Clinical History and Examination
A 34‐year‐old female presented with severe parkinsonism. She had axial and asymmetrical appendicular rigidity as well as spasticity of the lower limbs. Tonic‐clonic seizures started at 18 months of age after bacterial meningoencephalitis was diagnosed, and she was treated with antibiotics and antiepileptics until her last seizure at age 7.5 years. Speech and language development was markedly delayed, and she was diagnosed with severe mental disability of unknown etiology. Her motor function improved over time, and she could eat and dress independently by age 21 years. However, behavioral problems, personality changes, and psychotic episodes at age 21 years necessitated antipsychotic treatment with typical antipsychotic medications. She later experienced recurrent outbursts of anger, followed by motor impairments, including toe walking, postural rigidity, freezing of gait, falls, and mild tremor. Drug‐induced parkinsonism was suspected and the antipsychotics were stopped with the addition of baclofen and procyclidine, resulting in improvement of motor function. The outbursts of anger reappeared six months later and her gait became spastic. Atypical antipsychotics were prescribed, baclofen was stopped, and procyclidine was slowly tapered down, causing further motor deterioration. Night agitation and insomnia appeared during the following year for which she received benzodiazepines, worsening the extrapyramidal symptoms. Withdrawal of the antipsychotics and benzodiazepines in 2014 resulted in motor improvement, but the cognitive and behavioral deterioration persisted. It was at this time, we saw her for the first time, and quetiapine was initiated.
Investigations
A brain computerized tomogram revealed generalized atrophy.1 The typical “eye‐of‐the‐tiger” sign was absent on brain MRI,2 but there were bilateral T2 basal ganglia hypointensities, mainly in the globus pallidus (Figure 1). T1 showed hyperintensity in the substantia nigra. Electroencephalographic recordings disclosed no epileptic signs. The blood smear was negative for acanthocytes and serum ferritin, ceruloplasmin, albumin, and lipoproteins were within reference values. The CPK level reached 2577 IU/L (normal = 60 to 174 IU/L).
Figure 1.
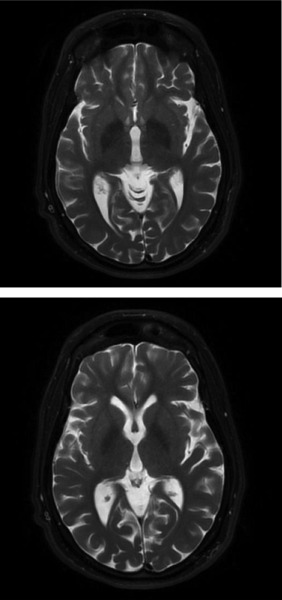
(Brain MRI) shows T2 basal ganglia hypointensities, mainly in the globus pallidus, bilaterally.
A preliminary diagnosis of NBIA was made. Analysis of the WDR45 gene revealed a novel, heterozygous deletion c.27dupG (hemi) p.10Aspfs*61, predicted to lead to the formation of a stop codon and protein truncation. The mutation was also found in the patient's asymptomatic mother.
The patient was started on low doses of levodopa/carbidopa, which resulted in a significant improvement of her gait and motor function (Video S1), although mild nondisabling dyskinesias appeared in the legs, arms, and face two months later. The patient is currently being treated with quetiapine, clonazepam, trazodone, procyclidine, and levodopa/carbidopa 125/12.5 mg three times daily. Despite the dyskinesias, the levodopa/carbidopa treatment resulted in a significant improvement in motor function.
Discussion
Beta‐propeller protein‐associated neurodegeneration (BPAN) is still in its early phase. It is characterized by global developmental delay, intellectual disability, and is frequently accompanied by early‐onset seizures, absent to limited expressive language, motor dysfunction (ataxia), and abnormal behaviors often similar to autism spectrum disorder. As seen in this patient, with age, seizures tend to resolve or become less prominent. In the second phase, usually starting in the second or third decade of life, cognitive deterioration and movement disorders (progressive parkinsonism and dystonia) emerge as characteristic findings. Treatment with levodopa/carbidopa may have a short lasting and/or partial beneficial effect on motor function.4, 5 Additional phenotypical features may be present, such as disordered sleep, high myopia, and incontinence.
With respect to behavioral characteristics, only anecdotal data are available that suggested aggressive behaviors in some patients.7 The neurological symptoms in here described highlights the interesting phenotype of a disease with a characteristic two‐phase course with, in its later stage, dystonia and marked cognitive deterioration. We would expect this patient to be wheelchair bound or have bedridden status by now, however after treatment with low dose levodopa/carbidopa, she has regained motor function. In addition (as expected), there were behavioral problems and challenging behaviors in the decades before motor deterioration, corresponding to the level of intellectual disability and dependent of contextual parameters.
Levodopa treatment had resulted in only partial and temporal improvement in previously described cases, and it was usually stopped after a few months due to debilitating dyskinesias.4, 5 In contrast, our patient is stable with relatively good motor function on levodopa treatment for the past 23 months.
An additional special feature of our case is that this is, to our knowledge, the second report of an asymptomatic WDR45 mutation carrier.3, 5, 6 In the other case, the mother was mosaic for the alteration, thus it is likely that this is also the case for our probands mother. Females in general could also present with milder phenotypes if they harbor a later somatic mutation, since the phenotype is determined at least in part by the stage of development at the time of mutation and by the tissue distribution of mutation‐containing cells. Since this is a previously unreported mutation, theoretically there is also a possibility that it has incomplete penetrance, but this is unlikely since the mutation occurs early in the transcript.
Our case may add unique phenotypical nuances to the rare but well‐defined syndrome of BPAN: good response to dopa with no exhausting dyskinesia and absence of the clinical features in the mother‐mutation carrier.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
J.Z.: 1A, 1B, 1C, 3A
N.G.: 1A, 3B
T.G.: 1B, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the approval of an institutional review board was not required for this work.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors have no disclosures to report.
Supporting information
Videos accompanying this article are available in the supporting information here.
Video S1. This video shows the patients gait before taking levodopa/carbidopa and how the gait improved after starting treatment.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kruer MC, Boddaert N, Schneider SA, et al. Neuroimaging features of neurodegeneration with brain iron accumulation. Am J Neuroradiol 2012;33:404e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayflick SJ, Westaway SK, Levinson B, et al. Genetic, clinical, and radiographic delineation of HallervordeneSpatz syndrome, N Engl J Med 2003;348:33e40. [DOI] [PubMed] [Google Scholar]
- 3. Hayflick S, Kruer MC, Gregory A, et al. Betapropeller protein‐associated neurodegeneration: a new X‐linked dominant disorder with brain iron accumulation. Brain 2013;136:1708e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haack TB, Hogarth P, Kruer MC, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X‐linked dominant form of NBIA. Am J Hum Genet 2012;91:1144e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath R. Brain iron takes off: a new propeller protein links neurodegeneration with autophagy. Brain 2013;136:1687e91. [DOI] [PubMed] [Google Scholar]
- 6. Zarate YA, Jones JA, Jones MA, et al. Lessons from a pair of siblings with BPAN. Eur J Hum Genet 2016;24(7):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saitsu H, Nishimura T, Muramatsu K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of children with neurodegeneration in adulthood. Nat Genet 2013;45:445e50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos accompanying this article are available in the supporting information here.
Video S1. This video shows the patients gait before taking levodopa/carbidopa and how the gait improved after starting treatment.


