Abstract
Access to essential medications and health products is critical to effective management of kidney disease. Using data from the ISN Global Kidney Health Atlas multinational cross-sectional survey, global access of patients with kidney disease to essential medications and health products was examined. Overall, 125 countries participated, with 118 countries, composing 91.5% of the world’s population, providing data on this domain. Most countries were unable to access eGFR and albuminuria in their primary care settings. Only one-third of low-income countries (LICs) were able to measure serum creatinine and none were able to access eGFR or quantify proteinuria. The ability to monitor diabetes mellitus through serum glucose and glycated hemoglobin measurements was suboptimal. Pathology services were rarely available in tertiary care in LICs (12%) and lower middle-income countries (45%). While acute and chronic hemodialysis services were available in almost all countries, acute and chronic peritoneal dialysis services were rarely available in LICs (18% and 29%, respectively). Kidney transplantation was available in 79% of countries overall and in 12% of LICs. While over one-half of all countries publicly funded RRT and kidney medications with or without copayment, this was less common in LICs and lower middle-income countries. In conclusion, this study demonstrated significant gaps in services for kidney care and funding that were most apparent in LICs and lower middle-income countries.
Keywords: acute kidney injury and chronic kidney disease care, funding for health care, funding for medications, global health care, health care service provision, renal replacement therapy
Equitable access to quality, affordable, safe, effective, and essential medications; health services; and health products or technologies that meet peoples’ priority health care needs without exposing them to financial hardship in paying for them is a key platform of the worldwide push for universal health coverage1, 2 and the World Health Organization (WHO) Sustainable Development Goal 3: Health.3 Such access is particularly important for people with chronic kidney disease (CKD) or acute kidney injury (AKI) or both given that kidney disease is a major global public health problem with extremely high morbidity and premature mortality4 and significant financial impacts for individuals, societies, and health care systems.5, 6 CKD is a common cause of noncommunicable disease with a mean global prevalence of 12% to 15%.7 It is strongly associated with excessive health care costs, high medication burden, kidney failure requiring renal replacement therapy (RRT), poor quality of life, and increased risks of both communicable and other noncommunicable diseases (particularly cardiovascular disease).4, 8, 9, 10 Similarly, AKI is common, not infrequently requires supportive RRT, and is associated with high rates of morbidity and mortality.11, 12 Furthermore, AKI is associated with an increased risk of CKD, and vice versa.13
Despite the public health importance of CKD and AKI, global access of people with kidney disease to essential medications and health products has not been comprehensively studied or described to date. The aim of the present study, which formed part of the International Society of Nephrology (ISN) Global Kidney Health Atlas project, was to characterize the availability, coverage, scope, capacity, and accessibility of health services for identification, monitoring, and management of kidney care; capacity and funding structure for acute and chronic RRT provision; and medication provision and reimbursement, across countries, ISN regions,14 and 2014 World Bank country classification as low-, lower middle-, upper middle-, and high-income nations.15
Results
Characteristics of participating countries
Of 130 countries surveyed, 125 countries participated, with 118 countries (17 low income, 33 lower middle income, 30 upper middle income, and 38 high income), composing 91.5% of the world’s population, providing data pertaining to this domain. The total percentage of gross domestic product spent on health care for each of these countries is presented in Supplementary Figure S1.16
Identification, monitoring, and management of CKD
The availability of 12 different health care services related to identification, monitoring, and management of CKD was examined at primary and secondary or tertiary care levels in all participating countries (Figures 1 and 2). Overall, there was a graded effect with greater availability observed in secondary or tertiary care compared with primary care and increasing levels of availability in countries through the progression from low-income to lower middle-income to upper middle-income to high-income categorizations (Figures 1 and 2).
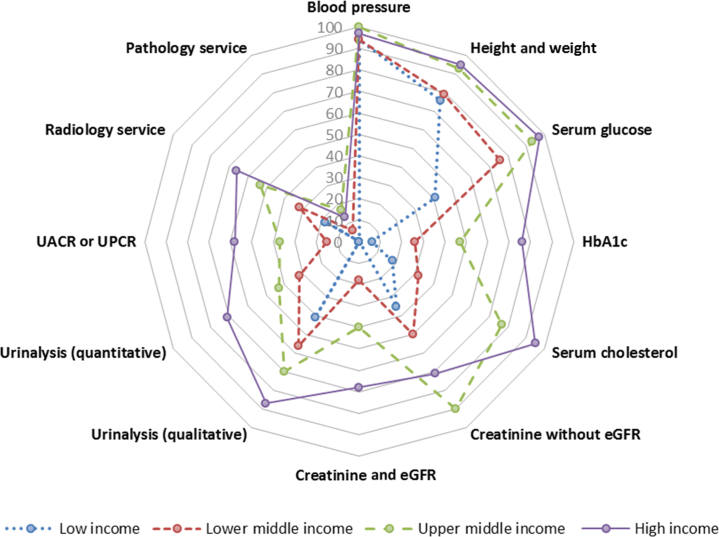
Figure 1.
Health care services for the identification and management of chronic kidney disease in primary care level by World Bank income groups. Capacities of primary health care services for chronic kidney disease care are reported as percentages of countries with particular services in each income group. eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio.
