Abstract
Internal RNA modifications have been known for decades, however their roles in mRNA regulation have only recently started to be elucidated. Here we investigated the most abundant mRNA modification, N6-methyladenosine (m6A) in transcripts from the hippocampus of HIV transgenic (Tg) rats. The distribution of m6A peaks within HIV transcripts in HIV Tg rats largely corresponded to the ones observed for HIV transcripts in cell lines and T cells. Host transcripts were found to be differentially m6A methylated in HIV Tg rats. The functional roles of the differentially m6A methylated pathways in HIV Tg rats is consistent with a key role of RNA methylation in the regulation of the brain transcriptome in chronic HIV disease. In particular, host transcripts show significant differential m6A methylation of genes involved in several pathways related to neural function, suggestive of synaptodendritic injury and neurodegeneration, inflammation and immune response, as well as RNA processing and metabolism, such as splicing. Changes in m6A methylation were usually positively correlated with differential expression, while differential m6A methylation of pathways involved in RNA processing were more likely to be negatively correlated with gene expression changes. Thus, sets of differentially m6A methylated, functionally-related transcripts appear to be involved in coordinated transcriptional responses in the context of chronic HIV. Altogether, our results support that m6A methylation represents an additional layer of regulation of HIV and host gene expression in vivo that contributes significantly to the transcriptional effects of chronic HIV.
Introduction
An extensive repertoire of modifications is believed to contribute to the regulation of RNA processing, metabolism and expression. It has long been known that transfer RNA (tRNA), ribosomal RNA (rRNA) and both mRNA and noncoding RNA (ncRNA) contain multiple modifications [1–5]. At least 10 distinct modified bases have now been identified in mammalian mRNAs, suggesting the existence of an “epitranscriptome” [6–9]. In addition to the 7-methylguanosine cap that is added at the 5’ end of all cellular mRNAs, several internal RNA modifications have been described, which include N6-methyladenosine (m6A), 2’-O-methyladenosine (Am), N6-2’-O-methyladenosine (m6Am), pseudouridine, and 5-methylcytosine, among others [6–10]. Of these, by far the most abundant RNA modification identified is N6-methyladenosine (m6A) [1, 2]. The occurrence of m6A in polyadenylated RNA was originally reported in 1974 [11, 12]. While m6A has been found in mRNAs from diverse tissue types, the brain has especially high levels of m6A [13]. mRNAs are modified by m6A preferentially in the 3′ untranslated regions (UTRs), near the stop codons within mRNAs and within long internal exons, and m6A modification appears to be conserved between humans and rodents [1].
Reminiscent of DNA epigenetics, the occurrence of both stable and dynamically regulated m6A-modified RNA sites has been suggested [1, 2, 14–16]. mRNAs encoding housekeeping genes are less likely to be m6A methylated, while highly regulated mRNAs often contain m6A residues [8, 10]. RNA is m6A methylated within the consensus motifs G(m6A)C and A(m6A)C [17]. RNA modification by m6A appears to contribute to the regulation of gene expression and is increasingly being implicated in disease. Evidence suggests that RNA m6A methylation contributes to the regulation of splicing, nuclear RNA export, mRNA stability, and translation [18–23]. RNA m6A methylation is at least in part dynamically regulated and potentially reversible [6–8]. Multiple proteins are involved in the regulation of m6A RNA methylation and the fate of m6A methylated RNA [6–8]. The fat mass and obesity associated gene (FTO), a m6A demethylase, has been linked to a variety of biological processes including dopaminergic neuron regulation [24] and a variety of human diseases including obesity, type 2 diabetes (T2DM), cancer, attention-deficit/hyperactivity disorder, and Alzheimer's disease [25–31]. The m6A contents in the RNAs of T2DM patients and diabetic rats are significantly lower than controls, while the level of FTO in T2DM patients was significantly higher [31]. Loss of ALKBH5, the other known mammalian demethylase, is associated with increased m6A content in mRNA and impaired fertility [32].
Multiple viral RNAs have long been known to be m6A modified. Classic studies by Shatkin and associates in the 70s showed specific patterns of m6A modification in transcripts from both DNA and RNA viruses such as influenza virus, adenovirus, papovavirus, and flavivirus, among others [3, 7, 33–35]. Retrovirus transcripts have also been found to be m6A modified [36, 37]. The phenotypic consequences of these modifications have recently begun to emerge and involve regulation of viral expression and infectivity, reviewed in [7, 38–41]. Recent studies showed m6A modification in HIV-1 transcripts contributes to regulate HIV-1 mRNA in vitro [42–44]. Another possible role of m6A modification of viral RNAs appears to be antiviral innate immune evasion, since the presence of m6A on viral RNA has been shown to reduce its activation of toll-like receptor 3 signaling [45].
Here we investigated m6A methylation of RNA in the hippocampus of HIV Tg rats. HIV-1 Tg rats harbor a non-replicating HIV-1 transgene and express multiple HIV-1 proteins under the viral LTR in macrophages, lymphocytes and disease-relevant glial cells, such as microglia and astrocytes, but not in neurons [46, 47]. HIV-1 Tg rats exhibit deficits in working memory [47], spatial [48] and reversal learning [49, 50], before the onset of motor deficits [47–49]. This is reminiscent of HAND in the cART era, which primarily impacts learning and executive functioning in a shift from the prominent slowed motor and speed of processing deficits in the pre-cART era [51].
