Abstract
Neuropsychiatric disorders (including substance misuse) are associated with the greatest burden of functional disability in young people, and contributory factors remain poorly understood. Early-onset substance use is one candidate risk factor which may inform functional prognosis and facilitate direction of interventions aiming to curtail impairment. Accordingly, we modelled associations between early-onset use of alcohol, tobacco, cannabis and amphetamine-type stimulants (ATSs) and longitudinal socio-occupational functioning (indexed by the Social and Occupational Functioning Assessment Scale) in an observational cohort presenting to early intervention mental health services. A clinical proforma collated demographic, clinical, and socio-occupational information for up to 60-months from presentation to services in young people aged 17–30. Of the wider cohort (n = 2398), 446 participants were selected with complete alcohol and substance use data. Latent class analysis was used to derive an ‘early-onset’ (n = 243) and ‘later-onset’ class (n = 203) based on age of first use of alcohol, tobacco, cannabis and ATSs. Maximum-likelihood multilevel analyses modelled functioning over time in care and tested associations with substance use latent class, age, gender and diagnosis. Membership in the ‘early-onset’ class (B = -1.64, p = 0.05), male gender (B = -3.27, p<0.001) and psychotic disorder diagnosis (B = -7.62, p<0.001) were associated with poorer functioning at presentation and at least one other time-point. To our knowledge, this is the first study to explore associations of early-onset substance use and longitudinal functioning in a cohort of young people with mental disorders. The identified factors may be useful for directing specific social (e.g. Social Recovery Therapy) or occupational (e.g. Individual Placement and Support) interventions to at-risk individuals, early in illness course.
Introduction
The emergence of a mental disorder during adolescence or early adulthood may profoundly and pervasively impact a young person’s educational achievement, workforce participation and social engagement [1–3]. Neuropsychiatric disorders (including substance misuse) are the greatest cause of years lived with disability for young people aged 10–24 [4], and disability-adjusted life years associated with common mental disorders (e.g. depression and anxiety) reach their peak between 10–29 years of age [5]. The reasons for this high burden of disability are complex, involving a coalescence of factors operating within a formative and sensitive phase of social, cognitive and neurobiological development [6–9]. Importantly, strong evidence from longitudinal cohort studies suggests that functional impairment is both a cause and a consequence of mental ill-health [2, 3, 10–19], underscoring the need to consider both domains in assessment and treatment. In keeping with these observations, there has been a gradual shift toward more holistic models of recovery which take into account an individual’s ability to adaptively and meaningfully participate in work and social relationships [20, 21]. This shift complements patient reports citing loneliness, social isolation, financial problems and unemployment as their top-ranked challenges, above symptoms [22]. Attending to functional impairment in young people is especially important, as efforts made early in the course of illness (when trajectories are most malleable) are more likely to be impactful [23, 24]. Accordingly, there is a critical need for identification of factors driving impairment in the early phases of mental disorders in order to direct interventions to at-risk individuals.
Indeed, functional impairment is common and substantial at presentation to early intervention mental health services across a wide array of anxious, psychotic and mood syndromes [25–30]. A recent report from our group described multiple empirical trajectories of functioning over time in care, with substantial variability in improvement, decline and stability among young patients [31]. While some factors associated with poor functioning in psychiatric cohorts have been identified, including male gender, younger age, suicidality, cognitive impairment, substance and illness comorbidity, and greater illness stage [25, 27, 28, 31–34], considerable variance remains unaccounted for. One candidate factor that has received little attention in youth mental health cohorts is early-onset substance use.
In general populations (e.g. school-, birth- and population-based cohorts), it is well-established that early-onset use of alcohol, tobacco, cannabis and amphetamine-type stimulants (ATSs) is associated with numerous poor outcomes. For instance, early-onset alcohol use (i.e. before age 15) is associated with increased risk for future alcohol-related problems and substance dependence, academic difficulties, and employment problems in early adulthood [35–38]. Early-onset tobacco use (i.e. before age 15) predicts persistent cigarette smoking and dependence, school-dropout and psychiatric morbidity, with adolescent-initiators who continue smoking into adulthood at especially high-risk of negative outcomes [39–44]. Early-onset cannabis use (i.e. before age 16) is related to an increased risk for psychosis, cannabis dependence, school-dropout, unemployment at age 18 and socio-occupational difficulties at age 25 [45–48]. Finally, data describing outcomes associated with early-onset ATS use (e.g. methamphetamine, cocaine, MDMA) is scarce, however, some work suggests that early-onset methamphetamine use increases risk for psychosis, dependence and criminal activity [49–51], and early-onset cocaine use is associated with greater legal and psychiatric problems [52, 53]. Unfortunately, the above research has largely been restricted to general population samples, limiting generalisability to treatment-seeking young people with common mental disorders.
As there is no agreed upon cut-point for early- versus later-onset substance use and a range of ages reported in the literature, we chose to empirically derive latent classes of substance users as a function of their age of first use across our four substances of interest (alcohol, tobacco, cannabis and ATSs). Our first aim was to determine whether an earlier-onset substance class was associated with poorer longitudinal socio-occupational functioning (up to five years) in an observational cohort of young people accessing early intervention mental health services in Sydney, Australia. As a secondary question, we aimed to test a putative developmental-psychosis typology of mental disorders [54] with respect to functioning and substance use. Specifically, would individuals with a neurodevelopmental or psychotic disorder have poorer longitudinal functioning relative to their peers without either disorder, and, would participants with a neurodevelopmental or psychotic disorder who also reported earlier-onset substance use have even poorer functioning?
We hypothesised that: (i) the latent class with the earliest onset of substance use across alcohol, tobacco, cannabis and ATSs would be associated with lower functioning at presentation relative to the other class(s); (ii) a diagnosed neurodevelopmental or psychotic disorder would be associated with lower functioning at presentation and longitudinally; (iii) younger age would be associated with lower functioning at presentation; and (iv) male gender would be associated with poorer functioning at presentation. An additional exploratory question was whether the earlier-onset class would be associated with poorer functioning over time in contact with clinical services.
Methods
Human ethics
This study and the consent procedure were approved by the University of Sydney Human Ethics Committee (Project numbers: 2012/1626 and 2012/1631) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from participants aged 16 years and older, and parental/guardian consent was obtained for participants younger than 16 years.
Participants
Participants were drawn from a naturalistic, longitudinal cohort of young people, the ‘Brain and Mind Centre Optymise Cohort’ (n = 2398, mean age 18.8 ± 3.8 years, 58.7% female), who were accessing ‘headspace’ and associated early intervention mental health clinics in Sydney, Australia. headspace is Australia’s youth mental health initiative, which aims to provide youth-friendly and highly-accessible early intervention services for young people with emerging mental and substance use disorders [55, 56]. Primarily attracting young people with a wide range of mental health problems (typically anxiety, mood and/or psychotic syndromes), headspace consists of an integrated mixed of primary-level services and more specialised services (e.g. psychiatric, drug and alcohol, occupational support).
With informed consent, study participants were recruited to a case register for mood, psychotic, developmental and other mental disorders between January 2005 and January 2018. All participants were receiving ongoing clinician-based case management and relevant psychosocial and/or medical interventions throughout the duration of care, which may have involved contact with a psychiatrist, psychologist, occupational therapist, support worker, or hospitalisation for those whose need exceeded the capacity of the primary care services.
