Abstract
Approximately 20% to 40% of patients with type 1 or type 2 diabetes mellitus develop diabetic kidney disease. This is a clinical syndrome characterized by persistent albuminuria (> 300 mg/24 h, or > 300 mg/g creatinine), a relentless decline in glomerular filtration rate (GFR), raised arterial blood pressure, and enhanced cardiovascular morbidity and mortality. There is a characteristic histopathology. In classical diabetic nephropathy, the first clinical sign is moderately increased urine albumin excretion (microalbuminuria: 30–300 mg/24 h, or 30–300 mg/g creatinine; albuminuria grade A2). Untreated microalbuminuria will gradually worsen, reaching clinical proteinuria or severely increased albuminuria (albuminuria grade A3) over 5 to 15 years. The GFR then begins to decline, and without treatment, end-stage renal failure is likely to result in 5 to 7 years. Although albuminuria is the first sign of diabetic nephropathy, the first symptom is usually peripheral edema, which occurs at a very late stage. Regular, systematic screening for diabetic kidney disease is needed in order to identify patients at risk of or with presymptomatic diabetic kidney disease. Annual monitoring of urinary albumin-to-creatinine ratio, estimated GFR, and blood pressure is recommended. Several new biomarkers or profiles of biomarkers have been investigated to improve prognostic and diagnostic precision, but none have yet been implemented in routine clinical care. In the future such techniques may pave the way for personalized treatment.
Keywords: albuminuria, biomarker, diabetic kidney disease, diabetic nephropathy, glomerular filtration rate
Diabetic kidney disease is a major cause of morbidity and mortality in diabetes. Indeed, the excess mortality of diabetes occurs mainly in individuals with diabetes and proteinuria, and results not only from end-stage renal disease (ESRD) but also from cardiovascular disease, with the latter being particularly common in patients with type 2 diabetes.1, 2, 3 Clinically, diabetic kidney disease is characterized by progressive kidney damage reflected by increasing albuminuria, impairment in renal function (decline in glomerular filtration rate [GFR]), elevated blood pressure, and excess morbidity and mortality due to cardiovascular complications. Diabetic kidney disease rarely develops in patients with type 1 diabetes before 10 years following diagnosis, whereas approximately 3% of patients with newly diagnosed type 2 diabetes already have overt nephropathy.4 Diabetic kidney disease is the single most common cause of ESRD in many parts of the world including Europe, Japan, and the USA, and patients with diabetes account for 25% to 45% of all patients enrolled in ESRD programs.5
Because not all individuals with diabetes develop all the possible complications of the condition, systematic screening for relevant complications has become a major part of diabetes care today. The early detection of complications allows for more focused preventive treatment, or specific treatment to delay progression of a complication in its early stages. The main focus of treatment for diabetes is preventive: in essence, the aim of reducing blood glucose levels and maintaining glucose control is to prevent classical micro- and macrovascular complications.
Screening, diagnosis, and treatment for diabetic kidney disease have advanced substantially over the last 3 decades, improving both time to diagnosis and life-years gained after diagnosis.6, 7 To further this progress, current research seeks to develop new methods for the early detection of diabetic kidney disease, as well as improved treatment.
Definition of diabetic kidney disease
Diabetic kidney disease (also termed “chronic kidney disease” [CKD] due to diabetes or diabetic nephropathy) is defined in both type 1 and type 2 diabetes as the presence of persisting severely elevated albuminuria of >300 mg/24 h (or >200 μg/min), or an albumin-to-creatinine ratio (ACR) of >300 mg/g, confirmed in at least 2 of 3 samples, with concurrent presence of diabetic retinopathy and absence of signs of other forms of renal disease.8 As such, this clinical diagnosis requires only basic clinical and laboratory evaluations. The normal range for albuminuria is <30 mg/g, and the abnormal range is >30 mg/g, but values within both these ranges may be associated with an elevated risk of renal and cardiovascular disease.9 The presence of moderately elevated urine albumin excretion (microalbuminuria) (30–300 mg/g) is widely regarded as a precursor of diabetic nephropathy, both indicating early risk and providing a target for intervention. However, in some cases microalbuminuria can display remission, either spontaneously or owing to treatment,10, 11, 12 resulting in a lower renal risk compared with progression of albuminuria.