We observed that distribution of peaks of m6A RNA methylation in HIV transcripts in HIV Tg rats largely corresponds to the ones reported for HIV transcripts in cell lines and T cells in vitro [42–44]. Further, we observed that host genes in HIV Tg rats show significant differential m6A methylation of genes involved in several pathways related to neural function, suggestive of synaptodendritic injury and neurodegeneration, consistent with our previous observations in both HIV Tg rats and humans with HIV [52, 53]. Transcripts with significant differential m6A methylation and expression are also involved in immune response and inflammation, also consistently with gene expression changes in HIV Tg rats and humans with HIV [52, 53]. We also observed differential m6A methylation in transcripts of pathways involved in RNA splicing and processing, which are processes previously implicated in the role of m6A RNA methylation [6–10].
Results
RNA m6A methylation in viral and host transcripts from the hippocampus of HIV Tg rats
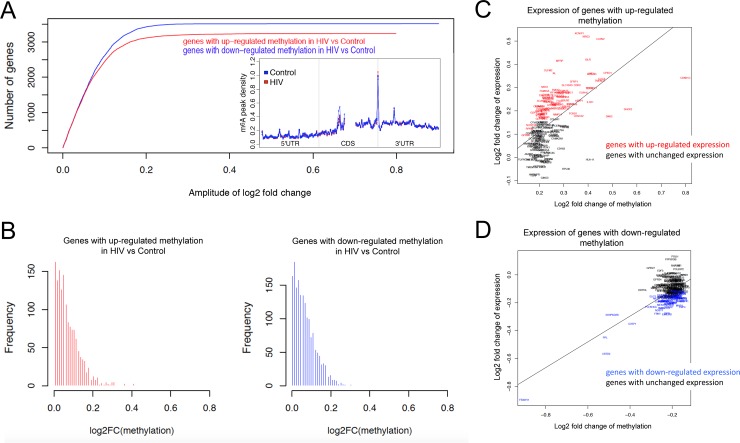
We carried out MeRIP from poly-A selected RNA extracted from the hippocampus of HIV Tg and control rats using an antibody specific for m6A. As shown in Fig 1A and 1B, the number of transcripts with significant increased and significant decreased m6A methylation was similar, although a slightly larger number of genes with down-regulation of m6A methylation was observed than genes with up-regulated methylation in the hippocampus of HIV Tg rats (201 genes with down-regulated methylation and 172 genes with significant up-regulated methylation; p-value<0.05, S1 Table). Analysis of the distribution of m6A peaks within host mRNAs in HIV Tg and control rat showed that m6A peaks were enriched (fold change of average coverage equals to 2.3) in the distal CDS and 3’UTR of the mRNAs and especially in the immediate vicinity of the stop codon (Fig 1A). Differential m6A methylation in poly-A selected transcripts mostly showed either positive correlated changes in m6A and mRNA levels or were not differentially expressed (Fig 1C and 1D).
Fig 1. Distribution of genes with differential m6A RNA methylation in the hippocampus of HIV Tg rats.
A) A larger number of genes with down-regulation of m6A methylation was observed in HIV Tg rats than the number of genes with up-regulated methylation, in particular, 201 genes with significant down-regulated methylation (p-value<0.05) while 172 genes were found with significant up-regulated methylation (p-value<0.05). Inset: Distribution of m6A peaks within hippocampal mRNAs of HIV Tg and control rat. Transcript architecture is shown underneath: 5’ untranslated region (UTR); coding sequence (CDS); and 3’ UTR. The density of m6A peaks was greater on the distal CDS and 3’UTR and showed the greatest enrichment in the immediate vicinity of the stop codon at the CDS-3’UTR boundary. B) The distribution of log2 fold change (log2FC) of genes with up-regulated and down-regulated methylation C,D) Correlation between the methylation and expression changes for genes with up-regulated and down-regulated methylation (Pearson correlation = 0.67 and 0.58 respectively). Among the 201 genes with down-regulated methylation, the expression of 127 genes are also down-regulated while the rest remain unchanged; Among the 172 genes with up-regulated methylation, the expression of 83 genes are also up-regulated while expression of 89 genes remain unchanged.