Individuals were included in the present study if they met the following criteria: (i) aged 17–30 years at the time of initial assessment (T1); and (ii) had completed the World Health Organization’s ‘Alcohol, Substance and Smoking Involvement Screening Test, Version 2’ (WHO-ASSIST-2). We added a further question to item 1 of the WHO-ASSIST-2 (lifetime use) to collect age of first use data: “If yes, at what age did you first use?”. Exclusion criteria included: (i) medical instability or lack of capacity to provide informed consent (determined by a treating psychiatrist); (ii) medical illness with cognitive sequelae (e.g. epilepsy, cancer); (iii) clinically-evident intellectual disability; and/or (iv) insufficient English-language ability. Of the wider Optymise cohort, 446 participants were included in analyses (see Participant Flow Diagram in S1 Fig).
Data collection
With consent, trained research psychologists and medical officers conducted a medical file audit to collate demographic, clinical and socio-occupational information at pre-specified intervals utilizing a specifically designed clinical proforma. These methods have been described previously in studies examining trajectories of functioning and suicidality [31, 34]. The earliest available comprehensive assessment at the service was represented as the initial timepoint (T1) for each participant, with T1 date determining the follow-up timepoints: 3-months (T2), 6-months (T3), 12-months (T4), 2-years (T5), 3-years (T6), 4-years (T7), and 5-years (T8). A “time-last-seen” entry was also recorded; however, this was not included in the current study. If no clinical notes were available within ±1-month of the 3- and 6-month timepoints, or ± 3-months of the remaining timepoints (T4-T8), then this particular entry was omitted. When data were available for a specified timepoint, all clinical notes collected after the preceding entry, up to and including the current entry, were used to complete the form.
Clinical proforma
The clinical proforma captures key information about the current presentation and specific illness course characteristics, with an earlier iteration previously reported [27, 57]. The proforma collects information regarding: (i) demographics; (ii) mental health diagnoses (based on Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria); (iii) clinical course (e.g. clinical stage, hospitalizations, childhood diagnoses); (iv) comorbidities (e.g. physical health problems, suicidal thoughts/behaviours); (v) and socio-occupational functioning, assessed using the Social and Occupational Functioning Assessment Scale (SOFAS), which is the outcome variable in this study. The SOFAS is a clinician-rated 100-point scale used to assess an individual’s level of social and occupational functioning along a continuum ranging from optimum functioning to important functional impairment (lower scores indicating poorer functioning). The SOFAS has been reported to have good construct validity (e.g. strong correlations with patient-reported difficulties in interpersonal relations and social adjustment [58]), excellent inter-rater reliability (i.e. intraclass correlation coefficient [ICC] > 0.74 [58]), and predictive validity (e.g. for length of initial psychiatric inpatient stay and two-year outcome [59]).
As we aimed to test a developmental-psychosis trajectory [54] hypothesis, participants were dichotomously coded at T1 with either the presence (1) or absence (0) of a psychotic disorder (including DSM-5 schizophrenia [n = 20]; schizoaffective disorder [n = 6]; substance/medication induced psychotic disorder [n = 7]; brief psychotic disorder [n = 6]; schizophreniform disorder [n = 3]; and psychotic disorder not otherwise specified [n = 8]) and presence (1) or absence (0) of a neurodevelopmental disorder (including DSM-5 autism-spectrum disorder [n = 8] and attention-deficit/hyperactivity disorder [n = 8]). Diagnoses were specified according to DSM-5 criteria [60], however, due to differences in the timing of presentation to clinical services clinical notes may have been based on previous iterations of the DSM.
Statistical analyses
Using the statistical program ‘Mplus’ [61], we conducted latent class (or latent profile) analyses (LCA) to derive empirical classes of substance users, with participants’ age of first use of alcohol, tobacco, cannabis and ATSs representing the input variables. As Mplus uses full-maximum-likelihood estimation to make use of all available data [62–64], participants with no lifetime use for a particular substance (and therefore no age of first use for that substance) were included in analyses. LCAs were run for 1–5 classes, with ample random starts and iterations used to arrive at a replicable best solution for each given number of classes (which was confirmed by a large number of replicated loglikelihoods for each model). Our choice of the number of classes that had a good balance of model fit and parsimony was informed by running 100 parametric bootstraps and comparing likelihood ratio test statistics, as well as inspecting the number of boundary conditions for each number of classes. Membership in a latent class was then dummy-coded (e.g. 1 = ‘member of class 1’; 0 = ‘not a member of class 1’) and used as a predictor variable in the next step of multilevel modelling.
Multilevel analyses were conducted using the ‘nlme’ package [65] for the statistical programming language R (version 3.4.2), utilizing full-maximum-likelihood estimation. This method represents a powerful way to assess change in a continuous dimension (e.g. SOFAS) longitudinally and within-participants, circumventing limitations associated with alternative repeated-measures techniques. Advantages of this method include: (i) tolerance of unbalanced assessment intervals; (ii) inclusion of participants with missing follow-up data (i.e. no list-wise deletion for missing timepoints); and (iii) does not assume independence of observations (which is unlikely to be met for within-participant repeated-measure data).
Our analyses were conducted sequentially. First, we constructed an unconditional model (i.e. no predictors) positing a linear change trajectory in SOFAS without attempting to predict inter-individual variation in parameters by between-subject factors. We additionally tested whether a non-linear term would provide a superior fit (as functional change is likely dynamic over time). Next, we fit a continuous autoregressive covariance structure, as we expected greater correlation in SOFAS scores at nearer timepoints than farther timepoints. We proceeded in conducting a set of conditional analyses examining systematic inter-individual differences in intercept and slope as a function of several pre-determined demographic and diagnostic factors (fixed effects), with the initial order entry substantively informed by the literature.
Normality of residuals was visually inspected using Q-Q plots, with an approximate normal distribution evident. Multicollinearity between predictors was assessed using the variation inflation factor (VIF), with no predictor variables observed to have a VIF exceeding 2.0. Model coefficients (B) are presented alongside standard errors, 95% confidence intervals (CI) and parameter-specific p-values. Deviance statistics are provided for each model, including the Akaike information criterion (AIC), Bayesian information criterion (BIC) and the Log-Likelihood. Goodness-of-fit between models was compared using the likelihood ratio test (LRT) statistic (which expresses how many times more likely the data are under one model relative to another) and p-values, with α level set at 0.05.
Results
Sample demographics and clinical characteristics
At T1, the included sample comprised four-hundred-and-forty-six young people (aged 17–30; M = 21.2; SD = 3.2), with 55.6% female gender. Presenting diagnoses, age of first use information, and sample size at each time-point are reported in Table 1. Baseline demographics of participants lost to follow up over 60-months are presented in Table 2.