The broader term “kidney disease in diabetes” is used for patients with CKD (impaired renal function: estimated GFR [eGFR] < 60 ml/min per 1.73 m2 or proteinuria) regardless of the background. Although impaired renal function with normal albuminuria (ACR < 30 mg/g) is prevalent, particularly in elderly individuals, it is much less likely to progress if albuminuria is not present.13, 14
The Italian Renal Insufficiency and Cardiovascular Events (RIACE) study of more than 15,000 participants with type 2 diabetes suggested that those with elevated albuminuria displayed the typical microvascular complications, whereas nonalbuminuric individuals with impaired renal function had a more cardiovascular or macrovascular phenotype.13
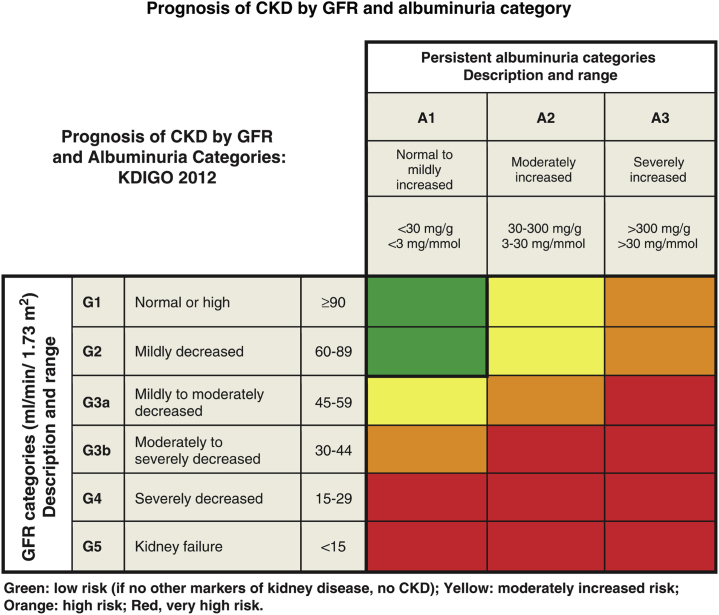
For CKD in general, including in patients with diabetes, it has been recommended to stage the severity of the condition using a combination of etiology (if known), level of urinary albumin excretion, and eGFR category (Figure 1).15 The National Kidney Foundation Kidney Disease: Outcomes Quality Initiative (KDOQI) working group for diabetes and CKD suggested that absence of retinopathy, fast deterioration of GFR, rapidly increasing or nephrotic-range albuminuria (>2500 mg/g), active urinary sediments, refractory hypertension, or signs or symptoms of other systemic diseases should raise suspicion of nondiabetic causes of CKD.16
Figure 1.
Staging of CKD.15 Green: low risk (if no other markers of kidney disease, no CKD); yellow: moderately increased risk; orange: high risk; red: very high risk. Reprinted with permission from Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. Copyright © 2013 KDIGO. CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes.
Pathology
If renal biopsies were feasible in all patients without safety considerations, many patients would probably be diagnosed with early stages of diabetic nephropathy. Morphological changes such as mesangial expansion and thickening of the glomerular and tubular basement membranes, as well as typical glomerulosclerosis with nodular mesangial lesions (Kimmelstiel-Wilson lesions), can be attributed to the impact of hyperglycemia and hyperfiltration. These changes may be observed after only a few years of disease, but their presence is variable, and patients with long-standing diabetes may display only minor changes. Because renal biopsy is not without risk of complications, the procedure is rarely used in routine clinical practice in uncomplicated cases, and is often reserved for cases with severe albuminuria, a fast decline in GFR, or where differential diagnoses are required.
