Abstract
Understanding capsid assembly is important because of its role in virus lifecycles and in applications to drug discovery and nanomaterial development. Many virus capsids are icosahedral, and assembly is thought to occur by the sequential addition of capsid protein subunits to a nucleus, with the final step completing the icosahedron. Almost nothing is known about the final (completion) step because the techniques usually used to study capsid assembly lack the resolution. In this work, charge detection mass spectrometry (CDMS) has been used to track the assembly of the T = 4 hepatitis B virus (HBV) capsid in real time. The initial assembly reaction occurs rapidly, on the time scale expected from low resolution measurements. However, CDMS shows that many of the particles generated in this process are defective and overgrown, containing more than the 120 capsid protein dimers needed to form a perfect T = 4 icosahedron. The defective and overgrown capsids self-correct over time to the mass expected for a perfect T = 4 capsid. Thus, completion is a distinct phase in the assembly reaction. Capsid completion does not necessarily occur by inserting the last building block into an incomplete, but otherwise perfect icosahedron. The initial assembly reaction can be predominently imperfect, and completion involves the slow correction of the accumulated errors.
INTRODUCTION
A capsid, the protein shell that surrounds the genetic material of a virus, is usually assembled from many, often hundreds, of identical proteins. Understanding capsid assembly may yield targets for drug development and provide a way to manipulate assembly to control the capsid size and stability. Such control would be valuable for potential applications of capsids as containers and templates.1
About half of known virus families have icosahedral capsids. For hepatitis B virus (HBV), the 183-residue core protein (Cp183) forms icosahedral capsids both in vivo and in vitro. A truncated form of the core protein (Cp149) also spontaneously assembles.2 Icosahedral capsids are defined by a triangulation number (T), where 60T is the number of capsid proteins present.3 For the HBV capsid, the building block is the core protein dimer and the T = 4 capsid consists of 120 dimers. In vitro, dimer–dimer interactions can be strengthened by increasing the ionic strength. The increased interaction energy promotes assembly. However, higher salt concentrations can lead to kinetically trapped reactions.
The first step in the assembly reaction is thought to be formation of a nucleus.4–8 The capsid is then thought to grow mainly by sequential addition of subunits to the nucleus,9 and finally insertion of the last subunit completes the icosahedron. However, no experimental information is available on the final step. On the theoretical side, there have been a number of model studies of capsid assembly performed with rigid building blocks. It is generally accepted that the rate of subunit addition should decrease as the capsid approaches completion due to steric effects.8 In the model of Nguyen et al. the incomplete capsid is flexible, and inserting the final subunit makes it stiffer.6 Thus, the free energy change for the final step is unfavorable under some conditions and an incomplete capsid is the thermodynamically preferred product.
Virus assembly is usually monitored by bulk techniques such as size exclusion chromatography and light scattering.9–13 These techniques provide an ensemble average of the species present. Time resolved small-angle X-ray scattering can provide size and temporal information about assembly intermediates as can fluorescence correlation spectroscopy.14–17 Electron microscopy (EM) and cryo-EM provide information on single particles, but lack time resolution.18 Resistive pulse sensing has been used to monitor HBV assembly though analysis of single particles in solution.19 However, all of these techniques lack the resolution needed to determine whether the capsids are complete or missing a single subunit.
Mass spectrometry (MS) has the necessary resolution and it has recently emerged as a useful tool to investigate virus assembly.20–23 However, the loss of charge state resolution in the m/z (mass to charge ratio) spectrum measured in conventional MS has often frustrated these efforts. Although early (relatively small) intermediates in HBV capsid assembly, as well as intact capsids, have been characterized using high-resolution MS and ion mobility MS,21–23 large intermediates could not be identified due to their size and heterogeneity.