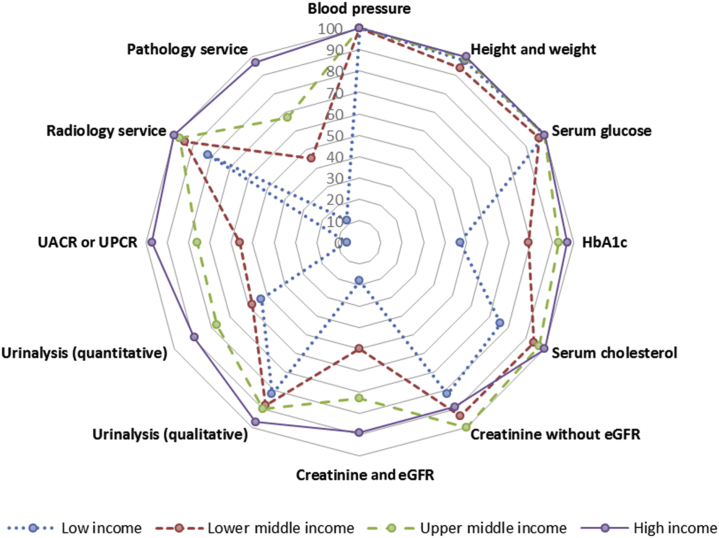
Figure 2.
Health care services for the identification and management of chronic kidney disease in secondary or tertiary care levels by World Bank income groups. Capacities of secondary or tertiary health care services for chronic kidney disease care are reported as percentages of countries with particular services in each income group. eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio.
Primary health care services in low-income countries, particularly in the region of Africa, had limited capacity to diagnose and monitor CKD, being primarily constrained to measurement of blood pressure (94%) and height and weight (73%). Only one-third of low-income countries were able to measure serum creatinine in primary care, and none was able to access estimated glomerular filtration rate (eGFR), quantitative urinalysis, urine albumin to creatinine ratio (UACR), or urine protein to creatinine ratio (UPCR). Qualitative urinalysis using test strips for albumin or protein or both was available in 41% of low-income countries, while 18% had access to radiology services, and 6% to glycated hemoglobin (HbA1c) measurements. Remarkably, only 58% of high-income countries had access to UACR or UPCR in primary care.
Secondary and tertiary health care services enjoyed greater access to CKD identification, monitoring, and management services, although limitations were commonly observed for proteinuria assessment, pathology services, and HbA1c measurement, particularly in low-income countries.
Capacity for provision of RRT
Chronic dialysis facilities
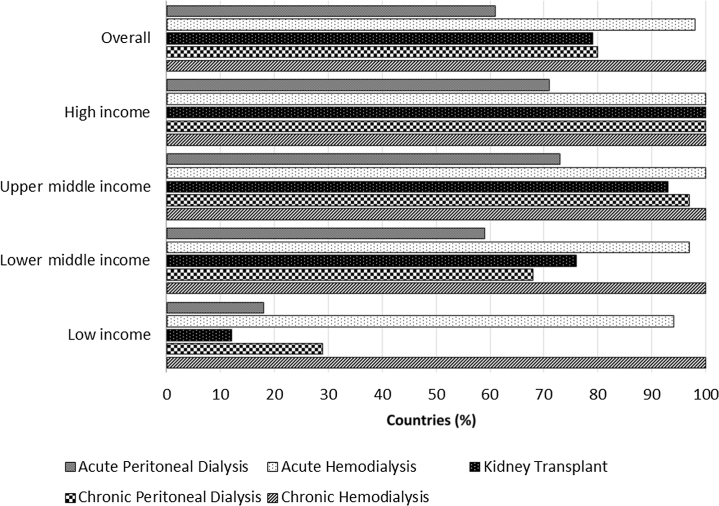
All participating countries (n = 118) had capacity to provide chronic hemodialysis (HD) services whereas only 80% (n = 95) of countries had capacity to provide chronic peritoneal dialysis (PD) services. Chronic PD services were rarely available in low-income countries, with only 29% (n = 5) of countries reporting such capacity (Figure 3). When analyzed according to ISN regions, 48% (n = 16) of African countries and 69% (n = 9) of Oceanian and Southeast Asian (OSEA) countries had capacity to provide chronic PD services (Supplementary Figure S2). The proportions of countries with capacity to provide chronic PD ranged from 69% to 100% in the remaining ISN regions (Supplementary Figure S2).
Figure 3.
Capacity for provision of renal replacement therapy services across countries classified by World Bank income groups. Capacities for renal replacement therapy services across countries are reported as percentages of countries with availability of particular services in each income group.
Transplant facilities
A total of 93 of 118 (79%) participating countries had the capacity to perform kidney transplantation. Only 12% (n = 2) of low-income countries had kidney transplantation services (Figure 3). The types of kidney transplantations performed varied across countries, with the majority of lower middle-income countries (62%, n = 16) and low-income countries (100%, n = 2) performing solely living donor kidney transplantations (Supplementary Figure S3). When analyzed according to ISN regions, the majority of countries in Africa (58%, n = 7) and South Asia (60%, n = 3) solely performed living donor kidney transplantations (Supplementary Figure S4). The presence of national transplant waiting lists also varied considerably according to income groups: low-income (0%, n = 0), lower middle-income (24%, n = 8), upper middle-income (47%, n = 14), and high-income (71%, n = 27).
When analyzed according to ISN regions, only 36% (n = 12) of African countries and 69% (n = 9) of OSEA countries had the capacity to perform kidney transplantations (Supplementary Figure S2). All countries in the remaining ISN regions had the capacity to perform kidney transplantations.
