Introduction
Rapid‐onset dystonia‐parkinsonism (RDP; DYT12; online Mendelian inheritance in man [OMIM] 128235) is a dystonia‐plus syndrome characterized by the abrupt manifestation of dystonia, mostly spreading in a rostro‐caudal pattern with prominent bulbar symptoms accompanied by parkinsonism features.1 Onset commonly follows a specific triggering event consisting of physical or psychological stress.2 The underlying causes are autosomal‐dominant or de novo mutations in the ATP1A3‐gene encoding the alpha‐3‐subunit of the Na/Ka‐ATPase.3 With about 100 cases described in the literature, it remains a rare disease that, along with its uncommon clinical presentation, may complicate diagnosis. Consequently, little is known about sufficient treatment options.
Case Report
The female patient first presented at 28 years of age with slowly progressing gait disturbance caused by dystonic movements of the right leg. Over the course of a few months, dystonia spread to the left leg and upper limbs, with more pronounced impairment of fine motor skills on her right side. Remarkably, three years later and several months after giving birth to a healthy child, the patient developed persistent severe speech impairment over a few hours, presenting with dysarthrophonia and orofacial dystonic movements. Symptoms did not progress further over seven years. In the neurological examination, her fingers showed a dystonic posture associated with overflow muscle activation. Overall, bradykinesia could be detected without signs of rigor or tremor. Walking was only possible with assistance or an orthosis. The dysarthrophonia improved a little with a typical sensory trick (i.e. clenching her fist to her chin). Notably, some symptoms vanished when she was distracted and were absent during sleep. Family history was negative, although her father had suffered from idiopathic Parkinson's disease from the age of 72 years.
Over the last few years, the patient underwent thorough investigations, including extensive laboratory testing of cerebrospinal fluid and blood samples, imaging with MRI, (123I) FP‐CIT‐SPECT and (18F) FDG‐PET, and genetic testing for DYT1 that all showed unremarkable results. After the vast negative diagnostics, the patient was treated over several years for psychogenic dystonia. Following unsuccessful treatment and the sudden onset of the speech disturbances, in combination with a possible trigger, another genetic testing was initiated in 2016 that revealed a heterozygote mutation in the ATP1A3 gene (p.Thr613Met).
Treatment with trihexyphenidyl (5 mg/d) and tetrabenazine (25 mg/d) alleviated dystonia to a small extent, while levodopa (200 mg/d) showed no effect. Higher doses of each of these treatments were not tolerated. Despite extensive discussion about the experimental character of deep brain stimulation (DBS) in RDP and the negative case reports of DBS in the internal segment of the globus pallidus (GPi) in DYT12 cases,4 the patient and her family actively decided to undergo DBS. Bilateral DBS in the subthalamic nucleus (STN) and field of Forel (FF) was performed in our center in July 2016 (Vercise RC; octopolar linear lead) (Figure 1). A small therapeutic range of STN stimulation resulted in mild improvement in speech, while gait disturbances due to aggravation of preexisting dystonia with impaired leg control occurred. Stimulation in FF appeared to improve speech and orofacial dystonia. So far, no functionally significant improvement has been achieved with STN‐DBS (BFMDRS before and after STN‐DBS: 57.5). However, the patient reports an improvement in subjective well‐being.
Figure 1.
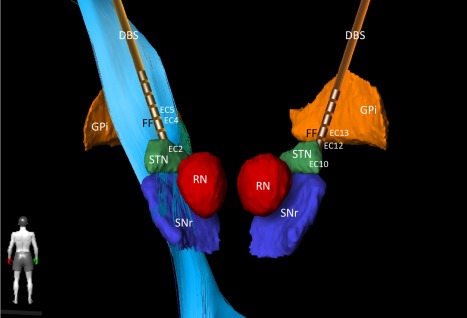
Bilateral DBS implantation sites. Target regions were STN and Field of Forel (FF) with pallido‐fugal fibers (not shown). Left corticospinal tract shown as light blue fibers. FF stimulation; left: case positive, EC4 negative, EC5 negative (1.4 mA, 0.6 mA; 90 us, 119 Hz), right: case positive, EC12 negative, EC13 negative (1.6 mA, 0.4 mA, 90 us, 119 Hz). STN stimulation was performed as follows; left: case positive, E2 negative, 1.8 mA, 90us, 119Hz, right case positive, EC10 negative, 1.8 mA, 90 us, 119 Hz (reduced frequency [60 Hz] did not lead to a better outcome, increased frequency and amplitude of STN DBS led to increased postural instability during gait).
Abbreviations: DBS, deep brain stimulation electrode; EC, effective contact; GPi, globus pallidus internus, (3D reconstruction with BrainLab Elements); RN, red nucleus; SNr, substantia nigra; STN, subthalamic nucleus.