Prevalence
The global Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes (DEMAND) study,17 published in 2006, used a dipstick method to assess the presence of albuminuria in a referred cohort of more than 24,000 patients with type 2 diabetes without known albuminuria from 33 countries. It found an overall global prevalence of severely elevated albuminuria (macroalbuminuria) of 10%, with some variation between regions; microalbuminuria was present in 39% of participants, demonstrating incipient or overt diabetic nephropathy in approximately 50% of this population. Furthermore, 22% of patients had an eGFR < 60 ml/min per 1.73 m2. Although the methodology cannot be regarded as robust, this trial provides one of a few global pictures of the prevalence of diabetic nephropathy.
A number of population-based cohorts and data from clinical centers have provided more detailed descriptions of nephropathy in both type 1 and type 2 diabetes. In short, the prevalence of macroalbuminuria in type 2 diabetes clinics is in the range of 5% to 48% (median 14%) and in type 1 diabetes is 8% to 22% (median 15%). Similarly, microalbuminuria is prevalent in a median of 13% and 20% of patients with type 1 and type 2 diabetes, respectively.8 Interestingly, however, the most recent publication from the US National Health and Nutrition Examination Survey (NHANES) points to a declining trend in albuminuria in the USA, which may be a result of more focused multifactorial treatment over the last few decades.1
Screening
Annual screening of all individuals with diabetes is recommended to detect abnormal and/or changing levels of albuminuria and renal function (i.e., eGFR), so that early renoprotective treatment may be initiated.16 Early-morning spot urine collections are sufficient for screening and monitoring, and are convenient for the patient.9, 18 To take account of wide intraday variability (30%–40%), 2 of 3 spot urine samples within 3 to 6 months must be elevated to confirm the diagnosis. A 24-hour urine collection has been considered the gold standard for albuminuria assessment and can provide additional important information on sodium and protein intake, but complete collection is often difficult for the patient, and so this method is usually restricted to those with established diabetic kidney disease. It should be noted that urinary albumin excretion may be elevated independent of kidney disease by factors such as severe exercise within 24 hours, severe urinary tract infection, menstruation, heart failure, and marked hyperglycemia.
The second clinical variable to assess in screening for diabetic kidney disease is eGFR, using creatinine-based formulae such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.19 If untreated, the “natural” course of diabetic nephropathy displays a continuing annual decline in eGFR of between 2 and 20 ml/min per 1.73 m2 (mean 12 ml/min per 1.73 m2), but effective treatment targeting glycemia, control of blood pressure, blocking of the renin-angiotensin system (RAS), reduction of blood cholesterol levels, and improving lifestyle factors can limit progression to 2 to 5 ml/min per 1.73 m2 per year, demonstrating the importance of screening and intervention.
Clinical quality of albuminuria testing and monitoring
Although screening for albuminuria and renal function in patients with diabetes has long been part of guidelines, it remains difficult in many areas to test and continuously monitor a reasonable fraction of patients. This is despite urinary testing being low-cost, noninvasive, and a relatively simple process. In Denmark, data from the National Diabetes Register in 2014 demonstrated that 85% of patients with diabetes had been screened for albuminuria within a 2-year period in general practice, and 96% in hospital-based outpatient clinics.20 In the Swedish National Diabetes Register in 2016, data on albuminuria were available for 73% of patients with diabetes seen by general practitioners. The Scottish Diabetes Survey 2015 found 71% of patients with type 2 diabetes had been screened within 15 months. All these registers most likely represent relatively successful areas where national quality monitoring is carried out. In the Groningen Initiative to Analyse Type-2 Diabetes Treatment (GIANTT) cohort of primary care patients in the Netherlands,21 57% of patients had albuminuria measurements in 2009 and only 24% had these measurements annually. Similar or even lower levels of screening are found in other countries.