Acute dialysis facilities
Acute HD services were available in almost all participating countries (Figure 3). However, acute PD services were only available in 18% (n = 3) of low-income, 59% (n = 20) of lower middle-income, 73% (n = 22) of upper middle-income, and 71% (n = 27) of high-income countries (Figure 3). When analyzed according to ISN regions, only 36% (n = 12) of African countries, 46% (n = 6) of OSEA countries, and 54% (n = 7) of Middle Eastern countries had acute PD services. The proportions of countries with acute PD services ranged from 67% to 100% in the remaining ISN regions (Supplementary Figure S2).
Funding structure for RRT services
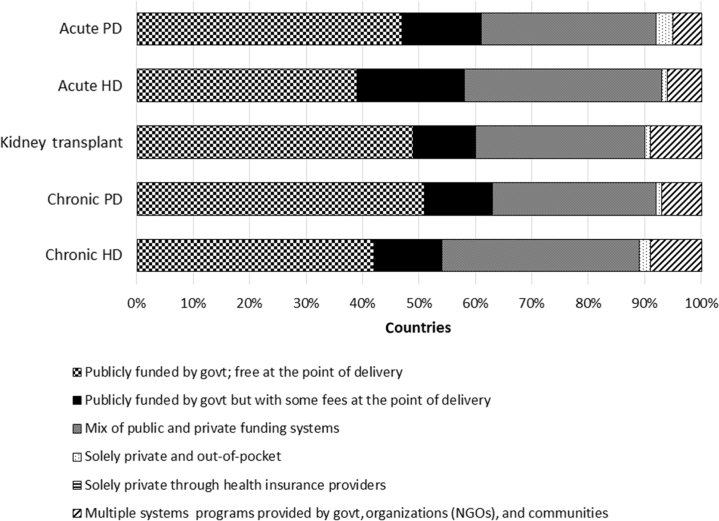
In general, funding for all RRT services across countries followed a similar pattern with the majority of countries funding RRT services through government with no fees at the point of delivery, followed by a mix of public and private funding systems, and then funding through government with some fees at the point of delivery. Only a minority of countries funded RRT services through solely private and out-of-pocket sources (Figure 4).
Figure 4.
Funding for different renal replacement therapy services across all countries. Funding structures for different renal replacement therapy services across all countries in the study are reported as a percentage of countries with a particular type of funding. govt, government; HD, hemodialysis; NGOs, nongovernmental organizations; PD, peritoneal dialysis.
Funding for chronic dialysis services
The distributions of sources of funding for chronic HD were examined according to both 2014 World Bank income group and ISN region classifications and are presented in Supplementary Figures S5 and S6, respectively. The majority of high-income countries (69%) and upper middle-income countries (60%) funded chronic HD through government, whereas only 48% of low-income countries and 36% of lower middle-income countries funded chronic HD through government. Twelve percent of low-income countries funded chronic HD solely through private and out-of-pocket sources (Supplementary Figure S5). All countries in the North America region and the majority of countries in the Eastern and Central Europe, Western Europe, Middle East, and Newly Independent States (NIS) and Russia regions funded chronic HD mainly through government with no fees at the point of delivery, whereas the majority of countries in Latin America and the Caribbean, OSEA, South Asia, and Africa funded chronic HD mainly through a mix of public and private funding systems. The majority of countries from North and East Asia funded chronic HD through government with some fees at the point of delivery.
The distributions of sources of funding for chronic PD according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S7 and S8, respectively. These funding sources followed a similar pattern to those of chronic HD, except that no chronic PD services in low-income countries were publicly funded through government and free at the point of delivery.
Funding for transplant services
The distributions of sources of funding for transplantation services according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S9 and S10, respectively. Kidney transplantation was most commonly funded through government with no fees at the point of delivery in high- and upper middle-income countries, while a mix of public and private funding systems predominated in lower middle- and low-income countries (Supplementary Figure S9). All countries in the North American and Eastern and Central Europe regions and the majority of countries in the Western Europe and NIS and Russia regions funded kidney transplantation through government with no fees, whereas all countries from the South Asia region and the majority of countries from Latin America and the Caribbean and OSEA funded kidney transplantation through a mix of public and private funding systems.
Funding for acute dialysis services
The distributions of sources of funding for acute HD according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S11 and S12, respectively. The patterns of these funding source distributions were comparable to those of chronic HD (Supplementary Figures S5 and S6).
Similarly, the patterns of funding source distributions for acute PD (Supplementary Figures S13 and S14) were comparable to those of chronic PD (Supplementary Figures S7 and S8), except that only 50% of countries from the North American region funded acute PD through government with no fees at the point of delivery and all countries in North and East Asia funded acute PD through government with some fees at the point of delivery (Supplementary Figure S14).
Access to essential medications and health products
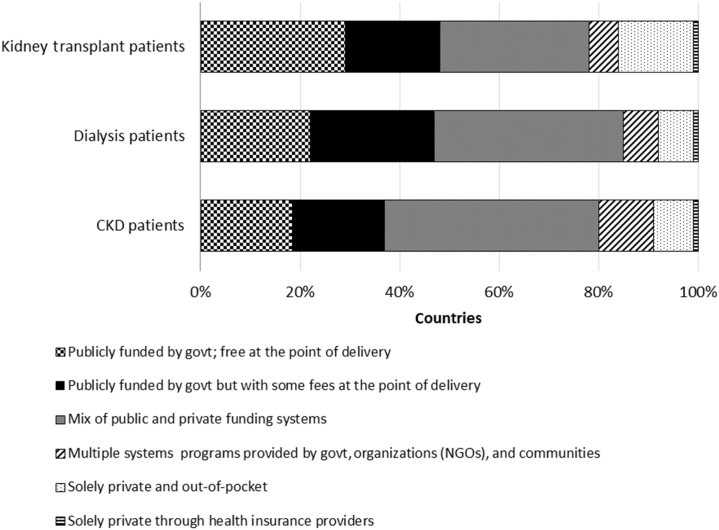
In general, fewer countries funded medications for CKD patients through government compared with medications for dialysis and transplant patients (Figure 5). Overall, less than one-half of countries funded medications for patients with kidney disease through government with or without fees at the point of delivery.