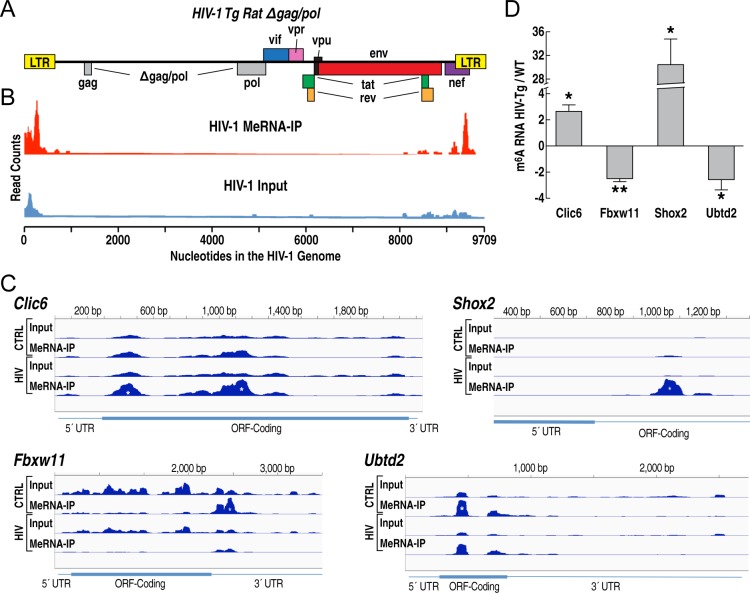
We then investigated the distribution of m6A modification in HIV-1 transcripts in HIV Tg rats (Fig 2). The HIV-1 Tg rats used in the present study harbor a gag/pol-deleted HIV-1 provirus under the control of the long-terminal repeat (LTR) promoter [46], resulting in the co-expression of multiple HIV-1 proteins in disease-relevant central nervous system (CNS) cells such as microglia and astrocytes, but not in neurons [46, 52, 54]. The organization of the HIV transgene in HIV Tg rats is shown in Fig 2A. The m6A methylation in HIV transcripts in the hippocampus of HIV Tg rats showed enrichment in the 5’ and 3’ of the provirus most prominently corresponding to the 5’ and 3’ UTRs and LTRs and the nef open reading frame (ORF), which partially overlaps with the 3’ LTR (Fig 2B). The location of this 3’ cluster of m6A methylation in the HIV genome can affect most of the virus transcripts [55]. The present distribution of m6A methylation is in general overall agreement with the pattern of m6A methylation of HIV transcripts in cell lines and lymphocytes acutely infected or transfected with replication-competent HIV-1 [42–44]. In particular, m6A RNA methylation corresponding to the nef ORF was observed in 3 studies using cell lines and lymphocytes in vitro [42–44]. Two of these studies also observed significant m6A methylation over the ORF of rev and/or env genes, among others [42, 44]. This pattern of m6A methylation was also seen in in vitro-infected CD4+ T cell lines and primary human CD4+ T cells [42, 44]. However, a previous study did not detect m6A methylation signal at the 5’ of the virus transcript in in vitro-infected human CD4+ CEM-SS T cell line [43]. RT-PCR validation of host genes with differential m6A methylation between HIV Tg and wild-type rats are shown in Fig 2C and 2D. Altogether, these results show that both HIV and host transcripts are differentially m6A methylated in HIV Tg rats.
Fig 2. Pattern of m6A methylation in HIV and host transcripts of HIV Tg rats.
A) Outline of HIV genome organization and gag/pol deletion in HIV Tg rats. B) m6A methylation in HIV RNA from HIV Tg rats (HIV-1 MeRNA-IP) is enriched in transcripts corresponding to the 5’ and 3’ of the virus with a similar overall distribution as observed in HIV transcripts in vitro [42–44]; signal obtained from RNA-Seq of input RNA from the hippocampus of HIV Tg rats (HIV-1 Input). C) Distribution of m6A methylation in representative host genes with significant differential m6A methylation (*significantly differentially methylated peaks). D) PCR validation of significantly differentially methylated intervals in the genes in D (*p<0.05, *p<0.01 by t test).
Pathway analysis of differential m6A RNA methylated genes in the hippocampus of HIV Tg rats
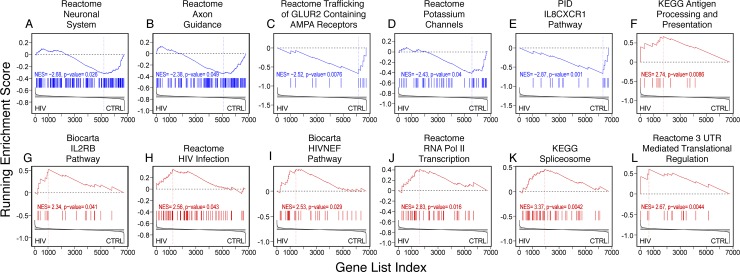
We used the Gene Set Enrichment Analysis (GSEA) algorithm [56] to identify molecular pathways that are differentially regulated by m6A RNA methylation in HIV-1 Tg rats (Fig 3, S2 Table). A total of 74 significantly differentially regulated pathways was observed (p-value < 0.05, and absolute normalized enriched score (NES) > 2, S2 Table) of which 44 showed increased m6A RNA methylation and 30 showed decreased m6A methylation (S2 Table). The top 40 differentially regulated pathways are shown in Fig 3. In host genes of HIV Tg rats, significant differential m6A methylation was seen in pathways relevant to neural function, suggestive of synaptodendritic injury [57–60], Fig 4. Other transcripts with significant differential m6A methylation were identified as involved in inflammation and immune response, also consistently with previous observations both in humans and HIV Tg rats [52, 53], Fig 4. Other genes belong to pathways involved in RNA splicing and processing (Fig 4). However, we did not observe significant differential expression of key transcripts involved in the regulation of m6A methylation by RT-PCR including FTO, Mettl3, Alkbh5, Ythdf1, Ythdf2, and Wtap (p>0.05 by t test; Supplementary Fig 1).
Fig 3. Pathway analysis of differential m6A RNA methylation in the hippocampus of HIV Tg rats.
Top 40 differential m6A methylation pathways in the hippocampus of HIV Tg rats by Gene Set Enrichment Analysis (GSEA) (Complete list in S2 Table).
Fig 4. Representative host pathways involving differentially m6A methylated genes in HIV Tg rats.
A-D) Several pathways containing genes differentially m6A modified are involved in neural function and are indicative of synaptodendritic injury [57–60], consistent with differential expression of genes in these ontology classes [52, 53]; E-G) pathways involved in inflammation and immune response were also differentially m6A methylated, consistent with previous observations both in humans and HIV Tg rats [52, 53]; H,I) differentially m6A methylated gene transcripts also included pathways related to HIV infection; J-L) and RNA metabolism and processing, e.g. splicing, which are processes in which m6A RNA methylation has been previously implicated [6–10]. Significance is indicated in each plot (Complete list in S2 Table).
