Abstract
The G8 questionnaire is a quick and easy-to-use screening tool. Several studies reported that the G8 questionnaire had a high sensitivity for predicting abnormalities in the full comprehensive geriatric assessment and predicted functional decline and survival in elderly cancer patients. The present study aimed to evaluate the role of the G8 questionnaire for predicting clinical outcomes and overall survival (OS) in elderly patients with lung cancer, who received chemotherapy or chemoradiotherapy. The data of 101 lung cancer patients aged ≥70 years, who were hospitalized between September 2011 and August 2014, were analyzed. Of these patients (median age, 77 years), 83 (82%) had impaired G8 scores. The proportion of patients with an impaired G8 score was significantly higher in patients aged ≥80 years than those aged <80 years (p = 0.04). All 18 patients with a normal G8 score possessed an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and none of the patients with a normal G8 score had an ECOG PS of ≥2 (p < 0.0001). An impaired G8 score tended to correlate with a relative dose intensity of <0.65 in patients who received chemotherapy or chemoradiotherapy (p = 0.05, odds ratio = 5.40). In the univariate analysis, an ECOG PS of ≥2 and an impaired G8 score were significantly associated with a poor OS (p = 0.009 and p = 0.003, respectively). Moreover, in the multivariate analysis, an ECOG PS of ≥2 (HR 2.55; 95% CI, 1.23–5.30; p = 0.01) and an impaired G8 score (HR 3.86; 95% CI, 1.44–13.36; p = 0.006) were remained independent prognostic factor for OS. G8 screening tool is useful for the prognostication of elderly lung cancer patients treated with chemotherapy. These finding suggest that the G8 questionnaire could be a useful tool in treatment decision-making to predict prognosis and prevent patients from receiving inappropriate anti-cancer treatment near the end of life.
Introduction
Alongside the aging of the global population, the number of elderly persons with lung cancer has been increasing. Recent data of the Surveillance, Epidemiology, and End Results program from the United States showed that patients aged ≥70 years accounted for 47% of all lung cancer cases [1]. This trend is similar to Japan, where more than 60% of new lung cancer cases are seen in individuals aged ≥65 years [2]. Furthermore, more than 75% of deaths from lung cancer in Japan are occurring in persons aged ≥65 years [3]. While the number of elderly patients with lung cancer is increasing, treatment guidelines are based on clinical trials conducted in healthy elderly participants. Therefore, these guidelines are difficult to apply to general clinical settings in which elderly, fragile, and heterogeneous populations are treated [4].
The Comprehensive Geriatric Assessment (CGA) is a widely used method to determine the medical, psychological, and functional capabilities of older patients and different components of CGA can be useful to predict toxicity [4, 5] and functional decline [6]. The International Society of Geriatric Oncology recommends that a CGA should be used in elderly cancer patients to select fit elderly patients who are able to receive standard treatment [7]. However, the CGA is time consuming [8, 9]. In contrast, the G8 questionnaire is a quick and easy-to-use screening tool that takes less than 5 minutes to administer. The G8 screening tool consists of 7 items from the mini nutritional assessment questionnaire and age (S1 Table) [10]. Several recent studies showed that the G8 questionnaire had a high sensitivity for predicting abnormalities in the full CGA [11]. Screening tools including G8 do not replace CGA but are recommended in a busy practice in order to identify those patients in need of full CGA [12]. Besides being a convenient screening tool for geriatric assessment, the G8 questionnaire has also been claimed to be a valuable tool for predicting survival. Several studies reported that the G8 detected functional decline and predicted survival in elderly cancer patients [13, 14]. Regarding lung cancer, a retrospective study conducted with 142 elderly lung cancer patients demonstrated that potentially frail patients, identified by an impaired G8 or Identification of Seniors at Risk for Hospitalized Patients (ISAR-HP), and higher disease stage had a significantly greater risk of 1-year mortality in the Cox regression analysis. Furthermore, analyzing the screening instruments separately showed that the G8 had an independent relation with 1-year mortality and the ISAR-HP did not [15]. Although the G8 tool has been extensively investigated in several elder cancers including solid and liquid tumors, its prediction ability in lung cancers is still elusive.