Figure 5.
Funding of medications for patients with kidney diseases. Funding of medications for patients with kidney diseases are reported as percentage of countries with a particular type of funding. CKD, chronic kidney disease; govt, government; NGOs, nongovernmental organizations.
Funding of medications for CKD patients
The distributions of sources of funding for medications for CKD patients according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S15 and S16, respectively. No low- or lower middle-income countries funded medications for CKD patients through government or provided medications free at the point of delivery (Supplementary Figure S15). Even among high-income countries, only a minority publicly funded medications for CKD patients, providing them free at the point of delivery. Although all countries from the North American region funded chronic RRT services through government with no fees at the point of delivery, none publicly funded medications for CKD patients through government, instead funding these through a mix of public and private funding systems (Supplementary Figure S16).
Funding of medications for dialysis patients
The distributions of sources of funding for medications for dialysis patients according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S17 and S18, respectively. These patterns of funding source distributions were similar to those of CKD patients.
Funding of medications for kidney transplant patients
The distributions of sources of funding for medications for kidney transplant patients according to both World Bank income group and ISN region classifications are presented in Supplementary Figures S19 and S20, respectively. For the majority of low-income countries (53%, n = 9), funding for medications for transplant patients was through solely private and out-of-pocket sources (Supplementary Figure S19). For the remaining countries, including high-income countries, only a minority publicly funded transplant medications through government and provided the medications free at the point of delivery (Supplementary Figure S19). The majority of countries from the regions of Eastern and Central Europe, NIS and Russia, and Middle East publicly funded medications for transplant patients, providing them free at the point of delivery (Supplementary Figure S20).
Discussion
The present study found evidence of considerable variation in access of patients with kidney disease to essential medications and health products between countries and within or between ISN regions and World Bank income groups. Specifically, basic and essential tests for identifying monitoring and managing CKD (such as serum creatinine and albuminuria or proteinuria measurements) were not widely available in primary health care services in many countries; PD and kidney transplantation were not available in approximately one-fifth of countries; and RRT and kidney care medications were publicly funded and free at the point of delivery in only a minority of countries. These gaps in kidney care were particularly marked in low- and lower middle-income countries, especially in the African and Southeast Asian regions.
A key global challenge and opportunity for closing the gap in kidney care identified in this study was addressing health care deficits in identifying monitoring and managing CKD. A recent systematic review estimated the global prevalence of CKD to be 13.4% (95% confidence interval: 11.7–15.1), with the majority of cases being in stage 3.7 Although early detection and management of CKD has the potential to yield marked public health benefits,17, 18 the present study found that most countries had inadequate CKD detection and surveillance systems to achieve this goal. The Kidney Disease Improving Global Outcomes Clinical Practice Guideline for the Evaluation and Management of CKD19 recommends measurement of both GFR and albuminuria for the detection, diagnosis, staging, and monitoring of CKD. However, the majority of countries in the present study were unable to access both eGFR and albuminuria in their primary health care settings. In particular, only one-third of low-income countries were able to measure serum creatinine and none were able to access eGFR, quantitative urinalysis, or UACR or UPCR. Remarkably, only just over one-half of high-income countries had access to either eGFR or UACR or UPCR measurements at the primary care level. Estimating GFR using serum creatinine is an essential component in diagnosis, staging, and management of CKD. Monitoring of eGFR with serum creatinine rather than serum creatinine alone is important as serum creatinine is an inaccurate marker of renal function and can be modified by several factors including age, sex, and race. Automated laboratory reporting of eGFR with serum creatinine requests has been identified as an important, low-cost strategy that has proven to be effective for improving CKD detection and management in the community.20, 21 Although eGFR can be easily obtained without additional cost, several low-income and lower middle-income countries reported unavailability of eGFR at both primary and secondary or tertiary care levels.
The present study also demonstrated that the ability of primary health care services to monitor diabetes mellitus, one of the commonest causes of CKD worldwide, was suboptimal in a majority of countries. In particular, serum glucose and HbA1c measurements were only available in 41% and 6% of low-income countries, respectively. It has been estimated that over 8% of the world’s population have diabetes mellitus and that 75% of people with diabetes mellitus live in low- and middle-income countries.22 Monitoring of HbA1c is important for predicting the complications of diabetes mellitus and assessing the efficacy of its treatment.23 Remarkably, one-quarter of high-income countries did not have HbA1c measurements available in primary care.
Secondary and tertiary health care services fared better than primary care in most countries, although a considerable number of countries did not have secondary or tertiary care access to eGFR, UACR or UPCR measurements, or pathology services (including renal biopsy). These gaps in CKD care were particularly marked in low- and lower middle-income countries, such that their abilities to diagnose important underlying causes of CKD (e.g., glomerulonephritis), monitor CKD progression, and institute appropriate treatment were extremely limited.