Representative differentially methylated m6A pathways related to neural function include “Reactome Neuronal Systems”, “KEGG Axon Guidance”, and “Reactome Trafficking of Glur2 containing AMPA Receptors” (Fig 4) and “Reactome Developmental Biology” (Fig 5), among others (S2 Table). These pathways are indicative of synaptodendritic injury and reduced trophic support [57–60], and are consistent with differential expression of genes in these ontology classes in both humans with HIV and HIV Tg rats [52, 53]. Among pathways involved in inflammation and immune response, differentially m6A methylated pathways are “MIPS TNF-alpha NF Kappa B Signaling Complex”, “PID IFNG Pathway”, “Reactome Class I MHC Mediates Antigen Processing and Presentation”, “PID IL8CXCR1 Pathways” (among others Figs 4 and 5 and S2 Table). Genesets related to HIV infection were also differentially methylated, such as Biocarta HIVNEF Pathway and Reactome HIV Infection (Fig 4). Additional pathways up-regulated in HIV Tg rats were pathways related to apoptosis (Fig 5). These pathways are consistent with previous observations of inflammation and immune dysregulation in both humans and HIV Tg rats [52, 53]. Interestingly, other differentially m6A methylated gene transcripts were in pathways involved in RNA splicing, metabolism and processing (Figs 4 and 5). Examples of the latter pathways are “Reactome Pol II Transcription”, Kegg Spliceosome, Reactome 3 UTR Mediated Translational Regulation”, “Reactome mRNA Splicing” (Figs 4 and 5 and S2 Table). These pathways are suggestive of a key role of m6A RNA methylation in RNA splicing, metabolism and processing [6–10].
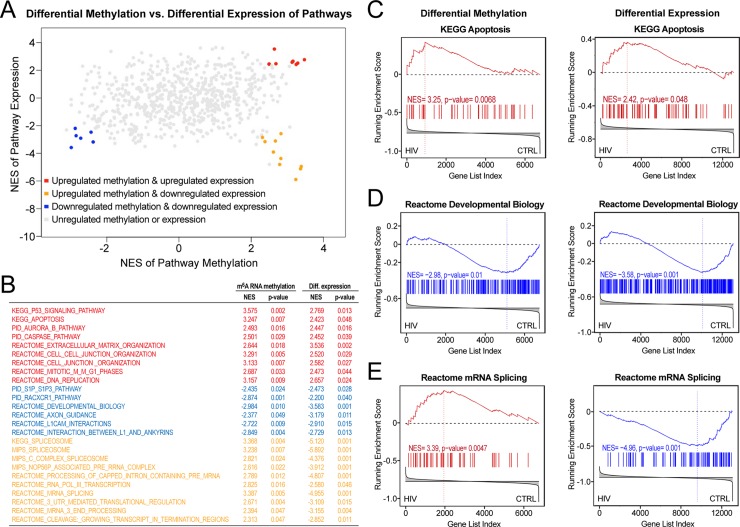
Fig 5. Correlation of m6A methylation and gene expression.
A) The normalized enrichment scores (NES) of pathways showing both significant differential m6A RNA methylation (NES of pathway methylation) and gene expression (NES of pathway expression). RNA m6A methylation was either directly or inversely correlated with gene expression. Pathways with increased m6A RNA methylation and expression are shown in red; pathways with decreased m6A RNA methylation and expression are shown in blue; pathways with increased m6A RNA methylation and decreased expression are shown in orange. B) Pathways showing increased differential m6A RNA methylation and expression included some pathways involved in apoptosis and tissue responses (red); pathways showing decreased differential m6A RNA methylation and expression included some pathways involved in neuronal trophisms and function (blue); pathways showing increased differential m6A RNA methylation and decreased expression included pathways involved in RNA processing and metabolism. C) Example of pathway showing increased differential m6A RNA methylation and expression, “KEGG Apopotosis”; D) Example of pathway showing decreased differential m6A RNA methylation and expression, “Reactome Developmental Biology”; E) Example of pathway showing increased differential m6A RNA methylation and decreased expression, “Reactome mRNA Splicing”.
Correlation of differential m6A RNA methylation and expression in pathways of the hippocampus of HIV Tg rats
Next we investigated the correlation of differential m6A RNA methylation and expression in pathways of the hippocampus of HIV Tg rats. As shown in Fig 5A, pathways that showed both significant differential m6A RNA methylation and expression by GSEA were either directly or inversely correlated with changes in m6A RNA methylation and gene expression. In particular, 9 pathways showed both increased m6A RNA methylation and transcription (Fig 5A, 5B and 5C); 6 pathways showed both decreased m6A RNA methylation and transcription (Fig 5A, 5B and 5D); and 10 pathways showed increased m6A RNA methylation but decreased transcription (Fig 5A, 5B and 5E). Pathways showing increased m6A RNA methylation and expression included some pathways involved in apoptosis and tissue responses, pathways showing decreased m6A RNA methylation and expression included some pathways involved in neuronal trophisms and function, while pathways showing increased m6A RNA methylation and decreased expression included pathways involved in RNA processing and metabolism (Fig 5A–5E).