The present study aimed to validate the role of the G8 questionnaire for predicting overall survival (OS) in elderly lung cancer patients, who received chemotherapy (CT). In addition, we aimed to evaluate the association between G8 scores and predictive factors for unfavorable clinical outcomes, such as severe adverse events (SAEs), cessation of treatment (COT), and relative dose intensity (RDI) <0.65.
Patients and methods
Study design and population
In this prospective cohort study, we enrolled 101 lung cancer patients aged ≥70 years who were candidates for CT, radiotherapy (RT), or chemoradiotherapy (CRT) and were hospitalized at Yokohama Municipal Citizen’s Hospital between September 2011 and August 2014. Since only hospitalized patients were included in this study, patients who were prescribed oral anti-cancer agents and initiated treatment in an outpatient setting were excluded. Furthermore, patients that physicians considered not suitable for receiving a G8 questionnaire were also excluded. This study was approved by the ethics committee of Yokohama Municipal Citizen’s Hospital (18-04-03). Participants provided their verbal informed consent to participate in this study. Because G8 examination was neither invasive nor interventional, our ethics committee recommended not to get written informed consent in this observational study.
G8 screening and other measures
All participants underwent G8 screening by physicians before the beginning of treatment (S1 Table). The maximum score of the G8 is 17 points, and a score of ≤14 is defined as an impaired G8 score, according to previous studies that analyzed the association between the G8 score and OS [10, 16].
In addition to the G8 scores, we collected the following patient characteristics: age, sex, histology, Eastern Cooperative Oncology Group performance status (ECOG PS) and stage. Furthermore, we investigated the associations between G8 scores and SAEs, RDI, COT, and OS in patients who received CT or CRT. A score of SAEs were defined as Grade 3–5 non-hematologic and Grade 4–5 hematologic adverse events. RDI was defined as the ratio of received to expected chemotherapy doses, and RDI was evaluated for initial 2 months of therapy. We set cut-off value of RDI to 0.65, because a recent study that reported a relationship between components of the CGA, chemotherapy dose intensity, and OS in colorectal cancer set cut-off value of RDI to 0.65 and 0.85 [17]. OS was defined as the period from the date of the diagnosis of lung cancer to the date of death from any cause or the last follow-up. The treatment strategy was decided based on the patient’s ECOG PS, age, and status of organ function, regardless of the G8 score.
Statistical analysis
The association between G8 score and patient characteristics was analyzed by the Fisher’s exact test. Determination of predictive factors for unfavorable clinical outcomes such as SAE, COT, and RDI <0.65 were performed using logistic regression analysis. Cumulative survival rates were calculated by the Kaplan-Meier method. The log-rank test was used to evaluate survival difference between the groups (normal G8 vs. impaired G8). Univariate analysis and multivariate analysis were performed using Cox regression analysis to evaluate the prognostic value of six clinically selected variable [G8 score, age (<80 vs. ≥80), sex, histology (NSCLC vs. SCLC), disease stage (I-III vs. IV)) and ECOG PS (0–1 vs. ≥2)]. The analyses of clinical outcomes were performed only in patients receiving CT or CRT. All data were analyzed with the JMP 9 software (SAS Institute Inc., Cary, NC, USA). Differences were considered significant when the p value was less than 0.05.