It is a limitation that methods of albuminuria assessment are not yet standardized, and there has been much discussion about quantitative versus qualitative methods and timed or spot urine collection. The precision of estimates of GFR based on creatinine levels has also been extensively debated. The major barrier that remains is that systematic screening, regardless of the method, is often not implemented, and diabetic kidney disease thus remains undetected.
Recent advances
The classification of diabetic kidney disease based on albuminuria and eGFR level is simple (Figure 1), provides prognostic information, and is helpful to guide therapeutic decisions; but it is not perfect. Not all patients with abnormal albuminuria progress to ESRD or cardiovascular disease, and the same is true of many patients with impaired renal function (eGFR < 60 ml/min per 1.73 m2). Therefore an intensive search for new biomarkers in blood or urine that could improve diagnostic and prognostic precision in early or later stages of diabetic kidney disease has been ongoing during the past decades. The underlying hypothesis is that the development from uncomplicated diabetes to renal damage, impaired renal function, and finally ESRD, cardiovascular events, or death takes years, and that an increased risk of progression or early changes in structure or function are reflected by changes in such biomarkers.22 Biomarkers may reflect cellular or systemic changes, changes in different compartments, glomeruli or tubuli,23, 24 or processes such as changes in extracellular matrix handling, fibrosis,25 inflammation,26 oxidative stress,27 glycemic damage, atherosclerosis,28 endothelial cell dysfunction, etc. Several studies including studies in type 1 and type 2 diabetes have found circulating tumor necrosis factor (TNF) receptors to be associated with renal outcome, although the underlying biology remains to be established.29, 30, 31
It has recently been suggested that attention should be focused on patients with a very fast decline in renal function corresponding to time to onset of ESRD of 2 to 6 years. These patients were characterized by elevated albuminuria and TNF receptor 1, based on observations from the Joslin Diabetes Center (Boston, MA), where a significant number of patients showed a very fast decline in eGFR (>15 ml/min/yr), while 80% had a slower decline in eGFR (>5 ml/min/yr). These findings remain to be confirmed in other cohorts.32
The search for biomarkers for increased risk of diabetic kidney disease has often been hypothesis-driven, but so far no biomarkers have been implemented in clinical care for reasons of lack of validation, and confirmation of their added value over that of the existing risk markers has yet to be proven.33
Future perspectives
Alternative research has focused on hypothesis-free multiple-marker approaches to find new markers or combinations of markers associated with progression of diabetic kidney disease.34 This has been described as the application of “-omics” platforms, including genomics, transcriptomics, metabolomics, and proteomics.
Genetics and genomics
Although familial clustering of diabetic kidney disease has been demonstrated, it has been difficult to identify clinically useful genetic markers. The angiotensin-converting enzyme insertion or deletion polymorphism was found in some but not all studies to indicate risk of progression of diabetic kidney disease, and interacted with the RAS-blocking intervention.35 More recently, genome-wide association studies have been performed in the search for genes linked to diabetic kidney disease, and although some areas of the genome have attracted attention, no major susceptibility genes have been identified so far.36, 37, 38, 39 This may reflect that the phenotypes studied to date have been too broad, and a more detailed phenotyping may be needed; alternatively, less common variants than those previously investigated may be involved.