Table 1. Demographic, age of substance use onset and presenting clinical diagnostic information (n = 446).
| M ± SD or N (%) | |
|---|---|
| Demographics | |
| Gender (female) | 248 (55.6) |
| Age at entry | 21.2 ± 3.2 |
| Substance use onset (age, yrs) | |
| Alcohol | 15.1 ± 2.4 |
| Tobacco | 15.6 ± 2.9 |
| Cannabis | 16.2 ± 2.6 |
| Amphetamine-type stimulant | 17.9 ± 2.6 |
| Presenting clinical diagnosis | |
| Depressive disorder | 202 (45.3) |
| Bipolar disorder | 69 (15.5) |
| Anxiety disorder | 77 (17.3) |
| Psychotic disorder | 50 (11.2) |
| Neurodevelopmental disorder | 16 (3.6) |
| Substance or addictive disorder | 8 (1.8) |
| Other | 22 (4.9) |
| No diagnosis | 2 (0.4) |
| Available timepoints | |
| T1 (Entry) | 446 (100.0) |
| T2 (3-months) | 275 (61.7) |
| T3 (6-months) | 238 (53.4) |
| T4 (12-months) | 218 (48.9) |
| T5 (2-years) | 172 (38.6) |
| T6 (3-years) | 128 (28.7) |
| T7 (4-years) | 97 (21.7) |
| T8 (5-years) | 56 (12.6) |
Table 2. Baseline demographics of participants lost to follow-up over 5 years (n = 446).
| Final timepoint with available data for each participant | ||||||||
|---|---|---|---|---|---|---|---|---|
| T1 (n = 72) |
T2 (n = 40) |
T3 (n = 55) |
T4 (n = 65) |
T5 (n = 63) |
T6 (n = 46) |
T7 (n = 49) |
T8 (n = 56) |
|
| Age at entry | 22.8 ± 3.3 | 20.7 ± 3.2 | 21.5 ± 3.1 | 21.0 ± 3.3 | 21.1 ± 3.3 | 20.9 ± 3.3 | 20.7 ± 2.6 | 20.4 ± 2.6 |
| Gender (female) | 33 (46%) | 20 (50%) | 30 (55%) | 42 (65%) | 28 (44%) | 25 (54%) | 34 (69%) | 35 (63%) |
| T1 SOFAS | 59.5 ± 13.6 | 61.2 ± 9.7 | 61.9 ± 8.7 | 58.8 ± 9.2 | 63.3 ± 9.0 | 58.4 ± 6.7 | 60.6 ± 9.0 | 60.2 ± 9.0 |
| T1 Psychotic dx | 23 (32%) | 3 (8%) | 5 (9%) | 4 (6%) | 5 (8%) | 3 (7%) | 3 (6%) | 4 (7%) |
| T1 ND dx | 3 (4%) | 4 (10%) | 0 (0%) | 2 (3%) | 2 (3%) | 3 (7%) | 2 (4%) | 0 (0%) |
| Alcohol AFU | 14.9 ± 2.5 | 15.4 ± 1.9 | 15.5 ± 2.2 | 15.1 ± 2.1 | 15.3 ± 2.4 | 14.4 ± 2.4 | 15.0 ± 2.5 | 15.1 ± 2.9 |
| Tobacco AFU | 15.9 ± 3.2 | 16.0 ± 2.3 | 14.8 ± 2.8 | 16.0 ± 2.6 | 16.3 ± 3.3 | 14.7 ± 2.5 | 15.1 ± 2.7 | 15.3 ± 2.7 |
| Cannabis AFU | 16.3 ± 3.0 | 16.2 ± 2.1 | 16.5 ± 2.6 | 16.3 ± 2.6 | 16.9 ± 2.6 | 15.1 ± 2.2 | 15.7 ± 2.0 | 16.4 ± 3.2 |
| ATS AFU | 18.1 ± 2.9 | 17.3 ± 2.7 | 18.3 ± 2.5 | 17.7 ± 2.7 | 18.3 ± 2.7 | 17.6 ± 2.6 | 17.6 ± 2.3 | 17.8 ± 2.5 |
T1 = service entry; T2 = 3-months; T3 = 6-months; T4 = 1-year; T5 = 2-years; T6 = 3-years; T7 = 4-years; T8 = 5-years; Psychotic dx = psychotic disorder; ND dx = neurodevelopmental diagnosis; ATS = amphetamine-type stimulant; AFU = age of first use
Latent class analyses
Analyses were run for 1–5 classes in order to arrive at the optimal number of classes representing the data. Information criteria and 100 parametric bootstrapped likelihood ratio tests (LRTs) were used to guide the decision of the number of classes. A sufficient number of random starts and iterations were used to arrive at a replicable solution, which was confirmed by a large number of replicated loglikelihoods for each model. All model estimations terminated normally.
A 3-class solution was found to be the best-fitting model with respect to the information criteria, LRT statistics (see Tables 3 and 4) and parsimony. However, a 2-class solution also provided a good fit to the data, comprised fewer boundary conditions than the 3-class solution, had an adequate sample size in each class to meaningfully model in longitudinal analyses, and was a more parsimonious solution with respect to our research question (i.e. early-onset versus later-onset substance users). We accordingly settled on a 2-class solution, which described an early-onset (n = 243) and later-onset (n = 203) substance use class (see Table 5 for class descriptives).
Table 3. Information criteria for 1–5 latent class estimations.
| AIC | BIC | Sample-size adjusted BIC | Entropy | |
|---|---|---|---|---|
| Number of latent classes | ||||
| 1 | 6089.80 | 6122.60 | 6097.21 | - |
| 2 | 5842.20 | 5895.50 | 5854.25 | 0.62 |
| 3 | 5707.71 | 5781.52 | 5724.40 | 0.71 |
| 4 | 5633.35 | 5727.66 | 5654.67 | 0.72 |
| 5 | 5583.08 | 5697.89 | 5609.03 | 0.75 |
AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion
Table 4. Model comparisons for 5 latent class estimations using 100 parametric bootstrapped likelihood ratio tests.
| Parametric boostrapped likelihood ratio test (2 times the Loglikelihood difference) | p | |
|---|---|---|
| Number of latent classes | ||
| 2 versus 1 | 257.60 | <0.001 |
| 3 versus 2 | 144.49 | <0.001 |
| 4 versus 3 | 84.36 | <0.001 |
| 5 versus 4 | 60.27 | <0.001 |
Table 5. Characteristics of early-onset and later-onset substance use latent classes.
| Latent class 1 Early-onset (n = 243) |
Latent class 2 Later-onset (n = 203) |
|
|---|---|---|
| M ± SD or N (%) | ||
| Age at entry | 21.1 ± 3.3 | 21.4 ± 3.1 |
| Gender (female) | 129 (53%) | 119 (59%) |
| Substance use onset (age, years) | ||
| Alcohol | 13.6 ± 1.9 | 16.9 ± 1.5 |
| Tobacco | 14.0 ± 1.8 | 18.3 ± 2.2 |
| Cannabis | 15.0 ± 1.9 | 18.7 ± 2.2 |
| Amphetamine-type stimulant | 17.0 ± 2.3 | 20.1 ± 2.0 |
Multilevel modelling: Unconditional analyses
Next, we began constructing our multilevel models by specifying an unconditional model (i.e. no predictors) with random intercepts. We then modelled the fixed relationship between SOFAS and ‘time’ with a linear term, which was significant and indicated a positive slope in SOFAS change over time across the sample (B = 0.31, p<0.001). We tested whether a quadratic trend in ‘time’ was a superior fit to the data, which was non-significant (p = 0.68) and did not improve model fit (LRT = 0.17, p = 0.68), indicating that a linear trend was appropriate. Next, slopes were randomly varied across participants, which is intuitive in that individuals are likely to be variable in their rate of improvement, decline or stability over time. The random slopes and random intercept model fit the data substantially better than the fixed slopes model (LRT = 111.21, p<0.001). We next determined whether there was autocorrelation in SOFAS scores across timepoints by fitting an autoregressive covariance structure to the data, which improved model fit (LRT = 27.99, p<0.001).
