for kidney transplantation should be evaluated for the presence of cirrhosis using either a noninvasive fibrosis-staging method or, on occasion, a liver biopsy. The choice of method is discussed in Chapter 1. In addition, measurement of hepatic-vein wedge-pressure gradient is useful when deciding whether single kidney transplantation or simultaneous liver-kidney transplantation should be proposed. Absence of varices on endoscopy and portal pressure gradient < 10 mm Hg suggests that cirrhosis is compensated.
In patients with compensated cirrhosis without portal hypertension, isolated kidney transplantation is recommended. HCV clearance halts the progression of liver disease and may even induce regression of liver fibrosis.217 The Consensus Conference Group on simultaneous liver-kidney transplantation proposed that combined liver-kidney transplantation should be performed if patients have decompensated cirrhosis and/or severe portal hypertension.218 Severe portal hypertension has been defined as a hepatic-vein wedge-pressure gradient of ≥ 10 mm Hg.40 The Portal Hypertension Collaborative Group stated that hepatic venous-pressure gradient predicts clinical decompensation in patients with compensated cirrhosis.219 Patients with cirrhosis who, despite having achieved SVR, have major hepatic complications such as ascites, hepatic encephalopathy, or worsening hepatocellular function should be evaluated for combined liver-kidney transplantation.
- 4.1.3: Timing of HCV treatment in relation to kidney transplantation (before vs. after) should be based on donor type (living vs. deceased donor), wait-list times by donor type, center-specific policies governing the use of kidneys from HCV-infected deceased donors, HCV genotype, and severity of liver fibrosis (Not Graded).
- 4.1.3.1: We recommend that all HCV-infected patients who are candidates for kidney transplantation be considered for DAA therapy, either before or after transplantation (1A).
- 4.1.3.2: We suggest that HCV-infected kidney transplant candidates with a living kidney donor can be considered for treatment before or after transplantation according to HCV genotype and anticipated timing of transplantation (2B).
- 4.1.3.3: We suggest that if receiving a kidney from an HCV-positive donor improves the chances for transplantation, the HCV NAT–positive patient can undergo transplantation with an HCV-positive kidney and be treated for HCV infection after transplantation (2B).
Until recently, only IFN-based therapy was available to treat HCV infection. The use of IFN was contraindicated after kidney transplantation (except in cases of fibrosing cholestatic hepatitis) because of its immunostimulatory properties, which increase the risk of graft rejection.220 Hence, it was recommended that candidates for kidney transplantation be treated with IFN before transplantation.34 The use of DAAs has completely changed this situation because HCV clearance is feasible in the vast majority of patients before and after kidney transplantation (see Chapter 2). The current issue is timing of HCV therapy in relationship to transplantation. Considerations for planning therapy include living versus deceased donor, wait-list time by donor type, center-specific policy for acceptance of organs from HCV-positive deceased donors, specific HCV GT, and severity of liver fibrosis (see Algorithm 3). Other factors such as candidate sensitization and patient preference can be also considered for choosing the timing of treatment.
Algorithm 3.
Proposed strategy in a hepatitis C virus (HCV)–infected kidney transplant candidate. SKLT, simultaneous kidney-liver transplantation.
In patients with compensated cirrhosis without portal hypertension, if living-donor kidney transplantation is anticipated without a long wait, HCV therapy can be deferred until after transplantation. If living-donor kidney transplantation is likely to be delayed more than 24 weeks (to allow 12 weeks of therapy and 12 weeks of follow-up to prove SVR), then HCV therapy can be offered before or after transplantation based on specific HCV GT and proposed treatment regimen.
In a potential recipient with compensated cirrhosis without portal hypertension and listed for kidney transplantation from a deceased donor at a center where it is possible to obtain a kidney allograft from an HCV-positive donor without a long wait, the potential recipient can defer antiviral therapy to allow receipt of an organ from an HCV-positive donor.221 However, the patient needs to provide written informed consent for this approach. In contrast, when kidney allografts from HCV-positive donors are not or cannot be used because of local policy, or when the anticipated time to obtain a kidney from an HCV-negative donor is long, the patient should be offered HCV therapy before transplantation.
Twice-yearly surveillance for HCC is indicated in cirrhotic patients. In addition, endoscopic surveillance for varices is indicated. Evaluation for complications of cirrhosis is indicated irrespective of whether the patient receives antiviral therapy or not.
Specific HCV GTs may also influence timing of HCV therapy, depending on the availability of individual drugs in some countries. If the pan-genotypic glecaprevir-pibrentasvir is available, the GT will not influence the timing of DAA treatment. If glecaprevir-pibrentasvir is not available, as discussed in Chapter 2, DAAs (grazoprevir plus elbasvir, daclatasvir plus asunaprevir, or 3D regimen) that are approved to treat HCV infections in CKD G4–G5 patients are efficacious in GTs 1 and 4. For other GTs, only a sofosbuvir-based therapy can be proposed. The off-label use of sofosbuvir-based therapy at reduced doses in CKD G4–G5 patients with GTs 2, 3, 5, or 6 has been reported, though it is not licensed for patients with an GFR < 30 ml/min per 1.73 m2 (see Chapter 2). Hence, in HCV-infected patients with GTs 2, 3, 5, and 6, if possible, treatment should be postponed until after transplantation.
4.2 Use of kidneys from HCV-infected donors
4.2.1: We recommend that all kidney donors be screened for HCV infection with both immunoassay and NAT (if NAT is available) (1A).
4.2.2: We recommend that transplantation of kidneys from HCV NAT–positive donors be directed to recipients with positive NAT (1A).
4.2.3: After the assessment of liver fibrosis, HCV-positive potential living kidney donors who do not have cirrhosis should undergo HCV treatment before donation; they can be accepted for donation if they achieve sustained virologic response (SVR) and remain otherwise eligible to be a donor (Not Graded).
Rationale
4.2.1: We recommend that all kidney donors be screened for HCV infection with both immunoassay and NAT (if NAT is available) (1A).
In 1991 Pereira et al. demonstrated that HCV was transmitted by organ transplantation.196 Several experiences published soon after the first description on the transplantation of kidneys from HCV RNA–positive donors corroborated unequivocally the transmission of HCV infection by organ transplantation.222 For this reason, organ procurement organizations and international guidelines have strongly recommended that all organ donors should be tested for HCV infection.34, 223
The diagnosis of HCV infection is made by the detection of anti-HCV by enzyme-linked immunosorbent assay.34, 223 The majority of patients who are seropositive for anti-HCV also have detectable HCV RNA in the serum. Performing NAT as an emergency test in potential deceased donors is optimal but is not widely available due to time constraints;34, 223 thus, in many cases, only anti-HCV is tested in potential organ donors prior to transplantation.
4.2.2: We recommend that transplantation of kidneys from HCV NAT–positive donors be directed to recipients with positive NAT (1A).
There has been a consensus that kidneys from HCV NAT–positive donors should not be transplanted into anti-HCV–negative recipients. Kidneys from donors with anti-HCV who are HCV NAT–negative can generally be used safely in negative anti-HCV patients. Nowak et al. recently reported a case series of 21 anti-HCV–positive kidneys (20 donors) who were HCV NAT–negative. In no case did the use of those kidneys lead to de novo HCV infection in HCV-negative recipients.224 However, there have been isolated cases of HCV transmission reported to Disease Transmission Advisory Committee (DTAC) from HCV aviremic (i.e., anti-HCV–positive and NAT-negative) donors; these are currently under investigation, but the risk of transmission is probably very low.225 The problem was and remains that the demand for kidney transplantation clearly surpasses the supply, and this is a particular concern in areas with a high prevalence of HCV infection.34 Universally discarding kidneys from HCV-positive donors could lead to the loss of up to 4.2% of organs.226 A recent retrospective study of 9290 donors for whom both anti-HCV and NAT data were available estimated that using anti-HCV–positive, NAT-negative donors at the same rate as anti-HCV–negative, NAT-negative donors could result in 48 more kidney donors. Thus, there is a potential for expanding donor pools by using organs from carefully selected anti-HCV–positive, NAT-negative donors.227
A related issue was whether organs harvested from HCV NAT–positive donors could be safely transplanted in HCV NAT–positive recipients.222 An experience in Spain of transplanting kidneys with positive HCV antibodies into HCV-positive recipients228, 229 provided some initial insights. When serum HCV RNA was retrospectively assessed in donor and recipients (by NAT) it was recognized that some HCV-positive recipients who were HCV NAT–negative had received organs from HCV NAT–positive donors.229 As a result of these findings, Spanish groups modified their policy, limiting the use of kidneys from HCV-positive donors to HCV NAT–positive recipients. This strategy was supported by international guidelines.34, 223 Therefore, the HCV RNA (i.e., NAT) status of the donor is critical for optimal allocation of HCV-positive organs.
Several studies from the US (registry or hospital data) have demonstrated that transplantation of kidneys from HCV-positive donors into HCV-positive recipients reduces the waiting time for transplantation,230, 231, 232, 233, 234, 235, 236 but is associated with a small increased risk of death, graft loss, and severe liver disease compared with HCV recipients who received kidneys from HCV-negative donors.235 Notably, despite this increase in risk, HCV-positive recipients transplanted with kidneys from HCV-positive donors have a better chance of survival than HCV-positive patients on the waiting list.232
Long-term results of transplantation with HCV-positive donors into HCV-positive recipients have demonstrated that donor anti-HCV seropositivity was not an independent risk factor for patient survival, graft loss, and liver disease.237 These results were comparable to a single-center experience in the US, showing that donor HCV status does not influence graft, patient survival or eGFR in HCV-positive recipients.238 Recent data from the US have corroborated these findings and demonstrated again that HCV patients who received kidneys from HCV-positive donors spent less time on the waiting list, which probably contributed to improved death-censored graft survival compared with HCV recipients from HCV-negative donors.239 The US experience using kidneys from HCV-positive donor demonstrated that the benefit of transplantation is limited to HCV-positive recipients older than 50 years (Supplementary Table S15).240 Recently, it has been shown that kidneys from anti-HCV–positive donors can be considered for transplant into HCV-infected recipients followed by early post-transplant treatment with DAA agents.241
Superinfection by another HCV GT can occur, and therefore matching donors and recipients according to their GT could be the next step to improve the safety of this policy.242 However, with the current availability of highly effective DAA regimens, matching by GT may be less of a serious concern.226
Despite international recommendations34, 223 currently there is underutilization of HCV-positive organs for a variety of reasons including concerns about HCV transmission, the fear of legal liability, the lack of acceptance of HCV-positive kidneys from another unit, and sometimes extensive recipient morbidities (e.g., long history of kidney disease and high immunological risk). Kucirka et al. have reported that kidneys from HCV-positive donors were 2.6 times more likely to be discarded than those from HCV-negative donors.122
In summary, the use of kidneys from HCV NAT–positive donors into HCV NAT–positive recipients (limiting the risk of transmission without loss of organs from the donor pool), is an acceptable approach. The capacity to use DAAs shortly after transplantation should allow safe use of these organs. Use of HCV NAT–positive kidneys for HCV NAT–positive recipients has been included in the algorithms to establish the policy of DAA therapy before or after transplantation.243, 244
4.2.3: After the assessment of liver fibrosis, HCV-positive potential living kidney donors who do not have cirrhosis should undergo HCV treatment before donation; they can be accepted for donation if they achieve sustained virologic response (SVR) and remain otherwise eligible to be a donor (Not Graded).
Potential living donors with HCV infection should be treated as in the general population. First, liver fibrosis should be assessed, and then, if there is no evidence of cirrhosis, they can receive DAAs based on GT (see Chapter 2).
SVR can then be assessed at 12 weeks with monitoring of kidney function and proteinuria during and after DAA therapy. In the absence of severe hepatic fibrosis, living donation is then feasible.
The scarcity of donor organs for transplantation results in long waiting times for kidney transplantation.34 In addition, individual patient characteristics, such as high sensitization, may contribute to delays in transplantation. Longer time on hemodialysis and on wait-list may be an independent risk factor for graft loss and mortality after transplantation. For these reasons kidney transplantation with expanded criteria donors has become a necessity.
A recent analysis of the US Organ Procurement and Transplant Network database through 2012 demonstrated inferior outcomes in HCV-negative recipients who had received an HCV-positive donor compared with HCV-negative recipients transplanted with HCV-negative donors.245 This practice has been considered unacceptable.34, 223 However, the availability of current DAAs for HCV infections has led to a reconsideration of this prohibition.
Treatment with DAAs is an established common practice in the general population and in liver transplant recipients.243 There is limited information about the use of DAAs in the early period after kidney transplantation.241 Preliminary information using DAAs in long-functioning kidney transplant patients with HCV infection indicates excellent SVR12 of 90% to 100%.118, 119 In liver transplantation, fibrosing cholestatic hepatitis has been successfully treated with DAAs.244 A clinical trial using HCV-positive kidneys into HCV-negative recipients has started very recently in Philadelphia.123 In this pilot study (THINKER), 10 patients with negative anti-HCV were given kidneys from donors who were HCV-NAT–positive for GT1.At day 3 post-transplantation, all patients had detectable HCV RNA and were given grazoprevir-elbasvir. SVR12 was observed in all patients.123 This novel strategy raises several questions regarding what the optimal informed consent process should be, the potential risk for viral complications, and the cost implications of post-transplant use of DAAs.246 Encouraging results from another trial (EXPANDER-1) of kidneys from HCV NAT–positive donors for HCV-negative recipients were also reported,124 but until more information is available regarding long-term safety of this approach, this practice should be considered strictly investigational.
4.3 Use of maintenance immunosuppressive regimens
4.3.1: We suggest that all conventional current induction and maintenance immunosuppressive regimens can be used in HCV-infected kidney transplant recipients (2C).
Rationale
In HCV-infected kidney transplant recipients, viral load increases after transplantation because immunosuppression facilitates viral replication.34 Roth et al. reported an increased rate of death by infection in HCV-positive patients in the first 6 months after kidney transplantation, a period when the impact of induction and high doses of maintenance immunosuppression therapy is greatest.216 These data suggest caution in the choice of immunosuppressive protocol in these patients34 given the frequent high immunological risk profile of HCV-infected recipients.
Antibody induction, particularly antilymphocyte preparations, had been associated with an increased risk of developing liver disease in HCV-infected transplant recipients.196 However, several studies have suggested that the use of antibody induction has no detrimental effect on survival in HCV-positive patients with post-transplantation chronic liver disease, even in African Americans (Supplementary Table S16).247, 248, 249, 250 In addition, the HR for death dropped from 2.51 over the first 6 months after transplant to 0.32 during the 7- to 84-month posttransplant period, in the study using induction therapy noted above.216
There are only limited data on the influence of steroids in kidney transplant patients with HCV infection. In a US study, mortality was not different among patients who received steroids as part of immunosuppression protocol versus those who did not.250 In the setting of liver transplantation, discontinuation of steroids after surgery was associated with a reduced rate of post-transplant diabetes.251 It is thus plausible that steroid withdrawal after kidney transplantation in HCV-positive selected patients could be beneficial to reduce post-transplant diabetes.
Concerning CNIs, there are no significant differences in outcomes with cyclosporine versus tacrolimus therapy in HCV-infected transplant recipients.34 However, it should be noted that the risk of post-transplant diabetes mellitus is higher in HCV-positive patients treated with tacrolimus,252 and cyclosporine inhibits HCV replication on cultured hepatocytes.253
Increased serum HCV RNA concentrations have been reported in patients who received MMF in place of azathioprine.254 However, MMF is considered part of the standard immunosuppression given to kidney transplant patients no matter what their HCV status is.216 Published information on clinical use of mTOR inhibitors (sirolimus and everolimus) in kidney transplant patients with HCV is scarce, and therefore the influence of mTOR inhibitors on HCV-positive patient survival after kidney transplantation is unknown.
One important concern with new DAAs for the treatment of HCV infection in kidney transplant patients is drug–drug interaction with immunosuppressive agents. Indeed, cyclosporine, tacrolimus, sirolimus, and everolimus are metabolized in the liver by the cytochrome P450. Thus, for most DAAs substrate competition can occur, influencing their elimination. The use of currently licensed DAAs can affect CNI levels and may require dose adjustment. As such, the Work Group suggests that the Hepatitis Drug Interactions website from the University of Liverpool (http://www.hep-druginteractions.org) be consulted for the latest guidance on potential drug–drug interactions prior to DAA use.
4.4 Management of HCV-related complications in kidney transplant recipients
4.4.1: We recommend that patients previously infected with HCV who achieved SVR before transplantation be tested by NAT 3 months after transplantation or if liver dysfunction occurs (1D).
4.4.2: Untreated HCV-positive kidney transplant recipients should have the same liver disease follow-up as HCV-positive non-transplant patients, as outlined in the American Association for the Study of Liver Diseases (AASLD) guidelines (Not Graded).
- 4.4.3: HCV-infected kidney transplant recipients should be tested at least every 6 months for proteinuria (Not Graded).
- 4.4.3.1: We suggest that patients who develop new-onset proteinuria (either urine protein-to-creatinine ratio > 1 g/g or 24-hour urine protein > 1 g on 2 or more occasions) have an allograft biopsy with immunofluorescence and electron microscopy included in the analysis (2D).
4.4.4: We recommend treatment with a DAA regimen in patients with post-transplant HCV-associated glomerulonephritis (1D).
Rationale
4.4.1: We recommend that patients previously infected with HCV who achieved SVR before transplantation be tested by NAT 3 months after transplantation or if liver dysfunction occurs (1D).
Kidney transplantation outcomes in patients with HCV without extensive fibrosis who are successfully treated before transplantation should be equivalent to those in uninfected transplant recipients. With achievement of SVR, viral relapse is unlikely, although kidney transplant recipients with unexplained hepatic dysfunction should undergo HCV and HBV testing.
4.4.2: Untreated HCV-positive kidney transplant recipients should have the same liver disease follow-up as HCV-positive non-transplant patients, as outlined in the American Association for the Study of Liver Diseases (AASLD) guidelines (Not Graded).
Kidney transplantation in patients with active HCV infection may be complicated by liver disease and also by extrahepatic complications.194 These patients exhibited a lower graft and patient survival and an increased risk of severe liver disease compared with HCV-negative recipients.34, 194, 223, 255 Therefore, patients with persistent HCV RNA because of lack of treatment before transplantation or due to failure of therapy before or after transplantation should be considered for liver disease reevaluation and re-treatment with DAAs. Preliminary publications of the use of DAAs in kidney transplant patients have exhibited SVR of almost 100% without important side effects.118, 119 More recently, a trial compared 12 and 24 weeks of sofosbuvir and ledipasvir in 114 kidney transplant recipients infected with HCV GTs 1 and 4 (96% GT1) with an eGFR of 40 ml/min per 1.73 m2 or greater (median eGFR: 56 ml/min per 1.73 m2). The therapy was very well tolerated, and SVR rates were close to 100% without differences between arms, suggesting that a 12-week regimen is also indicated in kidney transplant recipients.116
- 4.4.3: HCV-infected kidney transplant recipients should be tested at least every 6 months for proteinuria (Not Graded).
- 4.4.3.1: We suggest that patients who develop new-onset proteinuria (either urine protein-to-creatinine ratio > 1 g/g or 24-hour urine protein > 1 g on 2 or more occasions) have an allograft biopsy with immunofluorescence and electron microscopy included in the analysis (2D).
4.4.4: We recommend treatment with a DAA regimen in patients with post-transplant HCV-associated glomerulonephritis (1D).
HCV infection has been reported as a risk factor for the development of proteinuria in kidney transplant recipients.256 Several glomerular lesions have been described after kidney transplantation in HCV RNA–positive patients including recurrent or de novo cryoglobulinemic or non-cryoglobulinemic MPGN,257 membranous nephropathy (MN),258 acute transplant glomerulopathy,194 anti-cardiolipin related thrombotic microangiopathy,259 and chronic transplant glomerulopathy.260 MPGN and MN are the most frequent lesions related to HCV infection. The most common presentation is proteinuria with or without microhematuria, or nephrotic syndrome. The pathogenesis of MPGN and MN seems to be related to the deposition of immune complexes containing HCV RNA in the glomerulus.34
After HCV NAT–positive patients have undergone kidney transplantation, clinicians should screen for proteinuria and microhematuria. In the case of urine protein-to-creatinine ratio > 1 g/g or 24-hour urine protein (protein excretion rate) greater than 1 g on 2 or more occasions, a graft biopsy is indicated. Pathological examination should include immunofluorescence and electron microscopy. In the case of suspected transplant glomerulopathy, electron microscopy is mandatory to make the differential diagnosis with HCV-related MPGN.194, 260
For HCV-related glomerular disease, DAA therapy is indicated.261, 262, 263, 264, 265, 266, 267, 268, 269, 270 In severe HCV-related cryoglobulinemic MPGN, in addition to antiviral therapy with DAAs, rituximab and, in severe cases, plasmapheresis should be considered.194 This is discussed in detail in Chapter 5.
Research recommendations
-
•
Prospective studies should assess the best timing for HCV treatment in kidney transplant candidates: before or after transplantation?
-
•
Studies should examine whether accepting a kidney from an HCV-positive donor would reduce the time on the waiting list. Further studies are required in different countries because the prevalence of HCV in donors is highly variable worldwide.
-
•
Future research should evaluate the impact of delaying HCV treatment on HCV-induced morbidity (e.g., liver disease) and patient survival in HCV-positive kidney transplant candidates who are not given DAA therapy in order to be grafted with a kidney from a positive donor.
-
•
Prospective larger studies under investigational protocols should be conducted to corroborate the encouraging preliminary results obtained using kidneys from HCV-positive donors for HCV-negative recipients treated with DAAs. Studies should also examine the cost-effectiveness of this policy with different DAA treatment strategies.
-
•
SVR should be assessed in a large cohort of HCV-positive patients who receive a kidney allograft from a positive donor and who are given DAA therapy after transplantation. In this setting, the optimal timing for starting DAA therapy should be determined.
-
•
In patients presenting with an HCV-associated kidney disease after transplant, the effect of DAAs on the kidney graft should be assessed in a large series.
Chapter 5: Diagnosis and management of kidney diseases associated with HCV infection
In addition to chronic liver disease, HCV infection also leads to extrahepatic manifestations including kidney disease and mixed cryoglobulinemia.271 Although chronic HCV infection has been identified as an important cause of tubulo-interstitial injury in a large case-control study,272 HCV-associated glomerular disease is the most frequent type of kidney disease associated with HCV.
HCV-induced glomerular disease occurs frequently in the context of HCV-associated mixed cryoglobulinemia, a systemic vasculitis characterized by involvement of small and, less frequently, medium-size vessels.273, 274, 275, 276, 277 Mixed cryoglobulinemia represents 60% to 75% of all cryoglobulinemia cases and is observed in connective tissue diseases and infectious or lymphoproliferative disorders, all grouped under the term “secondary mixed cryoglobulinemia.” After its identification, HCV has been recognized as the cause of 80% to 90% of idiopathic mixed cryoglobulinemia.273, 276 In general, HCV is associated with type II mixed cryoglobulinemia (cryoglobulins consisting of polyclonal IgG and monoclonal IgM with rheumatoid factor activity), although it is also less frequently associated with type III mixed cryoglobulinemia (cryoglobulins consisting of polyclonal IgG and polyclonal IgM). In the absence of an identified etiology (currently <10% of mixed cryoglobulinemia), cryoglobulinemic vasculitis is defined as essential or idiopathic.
Immune complex glomerular diseases such as MPGN are the most frequent kidney diseases associated with chronic HCV infection.274, 275 The incidence of HCV-associated glomerular disease is probably low even if the available information is scanty. In an autopsy series of 188 consecutive patients with HCV infection, the frequency of MPGN was 11%, MN 2%, and mesangial proliferative GN 17%.278
A large survey has been conducted by El-Serag et al., who carried out a hospital-based case-control study among US male veterans from 1992 to 1999 and identified 34,204 patients infected with HCV (cases) and 136,816 randomly selected patients without HCV (controls).279 A greater fraction of HCV-infected patients had porphyria cutanea tarda (0.77% vs. 0.06%, P < 0.0001), vitiligo (0.17% vs. 0.10%, P = 0.0002), lichen planus (0.30% vs. 0.13%, P < 0.0001), and cryoglobulinemia (0.57% vs. 0.05%, P < 0.0001). A greater rate of MPGN (0.36% vs. 0.05%, P < 0.0001) but not MN (0.33% vs. 0.19%, P = 0.86) was found among patients with HCV. According to a prospective Norwegian study, the rate of CKD G5 due to MPGN was 0.2%.280 It has been further shown that anti-HCV seropositive status was more common in patients with non-cryoglobulinemic MPGN and MN (18%–20%) than that observed in the general population of the same area (7%) after correction for age.281 A large meta-analysis of 107,356 patients7 reported that anti-HCV–positive serology was an independent risk factor for proteinuria in the adult general population (adjusted OR: 1.51 [95% CI: 1.19–1.89)].65, 66, 282, 283, 284, 285 Another pooled analysis63 demonstrated that anti-HCV–positive serology was an independent risk factor for proteinuria among HIV-infected patients with an adjusted effect estimate of 1.23 (95% CI: 1.18–1.28).286, 287, 288, 289, 290, 291
5.1: We recommend that a kidney biopsy be performed in HCV-infected patients with clinical evidence of glomerular disease (Not Graded).
- 5.2: We recommend that patients with HCV-associated glomerular disease be treated for HCV (1A).
- 5.2.1: We recommend that patients with HCV-related glomerular disease showing stable kidney function and/or non-nephrotic proteinuria be treated initially with DAA (1C).
- 5.2.2: We recommend that patients with cryoglobulinemic flare, nephrotic syndrome, or rapidly progressive kidney failure be treated, in addition to DAA treatment, with immunosuppressive agents with or without plasma exchange (1C).
- 5.2.3: We recommend immunosuppressive therapy in patients with histologically active HCV-associated glomerular disease who do not respond to antiviral therapy, particularly those with cryoglobulinemic kidney disease (1B).
- 5.2.3.1: We recommend rituximab as the first-line immunosuppressive treatment (1C).
Rationale
5.1: We recommend that a kidney biopsy be performed in HCV-infected patients with clinical evidence of glomerular disease (Not Graded).
The main clinical manifestations of glomerular disease in HCV-infected patients are the presence of proteinuria and microscopic hematuria with or without reduction in GFR. It remains unclear why only a minority of patients with HCV infection develop kidney abnormalities. Glomerular diseases associated with HCV infection have been described in the presence or absence of significant liver disease; however, all patients with HCV-associated glomerular disease show detectable HCV RNA in serum.292, 293
The main reasons for recommending a kidney biopsy in patients with HCV infection and signs of glomerular disease are not markedly different from the usual reasons prompting a kidney biopsy for other glomerular diseases.294 Kidney biopsy remains invaluable to assess the precise histological picture of the disease and the probability that the observed lesions are causally related to HCV-infection. Other glomerular diseases (including diabetic nephropathy and other types) are indeed not infrequently reported among patients with HCV infection.295 In addition, the histology will provide an assessment of the extent of active or hyperactive lesions requiring urgent immunosuppressive treatment, and of chronic lesions that are unlikely to be reversible under immunosuppression. Thus, some patients might be spared from immunosuppression in the presence of severe chronic lesions when there is no extrarenal indication for immunosuppression.294
The most common type of HCV-related GN is immune complex-mediated MPGN usually in the context of type II cryoglobulinemia. Distinctive features of cryoglobulinemic GN, especially in patients with rapidly progressive deterioration of kidney function, include intraglomerular deposits, which are commonly seen in a subendothelial location, sometimes occluding the capillary lumen (intraluminal thrombi). Glomeruli may show prominent hypercellularity as a result of infiltration of glomerular capillaries by mononuclear and polymorphonuclear leucocytes. Glomeruli frequently show accentuation of lobulation of the tuft architecture with a combination of increased matrix and mesangial cells, capillary endothelial swelling, splitting of capillary basement membrane, and accumulation of eosinophilic material representing precipitated immune complexes or cryoglobulins. The glomerular basement membrane often shows double contours, which are caused by the interposition of monocytes between the basement membrane and the endothelium. On electron microscopy, large subendothelial deposits are present. Vasculitis of small renal arteries is present in 30% of cases.296
Of note, numerous intraluminal thrombi, vasculitis, or both are more commonly observed in patients with an acute nephritic syndrome and rapid progressive kidney failure. Histological features of exudative or lobular MPGN are associated with the occurrence of nephrotic and/or nephritic syndromes, whereas mesangial proliferation is prevalent in cases with intact kidney function and isolated proteinuria and/or microscopic hematuria.296
Some investigators have reported cases of HCV-associated MPGN without cryoglobulinemia.275 In these patients, the clinical picture, histological features and laboratory data are indistinguishable from “classical” idiopathic immune complex-mediated MPGN. Both subendothelial and mesangial immune complexes can be identified by electron microscopy typically without a distinctive substructure. In both forms of HCV-associated GN, immunofluorescence commonly reveals deposition of IgM, IgG, and C3 in the mesangium and capillary walls.
MN is also observed in association with chronic HCV infection.258 Whether this corresponds to a true association or a coincidence is unclear. The clinical presentation, outcome, and histopathology are similar to those observed in idiopathic MN. On light microscopy, the characteristic finding is a diffuse and uniform thickening of the glomerular basement membrane without mesangial or endothelial proliferation. Diffuse subepithelial immune deposits can be identified by electron microscopy, and immunofluorescence shows diffuse and granular deposits of IgG, IgA, and C3.
Other glomerular diseases that have been occasionally reported in association with chronic HCV infection are acute proliferative GN, focal segmental glomerulosclerosis,297 IgA nephropathy,298 thrombotic microangiopathy,259 rapidly progressive nephritis,299 fibrillary GN, and immunotactoid glomerulopathy.300 However, these likely correspond to sporadic cases and their pathogenic link with HCV remains even more uncertain than for MN.
The pathogenesis of glomerular disease associated with HCV infection is not completely understood. It appears that HCV binds and penetrates into the renal parenchymal cells via the CD81 and SR-B1 receptors.301 HCV RNA has been found in mesangial cells, tubular epithelial cells, and endothelial cells of glomerular and tubular capillaries. The deposition of immune complexes containing HCV proteins in the glomerular basement membrane has been cited in the pathogenesis of HCV-associated MN.301 HCV-related granular protein deposits located in the mesangium have been observed in patients with HCV-related MPGN; they are probably related to higher degrees of proteinuria.302 Viral antigens have been found by immunohistochemistry,303 in situ hybridization,303 and laser capture microdissection.304
5.2: We recommend that patients with HCV-associated glomerular disease be treated for HCV (1A).
In view of the role of HCV in the pathogenesis of cryoglobulinemic GN, antiviral therapy has been used to achieve clearance of HCV and ameliorate the renal injury. RCTs remain sparse; the evidence on the impact of antiviral treatment of HCV-related glomerular disease was until recently limited and consisted mostly of anecdotal reports and small-sized observational studies (Supplementary Tables S17 and S18). Initial reports adopted monotherapy with conventional IFN,305 but the combined regimen (pegylated IFN plus RBV) superseded monotherapy.306 With the arrivals of DAAs, IFN-based regimens are now considered obsolete, though these antiviral studies306, 307, 308 provided valuable insight on the etiological role of HCV in the pathogenesis of GN.
Some evidence supporting the antiviral therapy of HCV-associated glomerular disease has been provided by a meta-analysis of comparative studies of various study designs comparing antiviral versus immunosuppressive regimens for HCV-induced GN.309 However, even with pooling of study results, the effect of IFN (vs. corticosteroid therapy) on reducing proteinuria is highly imprecise: OR 1.92; 95% CI: 0.39–9.57. In a sensitivity analysis including only controlled trials using standard IFN doses, the OR was 3.86 (95% CI: 1.44–10.3). Of note, in all patients with proteinuria reduction, HCV RNA clearance was observed at the end of antiviral therapy.309
In another meta-analysis,78 antiviral therapy based on IFN-α decreased proteinuria in HCV-positive CKD patients. At the end of antiviral therapy, the summary estimate of the mean decrease in proteinuria was 2.71 g/24 hr (95% CI: 1.38–4.04). The decrease in proteinuria following antiviral therapy was associated with HCV RNA clearance. Serum creatinine was not significantly decreased with antiviral treatment; however, stabilization of serum creatinine was achieved. Patients receiving combination with IFN plus RBV achieved a higher SVR rate than did those with IFN monotherapy regardless of HCV GT.
Additional anecdotal reports on the antiviral treatment of HCV-associated glomerular disease in adults with native kidneys have been published, and a large variety of histological lesions was found.310 According to an updated review, a total of 36 reports based on 47 unique patients were retrieved.311, 312, 313, 314, 315, 316, 317 The majority of these patients had improvement of renal changes after clearance of HCV RNA, and this confirms the role of the virus in the pathogenesis of the kidney disease. One report emphasized the spontaneous remission of glomerular lesions; this cannot be excluded in a few cases.318 Additional, albeit limited, information on antiviral treatment of HCV-related glomerular disease in kidney,257 liver,319, 320, 321 and liver/kidney transplanted population322 and among pediatric individuals exists. Recombinant IFN given for treatment of HCV may exacerbate proteinuria in some patients with underlying glomerulopathies.323
Regardless of the regimens used (IFN-based or DAAs), antiviral treatment of HCV-related glomerular disease has limitations. First, the impact of antiviral therapy on the long-term outcomes of kidney disease remains uncertain. Second, the clinical benefit in patients who reached SVR may be transient and/or a dissociation between viral and renal responses can occur.275, 324, 325, 326 Two recent long-term (1- to 2-year) studies reported high rates of marked improvement on various cryoglobulinemia-related manifestations after SVR with DAAs, but confirmed that relapses of vasculitis may occur despite achieving SVR.327, 328
5.2.1: We recommend that patients with HCV-related glomerular disease showing stable kidney function and/or non-nephrotic proteinuria be treated initially with DAA (1C).
The development of kidney disease among patients with mixed cryoglobulinemia has particular importance because kidney involvement confers a poor prognosis to such patients.329, 330, 331 Clinically, HCV-associated mixed cryoglobulinemia is characterized by the triad of purpura, arthralgia, and weakness. The natural history of HCV-induced mixed cryoglobulinemia is clinically variable: some patients have an indolent course while others develop vasculitic lesions in various organs including kidneys. Extrarenal features of mixed cryoglobulinemia include neuropathy, hepatomegaly, sicca syndrome, and central nervous system and gut involvement. Overt pulmonary involvement is infrequent. Although extrarenal signs of mixed cryoglobulinemia vasculitis usually precede the kidney manifestations, often by years, in 29% of cases, kidney and extrarenal involvement are concurrent.331 Kidney disease occurs in 8% to 58% of patients with mixed cryoglobulinemia, and in a minority of cases can be the first manifestation of mixed cryoglobulinemia. Patients with HCV-associated cryoglobulinemic glomerular disease can present with nephritic syndrome, asymptomatic non-nephrotic proteinuria or hematuria, and/or reduced GFR. Acute nephritic and nephrotic syndrome can be a presenting feature in 25% and 20% of patients, respectively. Arterial hypertension is frequent (affecting >50% of patients at the time of diagnosis) and is often resistant to antihypertensive drugs; the severity of hypertension often mirrors the severity of kidney disease.330 Around 10% of patients present oliguric kidney failure.330, 331
Type II mixed cryoglobulinemia is most common in the fourth or fifth decade of life, and usually is characterized by periods of extrarenal symptoms alternating with periods of quiescence.332 The exacerbation of extrarenal symptoms often is associated with a flare-up of kidney disease, but can occur independently. Patients with cryoglobulinemic GN have a poor prognosis, mainly because of a high incidence of infections, end-stage liver disease, and cardiovascular diseases.330, 331
RCTs are lacking to help establish evidence-based recommendations to treat glomerular lesions associated with HCV infection. Until this information is available, the treatment of HCV-associated GN should probably be driven by the severity of proteinuria and kidney failure.
Given that remission of hematuria, proteinuria, and improvement of GFR in patients with HCV-associated GN who obtained sustained HCV RNA clearance by DAAs has been reported,261, 262, 263, 264, 265, 266, 267, 268, 269, 270 antiviral therapy with DAA regimens should be considered the first-line choice in patients with non-nephrotic proteinuria and relatively stable kidney function (Supplementary Tables S17 and S18). In addition, anti-proteinuric agents such as angiotensin-converting enzyme inhibitors/angiotensin receptor blockers should be given. Treatment including diuretics and antihypertensive agents should be used to achieve target blood pressure recommended in patients with CKD.
5.2.2: We recommend that patients with cryoglobulinemic flare, nephrotic syndrome, or rapidly progressive kidney failure be treated, in addition to DAA treatment, with immunosuppressive agents with or without plasma exchange (1C).
Immunosuppressive agents have been administered to patients with serious, life-threatening complications of mixed cryoglobulinemia, such as MPGN, severe neuropathy, or extensive skin disease. Cyclophosphamide has been selected to improve kidney disease by reducing stimulation of B lymphocytes and cryoglobulin synthesis; steroid pulses have been given to treat glomerular inflammation, and plasma exchange has been employed to remove circulating cryoglobulins from the plasma and consequently to reduce the deposition of immune complexes to the kidneys.
In patients with nephrotic-range proteinuria and/or rapidly progressive kidney failure and/or acute flare of cryoglobulinemia, control of disease by immunosuppressive agents, with or without plasma exchange (3 liters of plasma thrice weekly for 2–3 weeks), should be considered before the initiation of DAA therapy. Potential regimens include rituximab (375 mg/m2 weekly for 4 weeks) with or without corticosteroids (see below), or cyclophosphamide (2 mg/kg/d for 2–4 months) plus methylprednisolone pulses 0.5 to 1 g/d for 3 days. According to the decision of the clinician, immunosuppressive regimen alone or combined therapy (immunosuppressive agents plus DAA therapy) is suggested as the initial approach.
Until a few years ago, combined therapy with corticosteroids and immunosuppressive agents—for example, treatment using sequentially cyclophosphamide and azathioprine—has been used while awaiting the response, if any, to antiviral therapy. In one retrospective study, the clinical outcome of 105 patients with essential mixed cryoglobulinemia vasculitis and renal involvement was evaluated throughout a median follow-up of 72 months since kidney biopsy.330 Positive anti-HCV serologic status was reported in 85% of patients. About 80% of patients underwent treatment with oral or pulse intravenous steroids and/or cytotoxic agents, whereas 67% were treated with plasma exchange. Despite this aggressive treatment, patient survival was 49% at 10 years after kidney biopsy, and only 14% of patients had long-term remission of kidney disease.330 By multivariate analysis, age > 50 years, purpura, splenomegaly, cryocrit levels > 10%, C3 plasma levels < 54 mg/dl, and serum creatinine > 1.5 mg/dl (> 133 μmol/l) were independent risk factors for death or dialysis.330 Other case reports have also documented improvement following the administration of a combination of steroids and antivirals (IFN and RBV) or of the 3D regimen combined with plasmapheresis, corticosteroids, and rituximab.333, 334
- 5.2.3: We recommend immunosuppressive therapy in patients with histologically active HCV-associated glomerular disease who do not respond to antiviral therapy, particularly those with cryoglobulinemic kidney disease (1B).
- 5.2.3.1: We recommend rituximab as the first-line immunosuppressive treatment (1C).
Limited information exists on the use of DAAs in patients with HCV-associated glomerular disease. Nine patients with symptomatic mixed cryoglobulinemic disease (seven with MPGN) and HCV GT1 underwent triple antiviral therapy (pegylated IFN, RBV, and boceprevir [n = 2] or telaprevir [n = 5] or sofosbuvir [n = 2]).325, 335 All patients reached SVR, but serum cryoglobulins persisted in 3 patients; also, the benefits on renal signs were partial. MPGN remitted in 3 patients after further treatment with corticosteroids or corticosteroids plus rituximab.