Transcriptomics
Renal biopsies provide diagnostic information, with the typical histological findings described above. More recently it has been suggested that, similar to an oncologist characterizing a tumor based on histology as well as an analysis of typical markers and proteins or transcription of specific genes, this approach may in the future be relevant to diabetic nephropathy, at least for atypical cases or fast progressors, applying histology and transcriptomic analysis of renal tissue to characterize the subtype of disease and select optimal treatment.40 Transcriptomic profiles of renal tissue from patients with diabetic kidney disease and animal models of diabetic kidney disease suggested the importance of the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway as a key target. A clinical study in diabetes intervening with a JAK–STAT inhibitor subsequently demonstrated reduced albuminuria.41
Metabolomics
Metabolites have been investigated in blood and urine using platforms that capture hundreds or even thousands of metabolites. So far, metabolomics studies in diabetic nephropathy have been few, but Pena et al. demonstrated that a small number of metabolites in serum and urine could improve prediction of progression in albuminuria status in type 2 diabetes,42 while Solini et al. demonstrated in patients with type 2 diabetes that serum but not urine metabolites could improve prediction of progression of albuminuria and decline in GFR.43 Sharma et al. described a signature of 13 metabolites in urine that pointed towards mitochondrial dysfunction as a key feature in progression of diabetic kidney disease.27 Niewczas et al. demonstrated uremic solutes associated with development of ESRD in individuals with type 2 diabetes.44
Urinary proteomics
A CKD risk score based on a specific pattern of 273 peptides in urine has been developed by Good et al.45 and is associated with renal pathology and increased renal risk. In cohorts that included patients at an early stage of diabetes with a long follow-up, this classifier could detect patients at renal risk at a mean of 1.5 years before any clinical sign (microalbuminuria) was detected.46 In a cohort of patients with type 2 diabetes with albuminuria and eGFR information, this classifier predicted class change (normo- to micro- to macro-albuminuria), indicating that the prediction of progression in diabetic CKD can be improved.47 Importantly, in a population with mixed causes of CKD, the classifier was also associated with eGFR decline and improved risk prediction beyond that provided by eGFR and albuminuria.48 These studies are all retrospective and require further validation. Currently, the multicenter, prospective, randomized trial Proteomic Prediction and Renin Angiotensin Aldosterone System Inhibition Prevention of Early Diabetic Nephropathy in Type 2 Diabetic Patients With Normoalbuminuria (PRIORITY)49 is ongoing, using the classifier as a marker of renal risk in normoalbuminuric patients with type 2 diabetes. High-risk patients based on the classifier are then randomized to aldosterone blockade or placebo in addition to standard of care.
Another interesting finding regarding the use of urinary proteomics is that in a broad spectrum of renal diseases, the urinary proteome can be associated with renal biopsy findings, suggesting that urinary proteomics may provide noninvasive information on kidney pathology.50
Personalized medicine
Diabetic kidney disease has many phenotypes in terms of rate of progression, degree of comorbidity, and response to interventions. As we learn more about the value and usefulness of the different “omics”-based markers, as well as their limitations, it is expected that data from multiple platforms will be integrated using systems medicine models, thereby providing a better understanding of the underlying pathophysiology for the individual patient and leading to personalized medicine (Figure 2). This will obviously require the ability to identify specific subtypes of diabetic kidney disease, and that treatment options can be targeted toward the relevant pathophysiology, whether this means increased blockade of the RAS or antifibrotic, anti-inflammatory, or other interventions. New techniques are more expensive to use than screening for albuminuria and eGFR, thus the cost-effectiveness of the new methods must be analyzed taking into account the associated reduction in renal and/or cardiovascular risk, and the resulting delay or even prevention of ESRD.
Figure 2.
From albuminuria to personalized treatment.
In conclusion, the diagnosis of diabetic kidney disease relies on measurement and monitoring of urinary albumin excretion (i.e., ACR) and renal function (i.e., eGFR) in combination with clinical assessment. This guides classification, prognosis, and therapy but, although recommended in most guidelines, is still not fully implemented in global diabetes care. New markers and techniques have been suggested to improve diagnostic and prognostic precision and are currently being evaluated, but these are not yet fully validated and ready for use. The future may hold both an increased focus on early screening and a higher level of screening for diabetic kidney disease, as well as the implementation of new preventive measures, with the promise of earlier and more precise renal and cardiovascular risk prediction.