Discussion
Here we report for the first time patterns of RNA m6A methylation, the most abundant internal mRNA modification, in vivo in an animal model of HIV. Available data on m6A methylation of eukaryotic and HIV transcripts are primarily from in vitro studies. In particular, we profiled RNA m6A methylation in the hippocampus of HIV Tg rats, a small animal model of concomitant low level expression of multiple HIV-1 products in disease-relevant cells in the CNS [47], which also characterizes HIV-associated neurocognitive disorder (HAND) in the setting of viral suppression.
We found that both HIV and host transcripts of HIV Tg rats contain m6A methylation. The distribution of m6A peaks within host mRNAs in the hippocampus of HIV Tg and control rat was greater in the distal CDS and 3’UTR of the mRNAs with the greatest density in the immediate vicinity of the stop codon, reminiscent of the distribution of m6A peaks observed in cell lines in vitro and total brain RNA [1, 2, 61]. The distribution of m6A methylation in HIV transcripts of HIV Tg rats largely corresponded to the ones described for HIV transcripts in vitro in cell lines and lymphocytes acutely infected or transfected with replication-competent HIV-1 [42–44]. Minor differences with those studies as well as among them likely reflect the acute nature of the infection or transfection used in those studies vs. the chronic expressions of HIV transcripts in Tg rats in an in vivo setting and different cell lines or lymphocytes used in those studies vs. brain parenchyma in the present study where HIV is expressed in microglia and astrocytes [52].
Pathway analysis by GSEA of differential m6A RNA methylation and differential mRNA expression showed that pathways affected by m6A methylation is consistent with a key role of RNA methylation in the regulation of the brain transcriptome in chronic HIV disease [52, 53]. Host pathways identified by differential m6A methylation in the present study involve cellular functions that are key in the pathogenesis of neuroAIDS, such as pathways indicative of synaptodendritic injury, neurodegeneration and apoptosis as well as pathways involved in inflammation and immune response, overall consistent with our previous pathway analyses of both HIV Tg rats and humans with HIV [52, 53]. Additional pathways were involved in RNA splicing and processing, consistent with the emerging roles of m6A methylation in these processes [10, 13, 15, 62]. This suggests that m6A methylation plays a role as a broad regulator of gene expression in fine-tuning the regulation of host genes in HIV infection.
In the present setting of chronic HIV disease, both positively and negatively regulated differentially m6A methylated transcripts were observed. Thus, the present results do not suggest a global, unidirectional change in the level of m6A methylation in HIV Tg rats. Rather, GSEA pathway analysis indicates that multiple biological processes are affected by differential m6A methylation in a bidirectional manner. In particular, changes in m6A RNA methylation involving pathways related to neuronal function show reduced m6A RNA methylation and mRNA levels, consistent with synaptodendritic injury, neurodegenerative processes and reduced trophic support; conversely, pathways relevant to inflammatory processes and apoptosis show increased m6A RNA methylation and mRNA levels. Such direct correlation of m6A RNA methylation and mRNA levels in pathways involved in neurodegenerative and inflammatory processes is in keeping with the notion that m6A methylation promotes gene expression [21], and thus may serve to amplify transcriptional control of gene expression. Interestingly, we found changes in m6A methylation of several pathways related to RNA processing and metabolism to be negatively correlated with mRNA levels. m6A methylation has been shown to modulate several aspects of RNA metabolism [10, 63].
The present data suggest the regulation of genes involved in RNA processing as a possible new mechanism by which m6A RNA methylation can indirectly regulate RNA metabolism. The mechanisms through which m6A methylation regulates RNA processing and metabolism remain largely unclear. Interestingly, we did not observe significant differential expression of transcripts directly involved in the regulation of m6A methylation. This may indicate that small changes in levels of proteins involved in RNA modifications, or changes in their translational efficiency effect changes in the regulation of m6A RNA methylation in vivo. The present results also showed a considerable number of transcripts with differential m6A methylation that were not differentially expressed. Since m6A RNA methylation appears to mostly increase gene expression efficiency, these transcripts with increased m6A methylation but normal RNA levels may be mRNAs that are poised for greater induction or down-regulation should external stimuli result in activation or inhibition of their promoters. Alternatively, they may represent mRNAs with gene expression regulation at the translational level [9, 21].
The available evidence provides support for bidirectional regulation of m6A modification of mRNA. Addition and removal of m6A residues has been suggested to be dynamic, e.g. [14, 15]. This implies that modifications of RNA m6A content act as a separate layer of RNA regulation. Other evidence has suggested that m6A methylation occurs at the pre-mRNA level during transcription [16]. This scenario is consistent with coordinated differential expression and m6A modification of nascent mRNAs. Studies reported a direct correlation between m6A methylation and expression of both viral [43] and host proteins [1, 21]. It has been suggested that the coordinated actions of m6A writers, erasers and readers result in accelerating RNA processing and mRNA transport and result in more efficient but perhaps more transient translation [9, 21]. The m6A reader YTHDF1 in HeLa cells has been shown to localize mRNA to translation machinery and enhance translation efficiency [21]. Conversely the m6A reader YTHDF2 has been shown to localize m6A-methylated mRNA decay sites and reduce transcript stability [20]. Since m6A readers can play differential roles, it can be expected that the cellular and molecular context is key in determining the consequences of differential m6A methylation. Interaction of YTH domain ‘‘reader” proteins also regulates the efficiency of m6A methylated RNA processing and translation [20, 21]. However, it is important to note that these conclusions are based in part on studies of overexpression in cell lines and therefore it is possible that different results may be observed at different ratios of the proteins involved in m6A RNA methylation to mRNA and/or in different cell types. Changes in mRNA levels reflect the difference between transcription and degradation rates. Our results seem to indicate a role for m6A methylation in regulating mRNA levels in concert with transcriptional regulation as we observed a primarily positive correlation of the cellular processes affected by differential m6A methylation and differential gene expression.