More recently, encouraging results have been obtained with IFN-free DAA regimens for HCV-related glomerular disease; a small group of 7 patients with symptomatic mixed cryoglobulinemia and GN (5 had a biopsy-proven MPGN and 2 were diagnosed clinically) underwent sofosbuvir-based regimens (6 with sofosbuvir and simeprevir and 1 with sofosbuvir and RBV).265 Only 1 patient was receiving ongoing immunosuppression concurrent with antiviral therapy. All patients had an improvement in eGFR and a reduction in proteinuria, particularly in those whose onset of proteinuria was recent. Also, in all patients HCV RNA was undetectable by week 4 and remained undetectable while on treatment. SVR was reached in 6 of 7 patients.
In another cohort of 44 consecutive patients with HCV-associated mixed cryoglobulinemia, 4 patients had renal involvement.263 The treatment of HCV-associated mixed cryoglobulinemia with sofosbuvir-based DAA therapy appeared to be highly effective (SVR12, 100%) and safe with some improvement of kidney disease262, 263 These studies suggest that IFN-free therapies can give high viral and clinical responses in a difficult-to-treat condition such as HCV-associated mixed cryoglobulinemia with renal involvement. In fact, the SVR rates ranging between 83% and 100% are comparable to the SVR12 rates reported with similar regimens in other non-cryoglobulinemic real-world groups. However, it is clear that we need larger and controlled studies to confirm these results. Combining DAA therapy with rituximab and other immunosuppressants might be of value for cases with severe or obstinate manifestations of cryoglobulinemic vasculitis.
Immunosuppressive therapies are suggested typically for patients with HCV-associated mixed cryoglobulinemia showing severe disease manifestations, such as progressive glomerular disease. In addition to conventional immunosuppressants, which target inflammation at the glomerular level, encouraging results have been obtained with rituximab, a human-mouse chimeric monoclonal antibody that binds to the B-cell surface antigen CD20 and selectively targets B cells.336, 337, 338, 339, 340, 341 Rituximab interferes with synthesis of cryoglobulins, monoclonal IgM, and renal deposition of immune complexes. An important pathogenetic feature of mixed cryoglobulinemia (including cryoglobulinemic GN) is chronic stimulation of B lymphocytes by HCV and widespread auto-antibody synthesis related to HCV-induced lowering of cell activation threshold.
Two RCTs have demonstrated the superiority of rituximab monotherapy as compared with conventional immunosuppressive therapy (i.e., corticosteroids, azathioprine, cyclophosphamide, methotrexate, and plasma exchange) for the treatment of HCV-associated cryoglobulinemic vasculitis in patients for whom prior IFN therapy failed to induce disease remission, or in patients who were not eligible for IFN therapy. Admittedly, only a minority of the included patients showed renal involvement.339, 341 Rituximab was well tolerated and was effective in 71.4% to 83% of patients with HCV-associated cryoglobulinemic vasculitis. Frequent relapses may occur after rituximab when B cells re-emerge in the peripheral blood; in addition, repeated rituximab infusions may expose patients to opportunistic infections.
In a recent prospective, single-center study, 16 patients with cryoglobulinemic nephropathy (diffuse MPGN and mixed cryoglobulinemia) received rituximab at a dose of 375 mg/m2, according to a “4 + 2” protocol (days 1, 8, 15, and 22 plus one dose 1 and 2 months later).337 No other immunosuppressive drugs were used. Safety and efficacy of rituximab was evaluated over a long-term follow-up (mean: 72.5 months). A significant improvement of cryoglobulinemic GN was found, starting from the second month after rituximab (serum creatinine from 2.1 ± 1.7 mg/dl [186 ± 150 μmol/l] to 1.5 ± 1.6 mg/dl [133 ± 141 μmol/l], P < 0.05; and 24-hour proteinuria from 2.3 ± 2.1 to 0.9 ± 1.9 g/24 hr, P < 0.05).337 No clinically relevant side effects were recorded. Re-induction with rituximab was carried out in 9 patients who relapsed after a mean of 31.1 months, again with beneficial effects. In addition, complete remission of pre-treatment active manifestations was observed in all cases of purpuric lesions and non-healing vasculitic ulcers, and in 80% of the peripheral neuropathies.
A point of caution is important as rituximab, which selectively targets B cells, has been associated with severe infectious complications including reactivation of HCV,342 or more frequently, HBV. The risk of reactivation of HBV infection has been added to the existing black box warning on the rituximab label by the FDA in 2013.343 Infections with ominous course after rituximab therapy have been observed in kidney transplant recipients and in the non-transplant setting. However, these complications were mostly observed in patients under multiple immunosuppressive agents. Infectious episodes have been frequently reported in a patient subgroup (age > 70 years, GFR < 60 ml/min per 1.73 m2, and concomitant high-dose corticosteroids) and were fatal in some patients.344 Cholestatic liver disease due to HCV reactivation by rituximab has been also observed after kidney transplant.342
In addition to conventional or selective immunosuppressive agents, additional immunosuppressive agents, such as MMF, should be evaluated. Preliminary evidence suggests that MMF can be effective for maintaining remission of HCV-associated cryoglobulinemic GN.345, 346
In summary, a kidney biopsy should be performed in HCV-positive patients with clinical evidence of glomerular disease. Patients with mild or moderate forms of HCV-associated GN with stable kidney function and/or non-nephrotic proteinuria should be managed first with a DAA regimen. Patients with severe cryoglobulinemia or severe glomerular disease induced by HCV (i.e., nephrotic proteinuria or rapidly progressive kidney failure) should be treated with immunosuppressive agents (generally with rituximab as the first-line agent) and/or plasma exchange in addition to DAA therapies. Patients with HCV-related glomerular disease who do not respond to or are intolerant of antiviral treatment should also be treated with immunosuppressive agents. In all cases, achievement of SVR after DAA treatment, changes in kidney function, evolution of proteinuria, and side effects from antiviral therapy must be carefully monitored. Treatment with antiproteinuric agents such as angiotensin-converting enzyme inhibitors and/or angiotensin-receptor blockers should be given to patients with HCV-associated glomerular disease. When appropriate, diuretics and antihypertensive drugs should be administered to achieve recommended target blood pressure goals for patients with CKD.
Research recommendations
-
•
Occult HCV infection (detectable HCV RNA in peripheral blood mononuclear cells and/or in serum after centrifugation) could be involved in the pathogenesis of glomerular disease among patients negative for HCV RNA.347 We need large-sized studies with appropriate technology to assess the relationship between occult HCV and glomerular disease.
-
•
The efficacy and safety of DAA therapies and/or imumunosuppressive agents for the treatment of HCV-associated GN should be assessed, preferably in larger, controlled clinical studies, with longer follow-up.
-
•
The antiviral approach to the treatment of HCV-related glomerular disease is expected to improve with IFN-free and RBV-free regimens. However, some of these drugs are not currently approved in patients with low GFR; hence, further studies of various DAAs are warranted in late CKD/ESKD for various GTs in patients with HCV-associated GN. Typically, patients with HCV-related glomerular disease receive a high number of concomitant drugs, including cytotoxic agents. Potential drug–drug interaction is another challenge to clinicians using DAA regimens for HCV-induced GN.
-
•
The role of immunosuppressive agents in the management of aggressive HCV-related glomerular disease (i.e., nephrotic syndrome, rapidly progressive decline of GFR) needs to be further clarified in light of the rapid antiviral activity provided by DAA regimens.
-
•
Numerous questions regarding the use of rituximab in HCV-positive glomerular disease remain. For example, what is the optimal timing and dosing of periodic rituximab infusions for relapsers? The role of rituximab as first-line or rescue therapy needs to be defined further.
-
•
Severe infections after rituximab therapy frequently occur in patients who are older than 50 years, have kidney disease, and report concomitant use of high-dose corticosteroids. Future studies should delineate how best to avoid infections associated with immunosuppression regimens.
Methods for guideline development
Aim
The overall aim of this project was to develop an evidence-based clinical practice guideline (CPG) for the management of patients with CKD as pertains to HCV infection. The guideline consists of recommendation statements, rationale text, and a summary of systematically generated evidence on relevant pre-defined clinical topics. The general guideline development method is described below.
Overview of process
The development process for the KDIGO 2018 CPG for the Prevention, Diagnosis, Evaluation and Treatment of Hepatitis C in CKD included the following steps:
-
•
Appointing Work Group members and the evidence review team (ERT)
-
•
Discussing process, methods, and results
-
•
Developing and refining topics
-
•
Identifying populations, interventions or predictors, and outcomes of interest
-
•
Selecting topics for systematic evidence review
-
•
Standardizing quality assessment methodology
-
•
Developing and implementing literature search strategies
-
•
Screening abstracts and retrieving full-text articles on the basis of pre-defined eligibility criteria
-
•
Creating data extraction forms
-
•
Extracting data and performing critical appraisal of the literature
-
•
Grading the methodology and outcomes in individual studies
-
•
Tabulating data from individual studies into summary tables
-
•
Grading quality of evidence for each outcome across studies, and assessing the overall quality of evidence across outcomes with the aid of evidence profiles
-
•
Grading the strength of recommendations on the basis of the quality of evidence and other considerations
-
•
Finalizing guideline recommendations and supporting rationales
-
•
Sending the guideline draft for public review in February 2017
-
•
Editing the guideline
-
•
Publishing the final version of the guideline
The overall process for conducting the systematic reviews and developing the CPG follow international standards, including those from the Institute of Medicine.348, 349
The Work Group Co-Chairs and the ERT met for a 2-day meeting to go over the guideline development process, evidence review topics, and systematic review findings. Following this, the Work Group, ERT, and KDIGO support staff met for 2 separate 2-day meetings to finalize review topics, review the available evidence, formulate recommendation statements, evaluate the quality of the evidence and strength of recommendations, deliberate on rationale for recommendations, and develop consensus.
Commissioning of Work Group and ERT
The KDIGO Co-Chairs appointed the Work Group Co-Chairs, who then assembled the Work Group of domain experts, including individuals with expertise in adult nephrology, transplant nephrology, hepatology, virology, infection control, and public health. The Brown University Center for Evidence Synthesis in Health in Providence, Rhode Island, was contracted as the ERT to conduct systematic evidence review and provide expertise in guideline development methodology. The ERT consisted of physician-methodologists with expertise in nephrology and evidence-based clinical practice guideline development, and experienced research associates.
Defining scope and topics
The Work Group Co-Chairs and the ERT defined the overall scope and goals of the guideline (including a list of critical and important interventions and outcomes) and then drafted a preliminary list of topics and key clinical questions. The list of research and recommendation topics was based on the original KDIGO guideline on HCV,34 which the ERT also had helped to develop (when it was based at Tufts Medical Center in Boston, MA). The Work Group and ERT further developed and refined each topic and its eligibility criteria, literature search strategies, and data extraction forms (Table 8).
Table 8.
Systematic review topics and screening criteria
| Hepatitis C treatment | |
| Population | CKD G3a–5 (including dialysis and transplant) or equivalent; HCV infection |
| Intervention | DAA (except 1st generation: telaprevir, boceprevir), pegylated interferon ± ribavirin, immunosuppression including induction (in combination with DAA or as treatment of HCV-associated GN) |
| Comparator | Active or control or none (single-arm studies) |
| Outcome | Categorical: all-cause mortality, SVR (preferably 24-wk), hepatocellular carcinoma, graft loss, NODAT, QoL, adverse events (including treatment discontinuation), pharmacokinetics/dynamics Continuous (HCV-associated GN only): kidney function, proteinuria |
| Study design | RCT, nonrandomized comparative studies, single-group studies; prospective (all topics) or retrospective (immunosuppression or GN topics only). Interferon in dialysis: RCT only. |
| Minimum duration of follow-up | HCV treatment studies: 12 weeks post-treatment; Other topics: no minimum |
| Minimum N of subjects | ≥ 10; Immunosuppression topic: any, including case reports |
| Publication dates | All: ≥ 2008 (plus studies in 2008 KDIGO CPG); interferon and dialysis topic: Cochrane review350 and ≥ 2012 |
| Liver testing | |
| Population | Tests for cirrhosis: CKD (all stages); pre-transplant biopsy: CKD G4–G5 pre-transplantation (or equivalent) |
| Intervention/comparator | Noninvasive liver testing, including upper endoscopy (for varices), liver biopsy |
| Outcome | Noninvasive test performance characteristics, change in management strategy, patient mortality, graft loss |
| Design | Any |
| Minimum N of subjects | Noninvasive testing: N ≥ 10, pre-transplant biopsy: N ≥ 5 |
| Publication dates | Any |
| Dialysis isolation | |
| Population | Hemodialysis (patients or units) |
| Intervention | Isolation, quarantine, etc. |
| Comparator | No isolation, less stringent standard |
| Outcome | HCV transmission |
| Design | Any |
| Minimum duration of follow-up | None |
| Minimum N of subjects | N ≥ 30 patients |
| Publication dates | ≥ 2008 (plus studies in 2008 KDIGO CPG) |
| Early versus late transplantation | |
| Population | HCV-infected transplantation candidates |
| Intervention | Transplantation (“now”) |
| Comparator | Remaining on wait-list or awaiting HCV-negative status |
| Outcome | Patient mortality, graft loss |
| Design | Any, multivariable analysis |
| Minimum duration of follow-up | None |
| Minimum N of subjects | N ≥ 100 |
| Publication dates | ≥ 2008 (plus studies in 2008 KDIGO CPG) |
| HCV-infected donors | |
| Population | HCV-infected kidney transplant recipients |
| Intervention | HCV-infected donors |
| Comparator | HCV-negative donors |
| Outcome | Patient mortality, graft loss |
| Design | Longitudinal comparative, multivariable analysis |
| Minimum duration of follow-up | None |
| Minimum N of subjects | N ≥ 100 |
| Publication dates | Any |
| Predictor analyses | |
| Population | Predictors of CKD progression: any (including general population) except CKD G5D (dialysis); HCV as predictor: kidney transplant recipients |
| Predictor | HCV-infection (untreated), other predictors of CKD progression (if HCV-infected) |
| Outcome | CKD progression (change in GFR, SCr doubling, ESKD), proteinuria, patient mortality, graft loss, delayed graft function, kidney pathology (HCV-associated GN) |
| Design | Longitudinal, multivariable analyses; HCV-associated GN: any (except autopsy studies) |
| Minimum duration of follow-up | Any |
| Minimum N of subjects | ≥ 100 |
| Publication dates | Predictors of CKD progression: any; HCV as predictor: ≥ 2008 (plus studies in 2008 KDIGO CPG) |
2008 KDIGO CPG, 2008 KDIGO clinical practice guideline on hepatitis C34; CKD, chronic kidney disease; DAA, direct-acting antiviral; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; GN, glomerulonephritis; HCV, hepatitis C virus; NODAT, new-onset diabetes after transplantation; QoL, quality of life; RCT, randomized controlled trial; SCr, serum creatinine; SVR, sustained virologic response.
Establishing the process for guideline development
The ERT performed systematic literature searches and organized abstract and article screening. The ERT also coordinated the methodological and analytical processes and defined and standardized the methodology for performing literature searches, data extraction, and summarizing the evidence. The Work Group took the primary role of writing and grading the recommendation statements and rationales and retained final responsibility for their content. The Work Group Co-Chairs and the ERT prepared the first draft of the scope-of-work document as a series of open-ended questions to be considered by Work Group members.
Formulating questions of interest
Questions of interest were formulated according to the PICODD criteria (population, intervention, comparator, outcome, study design, and duration of follow-up). Details of the PICODD criteria are presented in Table 8.
Ranking of outcomes
The Work Group ranked outcomes of interest on the basis of their importance for informing clinical decision making (Table 9).
Table 9.
Hierarchy of outcomes
| Hierarchy | Outcome |
|---|---|
| Critical importance | Mortality, graft loss, ESKD |
| High importance | SVR, treatment discontinuation due to adverse events, serious adverse events, CKD incidence, quality of life, HCV seroconversion, test performance characteristics |
| Moderate importance | HCV relapse, kidney function, proteinuria, HCV positivity, hepatocellular carcinoma |
CKD, chronic kidney disease; ESKD, end-stage kidney disease; HCV, hepatitis C virus; SVR, sustained virologic response.
Literature searches and article selection
Systematic search strategies were developed by the ERT with input from the Work Group Co-Chairs. Modules were created for kidney disease, HCV, and study designs. Searches were conducted in Medline, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews. For topics covered in the 2008 KDIGO HCV CPG,34 searches were limited to 2008 and later to capture new evidence. For new topics, searches were not limited by publication date. The full literature search strategies are provided in Supplementary Appendix A. In addition, the ERT searched for existing relevant systematic reviews. The final searches were conducted in May 2017. The search yield was also supplemented by focused searches for DAAs, conference abstracts from the 2016 and 2017 American Society of Nephrology (ASN) and AASLD meetings, and articles provided by Work Group members through July 2018.
For selection of studies, all members of the ERT screened the abstracts in duplicate using an open-source online screening program, Abstrackr (http://abstrackr.cebm.brown.edu/). To establish relevance and consensus among reviewers, the entire team screened and achieved consensus on a series of initial batches of 100 abstracts. A total of 8703 citations from the databases were screened, in addition to 520 conference abstracts and 93 studies included in the 2008 KDIG HCV CPG (Figure 2). Journal articles reporting original data or systematic reviews were selected for evidence review, based on a priori criteria for eligible evidence. Of these, 487 were selected for consideration for inclusion. In total, 125 studies met eligibility criteria for extraction.
Figure 2.
Search yield. AASLD, American Association for the Study of Liver Diseases; ASN, American Society of Nephrology; CKD, chronic kidney disease; GL, guideline; HCV, hepatitis C virus; KDIGO HCV CPG, Kidney Disease: Improving Global Outcomes hepatitis C virus clinical practice guideline.
Data extraction
Data extraction was done by ERT research associates. Extracted data from each study was reviewed by another ERT member to confirm accuracy. The ERT designed forms to capture data on design, methodology, eligibility criteria, study participant characteristics, interventions, comparators, predictors, outcomes, and results of individual studies. Methodology and outcomes were also systematically assessed for risk of bias (see the section on risk of bias assessment below) and recorded during the data extraction process. Data were extracted into the online repository SRDR (Systematic Review Data Repository); the data are available for review at http://srdr.ahrq.gov/.
Summary tables
Summary tables were developed for each reviewed topic. Summary tables contain outcomes of interest, relevant population characteristics, description of intervention and comparator (or predictor), results, and quality grading for each outcome. Categorical outcomes and continuous outcomes were tabulated separately.
Work Group members reviewed and confirmed all summary table data and quality assessments. Summary tables are available as supplementary material at www.kisupplements.org.
Evidence profiles
Evidence profiles were constructed to assess the quality and record quality grades and descriptions of effect (or association) for each outcome across studies, as well as the quality of overall evidence and description of net benefits or harms of the intervention or comparator across all outcomes. These profiles aim to make the evidence synthesis process transparent. Decisions in the evidence profiles were based on data from the primary studies listed in corresponding summary tables and on judgments of the ERT and Work Group. When the body of evidence for a particular comparison of interest consisted of 2 or fewer studies, the summary table provided the final level of synthesis and an evidence profile was not generated. Each evidence profile was initially constructed by the ERT and then reviewed, edited, and approved by the Work Group. The work products created by the ERT for summarizing the evidence base are listed in Table 10, together with the number of included studies.
Table 10.
Work products for the guideline
| Topics | Summary table | Included studiesa, n | Evidence profile |
|---|---|---|---|
| 1. HCV testing | |||
| 1.1 Determining which CKD patients should be tested for HCV | − | (not searched) | |
| 1.2 HCV testing in CKD | − | (not searched) | |
| 1.3 Noninvasive versus invasive tests for cirrhosis in CKD | + | 11 | + |
| 1.4 HCV as predictor of CKD progression | + | 16 | + |
| 1.4 Other predictors of CKD progression | + | 1 | − |
| 2. HCV treatment | |||
| 2 HCV treatment (DAA, CKD nontransplant including hemodialysis) | + | 11 | + |
| 2 HCV treatment (peg-interferon, hemodialysis) | + | 6 | + |
| 2 HCV treatment (DAA, kidney transplant) | + | 5 | + |
| 2 HCV treatment (interferon, kidney transplant) | + | 4 | + |
| 2 DAA drug dosing | − | 10 PK studies | − |
| 3. HCV transmission | |||
| 3 Dialysis isolation | + | 7 | + |
| 4. Kidney transplantation | |||
| 4.1.1 Transplantation versus wait-list | + | 5 | + |
| 4.1.1 HCV as predictor, patient mortality | + | 5 | + |
| 4.1.1 HCV as predictor, graft loss | + | 7 | + |
| 4.1.2 Pre-transplant liver biopsy | − | 1 | − |
| 4.1.3 Timing of HCV treatment versus kidney transplantation | − | (based on GL 2) | − |
| 4.2 HCV-positive versus negative donor kidneys | + | 8 | − |
| 4.3 DAA and immunosuppression interaction | + | 4 | − |
| 4.4 HCV-related complications | − | (not searched) | − |
| 5. HCV-associated glomerulonephritis | |||
| 5.1 HCV-associated kidney disease prevalence | + | 5 | − |
| 5.2 HCV-associated glomerulonephritis management | + | 13 | + |
CKD, chronic kidney disease; DAA, direct-acting antiviral; GL, guideline; HCV, hepatitis C virus; peg, pegylated; PK, pharmacokinetic.
Plus 6 case reports on miscellaneous topics.
Grading of quality of evidence for outcomes of individual studies
Methodological quality (internal validity) refers to the design, conduct, and reporting of outcomes of a clinical study. A previously devised 3-level classification system for quality assessment was used to grade the overall study quality and quality of all relevant outcomes in the study (Table 11). Grading of individual studies was done by one of the reviewers, then confirmed by another, with discrepancies discussed in conference.
Table 11.
Classification of study quality
| Good quality Low risk of bias and no obvious reporting errors; complete reporting of data. Must be prospective. If study of intervention, must be RCT. |
| Fair quality Moderate risk of bias, but problems with study or paper are unlikely to cause major bias. If study of intervention, must be prospective. |
| Poor quality High risk of bias or cannot rule out possible significant biases. Poor methods, incomplete data, reporting errors. Prospective or retrospective. |
RCT, randomized controlled trial.
We based the methodological quality of each study on predefined criteria. For RCTs and other comparative studies, the ERT used the Cochrane risk of bias tool,351 which asks about risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. For observational studies, we also used selected questions from the Newcastle Ottawa Scale about comparability of cohorts, representativeness of the population, and adjustment for different lengths of follow-up.352 Based on these characteristics an overall assessment was made whether the study was of good, fair, or poor quality (Table 11).
Each reported outcome was then evaluated and given an individual grade depending on the quality of reporting and methodological issues specific to that outcome. However, the quality grade of an individual outcome could not exceed the quality grade for the overall study.
Grading the quality of evidence and the strength of a guideline recommendation
A structured approach, based on GRADE353, 354, 355 and facilitated by the use of evidence profiles, was used to grade the quality of the overall evidence and the strength of recommendations. For each topic, the discussion on grading of the quality of the evidence was led by the ERT, and the discussion regarding the strength of the recommendations was led by the Work Group Co-Chairs. The “strength of a recommendation” indicates the extent to which one can be confident that adherence to the recommendation will do more good than harm. The “quality of a body of evidence” refers to the extent to which our confidence in an estimate of effect is sufficient to support a particular recommendation.354
Grading the quality of evidence for each outcome across studies
Following GRADE, the quality of a body of evidence pertaining to a particular outcome of interest was initially categorized on the basis of study design. For each outcome, the potential grade for the quality of evidence for each intervention-outcome pair started at high but was then lowered if there were serious limitations to the methodological quality of the aggregate of studies, if there were important inconsistencies in the results across studies, if there was uncertainty about the directness of evidence including limited applicability of the findings to the population of interest, if the data were imprecise (a low event rate [0 or 1 event] in either arm or a CI spanning a range > 1) or sparse (only 1 study or total N < 500), or if there was thought to be a high likelihood of bias. The final grade for the quality of the evidence for an intervention-outcome pair could be one of the following 4 grades: high, moderate, low, or very low (Table 12).
Table 12.
GRADE system for grading quality of evidence
| Step 1: starting grade for quality of evidence based on study design | Step 2: reduce grade | Step 3: raise grade | Final grade for quality of evidence and definition |
|---|---|---|---|
| Randomized trials = high Observational study = low Any other evidence = very low |
Study quality −1 level if serious limitations −2 levels if very serious limitations Consistency −1 level if important inconsistency Directness −1 level if some uncertainty −2 levels if major uncertainty Other −1 level if sparse or imprecise datac −1 level if high probability of reporting bias |
Strength of association +1 level if strong,a no plausible confounders +2 levels if very strong,b no major threats to validity Other +1 level if evidence of a dose-response gradient +1 level if all residual plausible confounders would have reduced the observed effect |
High = further research is unlikely to change confidence in the estimate of the effect Moderate = further research is likely to have an important impact on confidence in the estimate of effect, and may change the estimate Low = further research is very likely to have an important impact on confidence in the estimate, and may change the estimate Very low = any estimate of effect is very uncertain |
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
Strong evidence of association is defined as “significant relative risk of > 2 (< 0.5)” based on consistent evidence from 2 or more observational studies, with no plausible confounders.
Very strong evidence of association is defined as “significant relative risk of > 5 (< 0.2)” based on direct evidence with no major threats to validity.
Sparse if there is only 1 study or if total N < 500, and imprecise if there is a low event rate (0 or 1 event) in either arm or confidence interval spanning a range > 1.
Adapted by permission from Uhlig K, Macleod A, Craig J, et al.353
Grading the overall quality of evidence
The quality of the overall body of evidence was then determined on the basis of the quality grades for all outcomes of interest, taking into account explicit judgments about the relative importance of each outcome. The resulting 4 final categories for the quality of overall evidence were A, B, C, or D (Table 13).
Table 13.
Final grade for overall quality of evidence
| Grade | Quality of evidence | Meaning |
|---|---|---|
| A | High | We are confident that the true effect lies close to that of the estimate of the effect. |
| B | Moderate | The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| C | Low | The true effect may be substantially different from the estimate of the effect. |
| D | Very low | The estimate of effect is very uncertain, and often will be far from the truth. |
Assessment of the net health benefit across all important clinical outcomes
The net health benefit was determined on the basis of the anticipated balance of benefits and harms across all clinically important outcomes (Table 14). The assessment of net benefit also involved the judgment of the Work Group and the ERT.
Table 14.
Balance of benefits and harms
When there was evidence to determine the balance of medical benefits and harms of an intervention to a patient, conclusions were categorized as follows:
|
Developing the recommendations
Draft recommendation statements were developed by the Work Group Co-Chairs and Work Group members with input from all Work Group members. The health benefits, side effects, and risks associated with each recommendation were considered when formulating the guideline, as well as information on patient preferences when available. Recommendation statements were revised in a multistep process during face-to-face meetings and by subsequent drafts by e-mail. Relevant recommendations from the AASLD and EASL guidelines on management of HCV were reviewed to maximize consistency between guidelines. The final draft was sent for external public review. Based on feedback, it was further revised by the Work Group Co-Chairs and members. All Work Group members provided feedback on initial and final drafts of the recommendation statements and guideline text and approved the final version of the guideline.
Grading the strength of the recommendations
The strength of a recommendation is graded as level 1 or level 2. Table 15 shows the KDIGO nomenclature for grading the strength of a recommendation and the implications of each level for patients, clinicians, and policy makers. Recommendations can be for or against doing something. Each recommendation includes an explicit link between the quality of the available evidence and the strength of that recommendation. However, Table 16 shows that the strength of a recommendation is determined not only by the quality of the evidence but also by other, often complex judgments regarding the size of the net medical benefit (potential risks vs. benefit), values, and preferences, and costs. Formal decision analyses including cost analysis were not conducted.
Table 15.
KDIGO nomenclature and description for grading recommendations
| Gradea | Implications |
||
|---|---|---|---|
| Patients | Clinicians | Policy | |
|
Level 1 “We recommend” |
Most people in your situation would want the recommended course of action and only a small proportion would not. | Most patients should receive the recommended course of action. | The recommendation can be evaluated as a candidate for developing a policy or a performance measure. |
|
Level 2 “We suggest” |
The majority of people in your situation would want the recommended course of action, but many would not. | Different choices will be appropriate for different patients. Each patient needs help to arrive at a management decision consistent with her or his values and preferences. | The recommendation is likely to require substantial debate and involvement of stakeholders before policy can be determined. |
KDIGO, Kidney Disease: Improving Global Outcomes.
The additional category “not graded” was used, typically, to provide guidance based on common sense or where the topic does not allow adequate application of evidence. The most common examples include recommendations regarding monitoring intervals, counseling, and referral to other clinical specialists. The ungraded recommendations are generally written as simple declarative statements. They should not be interpreted as being weaker recommendations than Level 1 or 2 recommendations.
Table 16.
Determinants of strength of recommendation
| Factor | Comment |
|---|---|
| Balance between desirable and undesirable effects | The larger the difference between the desirable and undesirable effects, the more likely a strong recommendation is warranted. The narrower the gradient, the more likely a weak recommendation is warranted. |
| Quality of the evidence | The higher the quality of evidence, the more likely a strong recommendation is warranted. |
| Values and preferences | The more variability in values and preferences, or the more uncertainty in values and preferences, the more likely a weak recommendation is warranted. Values and preferences were obtained from the literature where possible or were assessed in the judgment of the Work Group where robust evidence was not identified. |
| Costs (resource allocation) | The higher the costs of an intervention—that is, the more resources consumed—the less likely a strong recommendation is warranted. |
Ungraded statements
This category was designed to allow the Work Group to issue general advice. Typically an ungraded statement meets the following criteria: it provides guidance based on common sense; it provides reminders of the obvious; and it is not sufficiently specific to allow for application of evidence to the issue and therefore it is not based on systematic evidence review. As such, ungraded statements may be considered to be relatively strong recommendations; they should not be interpreted as weak recommendations based on limited or poor evidence. Common examples include recommendations about frequency of testing, referral to specialists, and routine medical care. We strove to minimize the use of ungraded recommendations.
This grading scheme, with 2 levels for the strength of a recommendation together with four levels of grading the quality of the evidence, as well as the option of an ungraded statement for general guidance, was adopted by the KDIGO Board in December 2008. The Work Group took on the primary role of writing the recommendations and rationale statements and retained final responsibility for the content of the guideline statements and the accompanying narrative. The ERT reviewed draft recommendations and grades for consistency with the conclusions of the evidence review.
Format for guideline recommendations
Each chapter contains 1 or more specific recommendations. Within each recommendation, the strength of recommendation is indicated as level 1 or level 2 and the quality of the supporting evidence is shown as A, B, C, or D. The recommendation statements and grades are followed by the rationale text summarizing the key points of the evidence base and the judgments supporting the recommendation. In relevant sections, considerations of the guideline statements in international settings and suggested audit criteria are also provided where applicable. Important key points and research recommendations suggesting future research to resolve current uncertainties are also outlined at the conclusion of each chapter.
Limitations of approach
Although the literature searches were intended to be comprehensive, they were not exhaustive. Medline, Embase, and Cochrane databases were searched, but other specialty or regional databases were not. Hand searches of journals were not performed, and review articles and textbook chapters were not systematically searched. Recent conference abstracts were screened from ASN and AASLD, but older conference abstracts and other conference meetings were not specifically screened. We relied on Work Group members to provide the ERT with conference abstracts from recent EASL meetings. However, any important studies known to domain experts that were missed by the electronic literature searches were added to retrieved articles and reviewed by the Work Group.
Review of guideline development process
The Conference on Guideline Standardization (COGS) checklist has been developed to assess the quality of the methodological process for systematic review and guideline development.356 Table 17 shows the criteria that correspond to the COGS checklist and how each one is addressed in this guideline. Similarly, Supplementary Appendix B demonstrates the level of concurrence with which this guideline corresponds to the Institute of Medicine’s standards for systematic reviews and guidelines.348, 349
Table 17.
The Conference on Guideline Standardization (COGS) checklist for reporting clinical practice guidelines
| Topic | Description | Discussed in 2018 KDIGO HCV in CKD CPG |
|---|---|---|
| 1. Overview material | Provide a structured abstract that includes the guideline’s release date, status (original, revised, updated), and print and electronic sources. | See Abstract and Methods for Guideline Development. |
| 2. Focus | Describe the primary disease/condition and intervention/service/technology that the guideline addresses. Indicate any alternative preventative, diagnostic, or therapeutic interventions that were considered during development. | Management of HCV in terms of treatment, monitoring, and prevention in adults with CKD, including both dialysis and transplant populations. |
| 3. Goal | Describe the goal that following the guideline is expected to achieve, including the rationale for development of a guideline on this topic. | This CPG is intended to assist the practitioner caring for patients with CKD and HCV and to prevent transmission, resolve the infection, and prevent adverse outcomes such as deaths, graft loss, and progression to kidney failure while optimizing patients’ quality of life. |
| 4. User/setting | Describe the intended users of the guideline (e.g., provider types, patients) and the settings in which the guideline is intended to be used. | Target audience is practicing nephrologists and other health care providers for adults with CKD and HCV infection. |
| 5. Target population | Describe the patient population eligible for guideline recommendations and list any exclusion criteria. | Adults with CKD and HCV infection; CKD patients on dialysis therapy. |
| 6. Developer | Identify the organization(s) responsible for guideline development and the names/credentials/potential conflicts of interest of individuals involved in the guideline’s development. | Organization: KDIGO. Names/credentials/potential conflicts of interest of individuals involved in the guideline’s development are disclosed in the Biographic and Disclosure Information. |
| 7. Funding source/sponsor | Identify the funding source/sponsor and describe its role in developing and/or reporting the guideline. Disclose potential conflict of interest. | This guideline is funded by KDIGO. Financial disclosures of Work Group members are published in Biographic and Disclosure Information section of the guideline. |
| 8. Evidence collection | Describe the methods used to search the scientific literature, including the range of dates and databases searched, and criteria applied to filter the retrieved evidence. | Topics were triaged either to (i) systematic review, (ii) systematic search followed by narrative summary, or (iii) narrative summary. For systematic reviews, we searched PubMed, Embase, Cochrane Central Registry for trials, and Cochrane database of systematic reviews. Screening criteria for this and other topics are outlined in the Methods for Guideline Development chapter. The search was updated through May 2017 and supplemented by articles identified by Work Group members through July 2018. We also searched for pertinent existing guidelines and systematic reviews. |
| 9. Recommendation grading criteria | Describe the criteria used to rate the quality of evidence that supports the recommendations and the system for describing the strength of the recommendations. Recommendation strength communicates the importance of adherence to a recommendation and is based on both the quality of the evidence and the magnitude of anticipated benefits and harms. | Quality of individual studies was graded in a 3-tiered grading system (see Table 11). Quality of evidence and strength of recommendations were graded following the GRADE approach (Table 12, Table 13, Table 15). The Work Group could provide general guidance in the form of ungraded statements. |
| 10. Method for synthesizing evidence | Describe how evidence was used to create recommendations, e.g., evidence tables, meta-analysis, decision analysis. | For systematic review topics, summary tables and evidence profiles were generated. For recommendations on interventions, the steps outlined by GRADE were followed. |
| 11. Prerelease review | Describe how the guideline developer reviewed and/or tested the guidelines prior to release. | The guideline had undergone external public review in February 2017. Public review comments were compiled and fed back to the Work Group, which considered comments in its revision of the guideline. |
| 12. Update plan | State whether or not there is a plan to update the guideline and, if applicable, an expiration date for this version of the guideline. | The requirement for an update will be assessed periodically from the publication date or earlier if important new evidence becomes available in the interim. Such evidence might, for example, lead to changes to the recommendations or may modify information provided on the balance between benefits and harms of a particular therapeutic intervention. |
| 13. Definitions | Define unfamiliar terms and those critical to correct application of the guideline that might be subject to misinterpretation. | See Abbreviations and Acronyms. |
| 14. Recommendations and rationale | State the recommended action precisely and the specific circumstances under which to perform it. Justify each recommendation by describing the linkage between the recommendation and its supporting evidence. Indicate the quality of evidence and the recommendation strength, based on the criteria described in Topic 9. | Each guideline chapter contains recommendations for the management of HCV in CKD patients. Each recommendation builds on a supporting rationale with evidence tables if available. The strength of the recommendation and the quality of evidence are provided in parenthesis within each recommendation. |
| 15. Potential benefits and harms | Describe anticipated benefits and potential risks associated with implementation of guideline recommendations. | The benefits and harm for each comparison of interventions are provided in summary tables and summarized in evidence profiles. The estimated balance between potential benefits and harm was considered when formulating the recommendations. |
| 16. Patient preferences | Describe the role of patient preferences when a recommendation involves a substantial element of personal choice or values. | Recommendations that are level 2, or “discretionary,” indicate a greater need to help each patient arrive at a management decision consistent with her or his values and preferences. |
| 17. Algorithm | Provide (when appropriate) a graphical description of the stages and decisions in clinical care described by the guideline. | Algorithms were developed where applicable (see Chapters 2 and 4). |
| 18. Implementation considerations | Describe anticipated barriers to application of the recommendations. Provide reference to any auxiliary documents for providers or patients that are intended to facilitate implementation. Suggest review criteria for measuring changes in care when the guideline is implemented. | These recommendations are global. Local versions of the guideline are anticipated to facilitate implementation and appropriate care. Review criteria were not suggested because implementation with prioritization and development of review criteria have to proceed locally. Most recommendations are discretionary, requiring substantial discussion among stakeholders before they can be adopted as review criteria. The decision whether to convert any recommendations to review criteria will vary globally. Research recommendations were also outlined to address current gaps in the evidence base. |
CKD, chronic kidney disease; CPG, clinical practice guideline; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HCV, hepatitis C virus; KDIGO, Kidney Disease: Improving Global Outcomes.
Biographic and disclosure information