Results
Patient characteristics
Patient characteristics are listed in Table 1. The median age of the patients was 79 years-old (range, 70–95 years-old), with 19.8% being women. Moreover, there were 45 (44.6%) and 56 (55.4%) patients aged ≥80 years and <80 years, respectively. Adenocarcinoma was the most common histologic type, accounting for 45.6% of the patients, followed by squamous cell carcinoma (29.7%), and small cell carcinoma (18.8%). The ECOG PS score was 0, 1, 2, and 3 in 8 (7.9%), 50 (49.5%), 33 (32.7%), and 10 (9.9%) patients, respectively. At the time of evaluation, 85 patients had newly diagnosed lung cancer; of these, 38 patients had stage I–III disease and 47 patients had stage IV disease. 16 patients had recurrent disease; of these, 2 patients had stage I–III disease and 14 patients had stage IV disease. An impaired G8 score was found in 82.2% of all patients. The most common treatment was CT (58.4% of the patients), followed by RT (24.8%), best supportive care (BSC) only (9.9%), and CRT (6.9%).
Table 1. Patient characteristics (n = 101).
| Clinical Characteristic | No. of Patients (n = 101) | % | |
|---|---|---|---|
| Age | Median age (range) | 79 (70–95) | |
| Sex | Male | 81 | 80.2 |
| Female | 20 | 19.8 | |
| Histology | Adenocarcinoma | 46 | 45.6 |
| Squamous cell carcinoma | 30 | 29.7 | |
| Large cell carcinoma | 1 | 1.0 | |
| NOS | 5 | 4.9 | |
| SCLC | 19 | 18.8 | |
| ECOG PS | 0 | 8 | 7.9 |
| 1 | 50 | 49.5 | |
| 2 | 33 | 32.7 | |
| 3 | 10 | 9.9 | |
| Stage | I | 5 | 5.0 |
| II | 7 | 6.9 | |
| III | 26 | 25.7 | |
| IV | 47 | 46.5 | |
| Recurrence | 16 | 15.9 | |
| G8 screening score | Median (range) | 12 (2–17) | |
| ≤14 | 83 | 82.2 | |
| Treatment Received | RT | 25 | 24.8 |
| CT | 69 | 58.4 | |
| CRT | 7 | 6.9 | |
| BSC | 10 | 9.9 | |
NOS, not otherwise specified; SCLC, small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, Performance status; RT, radiotherapy; CT, chemotherapy; CRT, chemoradiotherapy; BSC, best supportive care.
Association between G8 scores and patient characteristics
The associations between G8 score and patient characteristics are shown in Table 2. The proportion of patients with an impaired G8 score was significantly higher in patients aged ≥80 years than those aged <80 years (p = 0.04). All 18 patients with a normal G8 score possessed an ECOG PS of 0 or 1, and none of the patients with a normal G8 score had an ECOG PS of ≥2 (p < 0.0001). We observed no differences in sex, histology, stage, and the presence or absence of CT between patients with a normal and an impaired G8 score. Of all the analyzed patients, 76 (75%) received CT or CRT. The associations between G8 scores and those 76 patient characteristics are listed in Table 3. As with the analysis of the entire cohort, all 18 patients with a normal G8 score possessed an ECOG PS of 0 or 1, and none of the patients with a normal G8 score had an ECOG PS of ≥2 (p < 0.0001)
Table 2. G8 scores and patient characteristics (n = 101).
| Total | Normal G8 scores | Impaired G8 scores | p value | ||
|---|---|---|---|---|---|
| n = 101 | n = 18 | n = 83 | |||
| Age | 80> | 56 | 14 (25%) | 42 (75%) | 0.04* |
| ≥80 | 45 | 4 (9%) | 41 (91%) | ||
| Sex | Male | 81 | 17 (21%) | 64 (79%) | 0.11 |
| Female | 20 | 1 (5%) | 19 (95%) | ||
| Histology | NSCLC | 82 | 14 (17%) | 68 (83%) | 0.74 |
| SCLC | 19 | 4 (21%) | 15 (79%) | ||
| ECOG PS | 0–1 | 58 | 18 (31%) | 40 (69%) | <0.0001* |
| ≥2 | 43 | 0 (0%) | 43 (100%) | ||
| Stage | I–III | 40 | 6 (15%) | 34 (85%) | 0.61 |
| IV | 61 | 12 (20%) | 49 (80%) | ||
| Treatment received | Non-chemotherapy | 25 | 0 (0%) | 25 (100%) | 0.11 |
| Chemotherapy | 76 | 18 (24%) | 58 (76%) |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, Performance status.
