Summary:
Background:
Initiation of antiretroviral therapy (ART) following diagnosis at birth is an emerging area of paediatric HIV care. We present outcomes of HIV-infected infants identified at birth at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa.
Methods:
From September 2013 (Era 1), only “high risk” HIV-exposed infants were offered diagnostic HIV PCR tests at birth. From June 2014 (Era 2) all HIV-exposed infants were offered laboratory-based diagnostic PCR tests. From October 2014 (Era 3), point-of-care (POC) diagnostic PCR tests were added if staff availability allowed. We describe time to ART initiation, mortality, retention in care and viral suppression among the HIV-infected infants identified across these eras born through June 2016.
Findings:
Of 5449 HIV-exposed infants tested, 88 confirmed cases of HIV-infection were identified and included in the study; 86 (97·7%) started ART. Age at ART initiation decreased from a median of nine days in Eras 1 and 2 to two days in Era 3. In Era 3, the 35 HIV-infected neonates who were co-tested with POC started ART earlier (82·9% ≤48 hours after birth) than the 29 infants not co-tested (3·5% ≤ 48 hours; median of six days). The probability of mortality by twelve months was 0·14 (95% CI 0·08-0·24) and did not differ by eras. Of the infants who survived and initiated ART at the site, retention at twelve months was 77·8%. Of those infants retained in care, 71·3% had viral load <400 copies/ml at twelve months with no differences between eras.
Interpretation:
HIV-infected infants can be identified at birth and ART initiated within hours to days. However, reducing mortality and improving retention and viral suppression remain urgent priorities.
Introduction
In the absence of antiretroviral therapy (ART), HIV-infected infants are at high risk of early mortality. Almost a quarter may die before three months of age and up to half by two years of age.1 South African guidelines have recommended immediate initiation of ART among all HIV-infected infants upon diagnosis regardless of immunologic or clinical status since the landmark Children with HIV Early antiRetroviral therapy (CHER) study.2 However in 2017, ART coverage among HIV-infected children in South Africa was estimated to be only 55%.3 HIV-infected infant care through the cascade of diagnosis, result return, ART initiation, and successful retention in long-term care is challenging.4–6 We have demonstrated that even though birth testing misses intrapartum and post-natal infections, testing at this time identifies a larger total number of infected infants than when testing is done later due to high rates of death and loss to follow-up.7
Early initiation of ART for neonates requires consideration of many practical and biological challenges inherent in early infant diagnosis (EID). At Rahima Moosa Mother and Child Hospital (RMMCH) in Johannesburg, South Africa, we introduced birth testing with the aim of improving EID coverage, increasing rates of early ART initiation and reducing HIV-related mortality.8,9 Here we evaluate whether the introduction of different birth testing approaches was associated with improved infant outcomes including earlier ART initiation, survival, retention in care and viral suppression rates.
Methods
Study Design and Participants
We describe twelve month outcomes among confirmed HIV-infected infants identified at birth between September 2013 and June 2016 at RMMCH in Johannesburg, South Africa. RMMCH is an academic paediatric referral centre and functions as the main delivery facility and obstetric referral site for complicated cases of a sub-district of Johannesburg. Maternal HIV prevalence at RMMCH was 23% during the study period.8 We compared infant outcomes by changes in the EID programme that were implemented over this time.
From September 2013, the EID programme expanded beyond the, at the time, recommended six week PCR test to include a PCR test as close to birth as possible of “high risk” neonates (preterm infants [<37 weeks], low birth weight [<2,500 grams] and/or neonatal admissions – Era 1 – Targeted Testing). Thus during this era only a proportion of the HIV-exposed newborns were eligible for birth testing. From June 2014, the EID programme expanded further to test all HIV-exposed neonates born at the maternity service (Era 2 – Universal Testing).8 From October 2014, and where staff availability allowed, point-of-care (POC) testing was added (Era 3 – Universal Testing, POC available).9 POC staffing was limited during weekends and public holidays. EID coverage (proportion of eligible HIV-exposed infants tested) was ~66% during Era 1, and 93% for birth testing overall in Eras 2 and 3 and 51% for POC testing during Era 3.8,9
Procedures
HIV PCR testing was performed on whole blood sampled by venipuncture and tested at the national laboratory using COBAS® TaqMan® HIV-1 Qualitative Test Version 2·0 (Roche Molecular Systems, Inc., Branchburg, NJ). Version 1 of this assay was used during Era 1. We used the Cepheid Xpert® HIV-1 Qualitative assay (Cepheid, Sunnyvale, CA) for POC testing.9 In all eras, neonates with positive or indeterminate results on the standard laboratory-based PCR were actively traced and repeat tested to confirm diagnosis with a repeat diagnostic PCR and a quantitative HIV RNA viral load (COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 test, version 2·0 [Roche Molecular Systems, Inc., Branchburg, NJ]). HIV infection was considered confirmed when a second sample resulted in a positive diagnostic PCR or viral load >20 copies per mL.10
ART was started presumptively as soon as possible after the first available positive PCR result. The confirmatory sample was taken at this time but ART was not delayed to wait for the result. During Era 1 lopinavir/ritonavir, lamivudine and zidovudine was generally the preferred first-line regimen. In light of growing concerns about lopinavir/ritonavir use in preterm infants, hospital guidance was revised to recommend zidovudine, lamivudine and nevirapine as first-line ART for neonates. Nevirapine was replaced with lopinavir/ritonavir after 42 weeks post menstrual age and 14 days of calendar age or later based on patient readiness. Co-trimoxazole prophylaxis was initiated at 4-6 weeks of age. Weekly or fortnightly follow-up occurred in their first month of life, monthly until six months of age and then three monthly, unless clinical concerns necessitated more frequent visits. Loss to follow-up was defined as not returning to the site within 90 days of the last appointment.