Michel Jadoul, MD (Work Group Co-Chair), received his MD degree in 1983 at the Université Catholique de Louvain (UCL), Brussels, Belgium. Dr. Jadoul trained in internal medicine and nephrology under the mentorship of Professor Charles van Ypersele de Strihou. He has served as chair at the Department of Nephrology of the Cliniques Universitaires Saint-Luc since 2003 and is currently full clinical professor at UCL. Dr. Jadoul’s clinical activities focus on the follow-up of hemodialysis and CKD patients, and his main research interests include β2-microglobulin amyloidosis, hepatitis C, and other complications (e.g., falls, bone fractures, sudden death) in hemodialysis patients, as well as cardiovascular complications after kidney transplantation and various causes of kidney disease (e.g., drug-induced).
Dr. Jadoul has co-authored over 230 scientific papers, most of them published in major nephrology journals. He is currently serving as a theme editor of Nephrology Dialysis Transplantation, and he is also a country co-investigator for the Dialysis Outcomes and Practice Patterns Study (DOPPS) (2001–present). In 2008, he received the international distinguished medal from the US National Kidney Foundation. He was previously a member of the KDIGO Executive Committee (2010–2015) and the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Council (2013–2016). Presently, Dr. Jadoul is the KDIGO Co-Chair Elect.
Consultant: Astellas, GlaxoSmithKline, Merck Sharp & Dohme, Vifor Fresenius Medical Care Renal Pharma
Grant/research support: Alexion, Amgen, Janssen-Cilag, Merck Sharp & Dohme Otsuka, Roche
Speaker: AbbVie, Amgen, Menarini, Merck Sharp & Dohme, Vifor Fresenius Medical Care Renal Pharma
Travel: Amgen
All monies paid to institution.