Multilevel modelling: Conditional analyses
We then examined factors that might explain intercept variation. We first entered the presence of a psychotic disorder at presentation to the model, which was significant (B = -7.74, p<0.001) and improved fit (LRT = 30.77, p<0.001). Next, the presence of a neurodevelopmental disorder was added, which was neither significant at our a priori alpha of 0.05 (B = -4.19, p = 0.07) nor improved fit (LRT = 3.39, p = 0.07), and was therefore excluded from further modelling. We then added gender to the model, which was significant (male gender; B = -3.34, p<0.001) and improved model fit (LRT = 14.97, p<0.001), followed by age at each time-point which was non-significant (B = -0.01, p = 0.92), did not improve fit (LRT = 0.01, p = 0.92), and was not included in further modelling. We next added membership in the ‘early-onset’ latent class (with the ‘later-onset’ class serving as reference) to the model, which was significant (B = -1.65, p = 0.05) and improved model fit (LRT = 3.94, p = 0.05). There was no significant interaction between membership in the early-onset class and having a psychotic disorder (B = -1.24, p = 0.65).
Finally, we tested whether statistical interactions between predictor variables would be associated with variability in the rate of SOFAS change over time (i.e. slope). We observed a trend towards a significant ‘time’ by gender interaction (male gender; B = 0.44, p = 0.06), and a trend toward improved model fit (LRT = 3.51, p = 0.06), which would indicate that males had a greater rate of SOFAS improvement over time than females. There were no significant interactions between ‘time’ and the ‘early-onset’ latent class (B = -0.17, p = 0.46) or ‘time’ and psychotic disorder (B = 0.61, p = 0.17). Final model coefficients are presented in Table 6, and fitted models are plotted in Fig 1.
Table 6. Final linear multilevel model (n = 446).
| Predictor | Model | ||
|---|---|---|---|
| Fixed effects | Coefficients (95% CI) | t | p |
| Intercept | 63.67 (62.26, 65.07) *** | 89.01 | <0.001 |
| Time | 0.13 (-0.16, 0.42) | 0.88 | .378 |
| Psychotic disorder | -6.41 (-9.12, -3.70) *** | -4.64 | <0.001 |
| Gender (male) | -3.69 (-5.42, -1.96) *** | -4.18 | <0.001 |
| ‘Early-onset’ class | -1.66 (-3.28, -0.03) * | -2.00 | .046 |
| Interactions | |||
| Time x Gender | 0.44 (-0.02, 0.89) | 1.88 | .061 |
| Random effects | SD | ||
| Intercept | 7.29 | ||
| Time | 1.17 | ||
| Residual | 5.85 | ||
| Deviance statistics | |||
| AIC | 11092.95 | ||
| BIC | 11152.27 | ||
| logLik | -5535.47 |
* p<0.05
** p<0.01
*** p<0.001
AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; logLik = loglikelihood
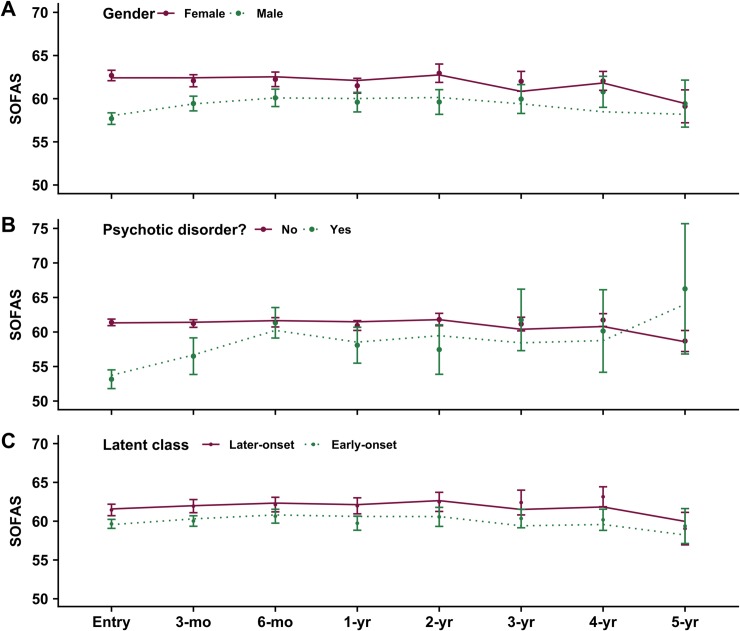
Fig 1. Observed data (± SE) and linear model fits for socio-occupational functioning (SOFAS) over 5-years in 446 young people with common mental disorders.
Note: filled circles = mean observed data; bars = standard error; lines = fitted model.
Discussion
Functional impairment is common and often pervasive in young people with mental health problems [31] and identification of factors predictive of longitudinal functioning is warranted in order to inform clinical prognosis and facilitate treatment selection. The present study sought to explore several candidate predictive factors of functioning at service entry and over time in contact with clinical services, observing that: i) membership in a latent class of early-onset substance users was associated with lower functioning at service entry and 3-, 12- and 48-months later (see Fig 1C); ii) male gender was associated with lower functioning throughout the first 6-months of care and at 2-years after service entry (see Fig 1A); and iii) a psychotic disorder at service entry was associated with lower functioning throughout the first 3-months in care (see Fig 1B). Against expectations, neither age nor having a neurodevelopmental disorder were associated with poorer functioning.
Our finding of poorer functioning among early-onset substance users may have several explanations. First, it is possible that early- and later-onset substance users may be neurocognitively or neurobiologically distinct, with differences mapping onto differential capacities for functioning. While a number of preclinical and human studies have revealed neurocognitive and neurobiological changes associated with heavy alcohol use during adolescence [66–69], few have investigated the effects of age of initiation. One recent preliminary study however reported associations between poorer processing speed and visual attention with earlier age of first drink, and poorer cognitive inhibition and working memory with earlier age of weekly drinking onset [70]. Importantly, these effects were robust to controlling for baseline neurocognition, severity of substance use and several family and social environment factors [70]. With respect to tobacco, a number of preclinical and human studies have suggested a neurotoxic effect of early exposure to nicotine (during adolescence) on brain and neurocognitive development [71–73]. Work in animal models has demonstrated long-lasting deficits in attention following administration of nicotine during adolescence [74], with lasting synaptic changes to dopaminergic and glutamatergic signalling in prefrontal cortex thought to represent two mechanisms underpinning attentional deficits [74, 75]. In humans, earlier initiation of tobacco smoking has been associated with deficits in response inhibition [76], sustained attention [76], and working memory [71]. Likewise, earlier use of cannabis during adolescence has been associated with poorer performance on a number of cognitive tasks indexing decision-making [77], verbal IQ [78], impulsivity [79], executive functions [80, 81] and memory [82], with suggestions that cannabis use during adolescence may perturb developmental processes such as white matter development and synaptic pruning [83]. Importantly, many of these studies are cross-sectional and collect retrospective age of onset data, and there is a need for prospective and longitudinal studies tracking adolescents before and after initiation of substance use to clarify the links between brain health and adolescent substance use [84, 85]. An alternative explanation may be that antecedent factors preceding substance use initiation may differentiate early- and later-onset users, which may signal shared liability for both early substance involvement and socio-occupational problems. For instance, Ellickson and colleagues [38] observed in a school-based cohort that early-onset and experimental drinkers were more likely than non-drinkers to have academic problems in school and employment problems in early adulthood, suggesting that early drinkers may not ‘mature’ out of problematic antecedent lifestyles that may represent shared risk for early and later difficulties. Other antecedent factors may include: i) early-onset mental health problems [86–88]; ii) socio-economic and family-level factors, including disrupted family structures, substance-misusing parents and siblings, social disadvantage, trauma-exposure, and poor parental monitoring and parent-child relationships [37, 89–94]; or iii) personality and behavioral factors, such as male gender, teacher-reported aggressive behaviour, conduct symptoms, positive alcohol expectancies, and reward-related personality traits [37, 95–99]. On balance, early-onset substance use may represent an associative (rather than causal) marker for the above confounding factors which may in turn increase risk for functional problems.