Mothers or legal guardians signed written informed consent for their own and their infant’s participation. The study was approved by the Institutional Review Boards of the University of the Witwatersrand and Columbia University.
Statistical Analysis
We compared outcomes between the three EID programme eras described above. The primary focus was on Era 3 which was further stratified into those who were and were not co-tested with POC. Baseline characteristics, age at ART initiation, and mortality and retention in care through 12 months were compared. Viral suppression was examined at visits closest to six (3-8) and twelve (9-14) months after ART initiation. We present characteristics of infants using descriptive statistics (median, inter-quartile ranges [IQR], proportions and 95% confidence intervals [95% CI]). Comparisons were tested using Chi-Square and Fisher Exact tests for proportions, and Student’s t- and Wilcoxon tests for continuous variables. Survival was analysed by Kaplan-Meier analysis and log-rank tests. Analyses were conducted using SAS (Version 9·4, SAS Institute Inc., Cary, NC). A case summary is provided for the cases of death.
Role of the funding source
The National Institutes of Health was involved in the study design and monitoring, but was not involved in analysis and interpretation of data, writing the report or decision to submit the paper for publication. We received a reduced price per cartridge from the POC manufacturer. The corresponding author had access to the data and takes final responsibility for the decision to submit.
Results:
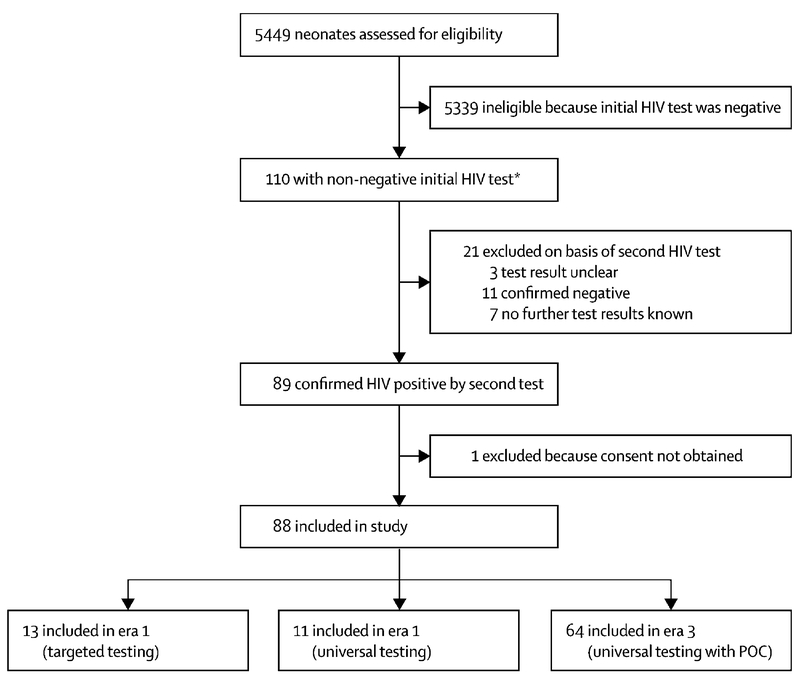
During Era 1, 201 HIV-exposed neonates were tested, 15 (7·5%) had either positive or indeterminate initial results, one infant with initial indeterminate result was confirmed negative, in one case no consent was available thus resulting in 13 cases (Figure 1). During Era 2, 798 HIV-exposed neonates were tested, 15 (1·9%) had either positive or indeterminate initial results, two infants with initial indeterminate result were confirmed negative and two with initial positive results did not return for confirmatory testing thus resulting in 11 cases. During Era 3, 4450 HIV-exposed neonates were tested, 80 (1·8%) tested positive or indeterminate initially and 64 were confirmed to be infected on later follow-up. Of the 16 remaining, five did not return for confirmatory testing (two indeterminate and three positive), three remained with uncertain diagnosis and eight with initial indeterminate were confirmed negative. Of the 64 confirmed positives in Era 3, 29 were not co-tested with POC and 35 were POC co-tested.
Figure 1: Study population.