Discussion
The present case demonstrates a clinical manifestation of RDP lacking the diagnostic hallmarks of a rostrocaudal gradient and a focus on bulbar symptoms described in literature.1, 2 The prominent dystonia of the lower limbs, in particular during the initial manifestation, is rare. Contrarily, rapid‐onset bulbar symptoms were the last site of manifestation. Retrospectively, the childbirth preceding bulbar dystonia can be presumed as the triggering event. The unusual clinical presentation supported the diagnostic odyssey and the misjudgement as a psychogenic dystonia that the patient and her family had to cope with for a long time. We also present for the first time bilateral STN‐DBS in an RDP patient. Although GPi‐DBS is known to be effective for primary dystonia,5 it has previously been reported that RDP patients showed no significant response to GPi‐DBS.4, 6, 7 However, STN‐DBS, which is commonly used in PD patients to reduce bradykinesia, has been shown to be effective for controlling dystonic symptoms in these patients, and further studies supported the STN as an alternative target for treating dystonia.8 We also considered the increasing evidence that cerebellar dysfunction contributes to the development of dystonia, supported by mouse studies in which RDP could be induced by blocking sodium pumps in the cerebellum, while an abnormal cerebellar output could be detected.9, 10 Octopolar linear electrodes were used in the DBS under the hypothesis of reaching and modulating the fields of Forel, where cerebellar output is integrated in a neuronal circuit for movement control.11 Unfortunately, the approach showed only limited effectiveness in our patient. Consequently, DBS in DYT12 patients might be seen critically in both GPi‐ and STN‐DBS.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
J.W., S.K.: 1C, 3A
T.P., M.R., B.J., P.C.R., C.W., V.A.C.: 3B
Disclosures
Ethical Compliance Statement: The index patient gave her informed consent prior to their inclusion in the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No specific funding was received for this work.
Financial Disclosures for the previous 12 months: VAC reports honoraria and travel support from Medtronic (USA/Europe) and Boston Scientific (USA) and Brainlab (Germany). VAC has ongoing IITs with limited funding from Medtronic (USA/Europe) and Boston Scientific (USA) and collaborative projects with BrainLab (Germany). VAC serves as a member of the scientific advisory board for CorTec (Freiburg, Germany). P.C.R reports Grants and Grants Pending from German Ministry for Economic Affairs and Energy (Berlin, Germany), Else Kröner‐Fresenius Foundation (Bad Homburg v.d.H., Germany) and Travel/Accommodations/Meeting Expenses/Speaking Fees from Boston Scientific (Natick, MA, USA), Brainlab AG (München, Germany). C.W. DFG Cluster of excellence: BrainLinksBrainTools, 1 Gerok‐position/year. J.W., B.J., T.P., S.K., M.R. report no additional disclosures.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. Video of the index patient with generalized dystonia before and after STN‐DBS (stimulation parameters: FF stimulation; left: case positive, EC4 negative, EC5 negative (1.4 mA, 0.6 mA; 90 us, 119Hz), right: case positive, EC12 negative, EC13 negative (1.6 mA, 0.4 mA, 90 us, 119Hz). STN stimulation: left: case positive, E2 negative, 1.8 mA, 90 us, 119 Hz, right case positive, EC10 negative, 1.8 mA, 90 us, 119 Hz).
Acknowledgements
The authors would like to thank their patient for participating in this study.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Dobyns WB, Ozelius LJ, Kramer PL, et al. Rapid‐onset dystonia‐parkinsonism. Neurology 1993;43:12:2596–2602. [DOI] [PubMed] [Google Scholar]
- 2. Brashear A, Dobyns WB, de Carvalho Aguiar P, et al. The phenotypic spectrum of rapid‐onset dystonia‐parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain 2007;130(Pt 3):828–835. [DOI] [PubMed] [Google Scholar]
- 3. de Carvalho Aguiar P, Sweadner KJ, Penniston JT, et al. Mutations in the Na+/K+ ‐ATPase alpha3 gene ATP1A3 are associated with rapid‐onset dystonia parkinsonism. Neuron 22;43(2):169‐175. [DOI] [PubMed] [Google Scholar]
- 4. Brücke C, Horn A, Huppke P, Kupsch A, Schneider G, Kühn AA. Failure of pallidal deep brain stimulation in a case of rapid‐onset dystonia parkinsonism (DYT12). Mov Disord Clin Pract 2015;2:76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5‐year follow‐up of a randomised trial. Lancet Neurol 2012;11(12):1029–1038. [DOI] [PubMed] [Google Scholar]
- 6. Kamm C, Fogel W, Wächter T. et al. Novel ATP1A3 mutation in a sporadic RDP patient with minimal benefit from deep brain stimulation. Neurology 2008;70(16 Pt 2):1501–1503. [DOI] [PubMed] [Google Scholar]
- 7. Deutschländer A, Asmus F, Gasser T, Steude U, Bötzel K. Sporadic rapid‐onset dystonia‐parkinsonism syndrome: failure of bilateral pallidal stimulation. Mov Disord 2005;20:2:254–257. [DOI] [PubMed] [Google Scholar]
- 8. Schjerling L, Hjermind LE, Jespersen B. et al. A randomized double‐blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. J Neurosurg 2013;119(6):1537–1545. [DOI] [PubMed] [Google Scholar]
- 9. Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high‐frequency burst firing of cerebellar neurons in rapid‐onset dystonia‐parkinsonism. J Neurosci 2014;34:35:11723–11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid‐onset Dystonia‐Parkinsonism. Nat Neurosci 2011;14:3:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pong M, Horn KM, Gibson AR. Pathways for control of face and neck musculature by the basal ganglia and cerebellum. Brain Res Rev 2008;58:2:249–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. Video of the index patient with generalized dystonia before and after STN‐DBS (stimulation parameters: FF stimulation; left: case positive, EC4 negative, EC5 negative (1.4 mA, 0.6 mA; 90 us, 119Hz), right: case positive, EC12 negative, EC13 negative (1.6 mA, 0.4 mA, 90 us, 119Hz). STN stimulation: left: case positive, E2 negative, 1.8 mA, 90 us, 119 Hz, right case positive, EC10 negative, 1.8 mA, 90 us, 119 Hz).