*Statistically significant (p < 0.05)
Table 3. G8 scores and patient characteristics in the patients treated with chemotherapy (n = 76).
| Total | Normal G8 scores | Impaired G8 scores | p value | ||
|---|---|---|---|---|---|
| n = 76 | n = 18 | n = 58 | |||
| Age | 80> | 48 | 14 (29%) | 34 (71%) | 0.13 |
| ≥80 | 28 | 4 (14%) | 24 (86%) | ||
| Sex | Male | 63 | 17 (27%) | 46 (73%) | 0.10 |
| Female | 13 | 1 (8%) | 12 (92%) | ||
| Histology | NSCLC | 58 | 14 (24%) | 44 (76%) | 0.86 |
| SCLC | 18 | 4 (22%) | 14 (78%) | ||
| ECOG PS | 0–1 | 52 | 18 (35%) | 34 (65%) | <0.0001* |
| ≥2 | 24 | 0 (0%) | 24 (100%) | ||
| Stage | I–III | 23 | 6 (26%) | 17 (74%) | 0.74 |
| IV | 53 | 12 (23%) | 41 (77%) |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, Performance status.
*Statistically significant (p < 0.05)
Prognostic value of the G8 and other clinical parameters
For those patients who received CT or CRT, we evaluated the predictive factors for clinical outcomes including SAE, COT caused by SAEs, and an RDI <0.65 (Table 4). Logistic regression analysis revealed that stage (I–III vs. IV) and an impaired G8 tended to be correlated with an RDI <0.65 (p = 0.09, OR = 3.41, 95% confidence interval [CI]: 0.84–23.14; p = 0.05, OR = 5.40, 95% CI: 0.97–101.76, respectively).
Table 4. Prognostic value of G8 and other patient characteristics in the patients treated with chemotherapy (n = 76).
| Cut-off | SAE OR (95% CI) |
P | COT because of AE OR (95% CI) |
P | RDI <0.65 OR (95% CI) |
P | |
|---|---|---|---|---|---|---|---|
| Age | ≥80 vs. <80 | 0.84 (0.32–2.25) | 0.73 | 1.52 (0.51–4.47) | 0.45 | 1.67 (0.52–5.28) | 0.38 |
| Sex | Male vs. Female | 0.72 (0.18–2.49) | 0.61 | 1.87 (0.44–12.94) | 0.42 | 1.43 (0.33–9.98) | 0.66 |
| Histology | SCLC vs. NSCLC | 1.70 (0.56–5.91) | 0.35 | 0.32 (0.04–1.33) | 0.13 | 0.43 (0.06–1.80) | 0.27 |
| ECOG PS | 2–3 vs. 0–1 | 0.83 (0.29–2.44) | 0.73 | 2.20 (0.69–6.82) | 0.18 | 2.24 (0.98–7.36) | 0.19 |
| Stage | IV vs. I–III | 1.50 (0.54–4.08) | 0.43 | 2.63 (0.76–12.32) | 0.13 | 3.41 (0.84–23.14) | 0.09 |
| G8 Score | ≤14 vs. >14 | 0.81 (0.25–2.43) | 0.72 | 3.05 (0.75–20.65) | 0.13 | 5.40 (0.97–101.76) | 0.05 |
SAE, severe adverse event; COT, cessation of treatment; AE, adverse event; RDI, relative dose intensity; OR, odds ratio; CI, confidence interval; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, Performance status.