In this work we use charge detection mass spectrometry (CDMS) to monitor HBV assembly in real time. CDMS is a single particle technique where the m/z and charge are simultaneously measured for each ion, circumventing the need to resolve charge states in the m/z spectrum.24–32 Electrospray is the preferred ionization method because the solution is sampled directly. However, volatile buffers such as ammonium acetate must be used to avoid adduct formation and signal suppression. Using electrospray CDMS, we have previously identified high- mass intermediates in HBV Cp149 assembly.33 In that study, the assembly reactions were performed in a concentrated sodium chloride solution where intermediates were kinetically trapped. A high salt concentration led to the formation of a large number of nuclei at the beginning of the assembly reaction, leaving too few dimers to complete them. The products and trapped intermediates were then dialyzed into ammonium acetate for analysis. The trapped intermediates identified in that work were incomplete capsids. Here, we perform HBV Cp149 assembly reactions in ammonium acetate under milder assembly conditions, so that kinetic trapping and the dialysis step are avoided and the reactions can be tracked in real time.
The protein–protein interactions between Cp149 dimers are identical to those between dimers of the full length protein (Cp183). Cp149 lacks the C-terminal RNA-binding domain so it only assembles empty capsids. Understanding the assembly of empty capsids is a necessary first step to understanding assembly with RNA. In addition, assembly of empty HBV particles is directly relevant to the virus lifecycle because around 90% of the virus particles generated in vivo are empty.34 The role of the empty particles, beyond the neutralization of antibodies, remains unclear.35 Empty particles are also abundant for some other viruses such as herpes viruses and picornaviruses (e.g., polio).
RESULTS
A series of light scattering and SEC experiments were performed to characterize HBV assembly thermodynamics and kinetics in ammonium acetate. Figure 1A shows a plot of the scattered light intensity against time for the assembly of 5 µM dimer in ammonium acetate with concentrations ranging from 200 mM to 1 M. The assembly reactions are faster at a higher salt concentration. The results are similar to those obtained previously for HBV assembly in sodium chloride where it was concluded that higher salt concentration increases the strength of dimer–dimer interactions. This contributes to making assembly reactions faster by decreasing intermediate dissociation.36 For a given salt concentration, assembly in ammonium acetate is faster than in sodium chloride.
Figure 1.
Ensemble and CDMS measurements provide a consistent view of capsid assembly. (A) Plot of the intensity of the light scattered by the capsid against time for the assembly of 5 µM dimer in ammonium acetate concentrations ranging from 200 mM to 1M. (B) Size exclusion chromatography (SEC) measurements for the assembly of 5 to 25 µM dimer in 210 mM ammonium acetate. The SEC measurements were performed 24 h after initiation of the assembly reaction. Dimer assigned to the free dimer pool (red squares) and dimer in capsid (blue circles) are plotted against the total dimer concentration. The lines show an equilibrium-based fit used to determine the pseudocritical concentration. (C) CDMS spectrum measured 24 h after initiating assembly with a dimer concentration of 5 µM in 210 mM ammonium acetate. The spectrum was generated by sorting the masses into 20 kDa bins. The inset shows an expanded view of the low mass region generated with 5 kDa bins.
Figure 1B shows the results of SEC measurements performed 24 h after initiation of the assembly reactions (i.e., ostensibly after equilibrium had been established). There are two peaks in the chromatograms which are attributed to dimer and capsid. The amount of dimer in the free dimer-form (red points) and capsid- form (blue points) are plotted against the total dimer concentration for assembly of 5 to 25 µM dimer in 210 mM ammonium acetate. The plot shows the expected pseudocritical behavior which is represented by the red and blue lines. Below the pseudocritical concentration (3.55 µM as determined from these results) the capsid concentration is vanishingly small. Above the pseudocritical concentration the free dimer concentration is almost constant and the capsid concentration increases almost linearly.
To slow the onset of capsid formation so that it could be tracked in real time by CDMS, we used a total dimer concentration of 5 µM (which is slightly larger than the pseudocritical concentration), along with an ammonium acetate concentration of 210 mM (which is close to the lowest concentration used in Figure 1A). In addition to slowing the assembly reaction, the combination of low salt concentration and low protein concentration also minimizes kinetic trapping. Figure 1C shows a typical CDMS spectrum measured 24 h after initiation of the assembly reaction. There is a broad distribution of ions below 0.3 MDa. The inset shows an expanded view of the low mass region sampled to show resolution of the dimer and small oligomers. The oligomers are denoted by dn where n is the number of dimers. Above d5 the intensities become relatively small. Moving to higher mass, there is a small low intensity distribution between 3.0 and 3.5 MDa and a peak at around 4.0 MDa which is attributed to the T = 4 capsid.