Another important gap in global kidney care identified in this study was that PD, particularly acute PD, was less available compared with HD, particularly in resource-poor countries. For example, while acute and chronic HD were available in the vast majority of low-income countries, acute and chronic PD were available in only 18% and 29%, respectively. This paradoxical observation is contrary to what might be expected given that PD is generally less expensive than HD in most of the countries where it is practised,24 is less technically demanding, affords greater patient autonomy and satisfaction,25 is more feasible when patients reside great distances from the nearest health care facility,26 is generally less challenging than HD to manage in the setting of natural disasters,27and has been shown to be associated with comparable or superior survival and quality of life compared with HD.28 Consequently, a number of countries, including Hong Kong,29 Thailand,30 USA,31 and China,32 have enacted public policies that actively promote and provide financial incentives for use of PD over HD to leverage its lower costs to the health care system.32 Nevertheless, only 48% of countries in the African region and 69% in OSEA had the capacity to provide chronic PD services. A review of the experiences of resource-limited countries attempting to implement good quality dialysis as part of universal health coverage reforms recommended that low- and middle-income countries should opt for PD as first-line treatment whenever there is limited budget allocation for dialysis programs, restricted human resources for health or significant geographical barriers to health care facility access or both.33 While the barriers and enablers for establishing PD in resource-limited countries were not specifically evaluated in the present study, practical solutions would likely include securing reliable local manufacture and distribution of low-cost PD solutions and local training of health care professionals in PD care. As an example, PD solutions and catheters are considerably more expensive in sub-Saharan Africa than in other parts of the world; this problem could be successfully addressed through partnership among governments, international health agencies, and industry, as occurred with the provision of low-cost antiretroviral therapy to African countries.34 Similarly, the Saving Young Lives program of ISN has built capacity and increased access to acute PD in some low-income countries.35, 36, 37
Similar to PD, approximately one-fifth of countries did not have the capacity to perform kidney transplantation, despite the fact that kidney transplantation is associated with superior survival, quality of life, and cost-effectiveness compared with other forms of RRT.38 This gap was most marked in low-income countries, where only 12% had kidney transplantation services available. When analyzed by region, kidney transplantation was most underrepresented in countries in Africa (36%). Strategies for addressing these important gaps in kidney care include creation of national health insurance schemes; public education regarding organ donation; local health professional training in kidney transplantation; procurement of low-cost immunosuppressive agents and therapeutic drug monitoring; and facilitation of partnerships among government, industry, and nongovernment and philanthropic organizations to promote kidney transplantataion.39
Funding structures for RRT also showed considerable variations between countries and regions. While just over one-half of countries publicly funded RRT with or without a patient copayment, this was much less common in low- and lower middle-income countries and countries in the African, South Asian, and OSEA regions where there was a large private contribution toward payment for RRT services. Similar findings were observed for funding for medications for nondialysis CKD, dialysis, and kidney transplant patients. It is also interesting to note that countries in the North American region publicly funded medications for all RRT modalities through government and provided the medications free at the point of delivery, but this did not extend to funding medications for CKD. Development of context-specific and adaptable strategies to make these care components (services for identification, monitoring, and management of kidney diseases; provision of RRT services; and essential kidney care medications) accessible and affordable to the burgeoning CKD populations at global, regional, and national levels is urgently required.40 These strategies should ideally be integrated into overarching noncommunicable disease strategies. Communities should advocate for the widespread uptake of the WHO Model List of Essential Medications41 and pursuit of WHO Sustainable Development Goal 3: Health, particularly 3.b, in providing affordable essential medicines.3
This is the largest, most comprehensive, and most up-to-date study of country and regional availability of services for identifying, monitoring, and managing CKD, capacity for acute and chronic RRT provision, and access to medications and medication reimbursement plans. Its major strengths include high external validity (involving 118 countries composing 91.5% of the world’s population with broad coverage across World Bank income groups and geographic regions), use of a rigorous survey instrument based on the widely applied WHO health system building blocks,42 and involvement of a broad range of key regional and national stakeholders (including nephrologist leaders, health care policymakers, and consumer representative organizations). These strengths should be balanced against the study’s limitations, including response biases such as social desirability bias and demand characteristics. Such biases were mitigated by corroboration and validation of findings at country levels with regional leaders and published and gray literature. The nature of the survey also meant that the information acquired depended largely on the knowledge, expertise, and perceptions of respondents. In order to optimize the quality of information obtained, respondents with a range of expertise and regional representation were carefully selected following liaison with ISN regional boards. Any discrepant responses between respondents within countries were resolved by teleconference with regional board representatives. It should also be noted that this study focused on RRT availability but did not evaluate RRT accessibility, quality, or outcomes.
In conclusion, the present study examined 1 of the core areas of care of patients with kidney disease, that is, access to essential medications and health products. It demonstrated significant gaps in services for identification, monitoring, and management of CKD; provision of RRT; and funding of RRT and essential kidney care medications that varied markedly between countries and regions and were most apparent in low- and lower middle-income countries. Providing affordable, robust kidney care programs that facilitate early detection and management of kidney disease in the community and provide universal health coverage with respect to affordable RRT and essential kidney care medications is crucial to addressing the burgeoning public health problem due to CKD and AKI. This will require the forging of partnerships among the international nephrology community, governments, international health agencies, and nongovernment and philanthropic organizations to develop innovative solutions to closing the gaps in kidney care, particularly in resource-limited settings. The findings of the present study can also provide important baseline information against which country progress can be benchmarked.