POC: Point of care testing by Cepheid Xpert® HIV-1 Qualitative assay; ART: antiretroviral treatment
Baseline characteristics of the 88 confirmed HIV-infected infants identified in the three eras are displayed in Table 1. Mean birth weight was low (1,846 grams) with a high percentage preterm (53·9%) in Era 1. In Era 3, those not co-tested by POC had lower mean birth weights, a higher proportion of prematurity and were more likely to require hospitalization at delivery than those co-tested by POC. Median pre-treatment viral load was highest in the HIV-infected neonates identified in Era 1 (Table 1, p=0.12). In Era 3, pre-treatment viral loads were similar in those with or without POC co-testing.
Table 1:
Characteristics of the cohort of 88 neonates with confirmed HIV infection
| Era 1: targeted testing (n=13) | Era 2: universal testing, no POC testing available (n=11) | Era 3: universal testing, POC testing available (n=64) | p value | Era 3: universal testing, not POC co-tested (n=29) | Era 3: universal testing, POC co-tested (n=35) | p value | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 6 (46%) | 9 (82%) | 33 (52%) | 0·14 | 18 (62%) | 15 (43%) | 0·13 |
| Male | 7 (54%) | 2 (18%) | 31 (48%) | .. | 11 (38%) | 20 (57%) | .. |
| Birthweight (g) | |||||||
| Range | 1080–2438 | 2110–3425 | 905–3805 | .. | 905–3805 | 1860–3710 | .. |
| Mean (SD) | 1846 (470) | 2776 (385) | 2664 (653) | 0·0003 | 2419 (760) | 2867 (468) | 0·008 |
| Missing data | 1 (8%) | 0 | 0 | .. | 0 | 0 | .. |
| Antiretroviral therapy during this pregnancy | |||||||
| Yes, started before pregnancy and continued | 2 (15%) | 1 (9%) | 6 (9%) | 0·89 | 4 (14%) | 2 (6%) | 0·42 |
| Yes, during pregnancy, treatment duration ≥12 weeks | 4 (31%) | 2 (18%) | 19 (30%) | .. | 8 (28%) | 11 (31%) | .. |
| Yes, during pregnancy, treatment duration <12 weeks | 5 (38%) | 6 (55%) | 21 (33%) | .. | 9 (31%) | 12 (34%) | .. |
| Yes, during pregnancy, unknown when | 0 | 0 | 1 (2%) | .. | 0 | 1 (3%) | .. |
| None | 0 | 1 (9%) | 10 (16%) | .. | 3 (10%) | 7 (20%) | .. |
| Unknown | 2 (15%) | 1 (9%) | 7 (11%) | .. | 5 (17%) | 2 (6%) | .. |
| Gestational age | |||||||
| ≥37 weeks (term) | 5 (38%) | 9 (82%) | 47 (73%) | 0·066 | 16 (55%) | 31 (89%) | 0·011 |
| <37 weeks (preterm) | 7 (54%) | 2 (18%) | 12 (19%) | .. | 9 (31%) | 3 (9%) | .. |
| Missing data | 1 (8%) | 0 | 5 (8%) | .. | 4 (14%) | 1 (3%) | .. |
| First PCR | |||||||
| Positive | 9 (69%) | 9 (82%) | 61 (95%) | 0·012 | 26 (90%) | 35 (100%) | 0·051 |
| Indeterminate | 4 (31%) | 2 (18%) | 3 (5%) | .. | 3 (10%) | 0 | .. |
| Pretreatment viral load | |||||||
| Median (IQR) | 190 546 (1995–467 735) |
2045 (530–36 180) |
34 357 (7602–262 493) |
0·12 | 40 015 (1778–229 087) |
32 027 (9849–284 175) |
0·92 |
| Range | 240–1 122 018 | 155–65 760 | 60–4 950 000 | .. | 220–4 861 620 | 60–4 950 000 | .. |
| Pretreatment viral load (copies per mL) | |||||||
| <100 000 | 6 (46%) | 9 (82%) | 35 (55%) | 0·061 | 14 (48%) | 21 (60%) | 0·037 |
| >100 000 | 7 (54%) | 0 | 21 (33%) | .. | 8 (28%) | 13 (37%) | .. |
| Missing | 0 | 2 (18%) | 8 (13%) | .. | 7 (24%) | 1 (3%) | .. |
| Admission immediately after delivery | |||||||
| Discharged to mother after delivery | 7 (54%) | 10 (91%) | 43 (67%) | 0·40 | 13 (45%) | 30 (86%) | 0·0019 |
| Required admission due to neonatal condition | 5 (38%) | 0 | 16 (25%) | .. | 13 (45%) | 3 (9%) | .. |
| Required admission due to maternal condition | 0 | 0 | 2 (3%) | .. | 2 (7%) | 0 | .. |
| Missing | 1 (8%) | 1 (9%) | 3 (5%) | .. | 1 (3%) | 2 (6%) | .. |
Data are n (%) unless otherwise specified. POC=point of care.