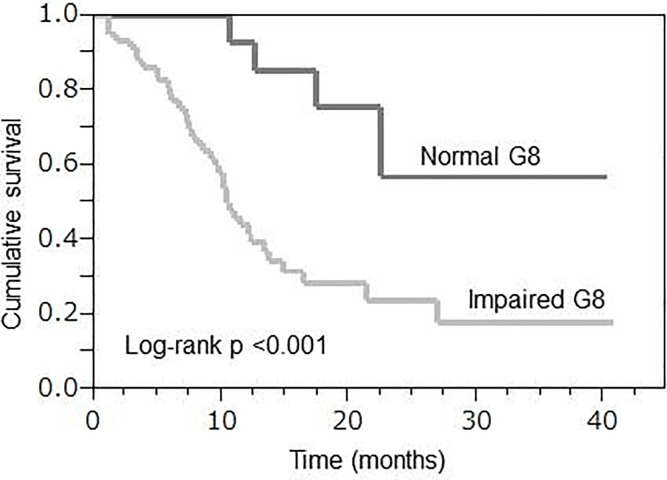
For evaluating OS, the median follow-up time was 12.8 months (range, 1.1–40.8 months). We compared OS between patients with normal and impaired G8 scores and found that it showed significant prognostic value (log-rank p <0.001, Fig 1). The median survival time was 10.6 months for patients with an impaired G8 score, whereas a median survival time was not achieved for patients with a normal G8 score. In the univariate analysis, ECOG PS of ≥2 (HR 2.54; 95% CI, 1.28–4.88; p = 0.009) and an impaired G8 score (HR 4.87; 95% CI, 1.94–16.32; p = 0.003) were significantly associated with a poor OS. In the multivariate analysis, ECOG PS of ≥2 (HR 2.55; 95% CI, 1.23–5.30; p = 0.01) and an impaired G8 score (HR 3.86; 95% CI, 1.44–13.36; p = 0.006) were remained independent prognostic factor for OS (Table 5).
Fig 1. Overall survival according to the G8 score in patients who received chemotherapy (n = 76).
Kaplan-Meier analyses for overall survival according to the G8 score.
Table 5. Univariate and multivariate survival analyses in patients who received chemotherapy (n = 76).
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Cut-off | OS HR (95% CI) |
p | OS HR (95% CI) |
p | |
| Age | ≥80 vs. <80 | 0.93 (0.48–1.73) | 0.82 | 0.90 (0.46–1.69) | 0.74 |
| Sex | Male vs. Female | 0.73 (0.35–1.71) | 0.45 | 1.10 (0.38–1.93) | 0.81 |
| Histology | SCLC vs. NSCLC | 0.83 (0.37–1.67) | 0.62 | 0.53 (0.23–1.15) | 0.11 |
| ECOG PS | 2–3 vs. 0–1 | 2.54 (1.28–4.88) | 0.009* | 2.55 (1.23–5.30) | 0.01* |
| Stage | IV vs. I–III | 1.61 (0.80–3.59) | 0.19 | 1.39 (0.68–3.14) | 0.38 |
| G8 Score | ≤14 vs. >14 | 4.87 (1.94–16.32) | 0.003* | 3.86 (1.44–13.36) | 0.006* |
OS, overall survival; HR, hazard ratio; CI, confidence interval; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, Performance status.
*Statistically significant (p < 0.05)
Discussion
This prospective cohort study was designed to evaluate the role of the G8 score in predicting clinical outcomes and OS in elderly patients with lung cancer. Moreover, this study was the first study to evaluate the association between G8 score and the predictive factors for unfavorable clinical outcomes such as SAE, COT, and RDI <0.65. As a result, we found that an impaired G8 score tended to correlate with an RDI <0.65. Moreover, our study revealed that the G8 score and ECOG PS were independent prognostic factors for OS.
