Abstract
Objectives:
Symptoms of benign prostatic hyperplasia (BPH) increasingly affect men as they age. Minimally invasive therapies for the treatment of BPH continue to evolve. We describe histotripsy, a non-invasive, non-thermal focused ultrasound technology for precise tissue ablation, and report initial results of using histotripsy for prostate tissue ablation in an in-vivo canine model.
Methods:
An annular 18-element, 750-kHz phased array ultrasound system delivered high intensity (22 kW/cm2), ultrasound pulses (15 cycles = 20 microsec) at pulse repetition frequencies of 100-500 Hz to canine prostates. Eight lateral lobe and 9 periurethral treatments were performed in 11 anesthetized dogs. Diagnostic ultrasound transducers provided in-line and transrectal imaging. Retrograde urethrograms (RUG) were performed before and after periurethral treatments. Following treatment, prostates were grossly examined, sectioned, and submitted for histology.
Results:
In lateral lobe treatments, a well-demarcated cavity containing liquefied material was present at the ablation site. Microscopically, the targeted volume was characterized by the presence of histotripsy paste (debris, absent cellular structure). A narrow margin of cellular injury was noted, beyond which there was no apparent tissue damage. Periurethral treatments resulted in total urethral ablation or significant urethral wall damage, with visible prostatic urethral defects on RUG. Real-time ultrasound imaging demonstrated a dynamic hyperechoic zone at the focus, indicative of cavitation and suggesting effective tissue ablation.
Conclusions:
Histotripsy is capable of precise prostate tissue destruction and results in subcellular fractionation of prostate parenchyma. Histotripsy can also produce prostatic urethral damage and thereby facilitate drainage of finely fractionated material per urethra, producing immediate debulking.
Introduction
The prevalence of histologic BPH increases from approximately 50% of the male population in their 50’s to greater than 80% of those in their 80’s [1, 2]. Based on the AUA symptom index, moderate to severe LUTS were found to occur in 33%, 41%, and 46% of men in their 50s, 60s, and 70s, respectively in the Olmsted County Study [3]. This high prevalence of BPH related symptoms coupled with associated deterioration in quality of life resulted in 4.5 million physician visits for a primary diagnosis of BPH among men in the United States in the year 2000 [4].
Current therapy for BPH is lacking in that transurethral resection of the prostate (TURP), albeit an effective therapy, is an invasive operative procedure with associated surgical risks. Pharmacologic management and minimally invasive treatments, although widely employed, are uniformly inferior to TURP [5]. Although some patients with mild LUTS may derive durable benefit from these therapies, many patients will eventually fail and require definitive tissue debulking therapy when they are older, have increased comorbidity, and greater surgical risk [4,6].
There is a substantial need for a less invasive, less morbid procedure for prostate tissue debulking that can produce equivalent results to TURP. Histotripsy is a promising extracorporeal ultrasound technology that produces nonthermal tissue fractionation by cavitational means (converting the targeted tissue into an acellular liquid). Cavitation occurs when intense ultrasound pulses induce extreme pressure changes within the targeted tissue resulting in the formation of a localized, highly dynamic cluster of microbubbles. These bubbles oscillate and violently collapse producing localized stresses that mechanically fractionate and subdivide the targeted tissue, resulting in cellular destruction [7-14].
Histotripsy, therefore, may be more suitable for tissue debulking in the setting of BPH than existing minimally invasive techniques that produce thermal coagulative necrosis. Our objective was to investigate the feasibility of histotripsy as a potential therapy for BPH using an in vivo canine prostate model. Specifically, we aimed to nonthermally ablate prostate tissue and to investigate the effects of histotripsy on the prostatic urethra.
Materials and Methods
Following institutional animal care committee approval, 11 dogs weighing 25-35 kg were treated. Each dog was pre-anesthetized with Acepromazine (0.1 mg/kg, 3 mg max dose), catheterized intravenously in both forearms (cephalic vein), administered Thiopental for anesthesia induction (3.5-5.5 mg/kg IV) and intubated. Digital disempaction and a warm soap and tap water enema were then performed. The suprapubic and abdominal regions were shaved and a depilatory cream was applied. Full anesthesia was maintained with inhaled 1-2% isoflurane and each animal was positioned supine and monitored with a pulse-oxymeter and ECG.