A limitation of the present study is that the present results do not allow us to determine at what stage in the life of RNA is m6A methylation regulated or if it is the result of a dynamic process. Another limitation of the present study is that here we explored the occurrence and regulation of the most abundant of the RNA modifications of m6A, while several other chemical marks can occur and their elucidation may reveal an epitranscriptome “code” that directs RNA processing, metabolism and expression [6–9]. Additionally, the antibody used in the present study, while highly specific, does not distinguish m6A from the highly similar m6Am modifications, which also contain a 2’-O-methyl in the ribose moiety [64] and whose function is less characterized.
In conclusion, we used a m6A-directed antibody in conjunction with RNA-Seq to explore RNA m6A methylation in vivo in HIV Tg rats. The density of m6A in hippocampal mRNAs in vivo was greater in the distal CDS and 3’UTR and the greatest density was in the immediate vicinity of the stop codon. The pattern of m6A methylation in HIV transcripts in HIV Tg rats overall resembled the ones reported in cell lines and T cells in vitro. The function of the host genes and pathways affected by changes in m6A in HIV Tg rats was reminiscent of the pathways differentially regulated in both HIV Tg rats and humans with HIV in our previous studies [52, 53]. These include pathways involved in neural function, suggestive of synaptodendritic injury and neurodegeneration, immune response and inflammation, and, interestingly, RNA splicing and processing. Correlation of m6A RNA methylation and differential expression indicates that RNA methylation is a significant contributor to gene expression regulation in neuroAIDS in a bidirectional manner with pathway-specific differential regulation in different categories of genes. Thus, sets of transcripts enriched in m6A appear to be involved in coordinated transcriptional host responses in the context of chronic HIV. Overall our results support that m6A methylation is a mechanism for widespread regulation of mRNA in vivo that affects both HIV transcripts and host genes orchestrating host adaptive and maladaptive transcriptional effects of chronic HIV.
Materials and methods
Animals
Adult male HIV Tg rats in Sprague Dawley background (Harlan Sprague-Dawley) 5–6 months of age were housed in groups of two per cage in a temperature-controlled (20–26 oC) vivarium on a 12h/12h light/dark cycle with ad libitum access to food and water. Rats were sacrificed by decapitation under deep CO2 anesthesia. All procedures adhered to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
mRNA isolation and m6A-modified RNA immunoprecipitation (MeRNA-IP)
Total RNA was extracted from dissected hippocampi of the wildtype and HIV Tg rats with RNEasy kit (Qiagen) and poly-A selected with Dynabeads Oligo(dT)25 (Thermo Fisher Scientific) following manufacturer’s instructions. Poly-A enriched RNA was recovered by ethanol precipitation overnight, fragmented using RNA Fragmentation Reagents (Thermo Fisher Scientific) and ethanol precipitation overnight. An aliquot of fragmented RNA was set aside serving as input or control for RNA sequencing. Twenty μg of fragmented RNAs were denatured at 75°C for 5 min, cooled on ice for 3 min, and then incubated for 2h at 4°C with 3 μg of pre-equilibrated anti-m6A (Synaptic Systems), which was pre-immobilized to M-270 Epoxy using the Dynabeads Antibody Coupling kit (Thermo Fisher Scientific) for easy capture of the m6A-modified RNA in subsequent elution with a Proteinase K-containing buffer. Eluate with the anti-m6A captured RNA was extracted by phenol/CHCl3 followed by ethanol precipitation.
RNA sequencing
Libraries for the anti-m6A-captured and control (fragmented, non-immunoprecipitated) RNAs were generated for next gen sequencing, following the protocol recommended in the ScriptSeq v2 RNA-Seq Library Preparation Kit (Illumina), and uniquely barcoded with the ScriptSeq Index PCR Primers (Illumina) to run a 16-library sample multiplex in a single flowcell on a NextSeq500 platform (Illumina) to generate ~30M 1x75 bp reads/sample.
Data analysis
Reads pre-processing and mapping
Sequences were first trimmed using Trimmomatic with default setting (version 0.33). All the samples passed the quality control by fastQC (version 0.11.3). Fastq files were aligned to a combined rat genome (Rnor_6.0) and HIV-1 genome (NC_001802) using STAR (v2.4) with default setting. The overall mapping rate range from 79.78% to 85.79% (S3 Table)
Detecting RNA methylation sites
RNA methylation sites were detected using exomePeak (R package, version 2.10.0) in HIV infected rat samples and control samples separately. Aligned bam files of m6A treated samples and non-treated samples were directly passed to exomePeak to test for statistically significant enrichment. A total of 9696 and 9051 consist RNA methylation sites with at least 100 bp were identified in HIV infected samples and control samples respectively (p-value < = 0.05, S4 Table). For the mapping of the distribution of m6A peaks within host mRNAs, per base coverage was generated for all the m6A treated samples using R package “RiboProfiling” for a fixed interval of 400 bases around the start codon and stop codon.