Overall 97.7% (86/88) of neonates confirmed to be infected initiated ART with no differences across eras. One neonate died before starting ART and one was lost to follow-up before ART could be initiated. Since confirmation of diagnosis required at least one further test, this statistic over-estimates the success of starting ART given that six neonates had no follow-up testing. Among those with a confirmed diagnosis who initiated ART, the median age of initiation was nine days (IQR 6-25) in Eras 1 and 2, and two days (IQR 1-8) in Era 3. Stratifying Era 3 into those not vs. co-tested by POC, the proportion initiating ART at the site was 25/29 (86.2%) and 2/29 (6.9%) initiating elsewhere if not co-tested by POC and 100% (35/35) initiating at the site if co-tested with POC. Among those initiating ART during Era 3 the median age at initiation was six days (IQR 5-10) if not co-tested by POC compared to one day (IQR: 1-2) if POC co-tested. In Era 3, of those initiating ART, 55·6% of those not co-tested by POC started ART within a week compared to 91·5% of the POC co-tested infants; 3·7% of those not co-tested by POC started ART within 48 hours compared to 82·9% of the POC co-tested infants (p<0.0001, Table 2).
Table 2:
Initiation of ART among the 86 neonates with confirmed HIV infection who started ART
| Era 1: targeted testing (n=13) | Era 2: universal testing (n=11) | Era 3: universal testing, POC testing available (n=62) | p value | Era 3: universal testing, not POC co-tested (n=27) | Era 3: universal testing, POC co-tested (n=35) | p value | |
|---|---|---|---|---|---|---|---|
| ART start location | |||||||
| On site | 12 (92%) | 9 (82%) | 60 (97%) | 0·31 | 25 (93%) | 35 (100%) | 0·07 |
| Not on site | 1 (8%) | 2 (18%) | 2 (3%) | .. | 2 (7%) | 0 | .. |
| Age at start of ART, days | |||||||
| Mean (SD) | 59 (150) | 21 (24) | 12 (25) | 0·0002 | 18 (26) | 8 (24) | 0·15* |
| Median (IQR) | 9 (6–25) | 9 (6–25) | 2 (1–7) | .. | 6 (5–10) | 1 (1–2) | .. |
| Range | 3–555 | 2–74 | 0–104 | .. | 2–95 | 0–104 | .. |
| Age category at start of ART | |||||||
| <48 h | 0 | 1 (9%) | 30 (48%) | 0·0007 | 1 (4%) | 29 (83%) | <0·0001 |
| 48 h to 7 days | 5 (38%) | 2 (18%) | 17 (27%) | .. | 14 (52%) | 3 (9%) | .. |
| ≥8 days | 8 (62%) | 8 (73%) | 15 (24%) | .. | 12 (44%) | 3 (9%) | .. |
| Starting regimen | |||||||
| Lopinavir-ritonavir, lamivudine, and abacavir | 0 | 0 | 5 (8%) | <0·0001 | 3 (11%) | 2 (6%) | 0·73 |
| Lopinavir-ritonavir, lamivudine, and zidovudine | 6 (46%) | 0 | 1 (2%) | .. | 0 | 1 (3%) | .. |
| Nevirapine, lamivudine, and zidovudine | 5 (38%) | 9 (82%) | 51 (82%) | .. | 21 (78%) | 30 (86%) | .. |
| Nevirapine, lamivudine, and stavudine | 0 | 0 | 2 (3%) | .. | 1 (4%) | 1 (3%) | .. |
| Other | 1 (8%) | 1 (9%) | 1 (2%) | .. | 1 (4%) | 0 | .. |
| Unknown | 1 (8%) | 1 (9%) | 2 (3%) | .. | 1 (4%) | 1 (3%) | .. |
Data are n (%) unless otherwise specified. Two of the 88 infants with confirmed HIV infection in era 3 who were not POC co-tested were not known to have started ART, so are not included here. POC=point of care. ART=antiretroviral therapy.
Wilcoxon two-sample test p<0.0001.
Of the 88 confirmed HIV-infected infants the probability of mortality by twelve months of age was 0·14 (95% CI 0·08-0·24). Among 86 infected infants who initiated ART, ten are known to have died. Nine of these deaths were in infants who initiated ART at the site and one had initiated elsewhere. A summary of the deaths is provided in Supplementary Table 1. The estimates censor those lost to follow-up at their last visit and may be under-estimates. The median age at death of infants after ART start was 73 days (IQR 33, 99; range 16,139 days). Probability of death by twelve months was higher in those with pre-treatment VL >100,000 copies per mL (0·28, 95% CI; 0·14, 0·49) vs. those with pre-treatment VL <100,000 copies per mL (0·07, 95% CI; 0·02, 0·19, p=0.01).
Probabilities of mortality by twelve months were 0·19 (95% CI: 0·05-0·58) in Era 1, no deaths in Era 2 and 0·14 (95% CI: 0·07-0·26) in Era 3. Although there were no significant differences in mortality when stratifying Era 3 by whether or not the child had been co-tested by POC (a factor which changed the timing of ART initiation), we observed double the probability of death 0·19 (95% CI; 0·08-0·39) in those not co-tested by POC than in those co-tested by POC 0·09 (95% CI; 0·03-0·27) p=0·31 (Figure 2). Two of five deaths in those not co-tested by POC were preterm whereas 0/3 deaths in those co-tested by POC were preterm.