Based on a putative typology of adolescent-onset mental disorders [54], we hypothesised that early substance users who also had a neurodevelopmental or psychotic disorder (i.e. a developmental-psychosis trajectory) would be at risk of poorer outcome. While main effects of early-onset substance use and psychotic disorder on functioning were evident, we did not observe a statistical interaction between them. Nevertheless, the clustering of male gender, early-onset substance use and psychosis with poor functioning is congruent with this putative typology [54], and warrants further examination with modelling of larger samples enriched with these factors.
Finally, male gender was associated with lower functioning across the first 6-months of care and at 2-years (Fig 1A). This dovetails with the wider literature and may result from greater impairment prior to illness-onset or help-seeking due to other risk factors (e.g. neurodevelopmental or cognitive risk factors more common in boys), delayed help-seeking behaviour associated with poor health literacy [100], or the lack of development of suitable healthcare environments engaging to young men [101].
There are several limitations and potential sources of bias in this study. First, the SOFAS indexes both social and occupational functioning within one scale, which while useful in characterising the ‘gestalt’ of the individual’s circumstances may also obfuscate specific strengths and weaknesses. Second, age of first substance use was self-reported and may suffer from recall bias or related inaccuracies. Moreover, our sample was biased toward young people engaged in help-seeking behaviour and may not be generalizable to individuals who do not seek help or enter clinical services due to poor insight, low support, or other factors. Finally, loss to follow-up within this subset of the wider cohort may have biased model estimates. However, characteristics presented in Table 2 suggest no substantial differences in T1 SOFAS, gender distribution or T1 age across participants with differing final timepoints with available data. With these limitations in mind, we recommend replication in a similar youth mental health cohort.
In sum, our work highlights a substantial need for enhanced socio-occupational intervention and assistance in young people with mental ill-health, especially as early disengagement may herald protracted problems. In a subset of our larger cohort, we show that early-onset substance use is associated with poorer functioning at service entry and at several time-points throughout care, highlighting an at-risk group which may benefit from additional social and occupational treatment and support (e.g. Individual Placement and Support, Social Recovery Therapy [102, 103]).
Supporting information
(TIF)
(CSV)
Acknowledgments
The authors graciously thank the young people who gave up their time to participate in this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
JC was supported by a postgraduate Research Training Program Stipend (provided by The University of Sydney, no stipend number). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. KC funding provided by NHMRC Early Career Fellowship Grant (number: 1122362). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. FI was supported by an Australian Postgraduate Award stipend (no stipend number). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AN was supported by Brain & Mind Centre, Clinical Research Officer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NZ was supported as Research Officer at the Brain and Mind Centre, University of Sydney. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. DW was employed by The University of Sydney. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AM was supported as Senior Lecturer, Western Sydney University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AG was supported by NHRMC Career Development Fellowship, The University of Sydney. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. DH was supported by Sunshine Coast Mind & Neuroscience Thompson Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ES was supported as Medical Director, Young Adult Mental Health Unit, St Vincent’s Hospital Darlinghurst, Discipline Leader of Adult Mental Health, School of Medicine, University of Notre Dame, Research Affiliate, The University of Sydney and Consultant Psychiatrist. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. IH was supported by (2018-2022) Optimising Personalised Care, at scale, for Young People with Emerging Mood Disorders, $951,005 over 5 years (APP1136259); NHMRC Research fellowship (no. 1046899). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kessler RC, Foster CL, Saunders WB, Stang PE. Social consequences of psychiatric disorders, I: Educational attainment. The American Journal of Psychiatry. 1995;152(7):1026–32. Epub 1995/07/01. 10.1176/ajp.152.7.1026 . [DOI] [PubMed] [Google Scholar]
- 2.Egan M, Daly M, Delaney L. Adolescent psychological distress, unemployment, and the Great Recession: Evidence from the National Longitudinal Study of Youth 1997. Social Science & Medicine. 2016;156:98–105. Epub 2016/03/29. 10.1016/j.socscimed.2016.03.013 . [DOI] [PubMed] [Google Scholar]
- 3.Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. The British Journal of Psychiatry. 2007;191:335–42. Epub 2007/10/02. 10.1192/bjp.bp.107.036079 . [DOI] [PubMed] [Google Scholar]
- 4.Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377(9783):2093–102. Epub 2011/06/10. 10.1016/S0140-6736(11)60512-6 . [DOI] [PubMed] [Google Scholar]
- 5.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820 Epub 2015/02/07. 10.1371/journal.pone.0116820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9(2):60–8. Epub 2005/01/26. 10.1016/j.tics.2004.12.008 . [DOI] [PubMed] [Google Scholar]
- 7.Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–77. Epub 2008/03/21. 10.1038/nrn2353 . [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry. 2007;20(4):359–64. Epub 2007/06/07. 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public-health challenge. Lancet. 2007;369(9569):1302–13. Epub 2007/04/17. 10.1016/S0140-6736(07)60368-7 . [DOI] [PubMed] [Google Scholar]
- 10.Fergusson DM, Horwood LJ, Lynskey MT. The effects of unemployment on psychiatric illness during young adulthood. Psychological Medicine. 1997;27(2):371–81. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 11.Gibb SJ, Fergusson DM, Horwood LJ. Burden of psychiatric disorder in young adulthood and life outcomes at age 30. The British Journal of Psychiatry. 2010;197(2):122–7. Epub 2010/08/04. 10.1192/bjp.bp.109.076570 . [DOI] [PubMed] [Google Scholar]
- 12.Morrell S, Taylor R, Quine S, Kerr C, Western J. A cohort study of unemployment as a cause of psychological disturbance in Australian youth. Social Science & Medicine. 1994;38(11):1553–64. Epub 1994/06/01. . [DOI] [PubMed] [Google Scholar]
- 13.Egan M, Daly M, Delaney L. Childhood psychological distress and youth unemployment: evidence from two British cohort studies. Social Science & Medicine. 2015;124:11–7. Epub 2014/12/03. 10.1016/j.socscimed.2014.11.023 . [DOI] [PubMed] [Google Scholar]
- 14.Goodman A, Joyce R, Smith JP. The long shadow cast by childhood physical and mental problems on adult life. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(15):6032–7. Epub 2011/03/28. 10.1073/pnas.1016970108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundborg P, Nilsson A, Rooth D. Early life health and adult earnings: Evidence from a large sample of sibling pairs and twins. IZA Dicussion Papers 5804, Institute for the Study of Labor (IZA. 2011).