Compared to the general population of elderly lung cancer patients in Japan, there are slightly more male than female in the male-to-female ratio observed in our study cohort. Generally, female patients have a high proportion of EGFR-positive lung cancer. Patients with EGFR-positive lung cancer commonly begin oral treatment as outpatients. These groups of patients are excluded from this study, since we examined hospitalized patients. Therefore, this is why there are slightly more male than female in this study. The histology of the patients in this study is representative of the general population of elderly lung cancer patients in Japan. In our study, 82.2% of the patients had impaired G8 scores; this is slightly higher or comparable to previous studies [10, 13, 18–20]. The results of the present study showed that the proportion of patients with an impaired G8 score was significantly higher in patients aged ≥80 years than those aged <80 years (p = 0.04). All 18 patients with a normal G8 score possessed an ECOG PS of 0 or 1, and none of the patients with a normal G8 score had an ECOG PS of ≥2 (p < 0.0001). When patients who received CT or CRT were analyzed, it was also shown that G8 score and ECOG PS score were significantly correlated to each other. This correlation between G8 score and ECOG PS score has been demonstrated by other studies [14, 21].
The G8 score was not found to be useful for predicting SAEs or COT, while an impaired G8 score tended to correlate with an RDI <0.65. We speculate the reason for this is as follows. When physicians determine the appropriate dosing for cytotoxic anticancer agents, they make decisions based not only on age and ECOG PS, but also functional decline. Age and ECOG PS alone may be insufficient to predict chemotherapy tolerance and clinical outcomes. Functional decline may be more important and it is associated with the G8 score in elderly patients and the reasons are as follows. The G8 screening tool puts weight on nutritional status and consists of mobility, neuropsychological problems, drugs prescribed, self-assessment health condition, and age, from the Mini-Nutritional Assessment questionnaire [22, 23]. We think that these items may be important factors for reflecting functional decline and predicting chemotherapy tolerance in elderly cancer patients. In order to prove this assumption is true, it is necessary to continue further research and increase the number of cases.
In the multivariate analysis, an ECOG PS score ≥2 and an impaired G8 score were remained independent prognostic factor for OS and an impaired G8 score (HR 2.55; 95% CI, 1.23–5.30; p = 0.01) was more strongly correlated with prognosis than PS score ≥2 (HR 3.86; 95% CI, 1.44–13.36; p = 0.006). It is reported that PS [24], weight loss [25], nutritional status [26], and inflammatory biomarkers [27] can predict survival in advanced lung cancer patients. As mentioned above, the G8 screening tool consists of assessments for food intake, weight loss, mobility, neuropsychological problems, drugs prescribed, self-assessment health condition, and age, from the Mini-Nutritional Assessment questionnaire [22, 23]. We speculate that evaluating patients using multiple aspects is reason why G8 was found to be a stronger prognostic factor than ECOG PS.
Each patient, and their associated cancer, has unique characteristics, among which lung cancer generally has a rapid disease course and poor overall survival [27, 28]. Therefore, it is important to specifically investigate each patient with lung cancer. Previously, Schulkes et al. found that potentially frail patients, identified by an impaired G8 or ISAR-HP, and higher disease stage had a significantly greater risk of 1-year mortality in the Cox regression analysis. Furthermore, analyzing the screening instruments separately showed that the G8 had an independent relation with 1-year mortality and the ISAR-HP did not [15]. The findings of our study are similar to this work, which supports G8 score as a prognostic factor of elderly lung cancer. However, our multivariate analysis did not include ISAR-HP score. In addition, several variables included into multivariate analysis were different from that of their studies. Nevertheless, similar results indicating that G8 was an independent prognostic factor in elderly lung cancer patients were obtained in both studies. Therefore, we believe that our results can add to the current knowledge.