Disclosure
Publication of this article was supported by Bayer AG. PR has equity interest in Novo Nordisk A/S; has research grants from Novo Nordisk, and AstraZeneca; and has been a consultant for AstraZeneca, Bayer AG, BMS, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Astellas, Abbvie, and Merck Sharp & Dohme. All honoraria go to PR’s institution. FP has received a research grant from Novartis, AstraZeneca, and Novo Nordisk; lecture fees from Novartis, Eli Lily, Boehringer Ingelheim, Novo Nordisk, Merck Sharp & Dohme, and AstraZeneca; and consultancy fees from Amgen; and he is a member of the advisory board for AstraZeneca and Merck Sharp & Dohme.
Acknowledgments
Editorial support was provided by Eric Southam of Oxford PharmaGenesis, funded by Bayer AG. The development of this supplement was supported by funding from Bayer AG. No person from the sponsor was involved in the development or review of any of the articles.
References
- 1.Afkarian M., Zelnick L.R., Hall Y.N. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K. The prognosis of insulin-dependent diabetes mellitus. An epidemiological approach. Dan Med Bull. 1989;39:336–349. [PubMed] [Google Scholar]
- 3.de Boer I.H., Gao X., Cleary P.A. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC Study. Clin J Am Soc Nephrol. 2016;11:1969–1977. doi: 10.2215/CJN.02870316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gall M.-A., Rossing P., Skøtt P. Prevalence of micro- and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34:655–661. doi: 10.1007/BF00400995. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle K.R., Bakris G.L., Bilous R.W. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andresdottir G., Jensen M.L., Carstensen B. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int. 2015;87:417–426. doi: 10.1038/ki.2014.206. [DOI] [PubMed] [Google Scholar]
- 7.Andresdottir G., Jensen M.L., Carstensen B. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care. 2014;37:1660–1667. doi: 10.2337/dc13-2036. [DOI] [PubMed] [Google Scholar]
- 8.Parving H.H., Mauer M., Fioretto P. Diabetic nephropathy. In: Brenner B., editor. Vol. 1. Elsevier; Philadelphia, PA: 2012. pp. 1411–1454. (Brenner and Rector's The Kidney). [Google Scholar]
- 9.American Diabetes Association Microvascular Complications and Foot Care. Diabetes Care. 2017;40:S88–S98. doi: 10.2337/dc17-S013. [DOI] [PubMed] [Google Scholar]
- 10.Perkins B.A., Ficociello L.H., Silva K.H. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 11.Hovind P., Tarnow L., Rossing P. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. Br Med J. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaede P., Tarnow L., Vedel P. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19:2784–2788. doi: 10.1093/ndt/gfh470. [DOI] [PubMed] [Google Scholar]
- 13.Solini A., Penno G., Bonora E. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:143–149. doi: 10.2337/dc11-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorn L.M., Gordin D., Harjutsalo V. The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2015;38:2128–2133. doi: 10.2337/dc15-0641. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes Group (KDIGO) Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 16.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Parving H.H., Lewis J.B., Ravid M. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 18.Lambers Heerspink H.J., Gansevoort R.T., Brenner B.M. Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol. 2010;21:1355–1360. doi: 10.1681/ASN.2010010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen M.E., Kristensen J.K., Reventlov Husted G. The Danish Adult Diabetes Registry. Clin Epidemiol. 2016;8:429–434. doi: 10.2147/CLEP.S99518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellemons M.E., Denig P., de Zeeuw D. Is albuminuria screening and treatment optimal in patients with type 2 diabetes in primary care? Observational data of the GIANTT cohort. Nephrol Dial Transplant. 2013;28:706–715. doi: 10.1093/ndt/gfs567. [DOI] [PubMed] [Google Scholar]
- 22.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 23.Araki S., Haneda M., Koya D. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36:1248–1253. doi: 10.2337/dc12-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S.E., Sugaya T., Hovind P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33:1320–1324. doi: 10.2337/dc09-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T.Q., Tarnow L., Jorsal A. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008;31:1177–1182. doi: 10.2337/dc07-2469. [DOI] [PubMed] [Google Scholar]