Figure 2: Mortality in the universal testing era (Era 3) by whether or not the HIV-infected infants were co-tested (n=35) or not co-tested (n=27) by the point-of-care test.

POC: Point of care testing by Cepheid Xpert® HIV-1 Qualitative assay.
Fifty-six (63·6%) of 88 confirmed HIV-infected infants were retained in care at the site by twelve months (Table 3). Of the 32 not retained in care at the site, five infants were reported to have initiated ART at other clinics (one death). Nine infants initiated ART at the site and died (not having disengaged from care). Sixteen started ART at the site but were not retained through twelve months but three of these were formally transferred to other services. Thus 18·1% (13/72) starting ART at RMMCH and not known to have died were lost to follow-up without transfer. If only those who initiated ART at the site and known not to have died are included in the denominator (N=72), the retention rate rises to 77·8%. In Era 3, overall rates of retention were similar in those not vs. co-tested by POC: 65·5% (19/29) and 68·6% (24/35) (Table 3).
Table 3:
Retention in care of the 88 neonates with confirmed HIV infection
| Era 1: targeted testing (n=13) | Era 2: universal testing (n=11) | Era 3: universal testing, POC testing available (n=64) | p value | Era 3: universal testing, not POC co-tested (n=29) | Era 3: universal testing, POC co-tested (n=35) | p value | |
|---|---|---|---|---|---|---|---|
| At 6 months (180 days) | |||||||
| Retained in care on site | 8 (62%) | 8 (73%) | 45 (70%) | 0·53 | 21 (72%) | 24 (69%) | 0·17 |
| Started or moved to care elsewhere before 6 months | 2 (15%) | 2 (18%) | 3 (5%) | .. | 2 (7%) | 1 (3%) | .. |
| Died | 2 (15%) | 0 | 8 (13%)* | .. | 5 (17%)* | 3 (9%) | .. |
| Lost to follow-up | 1 (8%) | 1 (9%) | 8 (13%) | .. | 1 (3%) | 7 (20%) | .. |
| At 12 months (365 days) | |||||||
| Retained in care on site | 6 (46%) | 7 (64%) | 43 (67%) | 0·27 | 19 (66%) | 24 (69%) | 0·23 |
| Started or moved to care elsewhere before 12 months | 3 (23%) | 2 (18%) | 3 (5%) | .. | 2 (7%) | 1 (3%) | .. |
| Died | 2 (15%) | 0 | 9 (14%)* | .. | 6 (21%)* | 3 (9%) | .. |
| Lost to follow-up | 2 (15%) | 2 (18%) | 9 (14%) | .. | 2 (7%) | 7 (20%) | .. |
Data are n (%). POC=point of care. ART=antiretroviral therapy.
One infant in this group died at another site at age 99 days shortly after starting ART during a hospitalisation having not returned to site to start ART after birth; one infant died on site at age 286 days before ART start having been lost to follow-up after birth.
Twelve months after ART start, the percentage virally suppressed <400 copies per mL among 56 infants who were alive, in care at RMMCH and had an available measurement was 71·3% (40/56). These proportions did not differ significantly across the three eras, but was highest (78·6%) in Era 3 (Table 4). Stratifying the latter era into those not co-tested vs. co-tested by POC testing, the percentage suppressed <400 copies per mL at twelve months was 68·4% vs. 87·0% in those not co-tested vs. co-tested by POC respectively (p=0·18).
Table 4:
HIV viral load of the surviving 72 neonates with confirmed HIV infection at 6 and 12 months after antiretroviral therapy initiation on site
| Era 1: targeted testing (n=10) | Era 2: universal testing (n=9) | Era 3: universal testing, POC testing available (n=53) | p value | Era 3: universal testing, not POC co-tested (n=21) | Era 3: universal testing, POC co-tested (n=32) | p value | |
|---|---|---|---|---|---|---|---|
| Measurement closest to 6 months (copies per mL) | |||||||
| <400 | 4/8 (50%) | 1/7 (14%) | 27/47 (57%) | 0·31 | 10/21 (48%) | 17/26 (65%) | 0·35 |
| 400-1000 | 1/8 (13%) | 2/7 (29%) | 5/47 (11%) | .. | 2/21 (10%) | 3/26 (12%) | .. |
| ≥1000 | 3/8 (38%) | 4/7 (57%) | 15/47 (32%) | .. | 9/21 (43%) | 6/26 (23%) | .. |
| Measurement closest to 12 months (copies per mL) | |||||||
| <400 | 4/7 (57%) | 3/7 (43%) | 33/42 (79%) | 0·23 | 13/19 (68%) | 20/23 (87%) | 0·27 |
| 400–1000 | 0/7 | 0/7 | 1/42 (2%) | .. | 1/19 (5%) | 0/23 | .. |
| ≥1000 | 3/7 (43%) | 4/7 (57%) | 8/42 (19%) | .. | 5/19 (26%) | 3/23 (13%) | .. |
Data are n/N (%). Denominators are number of infants for whom viral load results were available. POC=point of care.