Corre et al. reported a multicenter, open-label, phase III trial in 474 elderly patients aged ≥70 years with an ECOG PS score 0–2 and stage IV non-small cell lung cancer [29]. In this trial, patients were randomly assigned between chemotherapy allocation on the basis of PS and age (standard arm) and treatment allocation including chemotherapy and BSC on the basis of CGA (CGA arm). This study showed that treatment allocation on the basis of CGA failed to improve treatment failure free survival and OS, but slightly reduced treatment-related toxicity, although the CGA arm contained 23% of patients receiving BSC. Moreover, patients in the CGA arm, when compared to those in the standard arm, showed a significantly lower rate of all-grade toxicity (85.6% vs. 93.4%, p = 0.015) and a significantly lower rate of treatment failure resulting from toxicity (4.8% vs. 11.8%, p = 0.007). The fact that OS in the CGA arm was similar to that of the standard arm and the treatment-related toxicity was low despite the CGA arm containing 23% of patients receiving BSC alone, might indicate that frail patients in the CGA arm could avoid ineffective chemotherapy. What is revealed in this study is the possibility of using CGA to identify patients who should receive BSC without reducing their survival. However, since the CGA is time-consuming, economically low in rewards for most medical systems, and not necessary for all patients [30], applying this tool is burdensome and difficult to use for clinicians in their daily clinical practice. Therefore, it is important to examine whether the G8 questionnaire can be used as an alternative to the CGA to select patients who should receive BSC.
Our study has some limitations. First, our study included biases because the characteristics of the patients such as stage, histological type, and treatment received were heterogeneous. However, previous studies that reported the G8 questionnaire was a valuable predictive tool of survival also included patients with heterogeneous stages, treatments, and various types of solid cancers [19], various types of blood cancers [13], and both [14, 18]. Although bias cannot be excluded, it is interesting that the G8 score was a stronger prognostic factor than the ECOG PS; moreover, we were able to show that the prognostic value of the G8 remained in this heterogeneous population, which is a major strength of this study. Second, this study included a relatively low number of patients. Since this was an observational study, there was no sample size determination.
In conclusion, our prospective study found that an impaired G8 score tended to correlate with a RDI <0.65 in elderly lung cancer patients treated with chemotherapy. Moreover, we revealed that the G8 score was an independent prognostic factor for OS like ECOG PS. These finding suggest that the G8 questionnaire could be a useful tool in treatment decision-making to predict prognosis and prevent patients from receiving inappropriate anti-cancer treatment near the end of life. As this study was performed at a single institution and the number of cases was small, multi-center large-scale trials are necessary to confirm these results in elderly patients with lung cancer.
Supporting information
BMI, body mass index.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25(35):5570–7. 10.1200/JCO.2007.12.5435 [DOI] [PubMed] [Google Scholar]
- 2.Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2002: based on data from 11 population-based cancer registries. Jpn J Clin Oncol. 2008;38(9):641–8. 10.1093/jjco/hyn074 [DOI] [PubMed] [Google Scholar]
- 3.Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare, Vital Statistics of Japan http://wwwdbtk.mhlw.go.jp/toukei/cgi/sse_kensaku
- 4.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–65. 10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–86. Epub 2011/11/11. 10.1002/cncr.26646 . [DOI] [PubMed] [Google Scholar]
- 6.Hoppe S, Rainfray M, Fonck M, Hoppenreys L, Blanc JF, Ceccaldi J, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31(31):3877–82. 10.1200/JCO.2012.47.7430 Epub 2013 Sep 23. [DOI] [PubMed] [Google Scholar]
- 7.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241–52. 10.1016/j.critrevonc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 8.Aapro M, Extermann M, Repetto L. Evaluation of the elderly with cancer. Ann Oncol. 2000;3:223–9. [DOI] [PubMed] [Google Scholar]