Methods
This study formed part of the Global Kidney Health Atlas project, a multinational, cross-sectional study of global kidney care conducted by the ISN. All United Nations Member States were invited to participate, with a specific focus on 130 countries with ISN affiliated societies. An online questionnaire was distributed through the ISN’s 10 regional boards (Africa, Eastern and Central Europe, Latin America and the Caribbean, Middle East, North America, North and East Asia, OSEA, NIS and Russia, South Asia, and Western Europe) to a minimum of 3 key stakeholders in each country, including leaders of national nephrology societies, health care policymakers, and representatives of kidney disease patient advocacy organizations. Details regarding the sampling approach, development and validation of the survey, data handling, and statistical analysis have been previously published.43, 44 For the purpose of analysis, countries were grouped by 2014 World Bank income group15 and ISN region.14
The present study examined 1 of the main WHO health system building blocks: access to essential medications and health products.45 In this health system domain, the 3 major areas of kidney care evaluated included capacity for identification, monitoring, and management of CKD; capacity for acute and chronic RRT provision; and access to medications for kidney care and reimbursement plans.
The health care services examined under capacity for identification, monitoring, and management of CKD included capacity for monitoring of blood pressure, measurement of weight and height, monitoring of serum glucose, monitoring of HbA1c, measurement of serum cholesterol, monitoring of serum creatinine without eGFR, monitoring of serum creatinine with eGFR, qualitative monitoring of urine albumin, quantitative monitoring of urine albumin, monitoring of UACR or UPCR, radiology services, and pathology services. The availabilities of these services were assessed at both primary and secondary or tertiary care levels. An individual country was considered to have a particular service if such service was available in more than 50% of health care facilities within that country.
The health care services examined under capacity for RRT provision included availability and source(s) of funding for chronic HD, chronic PD, acute HD, acute PD, and kidney transplantation. The sources of funding for health care services were subclassified as publicly funded by government with no fee at the point of delivery; publicly funded by government with some fees at the point of delivery; a mix of publicly funded and private systems; multiple funding sources from government, nongovernment organizations, and communities; solely private and out-of-pocket sources; and other sources.
The health care services examined under access to medications and reimbursement plans included source of funding for medications for care of CKD patients, dialysis patients, and kidney transplant patients.
The data are presented as number (percentage) for categorical variables.
Disclosure
Publication of this article was supported by the International Society of Nephrology. EB-F declared seeing private patients on a part-time basis. MBG declared receiving lecture fees from Amgen, B Braun, Leo Pharma, Novartis, Novo-Nordisk, Promopharm, Roche, Sanofi, Servier, Sophadial, and Sothema. BB declared receiving consulting fees from Otsuka and currently receiving grant support from Amgen. DCH declared receiving lecture fees from Roche Myanmar and Otsuka. VJ declared receiving consulting fees from Baxter and Medtronic; and current grant support from the Department of Biotechnology, Government of India, Baxter, and GlaxoSmithKline. KK-Z declared receiving past and future consulting and lecture fees from Abbott, Abbvie, Alexion, Amgen, AstraZeneca, Aveo, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, and UpToDate; will receive future consulting and lecture fees from ZS-Pharma; currently receiving grant support from National Institutes of Health; and serving as an expert witness engagement for GranuFlo. RK declared receiving lecture fees from Baxter Healthcare. JP declared receiving consulting fees from Fresenius Medical Care, Baxter Healthcare, Otsuka, Boehringer Ingelheim; lecture fees from Baxter Healthcare; and current grant support from Canadian Institutes of Health Research and Baxter Healthcare. DWJ declared receiving consulting fees from AstraZeneca; lecture fees from Baxter Healthcare and Fresenius Medical Care; and support from Baxter Extramural and Clinical Evidence Council grants. All the other authors declared no competing interests.
Acknowledgments
We thank Drs. Marcello Tonelli and Valerie Luyckx for their contributions to the project and manuscript. We thank Sandrine Damster, research project manager at the International Society of Nephrology (ISN), and Alberta Kidney Disease Network staff (Ghennete Houston, Sue Szigety, Sophanny Tiv) for their support with the organization and conduct of the survey and project management. We thank the ISN staff (Louise Fox and Luca Segantini) for their support. We thank the executive committee of the ISN, the ISN regional leadership, and the leaders of the ISN affiliate societies at regional and country levels for their support toward the success of this initiative.
Footnotes
Figure S1. Health care expenditure as percentage of gross domestic product for participating countries classified by World Bank income groups.
Figure S2. Capacity for provision of renal replacement therapy services across countries in International Society of Nephrology regions. Capacities for renal replacement therapy services across countries are reported as percentages of countries with availability of particular services in each region.
Figure S3. Donor types across countries classified by World Bank income groups.
Figure S4. Donor types across countries in International Society of Nephrology regions.
Figure S5. Funding structure for chronic hemodialysis service across countries classified by World Bank income groups.
Figure S6. Funding structure for chronic hemodialysis service across countries in International Society of Nephrology regions.
Figure S7. Funding structure for chronic peritoneal dialysis service across countries classified by World Bank income groups.
Figure S8. Funding structure for chronic peritoneal dialysis service across countries in International Society of Nephrology regions.
Figure S9. Funding for kidney transplantation service across countries classified by World Bank income groups.
Figure S10. Funding for kidney transplantation service across countries in International Society of Nephrology regions.
Figure S11. Funding for acute hemodialysis service across countries classified by World Bank income groups.
Figure S12. Funding for acute hemodialysis service across countries in International Society of Nephrology regions.