- 16.Banks MH, Jackson PR. Unemployment and risk of minor psychiatric disorder in young people: cross-sectional and longitudinal evidence. Psychological Medicine. 1982;12(4):789–98. Epub 1982/11/01. . [DOI] [PubMed] [Google Scholar]
- 17.Paul KI, Moser K. Unemployment impairs mental health: Meta-analyses. Journal of Vocational Behavior. 2009;74(3):264–82. 10.1016/j.jvb.2009.01.001. [DOI] [Google Scholar]
- 18.Rodwell L, Romaniuk H, Nilsen W, Carlin JB, Lee KJ, Patton GC. Adolescent mental health and behavioural predictors of being NEET: a prospective study of young adults not in employment, education, or training. Psychological Medicine. 2018;48(5):861–71. Epub 2017/09/07. 10.1017/S0033291717002434 . [DOI] [PubMed] [Google Scholar]
- 19.Power E, Clarke M, Kelleher I, Coughlan H, Lynch F, Connor D, et al. The association between economic inactivity and mental health among young people: a longitudinal study of young adults who are not in employment, education or training. Irish Journal of Psychological Medicine. 2015;32(1):155–60. Epub 2015/03/01. 10.1017/ipm.2014.85 . [DOI] [PubMed] [Google Scholar]
- 20.Lin A, Wood SJ, Yung AR. Measuring psychosocial outcome is good. Current Opinion in Psychiatry. 2013;26(2):138–43. Epub 2013/01/16. 10.1097/YCO.0b013e32835d82aa . [DOI] [PubMed] [Google Scholar]
- 21.Juckel G, Morosini PL. The new approach: psychosocial functioning as a necessary outcome criterion for therapeutic success in schizophrenia. Current Opinion in Psychiatry. 2008;21(6):630–9. Epub 2008/10/15. 10.1097/YCO.0b013e328314e144 . [DOI] [PubMed] [Google Scholar]
- 22.Morgan VA, Waterreus A, Carr V, Castle D, Cohen M, Harvey C, et al. Responding to challenges for people with psychotic illness: Updated evidence from the Survey of High Impact Psychosis. The Australian and New Zealand Journal of Psychiatry. 2017;51(2):124–40. Epub 2016/12/04. 10.1177/0004867416679738 . [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Jimenez M, Gleeson JF, Henry LP, Harrigan SM, Harris MG, Killackey E, et al. Road to full recovery: longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychological Medicine. 2012;42(3):595–606. Epub 2011/08/23. 10.1017/S0033291711001504 . [DOI] [PubMed] [Google Scholar]
- 24.Tandberg M, Ueland T, Sundet K, Haahr U, Joa I, Johannessen JO, et al. Neurocognition and occupational functioning in patients with first-episode psychosis: a 2-year follow-up study. Psychiatry Research. 2011;188(3):334–42. Epub 2011/05/18. 10.1016/j.psychres.2011.04.021 . [DOI] [PubMed] [Google Scholar]
- 25.Hamilton BA, Naismith SL, Scott EM, Purcell S, Hickie IB. Disability is already pronounced in young people with early stages of affective disorders: data from an early intervention service. Journal of Affective Disorders. 2011;131(1–3):84–91. Epub 2010/11/30. 10.1016/j.jad.2010.10.052 . [DOI] [PubMed] [Google Scholar]
- 26.Scott EM, Hermens DF, Naismith SL, Guastella AJ, White D, Whitwell BG, et al. Distress and disability in young adults presenting to clinical services with mood disorders. International Journal of Bipolar Disorders. 2013;1:23 Epub 2013/01/01. 10.1186/2194-7511-1-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott J, Scott EM, Hermens DF, Naismith SL, Guastella AJ, White D, et al. Functional impairment in adolescents and young adults with emerging mood disorders. The British Journal of Psychiatry. 2014;205(5):362–8. Epub 2014/09/13. 10.1192/bjp.bp.113.134262 . [DOI] [PubMed] [Google Scholar]
- 28.Cotton SM, Lambert M, Schimmelmann BG, Filia K, Rayner V, Hides L, et al. Predictors of functional status at service entry and discharge among young people with first episode psychosis. Social Psychiatry and Psychiatric Epidemiology. 2017;52(5):575–85. Epub 2017/02/25. 10.1007/s00127-017-1358-0 . [DOI] [PubMed] [Google Scholar]
- 29.Lee RS, Hermens DF, Redoblado-Hodge MA, Naismith SL, Porter MA, Kaur M, et al. Neuropsychological and socio-occupational functioning in young psychiatric outpatients: a longitudinal investigation. PLoS One. 2013;8(3):e58176 Epub 2013/03/08. 10.1371/journal.pone.0058176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee RSC, Hermens DF, Scott J, O'Dea B, Glozier N, Scott EM, et al. A transdiagnostic study of education, employment, and training outcomes in young people with mental illness. Psychological Medicine. 2017;47(12):2061–70. Epub 2017/04/11. 10.1017/S0033291717000484 . [DOI] [PubMed] [Google Scholar]
- 31.Iorfino F, Hermens DF, Cross S, PM, Zmicerevska N, Nichles A, Badcock C-A, et al. Delineating the trajectories of social and occupational functioning of young people attending early intervention mental health services in Australia: a longitudinal study. BMJ Open. 2018;8(3). 10.1136/bmjopen-2017-020678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee RSC, Hermens DF, Naismith SL, Lagopoulos J, Jones A, Scott J, et al. Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Translational Psychiatry. 2015;5:e555 Epub 2015/04/29. 10.1038/tp.2015.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott EM, Hermens DF, Naismith SL, Guastella AJ, White D, Whitwell BG, et al. Distress and disability in young adults presenting to clinical services with mood disorders. International Journal of Bipolar Disorders. 2013;1(1):23 10.1186/2194-7511-1-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iorfino F, Hermens DF, Cross SP, Zmicerevska N, Nichles A, Groot J, et al. Prior suicide attempts predict worse functioning outcomes in young people attending a mental health service. Journal of Affective disorders. 2018; 238: 563–569. 10.1016/j.jad.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 35.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American Journal of Psychiatry. 2000;157(5):745–50. Epub 2000/04/28. 10.1176/appi.ajp.157.5.745 . [DOI] [PubMed] [Google Scholar]
- 36.Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13(4):493–504. Epub 2002/01/05. . [DOI] [PubMed] [Google Scholar]
- 37.Newton-Howes G, Boden JM. Relation between age of first drinking and mental health and alcohol and drug disorders in adulthood: evidence from a 35-year cohort study. Addiction. 2016;111(4):637–44. Epub 2015/11/15. 10.1111/add.13230 . [DOI] [PubMed] [Google Scholar]
- 38.Ellickson PL, Tucker JS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics. 2003;111(5 Pt 1):949–55. Epub 2003/05/03. . [DOI] [PubMed] [Google Scholar]
- 39.Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug and Alcohol Dependence. 1993;33(2):129–37. Epub 1993/09/01. . [DOI] [PubMed] [Google Scholar]
- 40.Georgiades K, Boyle MH. Adolescent tobacco and cannabis use: young adult outcomes from the Ontario Child Health Study. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48(7):724–31. Epub 2007/06/27. 10.1111/j.1469-7610.2007.01740.x . [DOI] [PubMed] [Google Scholar]