- 9.Baitar A, Kenis C, Moor R, Decoster L, Luce S, Bron D, et al. Implementation of geriatric assessment-based recommendations in older patients with cancer: A multicentre prospective study. J Geriatr Oncol. 2015;6(5):401–10. 10.1016/j.jgo.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Bellera CA, Rainfray M, Mathoulin-Pelissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–72. 10.1093/annonc/mdr587 [DOI] [PubMed] [Google Scholar]
- 11.Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12):e115060 10.1371/journal.pone.0115060 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendationsdagger. Ann Oncol. 2015;26(2):288–300. 10.1093/annonc/mdu210 Epub 2014 Jun 16. [DOI] [PubMed] [Google Scholar]
- 13.Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol. 2014;93(6):1031–40. 10.1007/s00277-013-2001-0 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M, Komine K, Yamada H, Kasahara Y, Chikamatsu S, Okita A, et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: A retrospective, single institutional study. PLoS One. 2017;12(6):e0179694 10.1371/journal.pone.0179694 eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulkes KJG, Souwer ETD, van Elden LJR, Codrington H, van der Sar-van der Brugge S, Lammers JJ, et al. Prognostic Value of Geriatric 8 and Identification of Seniors at Risk for Hospitalized Patients Screening Tools for Patients With Lung Cancer. Clin Lung Cancer. 2017;18(6):660–6 e1. Epub 2017/03/23. 10.1016/j.cllc.2017.02.006 . [DOI] [PubMed] [Google Scholar]
- 16.Dubruille S, Libert Y, Roos M, Vandenbossche S, Collard A, Meuleman N, et al. Identification of clinical parameters predictive of one-year survival using two geriatric tools in clinically fit older patients with hematological malignancies: Major impact of cognition. J Geriatr Oncol. 2015;6(5):362–9. 10.1016/j.jgo.2015.07.006 Epub Aug 12. [DOI] [PubMed] [Google Scholar]
- 17.Ramsdale E, Polite B, Hemmerich J, Bylow K, Kindler HL, Mohile S, et al. The Vulnerable Elders Survey-13 predicts mortality in older adults with later-stage colorectal cancer receiving chemotherapy: a prospective pilot study. J Am Geriatr Soc. 2013;61(11):2043–4. 10.1111/jgs.12536 [DOI] [PubMed] [Google Scholar]
- 18.Kenis C, Decoster L, Van Puyvelde K, De Greve J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32(1):19–26. 10.1200/JCO.2013.51.1345 [DOI] [PubMed] [Google Scholar]
- 19.Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liuu E, Canoui-Poitrine F, Tournigand C, Laurent M, Caillet P, Le Thuaut A, et al. Accuracy of the G-8 geriatric-oncology screening tool for identifying vulnerable elderly patients with cancer according to tumour site: the ELCAPA-02 study. J Geriatr Oncol. 2014;5(1):11–9. Epub Sep 16. 10.1016/j.jgo.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol. 2014;93(6):1031–40. 10.1007/s00277-013-2001-0 Epub 2014 Feb 2. [DOI] [PubMed] [Google Scholar]
- 22.Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–22. [DOI] [PubMed] [Google Scholar]
- 23.Valentini A, Federici M, Cianfarani MA, Tarantino U, Bertoli A. Frailty and nutritional status in older people: the Mini Nutritional Assessment as a screening tool for the identification of frail subjects. Clin Interv Aging. 2018;13:1237–1244. 10.2147/CIA.S164174 eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzoli CG, Temin S, Aliff T, Baker S Jr., Brahmer J, Johnson DH, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29(28):3825–31. 10.1200/JCO.2010.34.2774 Epub 1 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? British Journal Of Cancer. 2004;90:1905 10.1038/sj.bjc.6601781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannousi Z, Gioulbasanis I, Pallis AG, Xyrafas A, Dalliani D, Kalbakis K, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Supportive Care in Cancer. 2012;20(8):1823–9. 10.1007/s00520-011-1282-x [DOI] [PubMed] [Google Scholar]
- 27.Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer—A prospective study examining key clinicopathological factors. Lung Cancer. 2015;88(3):304–9. 10.1016/j.lungcan.2015.03.020 Epub Mar 28. [DOI] [PubMed] [Google Scholar]
- 28.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. 10.1016/j.ejca.2018.07.005 Epub Aug 9. [DOI] [PubMed] [Google Scholar]
- 29.Corre R, Greillier L, Le Caer H, Audigier-Valette C, Baize N, Berard H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. 2016;34(13):1476–83. 10.1200/JCO.2015.63.5839 Epub 2016 Feb 16. [DOI] [PubMed] [Google Scholar]
- 30.Extermann M, Meyer J, McGinnis M, Crocker TT, Corcoran MB, Yoder J, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49(1):69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BMI, body mass index.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.