Discussion
Between September 2013 and June 2016, we strengthened the EID programme at one large hospital in Johannesburg, South Africa; first by adding birth testing to high risk HIV-exposed neonates only, secondly by expanding the birth testing to all HIV-exposed neonates and thirdly, by introducing POC testing in addition to routine laboratory-based diagnostic testing. POC EID permitted earlier ART initiation in infants confirmed to be infected. However, even though mortality rates by 12 months for infants with POC EID and early ART were (non-significantly) lower than if only non-POC EID used, the 9% mortality by 12 months remains high. Retention in care in the POC EID-identified early-treated infants was no worse (68.6%) than among the non-POC EID identified infants (65.5%) during the same era. These results highlight that the advantages of the resource-intensive efforts for very early diagnosis and treatment are substantially limited by infants who are lost - many of whom likely died.
Over the full cohort of 88 HIV-infected infants, the probability of mortality by 12 months was 14% with a median age at death of 73 days. Some deaths, not known to us, may still have occurred in infants who became lost to follow-up. We excluded infants with unconfirmed status with two cases of very early death both presumably due to prematurity. The non-significantly lower rate of mortality in those co-tested by POC may be explained by differential selection of healthier children in the POC co-tested group. We previously reported that healthier infants (higher birth weight, fewer preterm, fewer neonatal admissions) were more likely to be co-tested with POC.9 This was due to the logistics of screening the HIV-exposed births at the hospital making the sicker newborns more difficult to access and their fragile health condition often precluded additional laboratory testing. Comparison of mortality rates with other cohorts is complicated since our cohort is likely comprising only of in utero infected infants shown in some studies to have higher mortality rates.11–13 Other cohorts including only those testing at later ages may underestimate mortality as infected infants may have died prior to inclusion in those cohorts. Our mortality rate was higher than the 4% mortality rate reported in CHER.2 However CHER included only those infants who did not meet clinical and immunological criteria to initiate ART in place at the time and therefore were likely to be healthier. Our mortality rate is similar to the mortality rate of 14% we observed in a prior study at the site of young children initiating ART under 2 years of age,14 in a large combined analysis of South African cohorts of infants initiating ART at a median of six months,15 and in a small cohort of neonates in Cape Town.16 It is lower than the mortality rate of 23·5% that we observed in a previous surveillance study at several sites in Johannesburg that we conducted in 2011 among newly-identified HIV-infected infants.17 Our study shows that earlier diagnosis and treatment is not sufficient to prevent all mortality in HIV-infected children. Mortality was still found to peak early consistent with pre-ART literature.1
We observed attrition at every step in the HIV care cascade reducing the net benefit of early diagnosis. There were many contributing factors. It needs to be recognised that these HIV-infected neonates were identified in the context of a <1·7% intrauterine transmission rate. PMTCT coverage is very high in this region. Thus there is a clustering of social and biological factors contributing to poor outcomes in HIV-infected neonates. For example, no or late access to antenatal care and inadequate maternal ART adherence contribute to increased risk of HIV transmission and are the same factors that contribute to challenges sustaining ART for the infant.18–20 Attrition prior to ART start was greater in those not co-tested with POC but attrition after ART start was somewhat greater in those co-tested with POC. This resulted in a net similarity in the overall rates of retention regardless of access to POC. The reasons for these differences are unclear. The short timeframe between testing and treatment initiation may leave families less well prepared for initiating and maintaining lifelong ART for their infants. It is also possible that this very early test and treat approach may simply be shifting the timing of loss to follow-up to a later point along the cascade. We previously showed that coverage of birth testing was high >90% but that program factors including weekend/holiday cover and availability of phlebotomists before the time of discharge of mother and child limited our ability to reach all infants with initial diagnosis.8 These were not related to follow-up of infected infants and result return after the diagnosis where maternal psycho-social factors seemed to play a larger role. The same complex factors that lead to loss between testing and receipt of results or ART initiation are likely to still be at play and contribute to high rates of loss post-ART initiation. We also hypothesize that the subgroup initiating ART in the non-POC group may be a selection of more adherent families who had voluntarily returned to site. Many of the individual and structural factors preventing families from staying in care with no POC continue to impact retention and adherence with POC. In addition, inadequate time for counselling in the immediate post-partum period may also contribute. Strategies to improve retention in care are urgently needed.