- 26.Astrup A.S., Tarnow L., Pietraszek L. Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: association with mortality and decline of glomerular filtration rate. Diabetes Care. 2008;31:1170–1176. doi: 10.2337/dc07-1960. [DOI] [PubMed] [Google Scholar]
- 27.Sharma K., Karl B., Mathew A.V. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenvinkel P., Carrero J.J., Axelsson J. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niewczas M.A., Gohda T., Skupien J. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsblom C., Moran J., Harjutsalo V. Added value of soluble tumor necrosis factor alpha receptor-1 as a biomarker of ESRD risk in patients with type 1 diabetes. Diabetes Care. 2014;37:2334–2342. doi: 10.2337/dc14-0225. [DOI] [PubMed] [Google Scholar]
- 31.Gohda T., Niewczas M.A., Ficociello L.H. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krolewski A.S., Skupien J., Rossing P. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int. 2017;91:1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mischak H., Ioannidis J.P., Argiles A. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest. 2012;42:1027–1036. doi: 10.1111/j.1365-2362.2012.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Looker H.C., Colombo M., Hess S. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015;88:888–896. doi: 10.1038/ki.2015.199. [DOI] [PubMed] [Google Scholar]
- 35.Parving H.H., de Zeeuw D., Cooper M.E. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol. 2008;19:771–779. doi: 10.1681/ASN.2007050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germain M., Pezzolesi M.G., Sandholm N. SORBS1 gene, a new candidate for diabetic nephropathy: results from a multi-stage genome-wide association study in patients with type 1 diabetes. Diabetologia. 2015;58:543–548. doi: 10.1007/s00125-014-3459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandholm N., Salem R.M., McKnight A.J. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandholm N., Van Zuydam N., Ahlqvist E. The genetic landscape of renal complications in type 1 diabetes. J Am Soc Nephrol. 2017;28:557–574. doi: 10.1681/ASN.2016020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kottgen A., Pattaro C., Boger C.A. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komorowsky C.V., Brosius F.C., III, Pennathur S. Perspectives on systems biology applications in diabetic kidney disease. J Cardiovasc Transl Res. 2012;5:491–508. doi: 10.1007/s12265-012-9382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosius F.C., Tuttle K.R., Kretzler M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia. 2016;59:1624–1627. doi: 10.1007/s00125-016-4021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pena M.J., Lambers Heerspink H.J., Hellemons M.E. Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with Type 2 diabetes mellitus. Diabet Med. 2014;31:1138–1147. doi: 10.1111/dme.12447. [DOI] [PubMed] [Google Scholar]
- 43.Solini A., Manca M.L., Penno G. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab. 2016;101:696–704. doi: 10.1210/jc.2015-3345. [DOI] [PubMed] [Google Scholar]
- 44.Niewczas M.A., Sirich T.L., Mathew A.V. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 2014;85:1214–1224. doi: 10.1038/ki.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Good D.M., Zurbig P., Argiles A. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zurbig P., Jerums G., Hovind P. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012;61:3304–3313. doi: 10.2337/db12-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roscioni S.S., de Zeeuw D., Hellemons M.E. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia. 2013;56:259–267. doi: 10.1007/s00125-012-2755-2. [DOI] [PubMed] [Google Scholar]
- 48.Schanstra J.P., Zurbig P., Alkhalaf A. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26:1999–2010. doi: 10.1681/ASN.2014050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindhardt M, Persson F, Zurbig P, et al. Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients, a sub-study of the DIRECT-Protect 2 study [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfw292. [DOI] [PubMed]
- 50.Siwy J, Zurbig P, Argiles A, et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfw337. [DOI] [PMC free article] [PubMed]