- 41.Ellickson P, Bui K, Bell R, McGuigan KA. Does early drug use increase the risk of dropping out of high school? Journal of Drug Issues. 1998;28(2):357–80. 10.1177/002204269802800205 [DOI] [Google Scholar]
- 42.Newcomb MD, Abbott RD, Catalano RF, Hawkins JD, Battin-Pearson S, Hill K. Mediational and deviance theories of late high school failure: Process roles of structural strains, academic competence, and general versus specific problem behavior. Journal of Counseling Psychology. 2002;49(2):172–86. 10.1037/0022-0167.49.2.172 [DOI] [Google Scholar]
- 43.Boys A, Farrell M, Taylor C, Marsden J, Goodman R, Brugha T, et al. Psychiatric morbidity and substance use in young people aged 13–15 years: results from the Child and Adolescent Survey of Mental Health. The British Journal of Psychiatry. 2003;182:509–17. Epub 2003/06/05. . [DOI] [PubMed] [Google Scholar]
- 44.Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(11):1294–305. Epub 2002/11/01. 10.1097/00004583-200211000-00010 . [DOI] [PubMed] [Google Scholar]
- 45.Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99(10):1333–41. Epub 2004/09/17. 10.1111/j.1360-0443.2004.00806.x . [DOI] [PubMed] [Google Scholar]
- 46.Chen CY, O'Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug and Alcohol Dependence. 2005;79(1):11–22. Epub 2005/06/10. 10.1016/j.drugalcdep.2004.11.014 . [DOI] [PubMed] [Google Scholar]
- 47.Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92(3):279–96. Epub 1997/03/01. . [PubMed] [Google Scholar]
- 48.Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103(6):969–76; discussion 77–8. Epub 2008/05/17. 10.1111/j.1360-0443.2008.02221.x . [DOI] [PubMed] [Google Scholar]
- 49.Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine. 2003;33. [DOI] [PubMed] [Google Scholar]
- 50.Hser YI, Huang D, Brecht ML, Li L, Evans E. Contrasting trajectories of heroin, cocaine, and methamphetamine use. Journal of Addictive Diseases. 2008;27(3):13–21. Epub 2008/10/30. 10.1080/10550880802122554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addictive Behaviors. 2007;32(1):24–38. Epub 2006/05/06. 10.1016/j.addbeh.2006.03.017 . [DOI] [PubMed] [Google Scholar]
- 52.Weiss LM, Petry NM. Substance abuse treatment patients with early onset cocaine use respond as well to contingency management interventions as those with later onset cocaine use. Journal of Substance Abuse Treatment. 2014;47(2):146–50. Epub 2014/05/29. 10.1016/j.jsat.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball SA, Carroll KM, Babor TF, Rounsaville BJ. Subtypes of cocaine abusers: support for a type A-type B distinction. Journal of Consulting and Clinical Psychology. 1995;63(1):115–24. Epub 1995/02/01. . [DOI] [PubMed] [Google Scholar]
- 54.Hickie IB, Hermens DF, Naismith SL, Guastella AJ, Glozier N, Scott J, et al. Evaluating differential developmental trajectories to adolescent-onset mood and psychotic disorders. BMC Psychiatry. 2013;13:303 Epub 2013/11/13. 10.1186/1471-244X-13-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGorry PD, Tanti C, Stokes R, Hickie IB, Carnell K, Littlefield LK, et al. headspace: Australia’s National Youth Mental Health Foundation—where young minds come first. Medical Journal of Australia. 2007;187(7 Supplement):S68. [DOI] [PubMed] [Google Scholar]
- 56.Rickwood DJ, Telford NR, Parker AG, Tanti CJ, McGorry PD. headspace—Australia’s innovation in youth mental health: who are the clients and why are they presenting? Medical Journal of Australia. 2014;200:1–4. [DOI] [PubMed] [Google Scholar]
- 57.Scott EM, Hermens DF, White D, Naismith SL, GeHue J, Whitwell BG, et al. Body mass, cardiovascular risk and metabolic characteristics of young persons presenting for mental healthcare in Sydney, Australia. BMJ Open. 2015;5(3). 10.1136/bmjopen-2014-007066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilsenroth MJ, Ackerman SJ, Blagys MD, Baumann BD, Baity MR, Smith SR, et al. Reliability and validity of DSM-IV axis V. The American Journal of Psychiatry. 2000;157(11):1858–63. Epub 2000/11/04. 10.1176/appi.ajp.157.11.1858 . [DOI] [PubMed] [Google Scholar]
- 59.Hay P, Katsikitis M, Begg J, Da Costa J, Blumenfeld N. A two-year follow-up study and prospective evaluation of the DSM-IV axis V. Psychiatric Services. 2003;54(7):1028–30. Epub 2003/07/10. 10.1176/appi.ps.54.7.1028 . [DOI] [PubMed] [Google Scholar]
- 60.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 61.Muthén LK, Muthén BO. Mplus User’s Guide (8th ed.). Los Angeles, CA: Muthén & Muthén; 1998–2017. [Google Scholar]
- 62.Little RJA, Rubin DB. Statistical Analysis with Missing Data (2nd ed.). John Wiley & Sons, Inc.; 2014. [Google Scholar]
- 63.Geiser C. Data Analysis with Mplus. New York, NY: Guildford Press; 2012. [Google Scholar]
- 64.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7(2):147–77. Epub 2002/07/02. . [PubMed] [Google Scholar]
- 65.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–137. 2018. [Google Scholar]
- 66.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism, Clinical and Experimental Research. 2000;24(2):164–71. Epub 2000/03/04. . [PubMed] [Google Scholar]
- 67.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. The American Journal of Psychiatry. 2015;172(6):531–42. Epub 2015/05/20. 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–25. Epub 2014/03/19. 10.1016/j.dcn.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacological Reviews. 2016;68(4):1074–109. 10.1124/pr.115.012138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, et al. Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcoholism, Clinical and Experimental Research. 2017;41(12):2082–92. Epub 2017/10/31. 10.1111/acer.13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57(1):56–66. Epub 2004/12/21. 10.1016/j.biopsych.2004.10.022 . [DOI] [PubMed] [Google Scholar]
- 72.Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: what we know and where we're headed. Neuroscience and Biobehavioral Reviews. 2014;45:323–42. Epub 2014/07/16. 10.1016/j.neubiorev.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harbor Perspectives in Medicine. 2012;2(12):a012120-a 10.1101/cshperspect.a012120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, et al. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. Epub 2008/06/27. 10.1038/npp.2008.96 . [DOI] [PubMed] [Google Scholar]
- 75.Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, et al. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nature Neuroscience. 2011;14:417 10.1038/nn.2770 [DOI] [PubMed] [Google Scholar]