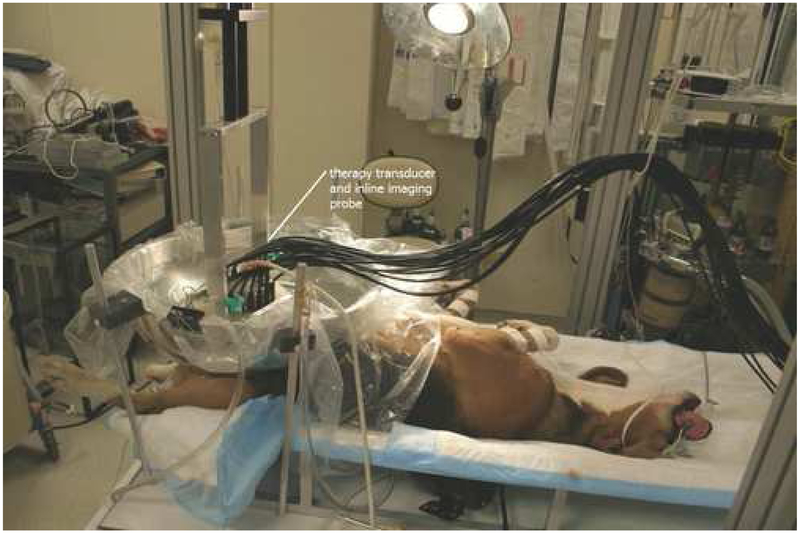
The therapeutic ultrasound unit consists of an 18 element piezo-composite phased array transducer (750kHz, 145mm diameter, and 100 mm focal length) (Imasonic, Besancon, France). A commercial diagnostic 5 MHz imaging probe (General Electric Medical Systems, Milwaukee, Wisconsin) aligned through the center of the phased array provided in-line real-time images during treatment (Figure 1). The circular opening in the bottom of a large metal bowl was centered over the targeted zone. The bowl was lined with clear plastic and filled with degassed, de-ionized water to provide acoustic coupling between the therapy transducer and targeted prostate tissue. Localization of the therapeutic focal zone was achieved by marking the location of the hyperechoic zone seen on the B-scan ultrasound image following a single 15 cycle pulse from the therapeutic transducer at 10 Hz pulse repetition frequency. Transrectal ultrasound was performed to visualize the prostate and surrounding pelvic anatomy.
Figure 1.
Picture of experimental setup. A diagnostic 5 MHz imaging probe was aligned through the center of the phased therapeutic. A circular opening in the bottom of the metal bowl was centered over the targeted zone. The bowl was lined with clear plastic and filled with degassed, de-ionized water.
Short pulses (7-40 microseconds) of ultrasound energy were delivered at pulse repetition frequencies of 100-500 Hz, resulting in duty cycles from 0.067% to 0.4%. Table 1 summarizes the treatment parameters. Preliminary experiments consisted of 8 treatments in the lateral prostatic lobes of 4 dogs. Subsequently, 9 periurethral ablations were performed in a total of seven dogs. Transrectal and transabdominal real time ultrasound images were used to localize the target area and to monitor treatment progress. A retrograde urethrogram (RUG) was performed through a 5 French urethral catheter before and after histotripsy treatment.
Table 1.
Summary of treatment parameters.
| lesion # | Power (V) |
Duty cycle (%) |
cycles/pulse | PRF (Hz) |
|---|---|---|---|---|
| Lateral lobe | ||||
| 1 | 200 | 0.2 | 15 | 100 |
| 2 | 150 | 0.2 | 15 | 100 |
| 3 | 200 | 0.13 | 10 | 100 |
| 4 | 150 | 0.067 | 5 | 100 |
| 5 | 150 | 0.067 | 5 | 100 |
| 6 | 150 | 0.4 | 30 | 100 |
| 7 | 150 | 0.4 | 30 | 100 |
| 8 | 150 | 0.4 | 30 | 100 |
| Periurethral | ||||
| 9 | 180 | 0.33 | 5 | 500 |
| 10 | 180 | 0.33 | 5 | 500 |
| 11 | 180 | 0.33 | 5 | 500 |
| 12 | 180 | 0.33 | 5 | 500 |
| 13 | 200 | 0.4 | 10 | 300 |
| 14 | 200 | 0.4 | 10 | 300 |
| 15 | 200 | 0.4 | 10 | 300 |
| 16 | 200 | 0.4 | 10 | 300 |
| 17 | 200 | 0.4 | 30 | 100 |
Following treatment, the animals were euthanized, periprostatic tissues were examined for changes, and the prostate was removed acutely. The prostate was fixed in formalin and processed with hematoxylin and eosin or Masson’s trichrome stains.