Paul Martin, MD, FRCP, FRCPI (Work Group Co-Chair), is professor of medicine, Mandel Chair of Gastroenterology, and chief of the Division of Gastroenterology and Hepatology at the University of Miami, USA. He graduated from medical school at University College, Dublin, Ireland and trained in internal medicine and gastroenterology in Dublin and in Canada. He was a medical staff fellow in the Liver Unit of the National Institutes of Health, where he trained with Dr. Jay Hoofnagle. He is Editor-in-Chief of Liver Transplantation and co-editor of Handbook of Liver Disease. Dr. Martin was previously a councilor for the American Society of Transplantation and has had a long-standing interest in viral hepatitis and organ transplantation. He was the Sheila Sherlock Lecturer for the Internal Association for the Study of Liver Disease in 2004 and received the Charles Trey Award from the American Liver Foundation in 2001.
Board member: AbbVie, Bayer, Bristol-Myers Squibb
Grant/research support: AbbVie*, Bristol-Myers Squibb*, Gilead*, Merck*
*Monies paid to institution.

Marina C. Berenguer, MD, is a consultant hepatologist at La Fe University Hospital in Valencia, Spain, and professor of medicine at the University of Valencia. She was trained in medicine at the University of Valencia before completing a fellowship at the Veterans Affairs Medical Center / University of California, San Francisco, with Dr. Teresa Wright.
Prof. Berenguer is well recognized for her important contributions to the field of post-transplantation HCV liver disease, where she has been involved in the creation of various consensus documents on viral hepatitis and liver disease. She is also an active committee member for several national and international hepatology and liver transplantation societies. Prof. Berenguer has also coordinated research within a national research network in hepato-gastroenterology (“Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas,” CIBER-ehd) since its creation in 2006.
Prof. Berenguer previously served as associate editor for the Journal of Hepatology and Liver Transplantation until December 2014, and is now deputy editor for Transplantation. She has authored more than 300 publications in peer-reviewed journals as well as over 70 chapters in international and national textbooks.
Consultant: AbbVie, Gilead, Merck Sharp & Dohme
Grant/research support: Gilead*
Speaker: AbbVie, Astellas, Gilead, Merck Sharp & Dohme, Novartis
*Monies paid to institution.

Wahid Doss, MD, is professor of hepatogastroenterolgy and endemic medicine at Cairo University, Egypt. Dr. Doss became the Head of the National Hepatology Institute, Cairo, from 2006 through 2015, and he is presently the Head of the National Committee for the Control of Viral Hepatitis since 2006. Together with his colleagues, he established and supervised one of the most comprehensive hepatitis treatment programs worldwide, which has received acclaim from local and international organizations including the World Health Organization. Dr. Doss is a founding member of the gastrointestinal endoscopy unit at Kasr El Aini Hospital, Cairo University, and maintains a special interest in therapeutic endoscopic procedures. A member of EASL, AASLD, and American Society for Gastrointestinal Endoscopy, he is also a founding member and current vice president of the Egyptian Liver Care Society.
Dr. Doss declared no competing interests.

Fabrizio Fabrizi, MD, is staff nephrologist and professor of medicine at Maggiore Policlinico Hospital and IRCCS Foundation, Milan, Italy. His research focus is aimed at the understanding of the epidemiology, natural history, and management of viral hepatitis (HBV and HCV) in the CKD population through laboratory work, clinical research studies, and clinical trials. He has received grants from the Italian Society of Nephrology and fellowships from the Society of Italian-American Nephrologists as support for his research projects. Dr. Fabrizi has actively participated in the development of numerous national and international guidelines regarding the management of viral hepatitis in CKD patients, including the inaugural 2008 KDIGO HCV guideline. He currently serves on the editorial board of the International Journal of Artificial Organs and the Journal of Nephrology, and has authored more than 250 publications in peer-reviewed journals such as Kidney International, the American Journal of Kidney Diseases, and the Clinical Journal of the American Society of Nephrology, among others.
Board member: AbbVie, Merck Sharp & Dohme
Consultant: AbbVie

Jacques Izopet, PharmD, PhD, is professor of virology at Toulouse University and head of the Federative Institute of Biology at Toulouse University Hospital, France. He is also head of a research team in the Pathophysiology Center of Toulouse-Purpan – INSERM UMR 1043/CNRS 5282. His primary research area has centered on viral persistence, host response, and pathophysiology, with a particular focus on HIV tropism and hepatitis E virus infection in immunocompetent and immunocompromised patients. Dr. Izopet has published over 450 papers in international journals.
Dr. Izopet declared no competing interests.

Vivekanand Jha, MBBS, MD, DM, FRCP, FRCP (Edin) FAMS, is the executive director at The George Institute for Global Health India, professor of nephrology at University of Oxford, UK, and the president-elect of the International Society of Nephrology. Prof. Jha received his internal medicine and nephrology training at the Postgraduate Institute of Medical Education and Research (PGIMER) in Chandigarh, India, and a research fellowship at Harvard University. He was a professor of nephrology, led the Stem Cell Research Facility, and was head of the Department of Translational and Regenerative Medicine at PGIMER.
Prof. Jha focuses on the study of emerging public health threats globally and in India, and in finding solutions using innovative methodologies appropriate for emerging countries. He currently spearheads research projects in more than 20 countries with a particular interest in the understanding of global burden of kidney diseases, the social- and disease-related drivers of diseases and their determinants of outcome. Prof. Jha is an expert in the effect of tropical ecology on kidney diseases and the impact of infections on patients with kidney diseases. He has served as a Work Group member on prior KDIGO guidelines including the management of patients with glomerulonephritis and the care of kidney transplant recipients.
Consultant: NephroPlus*
Grants/research support: Baxter Healthcare*, GlaxoSmithKline*
Speaker: Baxter Healthcare*
*Monies paid to institution.

Nassim Kamar, MD, PhD, is a professor of nephrology at Toulouse University Hospital in Toulouse, France, and is the head of the Department of Nephrology and Organ Transplantation at Toulouse University Hospital. Dr. Kamar received his medical degree from Dijon University, France. Thereafter, he received internship at Toulouse University, France, where he graduated with a specialty in nephrology. Dr. Kamar received additional training in kidney replacement therapy and medical pedagogy. He also completed a 1-year postdoctoral fellowship in basic research at the Department of Nephrology, La Charité Hospital, Berlin, Germany. Dr. Kamar was awarded his PhD in 2006.
Dr. Kamar’s interests include the studying of viral infection, particularly hepatitis E virus, HCV, and cytomegalovirus infections that develop after solid organ transplantation. He is also interested in immunosuppression after solid organ transplantation. Dr. Kamar has published over 450 papers in peer-reviewed journals and was a member of The Council of the International Transplant Infectious Disease Society. He has received numerous awards, including la Fondation du Rein (2008), the Grand Prix de Médecine from the Académie des Sciences, Inscriptions et Belles-Lettres de Toulouse (2009), and the Palme de Médecine des CHU (2015).
Board member: Astellas, Merck Sharp & Dohme, Novartis, Shire
Consultant: Novartis
Speaker: AbbVie, Amgen, Astellas, Chiesi, Fresenius, Gilead, Merck Sharp & Dohme, Neovii, Novartis, Roche, Sanofi, Shire

Bertram L. Kasiske, MD, FACP, obtained his undergraduate training at Michigan State University, East Lansing, MI, USA. He received his medical degree from the University of Iowa, Iowa City, IA, USA and completed an internal medicine residency and fellowship training in nephrology at Hennepin County Medical Center, an affiliate hospital of the University of Minnesota in Minneapolis, USA. Dr. Kasiske is former deputy director of the US Renal Data System, former Editor-in-Chief of the American Journal of Kidney Diseases, and former Co-Chair of Kidney Disease: Improving Global Outcomes (KDIGO). Currently he is director of nephrology at Hennepin County Medical Center and professor of medicine at the University of Minnesota. Dr. Kasiske is the principal investigator for a National Institutes of Health grant to study long-term effects of living kidney donation. He is also the director of the Scientific Registry of Transplant Recipients, which is a federal registry of solid organ transplants in the US.
Speaker: Novartis

Ching-Lung Lai, MD, FRCP, FRACP, FHKAM (Med), FHKCP, FAASLD, is the Simon K Y Lee Professor in Gastroenterology and the Chair Professor of Medicine and Hepatology at the Department of Medicine, University of Hong Kong, where he has been working since his graduation with honors from the university. For the last 4 decades he has been extensively involved in research on various aspects of HBV, including its molecular virology, natural history, treatment, and prevention. Prof. Lai is one of the lead investigators in the pivotal trials of various nucleos(t)ide analogues that have revolutionized the treatment of chronic hepatitis B. More recently he has been involved in studies for the treatment of chronic hepatitis C.
Prof. Lai has published over 500 peer-reviewed papers and reviews in international journals. His publications have been widely cited, and he is one of top scientists in the field of chronic hepatitis B infection. Prof. Lai was also invited to give the Leon Schiff State-of-the-Art Lecture at the 2005 annual meeting of the American Association for the Study of Liver Diseases (AASLD), entitled “The natural history and treatment of chronic hepatitis B: consensus and controversies,” and he has co-edited a book entitled Hepatitis B Virus.
Board member: Arrowhead Research Corporation*
Speaker: AbbVie, Gilead Sciences Hong Kong Limited
*Monies paid to institution.