Figure S13. Funding for acute peritoneal dialysis service across countries classified by World Bank income groups.
Figure S14. Funding for acute peritoneal dialysis service across countries in International Society of Nephrology regions.
Figure S15. Funding structure of medications for patients with chronic kidney disease across countries classified by World Bank income groups.
Figure S16. Funding structure of medications for patients with chronic kidney disease across countries in International Society of Nephrology regions.
Figure S17. Funding structure of medications for dialysis patients across countries classified by World Bank income groups.
Figure S18. Funding structure of medications for dialysis patients across countries in International Society of Nephrology regions.
Figure S19. Funding structure of medications for kidney transplant patients across countries classified by World Bank income groups.
Figure S20. Funding structure of medications for kidney transplant patients across countries in International Society of Nephrology regions.
Supplementary material is linked to the online version of the paper at http://www.kisupplements.org/.
Supplementary Material
Health care expenditure as percentage of gross domestic product for participating countries classified by World Bank income groups.
Capacity for provision of renal replacement therapy services across countries in International Society of Nephrology regions. Capacities for renal replacement therapy services across countries are reported as percentages of countries with availability of particular services in each region.
Donor types across countries classified by World Bank income groups.
Donor types across countries in International Society of Nephrology regions.
Funding structure for chronic hemodialysis service across countries classified by World Bank income groups.
Funding structure for chronic hemodialysis service across countries in International Society of Nephrology regions.
Funding structure for chronic peritoneal dialysis service across countries classified by World Bank income groups.
Funding structure for chronic peritoneal dialysis service across countries in International Society of Nephrology regions.
Funding for kidney transplantation service across countries classified by World Bank income groups.
Funding for kidney transplantation service across countries in International Society of Nephrology regions.
Funding for acute hemodialysis service across countries classified by World Bank income groups.
Funding for acute hemodialysis service across countries in International Society of Nephrology regions.
Funding for acute peritoneal dialysis service across countries classified by World Bank income groups.
Funding for acute peritoneal dialysis service across countries in International Society of Nephrology regions.
Funding structure of medications for patients with chronic kidney disease across countries classified by World Bank income groups.
Funding structure of medications for patients with chronic kidney disease across countries in International Society of Nephrology regions.
Funding structure of medications for dialysis patients across countries classified by World Bank income groups.
Funding structure of medications for dialysis patients across countries in International Society of Nephrology regions.
Funding structure of medications for kidney transplant patients across countries classified by World Bank income groups.
Funding structure of medications for kidney transplant patients across countries in International Society of Nephrology regions.
References
- 1.O’Connell T., Rasanathan K., Chopra M. What does universal health coverage mean? Lancet. 2014;383:277–279. doi: 10.1016/S0140-6736(13)60955-1. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Universal health coverage: supporting country needs. Available at: http://www.who.int/contracting/UHC_Country_Support.pdf. Accessed August 5, 2017.
- 3.WHO. Sustainable Development Goal 3: Health. 2016. Available at: http://www.who.int/topics/sustainable-development-goals/targets/en/. Accessed August 5, 2017.
- 4.Tonelli M., Wiebe N., Culleton B. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan J., Remuzzi G., Saran R. Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int. 2014;86:246–250. doi: 10.1038/ki.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey A.S., Atkins R., Coresh J. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 7.Hill N.R., Fatoba S.T., Oke J.L. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnak M.J., Levey A.S., Schoolwerth A.C. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 9.Herzog C.A., Asinger R.W., Berger A.K. Cardiovascular disease in chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort R.T., Matsushita K., van der Velde M. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lameire N.H., Bagga A., Cruz D. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 12.Mehta R.L., Burdmann E.A., Cerdá J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 13.Chawla L.S., Kimmel P.L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 14.ISN. Regions. Available at: https://www.theisn.org/about-isn/regions. Accessed August 5, 2017.
- 15.World Bank. World Bank country and lending groups—World Bank data help desk. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed August 25, 2017.
- 16.World Bank. Health expenditure, total (% of GDP). December 2016. Available at: http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed December 30, 2016.
- 17.Johnson D.W., Atai E., Chan M. KHA-CARI Guideline: early chronic kidney disease: detection, prevention and management: early chronic kidney disease guidelines. Nephrology. 2013;18:340–350. doi: 10.1111/nep.12052. [DOI] [PubMed] [Google Scholar]
- 18.Wouters O.J., O’Donoghue D.J., Ritchie J. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11:491–502. doi: 10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens P.E., Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 20.Noble E., Johnson D.W., Gray N. The impact of automated eGFR reporting and education on nephrology service referrals. Nephrol Dial Transplant. 2008;23:3845–3850. doi: 10.1093/ndt/gfn385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D.W., Jones G.R.D., Mathew T.H. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: new developments and revised recommendations. Med J Aust. 2012;197:224–225. doi: 10.5694/mja11.11329. [DOI] [PubMed] [Google Scholar]
- 22.International Diabetes Foundation. IDF diabetes atlas. Available at: http://www.diabetesatlas.org/. Accessed August 5, 2017.