- 76.Mashhoon Y, Betts J, Farmer SL, Lukas SE. Early onset tobacco cigarette smokers exhibit deficits in response inhibition and sustained attention. Drug and Alcohol Dependence. 2018;184:48–56. Epub 2018/02/07. 10.1016/j.drugalcdep.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alameda-Bailen JR, Salguero-Alcaniz P, Merchan-Clavellino A, Paino-Quesada S. Age of onset of cannabis use and decision making under uncertainty. PeerJ. 2018;6:e5201 Epub 2018/07/14. 10.7717/peerj.5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pope HG Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–10. Epub 2003/03/14. . [DOI] [PubMed] [Google Scholar]
- 79.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology. 2012;219(2):575–86. Epub 2011/09/23. 10.1007/s00213-011-2486-y . [DOI] [PubMed] [Google Scholar]
- 80.Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. The British Journal of Psychiatry. 2011;198(6):442–7. Epub 2011/06/02. 10.1192/bjp.bp.110.077479 . [DOI] [PubMed] [Google Scholar]
- 81.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012;26(3):496–506. Epub 2011/11/23. 10.1037/a0026269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216(1):131–44. Epub 2011/02/18. 10.1007/s00213-011-2203-x . [DOI] [PubMed] [Google Scholar]
- 83.Lubman DI, Cheetham A, Yucel M. Cannabis and adolescent brain development. Pharmacology & Therapeutics. 2015;148:1–16. Epub 2014/12/03. 10.1016/j.pharmthera.2014.11.009 . [DOI] [PubMed] [Google Scholar]
- 84.Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the risks and consequences of adolescent cannabis exposure. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56(3):214–25. Epub 2017/02/22. 10.1016/j.jaac.2016.12.014 . [DOI] [PubMed] [Google Scholar]
- 85.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry. 2016;73(3):292–7. Epub 2016/02/05. 10.1001/jamapsychiatry.2015.3278 . [DOI] [PubMed] [Google Scholar]
- 86.Birrell L, Newton NC, Teesson M, Tonks Z, Slade T. Anxiety disorders and first alcohol use in the general population. Findings from a nationally representative sample. Journal of Anxiety Disorders. 2015;31:108–13. Epub 2015/03/22. 10.1016/j.janxdis.2015.02.008 . [DOI] [PubMed] [Google Scholar]
- 87.Birrell L, Newton NC, Teesson M, Slade T. Early onset mood disorders and first alcohol use in the general population. Journal of Affective Disorders. 2016;200:243–9. Epub 2016/05/06. 10.1016/j.jad.2016.04.032 . [DOI] [PubMed] [Google Scholar]
- 88.Dahne J, Banducci AN, Kurdziel G, MacPherson L. Early adolescent symptoms of social phobia prospectively predict alcohol use. Journal of Studies on Alcohol and Drugs. 2014;75(6):929–36. Epub 2014/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayatbakhsh MR, Mamun AA, Najman JM, O'Callaghan MJ, Bor W, Alati R. Early childhood predictors of early substance use and substance use disorders: prospective study. The Australian and New Zealand journal of psychiatry. 2008;42(8):720–31. Epub 2008/07/16. 10.1080/00048670802206346 . [DOI] [PubMed] [Google Scholar]
- 90.Kuperman S, Chan G, Kramer JR, Bierut L, Bucholz KK, Fox L, et al. Relationship of age of first drink to child behavioral problems and family psychopathology. Alcoholism, Clinical and Experimental Research. 2005;29(10):1869–76. Epub 2005/11/05. . [DOI] [PubMed] [Google Scholar]
- 91.Macleod J, Hickman M, Bowen E, Alati R, Tilling K, Smith GD. Parental drug use, early adversities, later childhood problems and children's use of tobacco and alcohol at age 10: birth cohort study. Addiction. 2008;103(10):1731–43. Epub 2008/08/19. 10.1111/j.1360-0443.2008.02301.x . [DOI] [PubMed] [Google Scholar]
- 92.Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, et al. Genetic and environmental predictors of early alcohol use. Biological Psychiatry. 2007;61(11):1228–34. Epub 2006/11/25. 10.1016/j.biopsych.2006.06.039 . [DOI] [PubMed] [Google Scholar]
- 93.Chilcoat HD, Anthony JC. Impact of parent monitoring on initiation of drug use through late childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(1):91–100. Epub 1996/01/01. 10.1097/00004583-199601000-00017 . [DOI] [PubMed] [Google Scholar]
- 94.Guo J, Hill KG, Hawkins JD, Catalano RF, Abbott RD. A developmental analysis of sociodemographic, family, and peer effects on adolescent illicit drug initiation. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(7):838–45. Epub 2002/07/11. 10.1097/00004583-200207000-00017 . [DOI] [PubMed] [Google Scholar]
- 95.Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, et al. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37(4):986–95. Epub 2011/11/25. 10.1038/npp.2011.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Visser L, de Winter AF, Vollebergh WA, Verhulst FC, Reijneveld SA. Do child's psychosocial functioning, and parent and family characteristics predict early alcohol use? The TRAILS Study. European Journal of Public Health. 2015;25(1):38–43. Epub 2014/06/18. 10.1093/eurpub/cku072 . [DOI] [PubMed] [Google Scholar]
- 97.Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, et al. Neural predictors of initiating alcohol use during adolescence. The American Journal of Psychiatry. 2017;174(2):172–85. Epub 2016/08/20. 10.1176/appi.ajp.2016.15121587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Webb JA, Baer PE, McLaughlin RJ, McKelvey RS, Caid CD. Risk factors and their relation to initiation of alcohol use among early adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(4):563–8. Epub 1991/07/01. 10.1097/00004583-199107000-00006 . [DOI] [PubMed] [Google Scholar]
- 99.Mayzer R, Fitzgerald HE, Zucker RA. Anticipating problem drinking risk from preschoolers' antisocial behavior: evidence for a common delinquency-related diathesis model. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(8):820–7. Epub 2009/07/01. 10.1097/CHI.0b013e3181aa0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cotton SM, Wright A, Harris MG, Jorm AF, McGorry PD. Influence of gender on mental health literacy in young Australians. The Australian and New Zealand Journal of Psychiatry. 2006;40(9):790–6. Epub 2006/08/17. 10.1080/j.1440-1614.2006.01885.x . [DOI] [PubMed] [Google Scholar]
- 101.Ellis LA, Collin P, Hurley PJ, Davenport TA, Burns JM, Hickie IB. Young men’s attitudes and behaviour in relation to mental health and technology: implications for the development of online mental health services. BMC Psychiatry. 2013;13(1):119 10.1186/1471-244x-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Killackey E, Allott K. Utilising Individual Placement and Support to address unemployment and low education rates among individuals with psychotic disorders. The Australian and New Zealand Journal of Psychiatry. 2013;47(6):521–3. Epub 2013/05/31. 10.1177/0004867413476755 . [DOI] [PubMed] [Google Scholar]
- 103.Fowler D, Hodgekins J, French P, Marshall M, Freemantle N, McCrone P, et al. Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. The Lancet Psychiatry. 2018;5(1):41–50. Epub 2017/12/16. 10.1016/S2215-0366(17)30476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.