Jose M. Morales, MD, PhD, is professor of medicine and senior investigator of the Research Institute in the Hospital 12 de Octubre (Madrid, Spain), educational ambassador of the International Society of Nephrology, and associate editor of Clinical Transplantation.
He was medical director of the Renal Transplant Program of the Hospital 12 de Octubre in Madrid (one of the largest hospitals in Spain), chief of the Renal Transplant Office, and chief of the Section of Nephrology/Renal Transplantation. Prof. Morales also served as the past president of the Madrid Transplantation Society and a council member of the Spanish Transplantation Society. In addition, he was a council member of ERA-EDTA (1998–2001) and a medical coordinator of Forum Renal from Spain.
Prof. Morales has published over 300 articles in peer-reviewed journals, and he has served as reviewer in the main nephrology and transplantation journals. His principal areas of scientific interest include clinical nephrology/transplantation, and immunosuppression and HCV. Prof. Morales has also been a principal investigator for many important trials involving immunosuppressive agents such as tacrolimus, extended-release tacrolimus, rapamycin, mycophenolate mofetil, everolimus, and belatacept.
He was a member of the expert group that developed the European Best Practice Guidelines of Renal Transplantation (2000–2002) and a prior member of the Work Group that developed the 2008 KDIGO CPG on HCV in CKD. Recently, Madrid was chosen to host the Transplantation Society Congress in July 2018, and Dr. Morales is president of the local committee and vice chair of the 27th International Congress of the Transplantation Society.
Consultant: Merck Sharp & Dohme
Speaker: Astellas, Merck Sharp & Dohme

Priti R. Patel, MD, MPH, is a medical officer in the Division of Healthcare Quality Promotion at the US Centers for Disease Control and Prevention (CDC), where she leads CDC’s dialysis safety efforts. She is also adjunct assistant professor of family and preventive medicine at the Emory University School of Medicine. Dr. Patel earned a Master of Public Health degree from Columbia University and received her medical degree from Howard University College of Medicine (1999). She completed a residency in internal medicine at the University of Pennsylvania (2002) and residency in preventive medicine at CDC (2005). She received training as an officer in the Epidemic Intelligence Service (EIS) program assigned to CDC’s Division of Viral Hepatitis (2004). In her work at CDC, Dr. Patel has supervised numerous outbreak investigations in dialysis centers, has contributed to CDC guidance documents, and develops resources and strategies to help prevent infections among dialysis patients. Dr. Patel has authored more than 80 peer-reviewed publications, largely focused on health care–associated infection prevention and patient safety. She is the director of CDC’s Making Dialysis Safer for Patients Coalition and a member of the Nephrologists Transforming Dialysis Safety Project Committee.
Dr. Patel declared no competing interests.

Stanislas Pol, MD, PhD, is professor of hepatology and gastroenterology at Université Paris Descartes, Paris, France, and head of the liver department at Cochin Hospital, Paris, France. He completed hepatology and gastroenterology residency and chief residency at the Necker-Enfants Malades University, and a molecular enzymology fellowship in Henri Mondor Hospital. Dr. Pol completed his MD thesis on occult HBV infections in 1983 and his PhD thesis on the regulation of iso-enzymes of aspartate aminotransferase in liver disease in 1992. Dr. Pol’s current research interests involve studying the impact of immune deficiency, including HIV, on the natural history of viral hepatitis; the treatment of viral hepatitis; and the reversal of cirrhosis.
He is a co-leader of a research INSERM unit (U1223 of Institut Pasteur) studying the immune pathology of HCV infection.
Dr. Pol is the recipient of several research awards and fellowships and has published more than 350 primary and review articles in the field of liver diseases. He has previously chaired the coordinated action 24 (AC 24) of the French Agency for AIDS and Viral Hepatitis (ANRS: therapeutic trials in viral hepatitis), and he is presently the clinical head of the French ANRS HEPATHER cohort, which includes HBV and HCV patients. He is also the director of the Center of Translational Research of Institut Pasteur since 2015.
Board member: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharp & Dohme
Consultant: AbbVie, Gilead
Speaker: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharp & Dohme

Marcelo O. Silva, MD, is the head of hepatology and liver transplant units at Austral University Hospital in Pilar, Argentina. He earned his medical degree with honors from the University of Buenos Aires, and completed his post-graduate medical education in internal medicine and gastroenterology at the University of Buenos Aires Hospital. Dr. Silva obtained hepatology training with a research and clinical fellowship at the Center for Liver Diseases, University of Miami School of Medicine. Upon completion of his fellowship, Dr. Silva served as assistant professor of clinical medicine at the University of Miami, FL.
He has extensive experience in clinical trials involving chronic hepatitis B and C. Dr. Silva has published more than 60 papers in peer-reviewed journals, contributed over 100 abstracts and presentations in scientific meetings, and authored several book chapters. He also developed the Latin American Liver Research Education and Awareness Network to promote research education and awareness of liver diseases in the region. In January 2014, he was appointed as a board member of the World Health Organization Viral Hepatitis Scientific and Technical Advisory Committee Committee.
Board member: AbbVie, Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme
Grants/research support: AbbVie*, Bristol-Myers Squibb*, Gilead*, Merck Sharp & Dohme*
Speaker: AbbVie, Bristol-Myers Squibb, Merck Sharp & Dohme
Development of educational presentations: AbbVie, Bristol-Myers Squibb, Merck Sharp & Dohme
Travel: AbbVie, Bristol-Myers Squibb, Gador
KDIGO Co-Chairs

David C. Wheeler, MD, FRCP, is professor of kidney medicine at University College London, UK, an honorary consultant nephrologist at the Royal Free London NHS Foundation Trust, and an honorary professorial fellow of the George Institute for Global Health. He is a clinician scientist with an interest in the complications of CKD, specifically those that increase the burden of cardiovascular disease and/or accelerate progression of kidney failure. He has participated in the design, roll-out, and monitoring of several large-scale clinical trials including the Study of Heart and Renal Protection (SHARP) and the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE). He currently sits on the steering committee of Canaglifozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and is co-chief investigator of the Dapagliflozin in CKD (DAPA-CKD) study. He is clinical lead for Division 2 of the North Thames Clinical Research Network and heads a team of 10 clinical trial nurses and practitioners at the Centre for Nephrology, Royal Free Hospital in London. He has been involved in clinical practice guideline development for several organizations, most recently for KDIGO, of which he is currently Co-Chair. He is past president of the UK Renal Association and past chair of the UK Renal Registry. His other responsibilities include membership of the editorial board of the Journal of the American Society of Nephrology and of the Executive Committee of Standardised Outcomes in Nephrology (SONG).
Consultant: Akebia, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daichii-Sankyo, GlaxoSmithKline, Janssen, Vifor Fresenius Medical Care Renal Pharma
Grants/research support: AstraZeneca
Speaker: Amgen, Vifor Fresenius Medical Care Renal Pharma

Wolfgang C. Winkelmayer, MD, MPH, ScD, is the Gordon A. Cain Chair of Nephrology and professor of medicine at Baylor College of Medicine in Houston, TX, USA. Dr. Winkelmayer received his medical degree (1990) from the University of Vienna, Austria, and later earned a Master of Public Health in health care management (1999) and a Doctor of Science in health policy (2001) from Harvard University. He then spent 8 years on the faculty of Brigham and Women’s Hospital and Harvard Medical School, where he established himself as a prolific investigator and leader in the discipline of comparative-effectiveness research as it pertains to patients with kidney disease. From 2009 to 2014, he was the director of clinical research in the Division of Nephrology at Stanford University School of Medicine, Palo Alto, CA, USA. He assumed his current position as chief of nephrology at Baylor College of Medicine in September 2014. His main areas of research interest include comparative effectiveness and safety research of treatment strategies for anemia, as well as of various interventions for cardiovascular disease in patients with kidney disease. Dr. Winkelmayer is a member of the American Society of Clinical Investigation. His clinical passion lies in providing quality kidney care to the predominantly disadvantaged and un(der)insured population in the public safety net health system of Harris County, TX. Dr. Winkelmayer has authored over 300 peer-reviewed publications, and he has a particular interest in medical publishing. He currently serves as an associate editor for the Journal of the American Medical Association, was a co-editor of the American Journal of Kidney Disease from 2007 to 2016, and has been appointed to several other editorial boards of leading nephrology and epidemiology journals. He also volunteers his time toward important initiatives of the American Society of Nephrology (e.g., Public Policy Board).He joined KDIGO volunteer leadership as an executive committee member in 2015 and has served as its Co-Chair since 2016.
Consultant: Akebia, Amgen, AstraZeneca, Bayer, Daichii-Sankyo, Relypsa, Vifor Fresenius Medical Care Renal Pharma
Speaker: FibroGen
Evidence review team

Ethan M. Balk, MD, MPH, is associate director of the Center for Evidence Synthesis in Health and associate professor at Brown University School of Public Health in Providence, RI, USA. He is project director of the evidence review team and has collaborated on numerous KDIGO guidelines, and prior to that on Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines since 2000. As project director for this guideline, he played a role in providing methodological expertise in the guideline development process and assisted in the collection, evaluation, grading, and synthesis of evidence and the revisions of the final evidence report. Dr. Balk also provided methodological guidance and training of Work Group members regarding topic refinement, key question formulation, data extraction, study assessment, evidence grading, and recommendation formulation. His primary research interests are evidence-based medicine, systematic review, clinical practice guideline development, and critical literature appraisal.
Dr. Balk declared no competing interests.