The relatively low rate (71%) of viral suppression among infants treated and retained through 12 months likely speaks to suboptimal ART regimens/formulations for infants as well as caregiver difficulties in maintain high levels of adherence to twice daily administration of multiple drugs often in poorly palatable formulations. Rates of preterm birth and low birth weight even in the most recent era remained substantial, highlighting the importance of improving treatment options preterm and low birth weight neonates for whom drug dosing/disposition/safety may be different than for full term and average weight neonates.21 The relatively low rates of viral suppression occurred despite unexpectedly low pre-ART viral loads consistent with recent South African findings of declining baseline viraemia within the EID programme.22 High pre-ART viral load was strongly-related to mortality. Although this relationship has been described before early ART was available, it seems to persist even with very early ART and this requires further study taking into account other possible associations in order to reduce mortality more effectively.13,23,24
Our study has several limitations. Although we screened 5,449 HIV-exposed newborns we identified only 88 confirmed HIV-infected neonates; thus numbers are small and confidence intervals wide for all estimates. Our findings are further limited by the study taking place at a single relatively well-resourced site. This may limit generalisability. Standard laboratory-based HIV PCR testing turn-around time in our setting is two to three days which is more rapid than in many other settings. Although mothers and neonates are generally discharged by this time even at our site, we put in place active out-reach to trace those with positive results (telephonically and by home visit where needed). We may therefore have biased the study against POC EID. POC tests have the advantage of yielding results before discharge avoiding delays in disclosure and ART initiation inherent in EID systems that rely on return visits. Given our ability and experience of actively tracing families not co-tested with POC we are concerned that less-resourced settings, when reliant on routine laboratory-based EID, may experience worse outcomes if only passive systems to engage families in care are in place. Our estimates of retention in care on site may underestimate overall retention in care since at least some of the infants were formally transferred out to other services but data on retention in care, loss to follow-up and mortality at these other sites was difficult to obtain. There are several factors (maternal and child viral load interaction and effect on outcomes, adherence, social circumstances, co-morbidities) possibly related to the outcomes we include here but larger numbers are required in order to investigate independent associations with confidence.
In the light of these findings, attention needs to be given to how to improve outcomes in this high risk population of HIV-infected neonates. Sustained maternal adherence with infant ART is well-known to be extremely taxing. Support of mothers to provide ART for their infants must be linked with support for the mother for her own treatment and wellness. Adequate maternal counselling is essential and needs to include clear explanation about EID and infant ART starting during antenatal care and continuing through each of the steps in the care cascade. Because of lack of other alternatives, we used nevirapine as part of the ART regimen in the first few weeks. However, all of these infants had also been given nevirapine as prophylaxis prior to ART initiation and a high proportion had been exposed to maternal antiretroviral drugs usually including efavirenz during pregnancy. Single-point mutations confer resistance to nevirapine and are known to be selected in almost all exposed even to a single dose of this drug.25,26 Infants were transitioned to lopinavir/ritonavir at 42 weeks post menstrual age but this drug is known to present significant adherence challenges due to poor palatability. Better formulations of antiretroviral drugs with better tolerability and simplified dosing for this age group is an urgent research priority.16 The recent approval of raltegravir for use during the neonatal period brings hope that better regimens can be constructed.27,28 Our high rates of attrition are related to a highly mobile population and a fragmented health service. Improved patient-held records may help mothers to continue care elsewhere should the need arise and assist providers at different sites to coordinate care.
In conclusion, HIV-infected infants can be identified at birth and initiated on ART within hours or days. However, they are a challenging population to retain in care and we observed a stubbornly high rate of mortality and loss to follow-up and sub-optimal viral suppression. Although testing at birth can lead to earlier treatment, more research is needed on strategies to reduce poor outcomes and enhance viral suppression. We could not demonstrate that POC testing improved retention in care or viral suppression at twelve months. Significant efforts beyond ART initiation are required and should include adherence support, monitoring of maternal and infant health and better social services. Mothers require support with acceptance of results, family mobility and transfer to other health facilities.
Supplementary Material
Research in Context Panel:
Evidence before this study:
We searched PubMed and conference proceedings using the search terms “HIV”, “ART” and “early infant treatment” for English language articles published up to March 30, 2018. HIV infection in infants carries high mortality in the absence of suppressive antiretroviral treatment (ART). Early infant diagnosis has evolved to include diagnosis at birth which enables identification of infants at birth who are presumed to have acquired infection intrauterine and are thought to be at risk of poorer outcomes.
Added value of this study:
This study presents a large cohort of HIV-infected infants identified at birth at a single site in the context of high maternal ART coverage and a maturing PMTCT program. Prior cohorts have generally described HIV-infected infants identified later which inevitably excludes early mortality. Improvement of EID services and judicious use of point of care (POC) technology enables very early access to ART but ART initiation in the absence of POC is also achievable within days of birth. Mortality, retention and suppression require specific attention particularly in infants with high pre-treatment viral loads.
Implications of all the available evidence
ART can be initiated very early on in life but reduction of mortality and long term retention in care and viral suppression remains challenging. This is likely due to both biological and psycho-social factors including health seeking behaviour characteristics of these mothers and infants. POC technology and improvements in EID enable earlier initiation of ART but further efforts are needed to strengthen long term success.
Acknowledgements
We gratefully acknowledge the infants and families who participated in the study as well as the hard working study team. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (U01HD080441), USAID/PEPfAR and the South African National HIV Programme. Cepheid provided cartridges at discounted prices and loan of machines and technical support.