Results
Histotripsy was successfully performed in 8 lateral lobar areas and 9 periurethral areas. An immediate and transient hyperechoic region on real-time ultrasound imaging was observed during treatment at the marked focal volume. Immediately following treatment, a hypoechoic area was noted on real-time ultrasound images of the exposed area. In several of the periurethral ablations, ultrasound images after treatment revealed incomplete transition to a hypoechoic lesion in the expected location of urethral tissue.
Examination of prostates following ablation revealed only minimal surrounding hematoma in a few cases confined by the prostatic capsule. Superficial tissues in the path of the therapeutic ultrasound energy (skin, abdominal wall, periprostatic adipose) were undamaged. Periprostatic tissues were undamaged in all but one subject where a perforation in the right posterior-lateral capsule of the prostate was noted. Only mild submucosal ecchymosis was present in the opened bladders of all subjects and no intravesical blood clots were observed. No rectal injuries were seen.
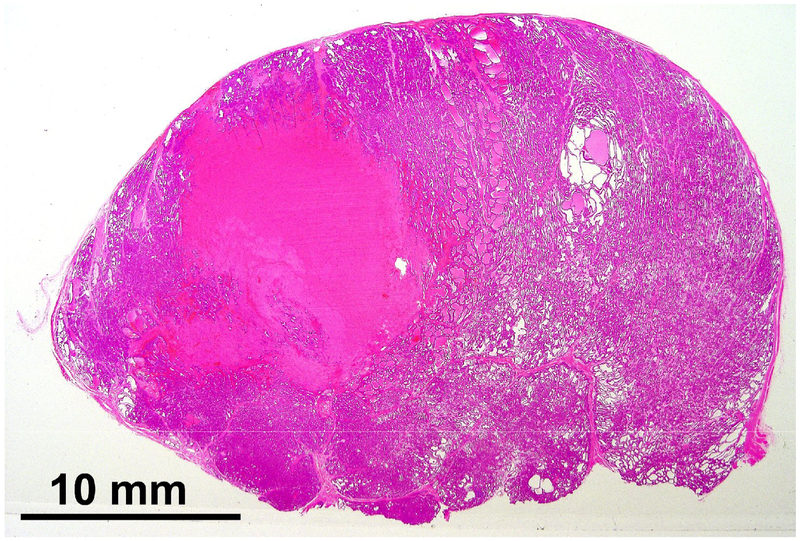
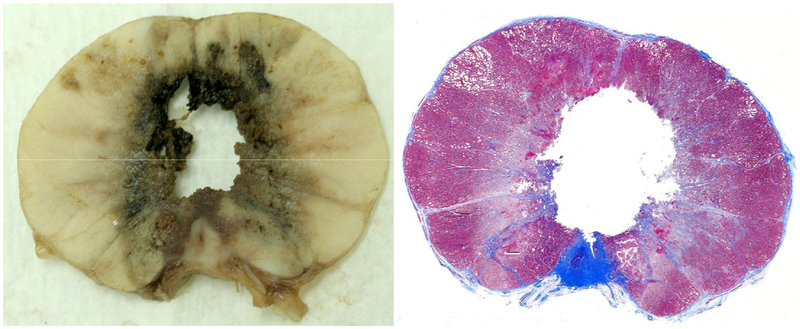
Upon sectioning prostates where ablation was performed in the lateral lobes, a, finely fractionated paste was observed in the targeted volume. On histologic analysis the paste corresponded to areas of acellular debris consistent with cytoplasm and homogenized cellular material (Figure 2). A sharp border between exposed and unexposed tissue was a consistent finding. In prostates where the prostatic urethra and periurethral parenchyma was targeted an acellular histotripsy paste was present in some specimens and absent in others, and likely was washed away by RUG. In 3 of the 7 periurethral treatment subjects urethral architecture was completely disrupted (Figure 3). Histologic sections of these treatments showed absence of fibrous or muscular urethral structure on at least one entire side of the urethra. In one treatment all urethral structure was completely absent. One subject showed no significant damage, and the remaining 3 showed less complete but still significant urethral wall damage with communication between the treatment zone and the urethral lumen.