Craig Gordon, MD, MS, is associate professor of medicine at Boston University Medical Center and training program director for the nephrology fellowship at Boston Medical Center, USA. Dr. Gordon graduated from New York University School of Medicine and received his master’s degree from the Tufts University Sackler School of Graduate Biomedical Sciences in Clinical Care Research. Dr. Gordon previously served as the assistant project director of the evidence review team for the 2008 KDIGO CPG on HCV in CKD. He served as the associate director of the evidence review team and assistant project director for the 2018 KDIGO CPG on HCV in CKD. Dr. Gordon provided methodologic expertise to the Work Group during the guideline development process and assisted in the collection, evaluation, grading, and synthesis of evidence for the guideline, as well providing guidance to Work Group members in the areas of topic refinement, key question formulation, data extraction, study assessment, evidence grading, and recommendation formulation. His primary research interests are in the management of HCV in patients with CKD, as well as evidence-based medicine and systematic review related to other areas of nephrology.
Board member: AbbVie
Consultant: Alexion
Mengyang Di, MD, PhD, is currently a medical resident at Rhode Island Hospital, Alpert Medical School, Brown University, Providence, RI, USA. She was a member of the evidence review team as a postdoctoral research associate at the Center for Evidence Synthesis in Health, Brown University School of Public Health. Dr. Di obtained her medical degree from the Chinese Academy of Medical Sciences and Peking Union Medical College, and her PhD in epidemiology from the Chinese University of Hong Kong. She was a core member of the evidence review team and performed key functions including study selection, data extraction, data analysis, drafting of evidence tables, and critical literature appraisals. Her research interests include systematic review, meta-analysis, and decision analysis.
Dr. Di declared no competing interests.
Amy Earley, BS, is a research associate with the evidence review team from the Center for Evidence Synthesis in Health at Brown University in Providence, RI, USA. She is key in conducting the evidence review, which includes running searches, screening, data extraction, drafting of tables and methods sections, proofing of guideline drafts, and critical literature appraisal. She also holds an important role in coordinating the guideline development activities within the evidence review team, especially in the development of the evidence reports for all guidelines. In addition to her role with the evidence review team, Ms. Earley works as a senior research associate at Evidera, where she is a lead researcher and principal investigator on qualitative and quantitative meta-research projects (meta-analyses and indirect treatment comparisons).
Ms. Earley declared no competing interests.
Acknowledgments
A special debt of gratitude is owed to the KDIGO Co-Chairs, David Wheeler and Wolfgang Winkelmayer, for their invaluable guidance throughout the development of this guideline. In particular, we thank Ethan Balk, Craig Gordon, and the ERT members for their substantial contribution to the rigorous assessment of the available evidence. We are also especially grateful to the Work Group members for their expertise throughout the entire process of literature review, data extraction, meeting participation, and the critical writing and editing of the statements and rationale, which made the publication of this guideline possible. The generous gift of their time and dedication is greatly appreciated. Finally, and on behalf of the Work Group, we gratefully acknowledge the careful assessment of the draft guideline by external reviewers. The Work Group considered all of the valuable comments made and, where appropriate, suggested changes were incorporated into the final publication. The following individuals provided feedback during the public review of the draft guideline:
Saeed M.G. Al-Ghamdi; Alsayed Alnahal; Mona Alrukhaimi; Andrea Angioi; Mustafa Arici; Mariano Arriola; Suheir Assady; Peter Bárány; Rashad S. Barsoum; Donald L. Batisky; Mohammed Benyahia; Roy D. Bloom; Boris Bogov; Rafael Burgos-Calderon; Maria Buti; Jianghua Chen; Rolando Claure-Del Granado; Andrew J. Crannage; Ana Maria Cusumano; Nida Dincel; Ute Eisenberger; Mohamed E. Elrggal; Patrícia Ferreira Abreu; Hélène Fontaine; Rebeca García-Agudo; Alvaro Garcia Garcia; Osama Gheith; HaiAn Ha Phan; Karin Hagen; Mohammed Haji Rashid Hassan; William E. Haley; Qiang He; Scott D. Holmberg; Eero Honkanen; Lai Seong Hooi; Jean-Michel Hougardy; Chandra Mauli Jha; Dario Jimenez Acosta; Holly J. Kramer; John R. Lake; Maria-Carlota Londoño; José Antó Lopes; Cesar Loza; Gerson Marques Pereira Junior; Gerardo Mogni; Anne Moorman; Sameh Morgan; Eugen Mota; Ricardo Mouzo; Reem A. Mustafa; Judit Nagy; Mustafa Nazzal; Armando Luis Negri; Abdou Niang; Julio Pascual; Nikil Patel; Ioan Mihai Paţiu; Saime Paydas; Jim Pearce; Ligia Petrica; Pradeep Kumar Rai; Harun Rashid; Hector Rodriguez; A. Blythe Ryerson; Deepak Sharma; Catherine Staffeld-Coit; Ekamol Tantisattamo; Yusuke Tsukamoto; Nosratola D. Vaziri; J. Todd Weber; Andrzej Więcek; Mai-Szu Wu; Chul-Woo Yang; Bahaa M. Zayed
Participation in the review does not necessarily constitute endorsement of the content of this report by the above individuals or the organizations or institutions they represent.
Michel Jadoul, MD
Paul Martin, MD
Work Group Co-Chairs
Supplementary Material
References
- 1.Wirtz V.J., Hogerzeil H.V., Gray A.L. Essential medicines for universal health coverage. Lancet. 2017;389:403–476. doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Gastroenterology Hepatology Access to HCV treatments: hurdles not barriers. Lancet Gastroenterol Hepatol. 2017;2:1. doi: 10.1016/S2468-1253(16)30175-3. [DOI] [PubMed] [Google Scholar]
- 3.Reau N, Kwo PY, Rhee S, et al. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection [e-pub ahead of print]. Hepatology. 10.1002/hep.30046. Accessed July 25, 2018. [DOI] [PMC free article] [PubMed]
- 4.World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. July 2018. Available at: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. Accessed July 27, 2018. [PubMed]
- 5.Bergman S., Accortt N., Turner A. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005;45:684–689. doi: 10.1053/j.ajkd.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Iwasa Y., Otsubo S., Sugi O. Patterns in the prevalence of hepatitis C virus infection at the start of hemodialysis in Japan. Clin Exp Nephrol. 2008;12:53–57. doi: 10.1007/s10157-007-0005-6. [DOI] [PubMed] [Google Scholar]
- 7.Fabrizi F., Verdesca S., Messa P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta-analysis. Dig Dis Sci. 2015;60:3801–3813. doi: 10.1007/s10620-015-3801-y. [DOI] [PubMed] [Google Scholar]
- 8.Crook E.D., Penumalee S., Gavini B. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187–2191. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- 9.Noureddine L.A., Usman S.A., Yu Z. Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol. 2010;32:311–316. doi: 10.1159/000319456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt C.M., Malvestutto C., Coca S.G. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS. 2008;22:1799–1807. doi: 10.1097/QAD.0b013e32830e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easterbrook P.J. Who to test and how to test for chronic hepatitis C infection - 2016 WHO testing guidance for low- and middle-income countries. J Hepatol. 2016;65:S46–S66. doi: 10.1016/j.jhep.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kamili S., Drobeniuc J., Araujo A.C. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis. 2012;55(Suppl 1):S43–S48. doi: 10.1093/cid/cis368. [DOI] [PubMed] [Google Scholar]
- 13.Cresswell F.V., Fisher M., Hughes D.J. Hepatitis C core antigen testing: a reliable, quick, and potentially cost-effective alternative to hepatitis C polymerase chain reaction in diagnosing acute hepatitis C virus infection. Clin Infect Dis. 2015;60:263–266. doi: 10.1093/cid/ciu782. [DOI] [PubMed] [Google Scholar]
- 14.Hu K.Q., Cui W. A highly specific and sensitive hepatitis C virus antigen enzyme immunoassay for One-step diagnosis of viremic hepatitis C virus infection. Hepatology. 2016;64:415–424. doi: 10.1002/hep.28663. [DOI] [PubMed] [Google Scholar]
- 15.Miedouge M., Saune K., Kamar N. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. J Clin Virol. 2010;48:18–21. doi: 10.1016/j.jcv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Fissell R.B., Bragg-Gresham J.L., Woods J.D. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 17.Saune K., Kamar N., Miedouge M. Decreased prevalence and incidence of HCV markers in haemodialysis units: a multicentric French survey. Nephrol Dial Transplant. 2011;26:2309–2316. doi: 10.1093/ndt/gfq696. [DOI] [PubMed] [Google Scholar]
- 18.Hmaied F., Ben Mamou M., Saune-Sandres K. Hepatitis C virus infection among dialysis patients in Tunisia: incidence and molecular evidence for nosocomial transmission. J Med Virol. 2006;78:185–191. doi: 10.1002/jmv.20526. [DOI] [PubMed] [Google Scholar]
- 19.Izopet J., Sandres-Saune K., Kamar N. Incidence of HCV infection in French hemodialysis units: a prospective study. J Med Virol. 2005;77:70–76. doi: 10.1002/jmv.20415. [DOI] [PubMed] [Google Scholar]
- 20.Mbaeyi C., Thompson N.D. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Semin Dial. 2013;26:439–446. doi: 10.1111/sdi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen D.B., Gutowski J., Ghiselli M. A large outbreak of hepatitis C virus infections in a hemodialysis clinic. Infect Control Hosp Epidemiol. 2016;37:125–133. doi: 10.1017/ice.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savey A., Simon F., Izopet J. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26:752–760. doi: 10.1086/502613. [DOI] [PubMed] [Google Scholar]
- 23.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 24.Moyer V.A. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 25.Barril G., Bartolome J., Sanz P. Effect of hemodialysis schedules and membranes on hepatocyte growth factor and hepatitis C virus RNA levels. J Med Virol. 2010;82:763–767. doi: 10.1002/jmv.21469. [DOI] [PubMed] [Google Scholar]
- 26.Midgard H., Weir A., Palmateer N. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65:S33–S45. doi: 10.1016/j.jhep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Hepatitis C, Acute 2016: Case Definition. https://wwwn.cdc.gov/nndss/conditions/hepatitis-c-acute/case-definition/2016/ Available at: Accessed July 27, 2018.
- 28.Centers for Disease Control and Prevention Health Alert Network CDC urging dialysis providers and facilities to assess and improve infection control practices to stop hepatitis C transmission in patients undergoing hemodialysis. https://emergency.cdc.gov/han/han00386.asp Available at: Accessed July 27, 2018.
- 29.Allander T., Medin C., Jacobson S.H. Hepatitis C transmission in a hemodialysis unit: molecular evidence for spread of virus among patients not sharing equipment. J Med Virol. 1994;43:415–419. doi: 10.1002/jmv.1890430417. [DOI] [PubMed] [Google Scholar]
- 30.Hmaied F., Ben Mamou M., Dubois M. Determining the source of nosocomial transmission in hemodialysis units in Tunisia by sequencing NS5B and E2 sequences of HCV. J Med Virol. 2007;79:1089–1094. doi: 10.1002/jmv.20877. [DOI] [PubMed] [Google Scholar]
- 31.Izopet J., Pasquier C., Sandres K. Molecular evidence for nosocomial transmission of hepatitis C virus in a French hemodialysis unit. J Med Virol. 1999;58:139–144. doi: 10.1002/(sici)1096-9071(199906)58:2<139::aid-jmv7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Kokubo S., Horii T., Yonekawa O. A phylogenetic-tree analysis elucidating nosocomial transmission of hepatitis C virus in a haemodialysis unit. J Viral Hepat. 2002;9:450–454. doi: 10.1046/j.1365-2893.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 33.Saab S., Martin P., Brezina M. Serum alanine aminotransferase in hepatitis C screening of patients on hemodialysis. Am J Kidney Dis. 2001;37:308–315. doi: 10.1053/ajkd.2001.21294. [DOI] [PubMed] [Google Scholar]
- 34.KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008:S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 35.Liu C.H., Liang C.C., Huang K.W. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6:1057–1065. doi: 10.2215/CJN.04320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadoul M., Horsmans Y. Impact of liver fibrosis staging in hepatitis C virus (HCV) patients with kidney failure. Nephrol Dial Transplant. 2014;29:1108–1110. doi: 10.1093/ndt/gfu047. [DOI] [PubMed] [Google Scholar]
- 37.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 38.Serpaggi J., Carnot F., Nalpas B. Direct and indirect evidence for the reversibility of cirrhosis. Hum Pathol. 2006;37:1519–1526. doi: 10.1016/j.humpath.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 39.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Tsao G., Abraldes J.G., Berzigotti A. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 41.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. [DOI] [PubMed]
- 43.Zampino R., Marrone A., Restivo L. Chronic HCV infection and inflammation: clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–540. doi: 10.4254/wjh.v5.i10.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacoub P., Comarmond C., Domont F. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis. 2016;3:3–14. doi: 10.1177/2049936115585942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodkin D., Bieber B., Jadoul M. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol. 2017;12:287–297. doi: 10.2215/CJN.07940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas G.M., Ross M.J., Stock P.G. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–e138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asrani S.K., Buchanan P., Pinsky B. Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol. 2010;8:79–84. doi: 10.1016/j.cgh.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butt A.A., Wang X., Fried L.F. HCV infection and the incidence of CKD. Am J Kidney Dis. 2011;57:396–402. doi: 10.1053/j.ajkd.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y.C., Lin H.Y., Li C.Y. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann J.N., Torner A., Chow W.H. Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: a nationwide register-based cohort study in Sweden. Eur J Cancer Prev. 2011;20:326–330. doi: 10.1097/CEJ.0b013e32834572fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.J., Lin M.Y., Chang J.S. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moe S.M., Pampalone A.J., Ofner S. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis. 2008;51:885–892. doi: 10.1053/j.ajkd.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molnar M.Z., Alhourani H.M., Wall B.M. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su F.H., Su C.T., Chang S.N. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kidney Dis. 2012;60:553–560. doi: 10.1053/j.ajkd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Tsui J.I., Vittinghoff E., Shlipak M.G. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 56.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 57.Dalrymple L.S., Koepsell T., Sampson J. Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol. 2007;2:715–721. doi: 10.2215/CJN.00470107. [DOI] [PubMed] [Google Scholar]
- 58.Lucas G.M., Jing Y., Sulkowski M. Hepatitis C viremia and the risk of chronic kidney disease in HIV-infected individuals. J Infect Dis. 2013;208:1240–1249. doi: 10.1093/infdis/jit373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park H, Chen C, Wang W, et al. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD [e-pub ahead of print]. Hepatology. 10.1002/hep.29505. [DOI] [PMC free article] [PubMed]
- 60.Soma J., Saito T., Taguma Y. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol. 2000;11:690–699. doi: 10.1681/ASN.V114690. [DOI] [PubMed] [Google Scholar]
- 61.Ble M., Aguilera V., Rubin A. Improved renal function in liver transplant recipients treated for hepatitis C virus with a sustained virological response and mild chronic kidney disease. Liver Transpl. 2014;20:25–34. doi: 10.1002/lt.23756. [DOI] [PubMed] [Google Scholar]
- 62.Norton B.L., Park L., McGrath L.J. Health care utilization in HIV-infected patients: assessing the burden of hepatitis C virus coinfection. AIDS Patient Care STDS. 2012;26:541–545. doi: 10.1089/apc.2012.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabrizi F., Dixit V., Martin P. Hepatitis C virus increases the risk of kidney disease among HIV-positive patients: Systematic review and meta-analysis. J Med Virol. 2016;88:487–497. doi: 10.1002/jmv.24353. [DOI] [PubMed] [Google Scholar]
- 64.Satapathy S.K., Lingisetty C.S., Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2012;6:369–378. doi: 10.1007/s12072-011-9284-9. [DOI] [PubMed] [Google Scholar]
- 65.Liangpunsakul S., Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int. 2005;67:285–290. doi: 10.1111/j.1523-1755.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 66.Tsui J.I., Vittinghoff E., Shlipak M.G. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–1174. doi: 10.1681/ASN.2005091006. [DOI] [PubMed] [Google Scholar]
- 67.Petta S., Adinolfi L.E., Fracanzani A.L. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J Hepatol. 2018;69:18–24. doi: 10.1016/j.jhep.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Rogal S.S., Yan P., Rimland D. Incidence and Progression of Chronic Kidney Disease After Hepatitis C Seroconversion: Results from ERCHIVES. Dig Dis Sci. 2016;61:930–936. doi: 10.1007/s10620-015-3918-z. [DOI] [PubMed] [Google Scholar]
- 69.Tsui J., Vittinghoff E., Anastos K. Hepatitis C seropositivity and kidney function decline among women with HIV: data from the Women's Interagency HIV Study. Am J Kidney Dis. 2009;54:43–50. doi: 10.1053/j.ajkd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu C.S., Kao J.H., Chao Y.C. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 71.van der Meer A.J., Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016;65:S95–S108. doi: 10.1016/j.jhep.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 72.Berenguer J., Rodriguez E., Miralles P. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55:728–736. doi: 10.1093/cid/cis500. [DOI] [PubMed] [Google Scholar]
- 73.Arase Y., Suzuki F., Kawamura Y. Development rate of chronic kidney disease in hepatitis C virus patients with advanced fibrosis after interferon therapy. Hepatol Res. 2011;41:946–954. doi: 10.1111/j.1872-034X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y.C., Hwang S.J., Li C.Y. A Taiwanese nationwide cohort study shows interferon-based therapy for chronic hepatitis C reduces the risk of chronic kidney disease. Medicine (Baltimore) 2015;94:e1334. doi: 10.1097/MD.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu Y.C., Ho H.J., Huang Y.T. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 76.Hsu Y.C., Lin J.T., Ho H.J. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 77.Leone S., Prosperi M., Costarelli S. Incidence and predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV-coinfected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis. 2016;35:1511–1520. doi: 10.1007/s10096-016-2692-y. [DOI] [PubMed] [Google Scholar]
- 78.Feng B., Eknoyan G., Guo Z.S. Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant. 2012;27:640–646. doi: 10.1093/ndt/gfr236. [DOI] [PubMed] [Google Scholar]
- 79.Reiss G., Keeffe E.B. Review article: hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther. 2004;19:715–727. doi: 10.1111/j.1365-2036.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- 80.Tung J., Carlisle E., Smieja M. A randomized clinical trial of immunization with combined hepatitis A and B versus hepatitis B alone for hepatitis B seroprotection in hemodialysis patients. Am J Kidney Dis. 2010;56:713–719. doi: 10.1053/j.ajkd.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Fabrizi F., Martin P., Messa P. Novel perspectives on the hepatitis B virus vaccine in the chronic kidney disease population. Int J Artif Organs. 2015;38:625–631. doi: 10.5301/ijao.5000458. [DOI] [PubMed] [Google Scholar]
- 82.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simmons B., Saleem J., Hill A. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Chronic hepatitis C virus infection: developing direct-acting antiviral drugs for treatment. Guidance for industry. 2017. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm225333.pdf. Accessed March 23, 2018.
- 85.Simmons B., Saleem J., Heath K. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. 2015;61:730–740. doi: 10.1093/cid/civ396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org. Accessed June 12, 2018. [DOI] [PMC free article] [PubMed]
- 88.Gamal N., Andreone P. Grazoprevir/elbasvir fixed-dose combination for hepatitis C. Drugs Today (Barc) 2016;52:377–385. doi: 10.1358/dot.2016.52.7.2510258. [DOI] [PubMed] [Google Scholar]
- 89.Reddy K.R., Roth D., Bruchfeld A. Elbasvir/grazoprevir does not worsen renal function in patients with hepatitis C virus infection and pre-existing renal disease. Hepatol Res. 2017;47:1340–1345. doi: 10.1111/hepr.12899. [DOI] [PubMed] [Google Scholar]
- 90.Roth D., Nelson D.R., Bruchfeld A. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 91.Bruchfeld A., Roth D., Martin P. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2017;2:585–594. doi: 10.1016/S2468-1253(17)30116-4. [DOI] [PubMed] [Google Scholar]
- 92.Alric L., Ollivier-Hourmand I., Berard E. Grazoprevir plus elbasvir in HCV genotype-1 or -4 infected patients with stage 4/5 severe chronic kidney disease is safe and effective. Kidney Int. 2018;94:206–213. doi: 10.1016/j.kint.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 93.Munoz-Gomez R., Rincon D., Ahumada A. Therapy with ombitasvir/paritaprevir/ritonavir plus dasabuvir is effective and safe for the treatment of genotype 1 and 4 hepatitis C virus infection (HCV) in patients with severe renal impairment: a multicenter experience. J Viral Hepat. 2017;24:464–471. doi: 10.1111/jvh.12664. [DOI] [PubMed] [Google Scholar]
- 94.Pockros P.J., Reddy K.R., Mantry P.S. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150:1590–1598. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 95.Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504. doi: 10.1016/j.jhep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Jacobson IM, Asante-Appiah E, Wong P, et al. Prevalence and impact of baseline NSA resistance associated variants (RAVs) on the efficacy of elbasvir/grazoprevir (EBR/GZR) against GT1a infection [Abstract LB-22]. In: 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), November 13-17, 2015. San Francisco, CA; 2015.
- 97.Gane E.J., Solà R., Cohen E. RUBY-II: Efficacy and Safety of a Ribavirin-free Ombitasvir/Paritaprevir/Ritonavir ± Dasabuvir Regimen in Patients with Severe Renal Impairment or End-Stage Renal Disease and HCV Genotypes 1a or 4 Infection. Hepatology. 2016;64(Suppl 1):470A. [Google Scholar]
- 98.Gane E., Lawitz E., Pugatch D. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 99.Kirby B.J., Symonds W.T., Kearney B.P. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet. 2015;54:677–690. doi: 10.1007/s40262-015-0261-7. [DOI] [PubMed] [Google Scholar]
- 100.Gane E.J., Robson R.A., Bonacini Safety, anti-viral efficacy and pharmacokinetics (PK) of sofosbuvir (SOF) in patients with severe renal impairment. [Abstract 966] Hepatology. 2014;60:667A. [Google Scholar]
- 101.Czul F., Schiff E., Peyton C. First ribavirin-free sofosbuvir and simeprevir treatment of Hepatitis C genotype 1 patients with severe renal impairment (GFR <30 ml/min or dialysis). [Abstract P0878] J Hepatol. 2015;62:S670–S671. [Google Scholar]
- 102.Nazario H.E., Ndungu M., Modi A. Safety and efficacy of sofosbuvir + simeprevir without ribavirin in hepatitis C genotype 1-infected patients with end-stage renal disease or GFR <30 ml/min. [Abstract P0802] J Hepatol. 2015;62:S635. [Google Scholar]
- 103.Perumpail R.B., Wong R.J., Pham E.A. A new standard of care? standard dose sofosbuvir in an HCV-infected liver transplant recipient undergoing hemodialysis. Dig Dis Sci. 2016;61:39–41. doi: 10.1007/s10620-015-3756-z. [DOI] [PubMed] [Google Scholar]
- 104.Perumpail R.B., Wong R.J., Ha L.D. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275–278. doi: 10.1111/tid.12348. [DOI] [PubMed] [Google Scholar]
- 105.Desnoyer A., Pospai D., Le M.P. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40–47. doi: 10.1016/j.jhep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 106.Bhamidimarri K.R., Czul F., Peyton A. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763–765. doi: 10.1016/j.jhep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 107.Saxena V., Koraishy F.M., Sise M.E. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dumortier J., Bailly F., Pageaux G.P. Sofosbuvir-based antiviral therapy in hepatitis C virus patients with severe renal failure. Nephrol Dial Transplant. 2017;32:2065–2071. doi: 10.1093/ndt/gfw348. [DOI] [PubMed] [Google Scholar]
- 109.Li T., Qu Y., Guo Y. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int. 2017;37:974–981. doi: 10.1111/liv.13336. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez-Parra E, Soledad PS, et al. Renal function evolution in patients infected with HCV and basal estimated glomerular filtration rate (GFRE) between 30-60 ml/min/1.73 m2 treated with ombitasvir/paritaprevir/ritonavir and dasabuvir (3D) vs regimens based on sofosbuvir (SOF). EASL Special Conference, September 23-24, 2016. Available at: http://www.easloffice.eu/office/paris2016/mobile/index.html#p=172. Accessed February 25, 2018.
- 111.Rosenblatt R., Mehta A., Wagner M., Kumar S. Baseline creatinine clearance is a predictor of worsening renal function while on HCV treatment with sofosbuvir-ledipasvir. J Hepatol. 2016;64(Suppl):S819. [Google Scholar]