- 23.Stratton I.M., Adler A.I., Neil H.A.W. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karopadi A.N., Mason G., Rettore E., Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 25.Kirchgessner J., Perera-Chang M., Klinkner G. Satisfaction with care in peritoneal dialysis patients. Kidney Int. 2006;70:1325–1331. doi: 10.1038/sj.ki.5001755. [DOI] [PubMed] [Google Scholar]
- 26.Wang V., Maciejewski M.L., Coffman C.J. Impacts of geographic distance on peritoneal dialysis utilization: refining models of treatment selection. Health Serv Res. 2017;52:35–55. doi: 10.1111/1475-6773.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson D.W., Hayes B., Gray N.A. Renal services disaster planning: lessons learnt from the 2011 Queensland floods and North Queensland cyclone experiences. Nephrology. 2013;18:41–46. doi: 10.1111/nep.12008. [DOI] [PubMed] [Google Scholar]
- 28.Mehrotra R., Devuyst O., Davies S.J., Johnson D.W. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P.K., Chow K.M. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62:993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Tantivess S., Werayingyong P., Chuengsaman P., Teerawattananon Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ. 2013;346:f462. doi: 10.1136/bmj.f462. [DOI] [PubMed] [Google Scholar]
- 31.Sedor J.R., Watnick S., Patel U.D. ASN End-Stage Renal Disease Task Force: perspective on prospective payments for renal dialysis facilities. J Am Soc Nephrol. 2010;21:1235–1237. doi: 10.1681/ASN.2010060656. [DOI] [PubMed] [Google Scholar]
- 32.Liu F.X., Gao X., Inglese G. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35:406–420. doi: 10.3747/pdi.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teerawattananon Y., Luz A., Pilasant S. How to meet the demand for good quality renal dialysis as part of universal health coverage in resource-limited settings? Heal Res Policy Syst. 2016;14:21. doi: 10.1186/s12961-016-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feehally J. The International Society of Nephrology (ISN): roles and challenges in Africa and other resource-limited communities. Clin Nephrol. 2016;86(suppl 1):3–7. doi: 10.5414/CNP86S101. [DOI] [PubMed] [Google Scholar]
- 35.ISN. PD for AKI: Saving Young Lives project. Available at: https://www.theisn.org/programs/saving-young-lives-project. Accessed August 25, 2017.
- 36.Abdou N., Antwi S., Koffi L.A. Peritoneal dialysis to treat patients with acute kidney injury—the Saving Young Lives experience in West Africa: proceedings of the Saving Young Lives Session at the First International Conference of Dialysis in West Africa, Dakar, Senegal, December 2015. Perit Dial Int. 2017;37:155–158. doi: 10.3747/pdi.2016.00178. [DOI] [PubMed] [Google Scholar]
- 37.Smoyer W.E., Finkelstein F.O., McCulloch M.I. “Saving Young Lives” with acute kidney injury: the challenge of acute dialysis in low-resource settings. Kidney Int. 2016;89:254–256. doi: 10.1016/j.kint.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 39.Ackoundou-N’Guessan C., Hoang A.D., Ben Abdallah T. Living kidney donor transplantation in a resource-limited country: the Ivory Coast experience. Transplant Proc. 2015;47:1580–1584. doi: 10.1016/j.transproceed.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Lee B.X., Kjaerulf F., Turner S. Transforming our world: implementing the 2030 agenda through sustainable development goal indicators. J Public Health Policy. 2016;37(suppl 1):13–31. doi: 10.1057/s41271-016-0002-7. [DOI] [PubMed] [Google Scholar]
- 41.WHO. WHO model lists of essential medicines. 2017. Available at: http://www.who.int/medicines/publications/essentialmedicines/en/. Accessed August 5, 2017.
- 42.Leowski J., Krishnan A. Capacity to control noncommunicable diseases in the countries of South-East Asia. Health Policy. 2009;92:43–48. doi: 10.1016/j.healthpol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Bello A.K., Levin A., Tonelli M. Assessment of global kidney health care status. JAMA. 2017;317:1864. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bello A.K., Johnson D.W., Feehally J. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl. 2017;7:145–153. doi: 10.1016/j.kisu.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Available at: http://www.who.int/healthinfo/systems/WHO_MBHSS_2010_full_web.pdf. Accessed August 5, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Health care expenditure as percentage of gross domestic product for participating countries classified by World Bank income groups.
Capacity for provision of renal replacement therapy services across countries in International Society of Nephrology regions. Capacities for renal replacement therapy services across countries are reported as percentages of countries with availability of particular services in each region.
Donor types across countries classified by World Bank income groups.
Donor types across countries in International Society of Nephrology regions.
Funding structure for chronic hemodialysis service across countries classified by World Bank income groups.
Funding structure for chronic hemodialysis service across countries in International Society of Nephrology regions.
Funding structure for chronic peritoneal dialysis service across countries classified by World Bank income groups.
Funding structure for chronic peritoneal dialysis service across countries in International Society of Nephrology regions.
Funding for kidney transplantation service across countries classified by World Bank income groups.
Funding for kidney transplantation service across countries in International Society of Nephrology regions.
Funding for acute hemodialysis service across countries classified by World Bank income groups.
Funding for acute hemodialysis service across countries in International Society of Nephrology regions.
Funding for acute peritoneal dialysis service across countries classified by World Bank income groups.
Funding for acute peritoneal dialysis service across countries in International Society of Nephrology regions.
Funding structure of medications for patients with chronic kidney disease across countries classified by World Bank income groups.
Funding structure of medications for patients with chronic kidney disease across countries in International Society of Nephrology regions.
Funding structure of medications for dialysis patients across countries classified by World Bank income groups.
Funding structure of medications for dialysis patients across countries in International Society of Nephrology regions.
Funding structure of medications for kidney transplant patients across countries classified by World Bank income groups.
Funding structure of medications for kidney transplant patients across countries in International Society of Nephrology regions.