HBV is dimorphic in vivo, forming both T = 3 and T = 4 capsids.37 In vivo, < 10% of the capsids are T = 3. In vitro the protein oxidation state, mutations and assembly conditions can modulate the relative abundances of T = 3 and T = 4.38–40 For ammonium acetate-induced assembly there is no peak at the mass expected for the T = 3 capsid (3.02 MDa based on the sequence mass) (see Figure 1C). Thus, in ammonium acetate at pH 7.5, the T = 3 capsid is not formed to any significant extent. This was confirmed by sucrose gradient centrifugation (see Supporting Information) and electron microscopy.
The intensities in the high mass region of Figure 1C are magnified 100× because the capsid is much less abundant than the dimer, a consequence of the dimer concentration being close to the pseudocritical value. To compare the relative intensities in the CDMS spectrum with the SEC results (Figure 1B), all ions in Figure 1C with masses greater than or equal to 2.5 MDa are associated with capsid peak in the size exclusion chromatogram, and all ions with masses less than 2.5 MDa are associated with the dimer peak. Given the fragility of dimer–dimer interactions, it is unlikely that the small oligomers present in the CDMS spectrum will stay intact during SEC. The ratio of the amount of dimer in “dimer”-form (i.e., dimer plus dimer oligomers) to the amount of dimer in capsid-form in the CDMS spectrum is 2.1:1. The corresponding ratio deduced from SEC results for identical dimer and salt concentrations is 2.45:1. The good agreement gives us confidence that the CDMS measurements provide an authentic representation of the relative concentrations of the species present in solution. However, very weakly bound intermediates may be disrupted during their transition into the gas phase and hence be underrepresented in the CDMS spectra. For time-resolved CDMS measurements, samples of 10 µM Cp149 dimer in 20 mM ammonium acetate were mixed with equal volumes of 400 mM ammonium acetate. The reaction mixture was immediately loaded into a chip-based nano- electrospray source, where the assembly reaction continues while the solution is electrosprayed. Each ion detected by CDMS is time-stamped, and so knowing when the assembly reaction was initiated it is possible to relate each ion to a particular time in the assembly reaction. The frequency of single ion trapping events is relatively low, 3–4 ions per second at best, with the 100 ms trapping period used here, so too few ions are detected in a single reaction to generate time-resolved spectra. However, the results of multiple reactions can be combined and sorted into time windows.