Funding:
Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health, USAID/PEPfAR and the South African National HIV Programme.
Footnotes
Declaration of interests
We declare no competing interests.
Ethics Approval
The Human Research Ethics Committee of the University of the Witwatersrand and the Institutional Review Board of Columbia University approved the study.
References
- 1.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. International journal of epidemiology 2011;40:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. The New England journal of medicine 2008;359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF Data: Monitoring the Situation of Children and Women. UNICEF, 2018. (Accessed 05/04/2018, at https://data.unicef.org/topic/hivaids/global-regional-trends/.) [Google Scholar]
- 4.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. Journal of acquired immune deficiency syndromes 2011;57:e40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC medicine 2011;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bulletin of the World Health Organization 2013;91:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. The Pediatric infectious disease journal 2013;32:1080–5. [DOI] [PubMed] [Google Scholar]
- 8.Technau KG, Kuhn L, Coovadia A, Carmona S, Sherman G. Improving early identification of HIV-infected neonates with birth PCR testing in a large urban hospital in Johannesburg, South Africa: successes and challenges. Journal of the International AIDS Society 2017;20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Technau KG, Kuhn L, Coovadia A, Murnane PM, Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. The lancet HIV 2017;4:e442–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Technau KG, Mazanderani AH, Kuhn L, et al. Prevalence and outcomes of HIV-1 diagnostic challenges during universal birth testing - an urban South African observational cohort. Journal of the International AIDS Society 2017;20:21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouet F, Sakarovitch C, Msellati P, et al. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics 2003;112:e289. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh K, Shevitz A, Zaknun D, et al. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. The Pediatric infectious disease journal 1996;15:1087–91. [DOI] [PubMed] [Google Scholar]
- 13.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. The New England journal of medicine 1997;336:1337–42. [DOI] [PubMed] [Google Scholar]
- 14.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. The Journal of infectious diseases 2010;201:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter M, Davies MA, Mapani MK, et al. Outcomes of Infants Starting Antiretroviral Therapy in Southern Africa, 2004-2012. Journal of acquired immune deficiency syndromes 2015;69:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigati L, Wynberg E, Maritz J, Holgate S, Cotton MF, Rabie H. Antiretroviral Treatment Initiated in the First Month of Life. The Pediatric infectious disease journal 2017;36:584–7. [DOI] [PubMed] [Google Scholar]
- 17.Abrams EJ, Woldesenbet S, Soares Silva J, et al. Despite Access to Antiretrovirals for Prevention and Treatment, High Rates of Mortality Persist Among HIV-infected Infants and Young Children. The Pediatric infectious disease journal 2017;36:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltzer K, Mlambo G. Factors determining HIV viral testing of infants in the context of mother-to-child transmission. Acta paediatrica 2010;99:590–6. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein L, Edmonds A, Okitolonda V, et al. Implementation and Operational Research: Maternal Combination Antiretroviral Therapy Is Associated With Improved Retention of HIV-Exposed Infants in Kinshasa, Democratic Republic of Congo. Journal of acquired immune deficiency syndromes 2015;69:e93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chetty T, Knight S, Giddy J, Crankshaw TL, Butler LM, Newell ML. A retrospective study of Human Immunodeficiency Virus transmission, mortality and loss to follow-up among infants in the first 18 months of life in a prevention of mother-to-child transmission programme in an urban hospital in KwaZulu-Natal, South Africa. BMC pediatrics 2012;12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotton MF, Holgate S, Nelson A, Rabie H, Wedderburn C, Mirochnick M. The last and first frontier--emerging challenges for HIV treatment and prevention in the first week of life with emphasis on premature and low birth weight infants. Journal of the International AIDS Society 2015;18:20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazanderani AH, Moyo F, Kufa T, Sherman GG. Brief Report: Declining Baseline Viremia and Escalating Discordant HIV-1 Confirmatory Results Within South Africa’s Early Infant Diagnosis Program, 2010-2016. Journal of acquired immune deficiency syndromes 2018;77:212–6. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. Jama 1998;279:756–61. [DOI] [PubMed] [Google Scholar]
- 24.Abrams EJ, Weedon J, Steketee RW, et al. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. New York City Perinatal HIV Transmission Collaborative Study Group. The Journal of infectious diseases 1998;178:101–8. [DOI] [PubMed] [Google Scholar]
- 25.Antunes F, Zindoga P, Gomes P, et al. Development of Nevirapine Resistance in Children Exposed to the Prevention of Mother-to-Child HIV-1 Transmission Programme in Maputo, Mozambique. PloS one 2015;10:e0131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn L, Hunt G, Technau KG, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. Aids 2014;28:1673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachman S, Alvero C, Acosta EP, et al. Pharmacokinetics and 48-Week Safety and Efficacy of Raltegravir for Oral Suspension in Human Immunodeficiency Virus Type-1-Infected Children 4 Weeks to 2 Years of Age. Journal of the Pediatric Infectious Diseases Society 2015;4:e76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV infection: the potential for cure. Nature reviews Immunology 2016;16:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.