Identifying genes with differential methylation and expression in HIV Tg rats vs. wild-type control rats
RNA-seq counts of 6,757 genes with at least one methylation site identified in the previous section Detecting RNA Methylation Sites were retrieved from m6A treated HIV infected samples and m6A treated controls using htseqcount2 with union mode. The rat genome was first humanized using ‘biomaRt’ package from R, only the gene with the highest counts were kept when multiple rat genes matched to one human gene. We then kept only the genes with an average raw count greater than 10. Differential methylation analysis was then performed using ‘DESeq2’ package in R (Wald method was used). 201 genes were identified with significant changes of methylation (p-value < 0.05) (S1 Table). Differential expression analysis was performed in a similar fashion for a total of 13,114 genes, where 383 genes were identified with significant differential expression (p-value < 0.01) (S5 Table). A set of 24 genes was both significantly modified in expression and methylation (S6 Table).
Identifying gene sets with enriched methylation and expression in HIV Tg rats vs. wild-type control rats
A total of 1,452 well-known gene sets from the MSigDB collection was tested with the GSEA algorithm. 166 gene sets were significantly changed in expression (p-value<0.05, 76 genes sets were up regulated in HIV infected samples while 90 gene sets were down regulated) (S7 Table) and 74 gene sets were significantly changed in methylation (p-value<0.05, 44 gene sets were up regulated in HIV infected samples while 30 gene sets were down regulated) (S2 Table). Among these gene sets, 6 were identified as significantly down regulated both at the level of expression and methylation while 9 were up regulated. The top 40 most enriched genes set are shown in Fig 3.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
We thank Celine Lefebvre, Head of Bioinformatics and Computational Biology, Servier, Paris, France, for valuable discussion and critical review of the manuscript.
Data Availability
RNA-Seq data can be accessed from the ENA database (https://www.ebi.ac.uk/ena/) under project accession: PRJEB28812.
Funding Statement
This work was supported by National Institutes of Health/National Institute on Drug Abuse grants DA041750 and DA043268, https://www.drugabuse.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6. 10.1038/nature11112 . [DOI] [PubMed] [Google Scholar]
- 2.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635–46. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavi S, Shatkin AJ. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975;72(6):2012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–5. 10.1038/nature14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19(12):1848–56. 10.1261/rna.041178.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Mason CE. The pivotal regulatory landscape of RNA modifications. Annu Rev Genomics Hum Genet. 2014;15:127–50. 10.1146/annurev-genom-090413-025405 . [DOI] [PubMed] [Google Scholar]
- 7.Kennedy EM, Courtney DG, Tsai K, Cullen BR. Viral Epitranscriptomics. J Virol. 2017;91(9). 10.1128/JVI.02263-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer KD, Jaffrey SR. Expanding the diversity of DNA base modifications with N(6)-methyldeoxyadenosine. Genome Biol. 2016;17:5 10.1186/s13059-016-0874-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169(7):1187–200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29(13):1343–55. 10.1101/gad.262766.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry R, Kelley D. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. [Google Scholar]
- 12.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterlee JS, Basanta-Sanchez M, Blanco S, Li JB, Meyer K, Pollock J, et al. Novel RNA modifications in the nervous system: form and function. J Neurosci. 2014;34(46):15170–7. 10.1523/JNEUROSCI.3236-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15(5):293–306. 10.1038/nrg3724 . [DOI] [PubMed] [Google Scholar]
- 15.Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol. 2016;6(4):160003 10.1098/rsob.160003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, Geula S, et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31(10):990–1006. 10.1101/gad.301036.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei CM, Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977;16(8):1672–6. . [DOI] [PubMed] [Google Scholar]
- 18.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–4. 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Ji X, Guo X, Ji S. Regulatory Role of N6 -methyladenosine (m6 A) Methylation in RNA Processing and Human Diseases. J Cell Biochem. 2017;118(9):2534–43. 10.1002/jcb.25967 . [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20. 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161(6):1388–99. 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61(4):507–19. 10.1016/j.molcel.2016.01.012 . [DOI] [PubMed] [Google Scholar]
- 23.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10(11):927–9. 10.1038/nchembio.1654 . [DOI] [PubMed] [Google Scholar]
- 24.Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, Bronneke HS, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16(8):1042–8. 10.1038/nn.3449 . [DOI] [PubMed] [Google Scholar]
- 25.Choudhry Z, Sengupta SM, Grizenko N, Thakur GA, Fortier ME, Schmitz N, et al. Association between obesity-related gene FTO and ADHD. Obesity (Silver Spring). 2013;21(12):E738–44. 10.1002/oby.20444 . [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45(4):392–8, 8e1-2. 10.1038/ng.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iles MM, Law MH, Stacey SN, Han J, Fang S, Pfeiffer R, et al. A variant in FTO shows association with melanoma risk not due to BMI. Nat Genet. 2013;45(4):428–32, 32e1. 10.1038/ng.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le PT, Mortensen RF. Induction and regulation by monokines of hepatic synthesis of the mouse serum amyloid P-component (SAP). J Immunol. 1986;136(7):2526–33. . [PubMed] [Google Scholar]