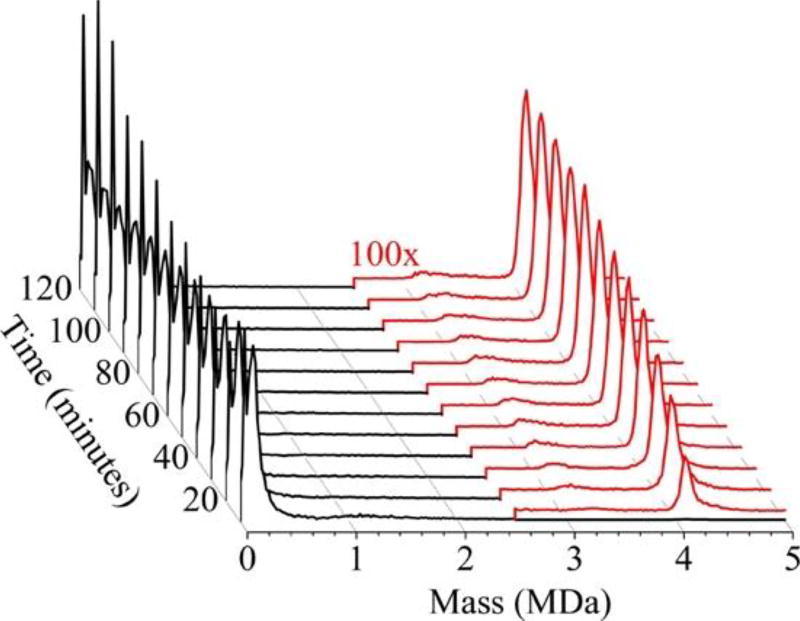
A series of time-resolved CDMS spectra (Figure 2) show the progression of capsid growth over the first 120 min of assembly. The results in Figure 2 were compiled from 28 individual experiments measured on five different days where each assembly experiment was recorded continuously for 120 min after initiation. The x-axis shows the mass binned in units of 20 kDa, the y-axis represents the intensity normalized by peak area, and the z-axis represents the reaction time binned into 10 min intervals. The earliest time interval (0–10 min) is at the front. The prominent peaks below 0.5 MDa correspond to the dimer and oligomers of up to five dimers. The oligomers are not well-resolved in these spectra in part because of the 20 kDa bins employed. The intensity of single dimer increases over 120 min while the intensity of the oligomers decreases. At early time points in these assembly reactions (<30 min) there is a broad, low abundance peak at around 1.1 MDa. In addition, a broad, low-abundance peak at around 3.2 MDa grows in over time and shifts to slightly higher masses as time progresses. The prominent peak at around 4.1 MDa is close to the mass expected for the empty T = 4 capsid. The assembly reaction appears to be complete around half way through the 120 min reaction time shown in Figure 2.
Figure 2.
Time-resolved CDMS spectra showing capsid growth over the first 120 min for assembly of 5 µM dimer in 210 mM ammonium acetate. The earliest time interval (0 to 10 min) is at the front. Under the conditions employed, the capsid concentration is much less than the free dimer concentration, and so the intensities of ions with masses >2.5 MDa (in red) have been scaled up by a factor of 100×. The spectra weregenerated using 20 kDa bins.
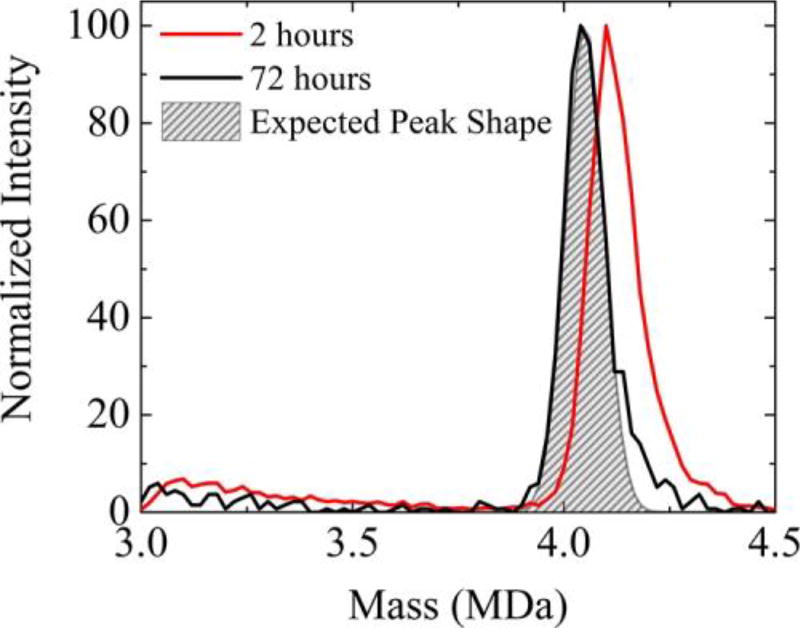
For a single species, the CDMS peak shape is expected to be Gaussian with a width determined by the uncertainties in the m/z and charge measurements, both of which are well characterized (see below).41 For the T = 4 capsid, the peak width at half height for a homogeneous sample is expected to be around 100 kDa. The expected peak width is larger than the mass of