- 112.Mallet V., Parlati L., Dorval O. Estimated glomerular filtration rate variations and direct acting antivirals treatment for chronic hepatitis C: a retrospective longitudinal study. J Hepatol. 2018;68:S22. [Google Scholar]
- 113.Sise M.E., Backman E., Ortiz G.A. Effect of sofosbuvir-based hepatitis C virus therapy on kidney function in patients with CKD. Clin J Am Soc Nephrol. 2017;12:1615–1623. doi: 10.2215/CJN.02510317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stark J.E., Cole J. Successful treatment of chronic hepatitis C virus infection in a patient receiving daily peritoneal dialysis. Am J Health Syst Pharm. 2017;74:1541–1544. doi: 10.2146/ajhp160729. [DOI] [PubMed] [Google Scholar]
- 115.Lin M.V., Sise M.E., Pavlakis M. Safety and efficacy of novel antivirals in kidney transplant recipients with chronic hepatitis C virus (HCV) infection. [Abstract LP42] Journal of Hepatol. 2015;62:S284–S285. [Google Scholar]
- 116.Colombo M., Aghemo A., Liu H. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med. 2017;166:109–117. doi: 10.7326/M16-1205. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez I., Munoz-Gomez R., Pascasio J.M. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol. 2017;66:718–723. doi: 10.1016/j.jhep.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 118.Kamar N., Marion O., Rostaing L. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant. 2016;16:1474–1479. doi: 10.1111/ajt.13518. [DOI] [PubMed] [Google Scholar]
- 119.Sawinski D., Kaur N., Ajeti A. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents. Am J Transplant. 2016;16:1588–1595. doi: 10.1111/ajt.13620. [DOI] [PubMed] [Google Scholar]
- 120.Agarwal K, Castells L, Mullhaupt B, et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1-4 HCV-infected liver transplant recipients [e-pub ahead of print]. J Hepatol. 10.1016/j.jhep.2018.05.039. Accessed July 28, 2018. [DOI] [PubMed]
- 121.Morelle J., Goffin E., Wallemacq P. Extended release tacrolimus and antiretroviral therapy in a renal transplant recipient: so extended! Transpl Int. 2010;23:1065–1067. doi: 10.1111/j.1432-2277.2010.01098.x. [DOI] [PubMed] [Google Scholar]
- 122.Kucirka L.M., Singer A.L., Ros R.L. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238–1246. doi: 10.1111/j.1600-6143.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 123.Goldberg D.S., Abt P.L., Blumberg E.A. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;376:2394–2395. doi: 10.1056/NEJMc1705221. [DOI] [PubMed] [Google Scholar]
- 124.Durand C.M., Bowring M.G., Brown D.M. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med. 2018;168:533–540. doi: 10.7326/M17-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen G., Wang C., Chen J. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology. 2017;66:13–26. doi: 10.1002/hep.29109. [DOI] [PubMed] [Google Scholar]
- 126.Mucke M.M., Backus L.I., Mucke V.T. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:172–180. doi: 10.1016/S2468-1253(18)30002-5. [DOI] [PubMed] [Google Scholar]
- 127.Bersoff-Matcha S.J., Cao K., Jason M. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug administration adverse event reporting system. Ann Intern Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 128.Fabrizi F., Martin P., Dixit V. Hepatitis C virus infection and kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2012;7:549–557. doi: 10.2215/CJN.06920711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schneeberger P.M., Keur I., van Loon A.M. The prevalence and incidence of hepatitis C virus infections among dialysis patients in the Netherlands: a nationwide prospective study. J Infect Dis. 2000;182:1291–1299. doi: 10.1086/315869. [DOI] [PubMed] [Google Scholar]
- 130.Vladutiu D.S., Cosa A., Neamtu A. Infections with hepatitis B and C viruses in patients on maintenance dialysis in Romania and in former communist countries: yellow spots on a blank map? J Viral Hepat. 2000;7:313–319. doi: 10.1046/j.1365-2893.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 131.Sun J., Yu R., Zhu B. Hepatitis C infection and related factors in hemodialysis patients in China: systematic review and meta-analysis. Ren Fail. 2009;31:610–620. doi: 10.1080/08860220903003446. [DOI] [PubMed] [Google Scholar]
- 132.Voiculescu M., Iliescu L., Ionescu C. A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania. J Gastrointestin Liver Dis. 2010;19:43–48. doi: 10.1007/s11749-009-0177-3. [DOI] [PubMed] [Google Scholar]
- 133.Selm S.B. Prevalence of hepatitis C virus infection among hemodialysis patients in a single center in Yemen. Saudi J Kidney Dis Transpl. 2010;21:1165–1168. [PubMed] [Google Scholar]
- 134.Ali I., Siddique L., Rehman L.U. Prevalence of HCV among the high risk groups in Khyber Pakhtunkhwa. Virol J. 2011;8:296. doi: 10.1186/1743-422X-8-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su Y., Norris J.L., Zang C. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17:532–541. doi: 10.1111/j.1542-4758.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 136.Marwaha N., Sachdev S. Current testing strategies for hepatitis C virus infection in blood donors and the way forward. World J Gastroenterol. 2014;20:2948–2954. doi: 10.3748/wjg.v20.i11.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thompson N.D., Novak R.T., White-Comstock M.B. Patient-to-patient hepatitis C virus transmissions associated with infection control breaches in a hemodialysis unit. J Nephrol Therapeutics. 2012;S10:002. [Google Scholar]
- 138.Aho-Glele L.S., Giraudon H., Astruc K. Investigation of a case of genotype 5a hepatitis C virus transmission in a french hemodialysis unit using epidemiologic data and deep sequencing. Infect Control Hosp Epidemiol. 2016;37:134–139. doi: 10.1017/ice.2015.263. [DOI] [PubMed] [Google Scholar]
- 139.Thompson N.D., Novak R.T., Datta D. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30:900–903. doi: 10.1086/605472. [DOI] [PubMed] [Google Scholar]
- 140.Fabrizi F., Messa P. Transmission of hepatitis C virus in dialysis units: a systematic review of reports on outbreaks. Int J Artif Organs. 2015;38:471–480. doi: 10.5301/ijao.5000437. [DOI] [PubMed] [Google Scholar]
- 141.Jadoul M., Poignet J.L., Geddes C. The changing epidemiology of hepatitis C virus (HCV) infection in haemodialysis: European multicentre study. Nephrol Dial Transplant. 2004;19:904–909. doi: 10.1093/ndt/gfh012. [DOI] [PubMed] [Google Scholar]
- 142.Centers for Disease Control and Prevention Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention, 2008-2015. https://www.cdc.gov/hepatitis/outbreaks/healthcarehepoutbreaktable.htm Available at: Accessed July 27, 2018.
- 143.The Centers for Disease Control and Prevention CDC urging dialysis providers and facilities to assess and improve infection control practices to stop hepatitis C virus transmission in patients undergoing hemodialysis. Am J Transplant. 2016;16:1633–1634. [Google Scholar]
- 144.Thompson N.D., Perz J.F., Moorman A.C. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med. 2009;150:33–39. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 145.de Lamballerie X., Olmer M., Bouchouareb D. Nosocomial transmission of hepatitis C virus in haemodialysis patients. J Med Virol. 1996;49:296–302. doi: 10.1002/(SICI)1096-9071(199608)49:4<296::AID-JMV7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 146.McLaughlin K.J., Cameron S.O., Good T. Nosocomial transmission of hepatitis C virus within a British dialysis centre. Nephrol Dial Transplant. 1997;12:304–309. doi: 10.1093/ndt/12.2.304. [DOI] [PubMed] [Google Scholar]
- 147.Bieber B., Goodkin D.A., Nwankwo C. Hepatitis C prevalence and clinical outcomes in the Dialysis Outcomes and Practice Patterns Study. Neph Dial Transpl. 2015;30(Suppl 3):iii314. [Google Scholar]
- 148.de Jesus Rodrigues de Freitas M., Fecury A.A., de Almeida M.K. Prevalence of hepatitis C virus infection and genotypes in patient with chronic kidney disease undergoing hemodialysis. J Med Virol. 2013;85:1741–1745. doi: 10.1002/jmv.23654. [DOI] [PubMed] [Google Scholar]
- 149.Santana R.R., Martínez Z., Martínez M.T., Mato J. Hepatitis C virus present in hemodialysis units from Cuban western region. Rev Cub Meda. 2009;48:28–35. [Google Scholar]
- Ashkani-Esfahani S., Alavian S.M., Salehi-Marzijarani M. Prevalence of hepatitis C virus infection among hemodialysis patients in the Middle-East: a systematic review and meta-analysis. World J Gastroenterol. 2017;23:151–166. doi: 10.3748/wjg.v23.i1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abou Rached A., El Khoury L., El Imad T. Incidence and prevalence of hepatitis B and hepatitis C viruses in hemodialysis patients in Lebanon. World J Nephrol. 2016;5:101–107. doi: 10.5527/wjn.v5.i1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alashek W.A., McIntyre C.W., Taal M.W. Hepatitis B and C infection in haemodialysis patients in Libya: prevalence, incidence and risk factors. BMC Infect Dis. 2012;12:265. doi: 10.1186/1471-2334-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ummate I., Denue B.A., Kida I.M. Risk factors for hepatitis C virus sero-positivity among haemodialysis patients receiving care at kidney centre in a tertiary health facility in Maiduguri, Nigeria. Pan Afr Med.J. 2014;19:305. doi: 10.11604/pamj.2014.19.305.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schiller A., Timar R., Siriopol D. Hepatitis B and C virus infection in the hemodialysis population from three romanian regions. Nephron. 2015;129:202–208. doi: 10.1159/000371450. [DOI] [PubMed] [Google Scholar]
- 154.Seck S.M., Dahaba M., Gueye S. Trends in hepatitis C infection among hemodialysis patients in Senegal: results of a decade of prevention. Saudi J Kidney Dis Transpl. 2014;25:1341–1345. doi: 10.4103/1319-2442.144319. [DOI] [PubMed] [Google Scholar]
- 155.Alfurayh O., Sabeel A., Al Ahdal M.N. Hand contamination with hepatitis C virus in staff looking after hepatitis C-positive hemodialysis patients. Am J Nephrol. 2000;20:103–106. doi: 10.1159/000013565. [DOI] [PubMed] [Google Scholar]
- 156.Bergervoet P.W., van Riessen N., Sebens F.W. Application of the forensic Luminol for blood in infection control. J Hosp Infect. 2008;68:329–333. doi: 10.1016/j.jhin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 157.Caramelo C., de Sequera P., Lopez M.D. Hand-borne mechanisms of dissemination of hepatitis C virus in dialysis units: basis for new addenda to the present preventive strategies. Clin Nephrol. 1999;51:59–60. [PubMed] [Google Scholar]
- 158.Froio N., Nicastri E., Comandini U.V. Contamination by hepatitis B and C viruses in the dialysis setting. Am J Kidney Dis. 2003;42:546–550. doi: 10.1016/s0272-6386(03)00787-x. [DOI] [PubMed] [Google Scholar]
- 159.Girou E., Chevaliez S., Challine D. Determinant roles of environmental contamination and noncompliance with standard precautions in the risk of hepatitis C virus transmission in a hemodialysis unit. Clin Infect Dis. 2008;47:627–633. doi: 10.1086/590564. [DOI] [PubMed] [Google Scholar]
- 160.Kamili S., Krawczynski K., McCaustland K. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28:519–524. doi: 10.1086/513727. [DOI] [PubMed] [Google Scholar]
- 161.Patel P.R., Thompson N.D., Kallen A.J. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 162.Paintsil E., Binka M., Patel A. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis. 2014;209:1205–1211. doi: 10.1093/infdis/jit648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Laporte F., Tap G., Jaafar A. Mathematical modeling of hepatitis C virus transmission in hemodialysis. Am J Infect Control. 2009;37:403–407. doi: 10.1016/j.ajic.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 164.Petrosillo N., Gilli P., Serraino D. Prevalence of infected patients and understaffing have a role in hepatitis C virus transmission in dialysis. Am J Kidney Dis. 2001;37:1004–1010. doi: 10.1016/s0272-6386(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 165.Shimokura G., Chai F., Weber D.J. Patient-care practices associated with an increased prevalence of hepatitis C virus infection among chronic hemodialysis patients. Infect Control Hosp Epidemiol. 2011;32:415–424. doi: 10.1086/659407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Centers for Disease Control and Prevention Dialysis safety audit tools and checklists. http://www.cdc.gov/dialysis/prevention-tools/audit-tools.html Available at: Accessed July 27, 2018.
- 167.Labriola L., Jadoul M. The decades-long fight against HBV transmission to dialysis patients: slow but definite progress. Nephrol Dial Transplant. 2010;25:2047–2049. doi: 10.1093/ndt/gfq238. [DOI] [PubMed] [Google Scholar]
- 168.Jadoul M. Should hemodialysis patients with hepatitis C virus antibodies be isolated? Semin Dial. 1995;8:1–3. [Google Scholar]
- 169.Sypsa V., Psichogiou M., Katsoulidou A. Incidence and patterns of hepatitis C virus seroconversion in a cohort of hemodialysis patients. Am J Kidney Dis. 2005;45:334–343. doi: 10.1053/j.ajkd.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 170.Bravo Zuniga J.I., Loza Munarriz C., Lopez-Alcalde J. Isolation as a strategy for controlling the transmission of hepatitis C virus (HCV) infection in haemodialysis units. Cochrane Database Syst Rev. 2016:CD006420. doi: 10.1002/14651858.CD006420.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shamshirsaz A.A., Kamgar M., Bekheirnia M.R. The role of hemodialysis machines dedication in reducing Hepatitis C transmission in the dialysis setting in Iran: a multicenter prospective interventional study. BMC Nephrol. 2004;5:13. doi: 10.1186/1471-2369-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karkar A., Abdelrahman M., Ghacha R. Prevention of viral transmission in HD units: the value of isolation. Saudi J Kidney Dis Transpl. 2006;17:183–188. [PubMed] [Google Scholar]
- 173.Harmankaya O., Cetin B., Erimez D. Patient isolation prevents the transmission of hepatitis C virus infection in hemodialysis units. Dialysis Transpl. 2002;31:859–861. [Google Scholar]
- 174.Dzekova-Vidimliski P., Pavleska-Kuzmanovska S., Trajceska L. Decreasing prevalence of hepatitis C virus infection in hemodialysis patients: Following KDIGO guidelines. Neph Dialysis Transpl. 2012;27(Suppl 2):ii294. [Google Scholar]
- 175.Agarwal S.K., Dash S.C., Gupta S. Hepatitis C virus infection in haemodialysis: the 'no-isolation' policy should not be generalized. Nephron Clin Pract. 2009;111:c133–c140. doi: 10.1159/000191208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gallego E., Lopez A., Perez J. Effect of isolation measures on the incidence and prevalence of hepatitis C virus infection in hemodialysis. Nephron Clin Pract. 2006;104:c1–c6. doi: 10.1159/000093252. [DOI] [PubMed] [Google Scholar]
- 177.Shebeb A.M., Kotkat A.M., Abd El Reheim S.M. An intervention study for prevention of HCV infection in some hemodialysis units in Alexandria. J Egypt Public Health Assoc. 2006;81:119–141. [PubMed] [Google Scholar]
- 178.Yang C.S., Chang H.H., Chou C.C. Isolation effectively prevents the transmission of hepatitis C virus in the hemodialysis unit. J Formos Med Assoc. 2003;102:79–85. [PubMed] [Google Scholar]
- 179.Schvarcz R., Johansson B., Nystrom B. Nosocomial transmission of hepatitis C virus. Infection. 1997;25:74–77. doi: 10.1007/BF02113578. [DOI] [PubMed] [Google Scholar]
- 180.Jadoul M., Cornu C., van Ypersele de Strihou C. Universal precautions prevent hepatitis C virus transmission: a 54 month follow-up of the Belgian Multicenter Study. The Universitaires Cliniques St-Luc (UCL) Collaborative Group. Kidney Int. 1998;53:1022–1025. doi: 10.1111/j.1523-1755.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 181.Mactier R., Davies S., Dudley C. Summary of the 5th edition of the Renal Association Clinical Practice Guidelines (2009-2012) Nephron Clin Pract. 2011;118(Suppl 1):c27–c70. doi: 10.1159/000328060. [DOI] [PubMed] [Google Scholar]
- 182.European Best Practice Guidelines Expert Group on Hemodialysis. European Renal Association Section VI. Haemodialysis-associated infection. Nephrol Dial Transplant. 2002;17(Suppl 7):72–87. [PubMed] [Google Scholar]
- 183.Jadoul M. Transmission routes of HCV infection in dialysis. Nephrol Dial Transplant. 1996;11(Suppl 4):36–38. doi: 10.1093/ndt/11.supp4.36. [DOI] [PubMed] [Google Scholar]
- 184.Finelli L., Miller J.T., Tokars J.I. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 185.dos Santos J.P., Loureiro A., Cendoroglo Neto M. Impact of dialysis room and reuse strategies on the incidence of hepatitis C virus infection in haemodialysis units. Nephrol Dial Transplant. 1996;11:2017–2022. doi: 10.1093/oxfordjournals.ndt.a027090. [DOI] [PubMed] [Google Scholar]
- 186.Patel P.R., Yi S.H., Booth S. Bloodstream infection rates in outpatient hemodialysis facilities participating in a collaborative prevention effort: a quality improvement report. Am J Kidney Dis. 2013;62:322–330. doi: 10.1053/j.ajkd.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 187.Yi S.H., Kallen A.J., Hess S. Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effort. Infect Control Hosp Epidemiol. 2016;37:863–866. doi: 10.1017/ice.2016.22. [DOI] [PubMed] [Google Scholar]
- 188.Arenas M.D., Sanchez-Paya J., Barril G. A multicentric survey of the practice of hand hygiene in haemodialysis units: factors affecting compliance. Nephrol Dial Transplant. 2005;20:1164–1171. doi: 10.1093/ndt/gfh759. [DOI] [PubMed] [Google Scholar]
- 189.Shimokura G., Weber D.J., Miller W.C. Factors associated with personal protection equipment use and hand hygiene among hemodialysis staff. Am J Infect Control. 2006;34:100–107. doi: 10.1016/j.ajic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 190.Ball L.K., George C.A., Duval L. Reducing blood stream infection in patients on hemodialysis: Incorporating patient engagement into a quality improvement activity. Hemodial Int. 2016;20(Suppl 1):S7–S11. doi: 10.1111/hdi.12463. [DOI] [PubMed] [Google Scholar]
- 191.Sanchez-Carrillo L.A., Rodriguez-Lopez J.M., Galarza-Delgado D.A. Enhancement of hand hygiene compliance among health care workers from a hemodialysis unit using video-monitoring feedback. Am J Infect Control. 2016;44:868–872. doi: 10.1016/j.ajic.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 192.CDC recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Morb Mortal Wkly Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 193.Frieden T.R. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100:590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morales J.M., Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172–182. doi: 10.1038/nrneph.2015.5. [DOI] [PubMed] [Google Scholar]
- 195.Fabrizi F., Martin P., Dixit V. Acquisition of hepatitis C virus in hemodialysis patients: a prospective study by branched DNA signal amplification assay. Am J Kidney Dis. 1998;31:647–654. doi: 10.1053/ajkd.1998.v31.pm9531181. [DOI] [PubMed] [Google Scholar]
- 196.Pereira B.J., Milford E.L., Kirkman R.L. Transmission of hepatitis C virus by organ transplantation. N Engl J Med. 1991;325:454–460. doi: 10.1056/NEJM199108153250702. [DOI] [PubMed] [Google Scholar]
- 197.Kamar N., Ribes D., Izopet J. Treatment of hepatitis C virus infection (HCV) after renal transplantation: implications for HCV-positive dialysis patients awaiting a kidney transplant. Transplantation. 2006;82:853–856. doi: 10.1097/01.tp.0000238898.14393.c9. [DOI] [PubMed] [Google Scholar]
- 198.Knoll G.A., Tankersley M.R., Lee J.Y. The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis. 1997;29:608–614. doi: 10.1016/s0272-6386(97)90345-0. [DOI] [PubMed] [Google Scholar]
- 199.Stehman-Breen C.O., Emerson S., Gretch D. Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis. 1998;32:629–634. doi: 10.1016/s0272-6386(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 200.Legendre C., Garrigue V., Le Bihan C. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65:667–670. doi: 10.1097/00007890-199803150-00011. [DOI] [PubMed] [Google Scholar]
- 201.Mathurin P., Mouquet C., Poynard T. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 202.Bruchfeld A., Wilczek H., Elinder C.G. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation. 2004;78:745–750. doi: 10.1097/01.tp.0000131948.29742.24. [DOI] [PubMed] [Google Scholar]
- 203.Fabrizi F., Takkouche B., Lunghi G. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 204.Scott D.R., Wong J.K., Spicer T.S. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165–1171. doi: 10.1097/TP.0b013e3181f92548. [DOI] [PubMed] [Google Scholar]
- 205.Port F.K., Wolfe R.A., Mauger E.A. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. [PubMed] [Google Scholar]
- 206.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 207.Pereira B.J., Natov S.N., Bouthot B.A. Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. The New England Organ Bank Hepatitis C Study Group. Kidney Int. 1998;53:1374–1381. doi: 10.1046/j.1523-1755.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 208.Bloom R.D., Sayer G., Fa K. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139–144. doi: 10.1111/j.1600-6143.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 209.Kamar N., Toupance O., Buchler M. Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol. 2003;14:2092–2098. doi: 10.1097/01.asn.0000079613.81511.3c. [DOI] [PubMed] [Google Scholar]
- 210.Nicot F., Kamar N., Mariame B. No evidence of occult hepatitis C virus (HCV) infection in serum of HCV antibody-positive HCV RNA-negative kidney-transplant patients. Transpl Int. 2010;23:594–601. doi: 10.1111/j.1432-2277.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 211.Cruzado J.M., Casanovas-Taltavull T., Torras J. Pretransplant interferon prevents hepatitis C virus-associated glomerulonephritis in renal allografts by HCV-RNA clearance. Am J Transplant. 2003;3:357–360. doi: 10.1034/j.1600-6143.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 212.Forman J.P., Tolkoff-Rubin N., Pascual M. Hepatitis C, acute humoral rejection, and renal allograft survival. J Am Soc Nephrol. 2004;15:3249–3255. doi: 10.1097/01.ASN.0000145896.16153.43. [DOI] [PubMed] [Google Scholar]
- 213.Alric L., Di-Martino V., Selves J. Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology. 2002;123:1494–1499. doi: 10.1053/gast.2002.36610. [DOI] [PubMed] [Google Scholar]
- 214.Fehr T., Riehle H.M., Nigg L. Evaluation of hepatitis B and hepatitis C virus-infected renal allograft recipients with liver biopsy and noninvasive parameters. Am J Kidney Dis. 2003;42:193–201. doi: 10.1016/s0272-6386(03)00423-2. [DOI] [PubMed] [Google Scholar]
- 215.Kamar N., Rostaing L., Selves J. Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704–1712. doi: 10.1111/j.1600-6143.2005.00918.x. [DOI] [PubMed] [Google Scholar]
- 216.Roth D., Gaynor J.J., Reddy K.R. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152–1160. doi: 10.1681/ASN.2010060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pol S., Carnot F., Nalpas B. Reversibility of hepatitis C virus-related cirrhosis. Hum Pathol. 2004;35:107–112. doi: 10.1016/j.humpath.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 218.Eason J.D., Gonwa T.A., Davis C.L. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 219.Ripoll C., Groszmann R., Garcia-Tsao G. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 220.Rostaing L., Izopet J., Baron E. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. 1995;59:1426–1431. doi: 10.1097/00007890-199505270-00012. [DOI] [PubMed] [Google Scholar]
- 221.Chascsa DM, Mousa OY, Pungpapong S, et al. Clinical outcomes of hepatitis C treatment before and after kidney transplantation and its impact on time to transplant: a multi-center study [e-pub ahead of print]. Am J Transplant. 10.1111/ajt.14931. [DOI] [PubMed]
- 222.Diethelm A.G., Roth D., Ferguson R.M. Transmission of HCV by organ transplantation. N Engl J Med. 1992;326:410–411. [PubMed] [Google Scholar]
- 223.European Best Practice Guidelines for Renal Transplantation Section I: Evaluation, selection and preparation of the potential recipient. Nephrol Dial Transplant. 2000;15(suppl 7):3–38. [PubMed] [Google Scholar]
- 224.Nowak K.M., Witzke O., Sotiropoulos G.C. Transplantation of renal allografts from organ donors reactive for HCV antibodies to HCV-negative recipients: safety and clinical outcome. Kidney Int Rep. 2016;2:53–59. doi: 10.1016/j.ekir.2016.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wolfe C.R., Michaels M.G. Donor-derived hepatitis C transmission from NAT negative donors-Still an unexpected event! Am J Transplant. 2017;17:2989. doi: 10.1111/ajt.14483. [DOI] [PubMed] [Google Scholar]
- 226.Levitsky J., Formica R.N., Bloom R.D. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17:2790–2802. doi: 10.1111/ajt.14381. [DOI] [PubMed] [Google Scholar]
- 227.Kling C.E., Perkins J.D., Landis C.S. Utilization of organs from donors according to hepatitis C antibody and nucleic acid testing status: time for change. Am J Transplant. 2017;17:2863–2868. doi: 10.1111/ajt.14386. [DOI] [PubMed] [Google Scholar]
- 228.Morales J.M., Andres A., Campistol J.M. Hepatitis C virus and organ transplantation. N Engl J Med. 1993;328:511–512. doi: 10.1056/NEJM199302183280714. [DOI] [PubMed] [Google Scholar]
- 229.Morales J.M., Campistol J.M., Castellano G. Transplantation of kidneys from donors with hepatitis C antibody into recipients with pre-transplantation anti-HCV. Kidney Int. 1995;47:236–240. doi: 10.1038/ki.1995.29. [DOI] [PubMed] [Google Scholar]
- 230.Abbott K.C., Lentine K.L., Bucci J.R. The impact of transplantation with deceased donor hepatitis C-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032–2037. doi: 10.1046/j.1600-6143.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 231.Ali M.K., Light J.A., Barhyte D.Y. Donor hepatitis C virus status does not adversely affect short-term outcomes in HCV+ recipients in renal transplantation. Transplantation. 1998;66:1694–1697. doi: 10.1097/00007890-199812270-00021. [DOI] [PubMed] [Google Scholar]
- 232.Bucci J.R., Lentine K.L., Agodoa L.Y. Outcomes associated with recipient and donor hepatitis C serology status after kidney transplantation in the United States: analysis of the USRDS/UNOS database. Clin Transpl. 2004:51–61. [PubMed] [Google Scholar]