- 29.Reitz C, Tosto G, Mayeux R, Luchsinger JA, Group N-LNFS, Alzheimer's Disease Neuroimaging I. Genetic variants in the Fat and Obesity Associated (FTO) gene and risk of Alzheimer's disease. PLoS One. 2012;7(12):e50354 10.1371/journal.pone.0050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung YC, Yeo GS. From GWAS to biology: lessons from FTO. Ann N Y Acad Sci. 2011;1220:162–71. 10.1111/j.1749-6632.2010.05903.x . [DOI] [PubMed] [Google Scholar]
- 31.Shen F, Huang W, Huang JT, Xiong J, Yang Y, Wu K, et al. Decreased N(6)-methyladenosine in peripheral blood RNA from diabetic patients is associated with FTO expression rather than ALKBH5. J Clin Endocrinol Metab. 2015;100(1):E148–54. 10.1210/jc.2014-1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Huang W, Huang JT, Shen F, Xiong J, Yuan EF, et al. Increased N6-methyladenosine in Human Sperm RNA as a Risk Factor for Asthenozoospermia. Sci Rep. 2016;6:24345 10.1038/srep24345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krug RM, Morgan MA, Shatkin AJ. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976;20(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer S, Salditt-Georgieff M, Bachenheimer S, Darnell JE, Furuichi Y, Morgan M, et al. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3(3):749–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gokhale NS, McIntyre AB, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, et al. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe. 2016;20(5):654–65. 10.1016/j.chom.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimock K, Stoltzfus CM. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977;16(3):471–8. . [DOI] [PubMed] [Google Scholar]
- 37.Stoltzfus CM, Dimock K, Horikami S, Ficht TA. Stabilities of avian sarcoma virus RNAs: comparison of subgenomic and genomic species with cellular mRNAs. J Gen Virol. 1983;64 (Pt 10):2191–202. 10.1099/0022-1317-64-10-2191 . [DOI] [PubMed] [Google Scholar]
- 38.Kok YL, Ciuffi A, Metzner KJ. Unravelling HIV-1 Latency, One Cell at a Time. Trends Microbiol. 2017. 10.1016/j.tim.2017.06.002 . [DOI] [PubMed] [Google Scholar]
- 39.Pereira-Montecinos C, Valiente-Echeverria F, Soto-Rifo R. Epitranscriptomic regulation of viral replication. Biochim Biophys Acta. 2017;1860(4):460–71. 10.1016/j.bbagrm.2017.02.002 . [DOI] [PubMed] [Google Scholar]
- 40.Stoltzfus CM, Dane RW. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982;42(3):918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkel D, Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology. 1983;131(2):409–25. . [DOI] [PubMed] [Google Scholar]
- 42.Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011 10.1038/nmicrobiol.2016.11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy EM, Bogerd HP, Kornepati AV, Kang D, Ghoshal D, Marshall JB, et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host Microbe. 2016;19(5):675–85. 10.1016/j.chom.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirumuru N, Zhao BS, Lu W, Lu Z, He C, Wu L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 2016;5 10.7554/eLife.15528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–75. 10.1016/j.immuni.2005.06.008 . [DOI] [PubMed] [Google Scholar]
- 46.Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98(16):9271–6. 10.1073/pnas.161290298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob G, et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Molecular neurodegeneration. 2014;9(1):26 Epub 2014/07/02. 10.1186/1750-1326-9-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2(4):319–28. Epub 2007/11/28. 10.1007/s11481-007-9078-y . [DOI] [PubMed] [Google Scholar]
- 49.Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15(1):14–24. 10.1080/13550280802232996 . [DOI] [PubMed] [Google Scholar]
- 50.Festa L, Gutoskey CJ, Graziano A, Waterhouse BD, Meucci O. Induction of Interleukin-1beta by Human Immunodeficiency Virus-1 Viral Proteins Leads to Increased Levels of Neuronal Ferritin Heavy Chain, Synaptic Injury, and Deficits in Flexible Attention. J Neurosci. 2015;35(29):10550–61. 10.1523/JNEUROSCI.4403-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS. 2014;9(6):517–20. Epub 2014/09/10. 10.1097/COH.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:26 10.1186/1750-1326-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanna PP, Repunte-Canonigo V, Masliah E, Lefebvre C. Gene expression patterns associated with neurological disease in human HIV infection. PLoS One. 2017;12(4):e0175316 10.1371/journal.pone.0175316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royal W 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247(1–2):16–24. Epub 2012/04/17. 10.1016/j.jneuroim.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purcell DF, Martin MA. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J Virol. 1993;67(11):6365–78. Epub 1993/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. Epub 2005/10/04. 0506580102 [pii] 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33–44. 10.1038/nrn2040 . [DOI] [PubMed] [Google Scholar]
- 58.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–72. 10.1002/ana.410420618 . [DOI] [PubMed] [Google Scholar]
- 59.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009:1–11. Epub 2009/09/10. 914601275 [pii] 10.1080/13550280903131915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leng SX, Margolick JB. Understanding Frailty, Aging, and Inflammation in HIV Infection. Curr HIV/AIDS Rep. 2015. 10.1007/s11904-014-0247-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29(19):2037–53. 10.1101/gad.269415.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lev Maor G, Yearim A, Ast G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015;31(5):274–80. 10.1016/j.tig.2015.03.002 . [DOI] [PubMed] [Google Scholar]
- 63.Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15(5):313–26. 10.1038/nrm3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei C, Gershowitz A, Moss B. N6, O2'-dimethyladenosine a novel methylated ribonucleoside next to the 5' terminal of animal cell and virus mRNAs. Nature. 1975;257(5523):251–3. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
RNA-Seq data can be accessed from the ENA database (https://www.ebi.ac.uk/ena/) under project accession: PRJEB28812.