Figure 2.
Hematoxylin and eosin staining of a lateral prostatic lesion. The homogenous pink area devoid of cellular structure corresponds to the treated volume.
Figure 3.
Gross pathology and Masson’s trichrome staining of a periurethral treatment demonstrating complete ablation of the urethra and periurethral tissue.
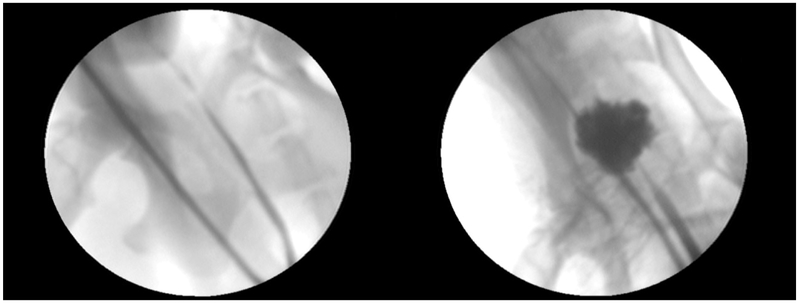
Post-treatment RUG was compared to pretreatment RUG in 6 subjects (8 treatments targeting the prostatic urethra and periurethral parenchyma). A large prostatic defect, was present in 4 of these subjects (Figure 4). No defect was noted on post-treatment RUG in 2 subjects. One post-treatment RUG demonstrated extravasation of contrast into the canine pelvis. Catheter drainage of the urinary bladder following treatment revealed only clinically insignificant hematuria.
Figure 4.
Pre-treatment (left) and Post-treatment (right) retrograde urethrogram images. Following histotripsy, contrast outlines a cavity corresponding to the treated volume of prostate tissue.
Discussion
Current treatments for BPH include open prostatectomy, transurethral removal of prostate tissue (TURP, HOLEP, PVP), minimally invasive thermotherapies (TUNA, TUMT, interstitial laser), and pharmacologic management (currently the only true non-invasive treatment). The gold standard of therapy is TURP in which periurethral prostate tissue is mechanically removed and immediate tissue debulking results. TURP requires anesthesia, and carries risks of urinary retention (6.5%), acute blood loss anemia requiring transfusion (3.9%), and a mortality rate in modern series of 0.2-1.5% [15,16]. Less invasive therapies for BPH such as transurethral needle ablation (TUNA) and transurethral microwave therapy (TUMT), are both methods of energy delivery that result in thermal ablation and induction of coagulative necrosis. No immediate tissue debulking is accomplished, rather treated prostate tissue remains in situ and undergoes a variable degree of sloughing or resorption over time. These minimally invasive modalities are less morbid than TURP and are most useful for select patients with favorable prostate anatomy. In general, they provide a lesser degree of relief, with higher urinary retention rates and a significant chance of requiring further treatment [5].
Ablation of prostate tissue by histotripsy was readily accomplished at various treatment settings in the preliminary work on the lateral prostatic lobes. These ablations revealed several unique qualities of histotripsy. Due to the mechanical mechanism of tissue destruction, histotripsy produces a pattern of ablation distinct from coagulative necrosis seen with thermal ablative modalities. Typical tissue response to this mechanical ablation reveals the presence of a distinctive, liquefied, acellular paste, and sharp borders between treated and untreated tissue.