- 233.Bucci J.R., Matsumoto C.S., Swanson S.J. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol. 2002;13:2974–2982. doi: 10.1097/01.asn.0000034944.90425.75. [DOI] [PubMed] [Google Scholar]
- 234.Maluf D.G., Archer K.J., Mas V.R. Kidney grafts from HCV-positive donors: advantages and disadvantages. Transplant Proc. 2010;42:2436–2446. doi: 10.1016/j.transproceed.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 235.Mandal A.K., Kraus E.S., Samaniego M. Shorter waiting times for hepatitis C virus seropositive recipients of cadaveric renal allografts from hepatitis C virus seropositive donors. Clin Transplant. 2000;14:391–396. doi: 10.1034/j.1399-0012.2000.14040602.x. [DOI] [PubMed] [Google Scholar]
- 236.Singh N., Neidlinger N., Djamali A. The impact of hepatitis C virus donor and recipient status on long-term kidney transplant outcomes: University of Wisconsin experience. Clin Transplant. 2012;26:684–693. doi: 10.1111/j.1399-0012.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 237.Morales J.M., Campistol J.M., Dominguez-Gil B. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10:2453–2462. doi: 10.1111/j.1600-6143.2010.03280.x. [DOI] [PubMed] [Google Scholar]
- 238.Jawa P., Knorr J., Torres E. Donor hepatitis C status does not impact outcomes in hepatitis C positive kidney transplant recipients [abstract] Am J Transplant. 2013;13(suppl 5):403. [Google Scholar]
- 239.Scalea J.R., Barth R.N., Munivenkatappa R. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation. 2015;99:1192–1196. doi: 10.1097/TP.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 240.Myint T., Wright A., Rose C. The benefit of hepatitis C donor kidney transplantation is limited to hepatitis C positive patients over 50 years of age [abstract] Am J Transplant. 2015;15(suppl 3) https://atcmeetingabstracts.com/abstract/the-benefit-of-hepatitis-c-donor-kidney-transplantation-is-limited-to-hepatitis-c-positive-patients-over-50-years-of-age/ Available at: Accessed July 27, 2018. [Google Scholar]
- 241.Bhamidimarri K.R., Ladino M., Pedraza F. Transplantation of kidneys from hepatitis C-positive donors into hepatitis C virus-infected recipients followed by early initiation of direct acting antiviral therapy: a single-center retrospective study. Transpl Int. 2017;30:865–873. doi: 10.1111/tri.12954. [DOI] [PubMed] [Google Scholar]
- 242.Widell A., Mansson S., Persson N.H. Hepatitis C superinfection in hepatitis C virus (HCV)-infected patients transplanted with an HCV-infected kidney. Transplantation. 1995;60:642–647. doi: 10.1097/00007890-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 243.Ladino M., Pedraza F., Roth D. Hepatitis C virus infection in chronic kidney disease. J Am Soc Nephrol. 2016;27:2238–2246. doi: 10.1681/ASN.2016010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sawinski D., Bloom R.D. Novel hepatitis C treatment and the impact on kidney transplantation. Transplantation. 2015;99:2458–2466. doi: 10.1097/TP.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 245.Limkemann A., Ramanathan R., Benhke M. Inferior outcomes in hepatitis C virus positive donors to hepatitis C virus negative kidney recipients: analysis from National data 2015 [abstract] Am J Transplant. 2015;suppl 1:76. [Google Scholar]
- 246.Reese P.P., Abt P.L., Blumberg E.A. Transplanting hepatitis C-positive kidneys. N Engl J Med. 2015;373:303–305. doi: 10.1056/NEJMp1505074. [DOI] [PubMed] [Google Scholar]
- 247.Cortijo C. Impacto de la terapia de induccion con agents biologicos en los resultados del trasplante renal en pacientes con infeccion por le virus de la hepatitis C. Doctoral Thesis, Universidad Europea de Madrid, Madrid (Spain) 2012.
- 248.Sureshkumar K.K., Hussein S.M., Thai N.I., Marcus R.J. Kidney transplant outcomes in African American patients with hepatitis C: influence of induction agent [abstract] Am J Transplant. 2012;suppl 3:320. [Google Scholar]
- 249.Linatoc Q., Ren M., Behnke Effect of induction therapy with thymoglobulin on outcome in hepatitis C infected kidney transplant recipients: a single center experience [abstract] Am J Transplant. 2013;suppl 3:85. [Google Scholar]
- 250.Luan F.L., Schaubel D.E., Zhang H. Impact of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation. 2008;85:1601–1606. doi: 10.1097/TP.0b013e3181722f3a. [DOI] [PubMed] [Google Scholar]
- 251.Manuel O., Baid-Agrawal S., Moradpour D. Immunosuppression in hepatitis C virus-infected patients after kidney transplantation. Contrib Nephrol. 2012;176:97–107. doi: 10.1159/000332387. [DOI] [PubMed] [Google Scholar]
- 252.Bloom R.D., Rao V., Weng F. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 253.Watashi K., Hijikata M., Hosaka M. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- 254.Rostaing L., Izopet J., Sandres K. Changes in hepatitis C virus RNA viremia concentrations in long-term renal transplant patients after introduction of mycophenolate mofetil. Transplantation. 2000;69:991–994. doi: 10.1097/00007890-200003150-00055. [DOI] [PubMed] [Google Scholar]
- 255.Gentil Govantes M.A., Esforzado N., Cruzado J.M. Harmful effects of viral replication in seropositive hepatitis C virus renal transplant recipients. Transplantation. 2012;94:1131–1137. doi: 10.1097/TP.0b013e31826fc98f. [DOI] [PubMed] [Google Scholar]
- 256.Hestin D., Guillemin F., Castin N. Pretransplant hepatitis C virus infection: a predictor of proteinuria after renal transplantation. Transplantation. 1998;65:741–744. doi: 10.1097/00007890-199803150-00024. [DOI] [PubMed] [Google Scholar]
- 257.Cruzado J.M., Gil-Vernet S., Ercilla G. Hepatitis C virus-associated membranoproliferative glomerulonephritis in renal allografts. J Am Soc Nephrol. 1996;7:2469–2475. doi: 10.1681/ASN.V7112469. [DOI] [PubMed] [Google Scholar]
- 258.Morales J.M., Pascual-Capdevila J., Campistol J.M. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63:1634–1639. doi: 10.1097/00007890-199706150-00017. [DOI] [PubMed] [Google Scholar]
- 259.Baid S., Pascual M., Williams W.W., Jr. Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10:146–153. doi: 10.1681/ASN.V101146. [DOI] [PubMed] [Google Scholar]
- 260.Baid-Agrawal S., Pascual M., Moradpour D. Hepatitis C virus infection and kidney transplantation in 2014: what's new? Am J Transplant. 2014;14:2206–2220. doi: 10.1111/ajt.12835. [DOI] [PubMed] [Google Scholar]
- 261.Bonacci M., Lens S., Londono M.C. Virologic, clinical, and immune response outcomes of patients with hepatitis C virus-associated cryoglobulinemia treated with direct-acting antivirals. Clin Gastroenterol Hepatol. 2017;15:575–583. doi: 10.1016/j.cgh.2016.09.158. [DOI] [PubMed] [Google Scholar]
- 262.Gragnani L., Piluso A., Urraro T. Virological and clinical response to interferon-free regimens in patients with HCV-related mixed cryoglobulinemia: preliminary results of a prospective pilot study. Curr Drug Targets. 2017;18:772–785. doi: 10.2174/1389450117666160208145432. [DOI] [PubMed] [Google Scholar]
- 263.Gragnani L., Visentini M., Fognani E. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology. 2016;64:1473–1482. doi: 10.1002/hep.28753. [DOI] [PubMed] [Google Scholar]
- 264.Saadoun D., Thibault V., Si Ahmed S.N. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann Rheum Dis. 2016;75:1777–1782. doi: 10.1136/annrheumdis-2015-208339. [DOI] [PubMed] [Google Scholar]
- 265.Sise M.E., Bloom A.K., Wisocky J. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63:408–417. doi: 10.1002/hep.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cacoub P., Vautier M., Desbois A.C. Effectiveness and cost of hepatitis C virus cryoglobulinaemia vasculitis treatment: From interferon-based to direct-acting antivirals era. Liver Int. 2017;37:1805–1813. doi: 10.1111/liv.13465. [DOI] [PubMed] [Google Scholar]
- 267.Emery J.S., Kuczynski M., La D. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. 2017;112:1298–1308. doi: 10.1038/ajg.2017.49. [DOI] [PubMed] [Google Scholar]
- 268.Lauletta G., Russi S., Pavone F. Direct-acting antiviral agents in the therapy of hepatitis C virus-related mixed cryoglobulinaemia: a single-centre experience. Arthritis Res Ther. 2017;19:74. doi: 10.1186/s13075-017-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mazzaro C., Dal Maso L., Quartuccio L. Long-term effects of the new direct antiviral agents (DAAs) therapy for HCV-related mixed cryoglobulinaemia without renal involvement: a multicentre open-label study. Clin Exp Rheumatol. 2018;36 Suppl 111:107–114. [PubMed] [Google Scholar]
- 270.Saadoun D., Pol S., Ferfar Y. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology. 2017;153:49–52. doi: 10.1053/j.gastro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 271.Gill K., Ghazinian H., Manch R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10:415–423. doi: 10.1007/s12072-015-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kasuno K., Ono T., Matsumori A. Hepatitis C virus-associated tubulointerstitial injury. Am J Kidney Dis. 2003;41:767–775. doi: 10.1016/s0272-6386(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 273.Agnello V., Chung R.T., Kaplan L.M. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 274.Fabrizi F., Colucci P., Ponticelli C. Kidney and liver involvement in cryoglobulinemia. Semin Nephrol. 2002;22:309–318. [PubMed] [Google Scholar]
- 275.Fabrizi F., Plaisier E., Saadoun D. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623–637. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 276.Ferri C., Greco F., Longombardo G. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606–1610. doi: 10.1002/art.1780341221. [DOI] [PubMed] [Google Scholar]
- 277.Zignego A.L., Craxi A. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 2008;12:611–636. doi: 10.1016/j.cld.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 278.Arase Y., Ikeda K., Murashima N. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836–840. doi: 10.2169/internalmedicine.37.836. [DOI] [PubMed] [Google Scholar]
- 279.El-Serag H.B., Hampel H., Yeh C. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 280.Kristiansen M.G., Gutteberg T.J., Mortensen L. Clinical outcomes in a prospective study of community-acquired hepatitis C virus infection in Northern Norway. Scand J Gastroenterol. 2010;45:746–751. doi: 10.3109/00365521003690699. [DOI] [PubMed] [Google Scholar]
- 281.Fabrizi F., Pozzi C., Farina M. Hepatitis C virus infection and acute or chronic glomerulonephritis: an epidemiological and clinical appraisal. Nephrol Dial Transplant. 1998;13:1991–1997. doi: 10.1093/ndt/13.8.1991. [DOI] [PubMed] [Google Scholar]
- 282.Huang J.F., Chuang W.L., Dai C.Y. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med. 2006;260:255–262. doi: 10.1111/j.1365-2796.2006.01686.x. [DOI] [PubMed] [Google Scholar]
- 283.Ishizaka N., Ishizaka Y., Seki G. Association between hepatitis B/C viral infection, chronic kidney disease and insulin resistance in individuals undergoing general health screening. Hepatol Res. 2008;38:775–783. doi: 10.1111/j.1872-034X.2008.00334.x. [DOI] [PubMed] [Google Scholar]
- 284.Lee J.J., Lin M.Y., Yang Y.H. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis. 2010;56:23–31. doi: 10.1053/j.ajkd.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 285.Yanik E.L., Lucas G.M., Vlahov D. HIV and proteinuria in an injection drug user population. Clin J Am Soc Nephrol. 2010;5:1836–1843. doi: 10.2215/CJN.01030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ando M., Yanagisawa N., Ajisawa A. Urinary albumin excretion within the normal range is an independent risk for near-term development of kidney disease in HIV-infected patients. Nephrol Dial Transplant. 2011;26:3923–3929. doi: 10.1093/ndt/gfr129. [DOI] [PubMed] [Google Scholar]
- 287.Banerjee T., Scherzer R., Powe N.R. Race and other risk factors for incident proteinuria in a national cohort of HIV-infected veterans. J Acquir Immune Defic Syndr. 2014;67:145–152. doi: 10.1097/QAI.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Estrella M.M., Wyatt C.M., Pearce C.L. Host APOL1 genotype is independently associated with proteinuria in HIV infection. Kidney Int. 2013;84:834–840. doi: 10.1038/ki.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Reynes J., Cournil A., Peyriere H. Tubular and glomerular proteinuria in HIV-infected adults with estimated glomerular filtration rate >/= 60 ml/min per 1.73 m2. AIDS. 2013;27:1295–1302. doi: 10.1097/QAD.0b013e32835fac51. [DOI] [PubMed] [Google Scholar]
- 290.Szczech L.A., Gange S.J., van der Horst C. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 291.Yanagisawa N., Ando M., Ajisawa A. Clinical characteristics of kidney disease in Japanese HIV-infected patients. Nephron Clin Pract. 2011;118:c285–c291. doi: 10.1159/000322278. [DOI] [PubMed] [Google Scholar]
- 292.Morales J.M., Morales E., Andres A. Glomerulonephritis associated with hepatitis C virus infection. Curr Opin Nephrol Hypertens. 1999;8:205–211. [Google Scholar]
- 293.Meyers C.M., Seeff L.B., Stehman-Breen C.O. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631–657. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 294.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 295.Kupin W.L. Viral-Associated GN: Hepatitis C and HIV. Clin J Am Soc Nephrol. 2017;12:1337–1342. doi: 10.2215/CJN.04320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 297.Stehman-Breen C., Alpers C.E., Fleet W.P. Focal segmental glomerular sclerosis among patients infected with hepatitis C virus. Nephron. 1999;81:37–40. doi: 10.1159/000045243. [DOI] [PubMed] [Google Scholar]
- 298.Dey A.K., Bhattacharya A., Majumdar A. Hepatitis C as a potential cause of IgA nephropathy. Indian J Nephrol. 2013;23:143–145. doi: 10.4103/0971-4065.109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Usalan C., Erdem Y., Altun B. Rapidly progressive glomerulonephritis associated with hepatitis C virus infection. Clin Nephrol. 1998;49:129–131. [PubMed] [Google Scholar]
- 300.Markowitz G.S., Cheng J.T., Colvin R.B. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998;9:2244–2252. doi: 10.1681/ASN.V9122244. [DOI] [PubMed] [Google Scholar]
- 301.Barsoum R.S. Hepatitis C virus: from entry to renal injury–facts and potentials. Nephrol Dial Transplant. 2007;22:1840–1848. doi: 10.1093/ndt/gfm205. [DOI] [PubMed] [Google Scholar]
- 302.Wornle M., Schmid H., Banas B. Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168:370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sansonno D., Gesualdo L., Manno C. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. 1997;25:1237–1244. doi: 10.1002/hep.510250529. [DOI] [PubMed] [Google Scholar]
- 304.Sansonno D., Lauletta G., Montrone M. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol. 2005;140:498–506. doi: 10.1111/j.1365-2249.2005.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Johnson R.J., Gretch D.R., Couser W.G. Hepatitis C virus-associated glomerulonephritis. Effect of alpha-interferon therapy. Kidney Int. 1994;46:1700–1704. doi: 10.1038/ki.1994.471. [DOI] [PubMed] [Google Scholar]
- 306.Mazzaro C., Panarello G., Mauro E. Efficacy and safety of pegylated interferon plus ribavirin for the treatment of hepatitis C virus-positive cryoglobulinemic glomerulonephritis. Dig Liver Dis. 2015;47:613–616. doi: 10.1016/j.dld.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 307.Mazzaro C., Monti G., Saccardo F. Efficacy and safety of peginterferon alfa-2b plus ribavirin for HCV-positive mixed cryoglobulinemia: a multicentre open-label study. Clin Exp Rheumatol. 2011;29:933–941. [PubMed] [Google Scholar]
- 308.Saadoun D., Resche-Rigon M., Thibault V. Antiviral therapy for hepatitis C virus–associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696–3706. doi: 10.1002/art.22168. [DOI] [PubMed] [Google Scholar]
- 309.Fabrizi F., Bruchfeld A., Mangano S. Interferon therapy for HCV-associated glomerulonephritis: meta-analysis of controlled trials. Int J Artif Organs. 2007;30:212–219. doi: 10.1177/039139880703000306. [DOI] [PubMed] [Google Scholar]
- 310.Fabrizi F., Martin P., Cacoub P. Treatment of hepatitis C-related kidney disease. Expert Opin Pharmacother. 2015;16:1815–1827. doi: 10.1517/14656566.2015.1066333. [DOI] [PubMed] [Google Scholar]
- 311.De Nicola S., Aghemo A., Campise M.R. Telaprevir in a patient with chronic hepatitis C and cryoglobulinemic glomerulonephritis. Antivir Ther. 2014;19:527–531. doi: 10.3851/IMP2684. [DOI] [PubMed] [Google Scholar]
- 312.Ennaifer R., Sabbah M., Hefaiedh R. Antiviral therapy for hepatitis C virus infection, cryoglobulinemic glomerulonephritis and low-grade malignant lymphoma: A challenge? Tunis Med. 2015;93:203–204. [PubMed] [Google Scholar]
- 313.Iliescu L., Herlea V., Toma L. Association between chronic HCV hepatitis, membranoproliferative glomerulopathy and cutaneous sarcoidosis. J Gastrointestin Liver Dis. 2015;24:8. doi: 10.15403/jgld.2014.1121.lil. [DOI] [PubMed] [Google Scholar]
- 314.Mauro E., Gattei V., Mazzaro C. Recombinant human erythropoietin (RHuEpo) and granular colony stimulating factor (G-CSF) in hepatitis C virus (HCV) related to mixed cryoglobulinaemia associated to membranoproliferative glomerulonephritis type I: a case report description. Infez Med. 2014;22:337–341. [PubMed] [Google Scholar]
- 315.Otsuka T., Sakai Y., Ohno D. A case of cryoglobulinemic membranoproliferative glomerulonephritis induced by hepatitis C virus. J Nippon Med Sch. 2015;82:193–201. doi: 10.1272/jnms.82.193. [DOI] [PubMed] [Google Scholar]
- 316.Wu H., Zou H.B., Xu Y. Hepatitis C virus-related heat-insoluble cryoglobulinemia and thrombotic microangiopathy. Am J Med Sci. 2013;346:345–348. doi: 10.1097/MAJ.0b013e318293cdee. [DOI] [PubMed] [Google Scholar]
- 317.Zhao L.J., Chen F., Li J.G. Hepatitis C virus-related mixed cryoglobulinemic endocapillary proliferative glomerulonephritis and B-cell non-Hodgkin lymphoma: a case report and literature review. Eur Rev Med Pharmacol Sci. 2015;19:3050–3055. [PubMed] [Google Scholar]
- 318.Dussol B., Moal V., Daniel L. Spontaneous remission of HCV-induced cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2001;16:156–159. doi: 10.1093/ndt/16.1.156. [DOI] [PubMed] [Google Scholar]
- 319.Davis C.L., Gretch D.R., Perkins J.D. Hepatitis C–associated glomerular disease in liver transplant recipients. Liver Transpl Surg. 1995;1:166–175. doi: 10.1002/lt.500010306. [DOI] [PubMed] [Google Scholar]
- 320.Donato M.F., Fabrizi F., Fogazzi G.B. Remission of HCV-associated glomerulonephritis with pegylated ifn and ribavirin therapy after liver transplantation: case report and literature review. Int J Artif Organs. 2013;36:63–68. doi: 10.5301/ijao.5000166. [DOI] [PubMed] [Google Scholar]
- 321.Francesca Donato M., Banfi G., Cresseri D. Antiviral therapy of symptomatic HCV-mixed cryoglobulinemia after liver transplant: case report and literature review. Int J Artif Organs. 2013;36:367–372. doi: 10.5301/ijao.5000199. [DOI] [PubMed] [Google Scholar]
- 322.Montalbano M., Pasulo L., Sonzogni A. Treatment with pegylated interferon and ribavirin for hepatitis C virus-associated severe cryoglobulinemia in a liver/kidney transplant recipient. J Clin Gastroenterol. 2007;41:216–220. doi: 10.1097/01.mcg.0000225569.04773.8b. [DOI] [PubMed] [Google Scholar]
- 323.Fabrizi F., Aghemo A., Fogazzi G.B. Acute tubular necrosis following interferon-based therapy for hepatitis C: case study with literature review. Kidney Blood Press Res. 2013;38:52–60. doi: 10.1159/000355753. [DOI] [PubMed] [Google Scholar]
- 324.Artemova M., Abdurakhmanov D., Ignatova T. Persistent hepatitis C virus-associated cryoglobulinemic vasculitis following virus eradication after direct-acting antiviral therapy. Hepatology. 2017;65:1770–1771. doi: 10.1002/hep.28981. [DOI] [PubMed] [Google Scholar]
- 325.Cornella S.L., Stine J.G., Kelly V. Persistence of mixed cryoglobulinemia despite cure of hepatitis C with new oral antiviral therapy including direct-acting antiviral sofosbuvir: A case series. Postgrad Med. 2015;127:413–417. doi: 10.1080/00325481.2015.1021660. [DOI] [PubMed] [Google Scholar]
- 326.Sollima S., Milazzo L., Peri A.M. Persistent mixed cryoglobulinaemia vasculitis despite hepatitis C virus eradication after interferon-free antiviral therapy. Rheumatology (Oxford) 2016;55:2084–2085. doi: 10.1093/rheumatology/kew268. [DOI] [PubMed] [Google Scholar]
- 327.Cacoub P, Si Ahmed SN, Ferfar Y, et al. Long-term efficacy of interferon-free antiviral treatment regimens in patients with hepatitis C virus-associated cryoglobulinemia vasculitis [e-pub ahead of print]. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2018.05.021. [DOI] [PubMed]
- 328.Bonacci M., Lens S., Marino Z. Long-term outcomes of patients with HCV-associated cryoglobulinemic vasculitis after virologic cure [e-pub ahead of print] Gastroenterology. 2018;155:311–315. doi: 10.1053/j.gastro.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 329.Ferri C., Sebastiani M., Giuggioli D. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 330.Tarantino A., Campise M., Banfi G. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618–623. doi: 10.1038/ki.1995.78. [DOI] [PubMed] [Google Scholar]
- 331.Tarantino A., De Vecchi A., Montagnino G. Renal disease in essential mixed cryoglobulinaemia. Long-term follow-up of 44 patients. Q J Med. 1981;50:1–30. [PubMed] [Google Scholar]
- 332.Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. doi: 10.1186/1750-1172-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ahmed M.S., Wong C.F., Shawki H. Rapidly deteriorating renal function with membranoproliferative glomerulonephritis Type 1 associated with hepatitis C treated successfully with steroids and antiviral therapy: a case report and review of literature. Clin Nephrol. 2008;69:298–301. doi: 10.5414/cnp69298. [DOI] [PubMed] [Google Scholar]
- 334.Palombo S.B., Wendel E.C., Kidd L.R. MPGN and mixed cryoglobulinemia in a patient with hepatitis C - new treatment implications and renal outcomes. Clin Nephrol Case Stud. 2017;5:66–69. doi: 10.5414/CNCS109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Humphries K., Darling J.M., Barritt ASt. Membranoproliferative glomerulonephritis, type II cryoglobulinemia and triple therapy for hepatitis C: a case series and review of the literature. Dig Dis Sci. 2014;59:2007–2012. doi: 10.1007/s10620-014-3085-7. [DOI] [PubMed] [Google Scholar]
- 336.Roccatello D., Baldovino S., Rossi D. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia. Clin Rev Allergy Immunol. 2008;34:111–117. doi: 10.1007/s12016-007-8019-0. [DOI] [PubMed] [Google Scholar]
- 337.Roccatello D., Sciascia S., Baldovino S. Improved (4 plus 2) rituximab protocol for severe cases of mixed cryoglobulinemia: a 6-year observational study. Am J Nephrol. 2016;43:251–260. doi: 10.1159/000445841. [DOI] [PubMed] [Google Scholar]
- 338.Saadoun D., Resche Rigon M., Sene D. Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood. 2010;116:326–334. doi: 10.1182/blood-2009-10-248518. [DOI] [PubMed] [Google Scholar]
- 339.De Vita S., Quartuccio L., Isola M. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 340.Quartuccio L., Zuliani F., Corazza L. Retreatment regimen of rituximab monotherapy given at the relapse of severe HCV-related cryoglobulinemic vasculitis: Long-term follow up data of a randomized controlled multicentre study. J Autoimmun. 2015;63:88–93. doi: 10.1016/j.jaut.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 341.Sneller M.C., Hu Z., Langford C.A. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:835–842. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Fabrizi F., Martin P., Elli A. Hepatitis C virus infection and rituximab therapy after renal transplantation. Int J Artif Organs. 2007;30:445–449. doi: 10.1177/039139880703000513. [DOI] [PubMed] [Google Scholar]
- 343.US Food and Drug Administration FDA Drug Safety Communication: Boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) https://www.fda.gov/drugs/drugsafety/ucm366406.htm Available at: Accessed March 23, 2018.
- 344.Terrier B., Launay D., Kaplanski G. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res (Hoboken) 2010;62:1787–1795. doi: 10.1002/acr.20318. [DOI] [PubMed] [Google Scholar]
- 345.Colucci G., Manno C., Grandaliano G. Cryoglobulinemic membranoproliferative glomerulonephritis: beyond conventional therapy. Clin Nephrol. 2011;75:374–379. doi: 10.5414/cnp75374. [DOI] [PubMed] [Google Scholar]
- 346.Reed M.J., Alexander G.J., Thiru S. Hepatitis C-associated glomerulonephritis–a novel therapeutic approach. Nephrol Dial Transplant. 2001;16:869–871. doi: 10.1093/ndt/16.4.869-a. [DOI] [PubMed] [Google Scholar]
- 347.Castillo I., Martinez-Ara J., Olea T. High prevalence of occult hepatitis C virus infection in patients with primary and secondary glomerular nephropathies. Kidney Int. 2014;86:619–624. doi: 10.1038/ki.2014.68. [DOI] [PubMed] [Google Scholar]
- 348.IOM (Institute of Medicine) The National Academies Press; Washington, DC: 2011. Clinical Practice Guidelines We Can Trust. [Google Scholar]
- 349.IOM (Institute of Medicine) The National Academies Press; Washington, DC: 2011. Finding What Works in Health Care: Standards for Systematic Reviews. [PubMed] [Google Scholar]
- 350.Prabhu R.A., Nair S., Pai G. Interventions for dialysis patients with hepatitis C virus (HCV) infection. Cochrane Database Syst Rev. 2015:CD007003. doi: 10.1002/14651858.CD007003.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wells G.A., Shea B., O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at: Accessed July 27, 2018.
- 353.Uhlig K., Macleod A., Craig J. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;70:2058–2065. doi: 10.1038/sj.ki.5001875. [DOI] [PubMed] [Google Scholar]
- 354.Guyatt G.H., Oxman A.D., Kunz R. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Atkins D., Best D., Briss P.A. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shiffman R.N., Shekelle P., Overhage J.M. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139:493–498. doi: 10.7326/0003-4819-139-6-200309160-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.