Treatment of urethral and periurethral tissue gave mixed results. Some treatments were very successful, resulting in complete ablation of urethra and periurethral tissue. In some cases the histotripsy paste was present on histologic analysis. In other cases, this thin paste drained via catheter and/or urethra during treatment or was flushed out with post-procedure RUG and resulted in immediate debulking of periurethral prostatic tissue. The urethra was found to be a more resilient target than the prostatic glandular tissue. A differential treatment effect between tissue types has been observed previously in a kidney model [17], and perhaps offers some insight into the incomplete prostatic urethral ablation observed in several subjects. In addition, some treatments were not centered on the urethra and periurethral tissue, but rather were noted on both gross and histologic examination to be skewed laterally, posteriorly, or anteriorly in the gland. This is likely due to suboptimal imaging and targeting. While the transrectal imaging probe provided good visualization of the gland and its architecture, it was not directly linked to the therapeutic transducer. Targeting relied on the in-line probe with a 6cm offset from the treatment area. With this probe, visualization of the urethra was difficult, and centering a treatment grid on the urethra was sometimes inaccurate.
No damage to periprostatic tissues or surrounding organs was noted on gross inspection of the treated prostates. In one case, pelvic extravasation of contrast was observed on RUG following a treatment which produced a lesion adjacent to the posterior prostatic capsule. No extravasation was noted initially on post-treatment retrograde images, but became apparent after successive pressure increases during injection of contrast.
Although initial results appear promising, several potential issues warrant further investigation, specifically: collateral damage to surrounding tissues assessed in the chronic setting, bleeding, urinary retention, erectile dysfunction, and possible effects histotripsy treatment may have on an occult prostate cancer.
Conclusions
This initial study demonstrates the feasibility of non-thermal histotripsy ablation of prostate tissue. Furthermore, histotripsy is noninvasive, in this case being performed transabdominally from an extracorporeal source. Real-time feedback during treatment allows adjustment of parameters during treatment to optimize results. The margin between treated, fractionated tissue and healthy tissue is exceedingly sharp. The liquid consistency of the fractionated, treated material facilitates drainage and results in immediate prostate debulking. As such histotripsy is a potential and promising noninvasive therapy for BPH.
Acknowledgments
This research was supported in part by NIH P50 CA069568 and a grant from the Wallace H. Coulter Foundation. Equipment support was provided by General Electric (GE) Healthcare.
Footnotes
No disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 132: 474–9, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Roehrborn CG, McConnell JD: Campbell-Walsh Urology, 9th ed, Philadelphia, Saunders Company, p 2739–42. [Google Scholar]
- 3.Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, et al. The prevalence of prostatism: A population-based survey of urinary symptoms. J Urol 150: 85–9, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol 173, 1256–61, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Tunuguntla HS, Evans CP. Minimally invasive therapies for benign prostatic hyperplasia. World J Urol 20(4):197–206, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Elhilali MM: Minimally invasive therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia, J Urol 177: 820–1, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Fry FJ, Kossoff G, Eggleton RC, Dunn F: Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am 6: 1413–7, 1970. [DOI] [PubMed] [Google Scholar]
- 8.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control 50: 1296, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JM, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelctr Freq Control 51: 726, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons JE, Cain CA, Fowlkes JB. Characterizing pulsed ultrasound therapy for production of cavitationally-induced lesions In: 4th International Symposium on Therapeutic Ultrasound, Kyoto, Japan, 18-20 Sept 2004. Berlin: Springer-Verlag, p. 178, 2004. [Google Scholar]
- 11.Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Accoust Soc Am 117: 424, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts WW, Hall TL, Ives K, Wolf JS Jr, Fowlkes JB, Cain CA. Pulsed Cavitational Ultrasound: A noninvasive Technology for Controlled Tissue Ablation (Histotripsy) in the Rabbit Kidney. J Urol 175: 734–38, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Kieran K, Hall TL, Parson JE, Wolf JS, Fowlkes JB, Cain CA, et al. Refining Histotripsy: Defining the parameter space for the creation of non-thermal lesions with high intensity pulsed ultrasound in the in vitro kidney. J Urol 178: 672–6, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hall TL, Kieran K, Ives K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy of rabbit renal tissue in vivo: Temporal histologic trends. J Endourol 21: 1159–66, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Roos NP, Wennberg JE, Malenka DJ, et al. Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N Engl J Med 320: 1120–3, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Mebust WK, Holtgrewe HL, Cockett AT, and Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol 141(2):243–7, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Lake AL, Xu Z, Wilkinson JE, Cain CA, Roberts WW. Renal Ablation by Histotripsy: Does it Spare Collecting System? J Urol March 2008. (accepted for publication) [DOI] [PubMed] [Google Scholar]