a Cp149 dimer (33 540 Da) so we are not able to resolve individual peaks corresponding to species with different numbers of dimers for the capsid. Two hours into the assembly reaction, the capsid peak in Figure 2 is significantly broader than the expected peak width, suggesting that the capsid peak is heterogeneous. An expanded view of the capsid peak 2 h into the assembly reaction is shown in Figure 3. In addition to having a high mass tail, the peak is centered at around 4.11 MDa which is 2.2% higher than the sequence mass of 4.02 MDa for the T = 4 capsid with 120 dimers. The black line in Figure 3 is the capsid peak measured for the same sample 72 h into the assembly reaction. The center of the capsid peak has receded to 4.04 MDa, which is only 0.6% higher than the sequence mass. We expect that the measured mass may be slightly (up to around 1%) larger than the sequence mass because of incomplete removal of solvent (water and ammonium acetate) and nonspecific aggregation during electrospray (see Supporting Information). Therefore, the peak center at 72 h is in line with expectations for the T = 4 capsid. The width of the capsid peak has also diminished with time. The shaded region in Figure 3 shows the expected peak width for a homogeneous sample. 72 h after initiation of the assembly reaction the measured peak is only slightly broader than expected, though it still has a small high mass tail. Thus, long after the initiation of the assembly reaction, both the peak center and peak width are consistent with the capsid containing predominantly 120 dimers.
Figure 3.
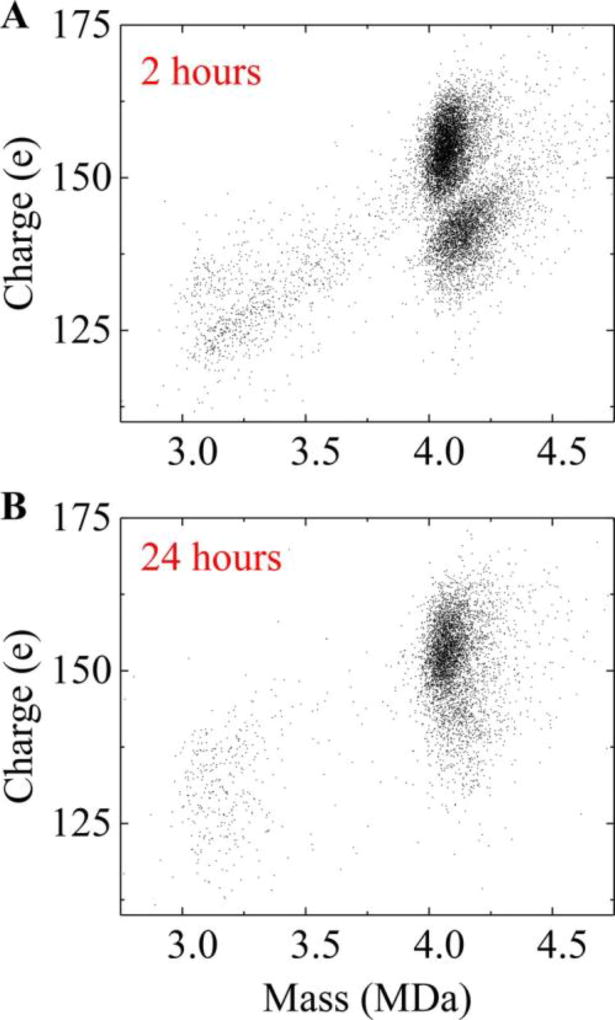
Expanded view of the CDMS spectrum showing the capsid peak at different times. The red trace shows the high mass range of the the assembly reaction. Each point represents a single ion. There are two clusters of ions with average charges centered around 140 and 155 e (elementary charges). Both clusters have masses around 4.1 MDa, though the lower charge cluster has a slightly broader distribution of masses and a slightly larger average mass. The higher charge cluster has around twice as many ions as the lower. Figure 4B shows the scatterplot for ions measured 24 h after initiation of the assembly reaction. At this time point, the low charge cluster has largely disappeared. Thus, the more highly charged cluster of ions that persists after 24 h is attributed to the T = 4 capsid.
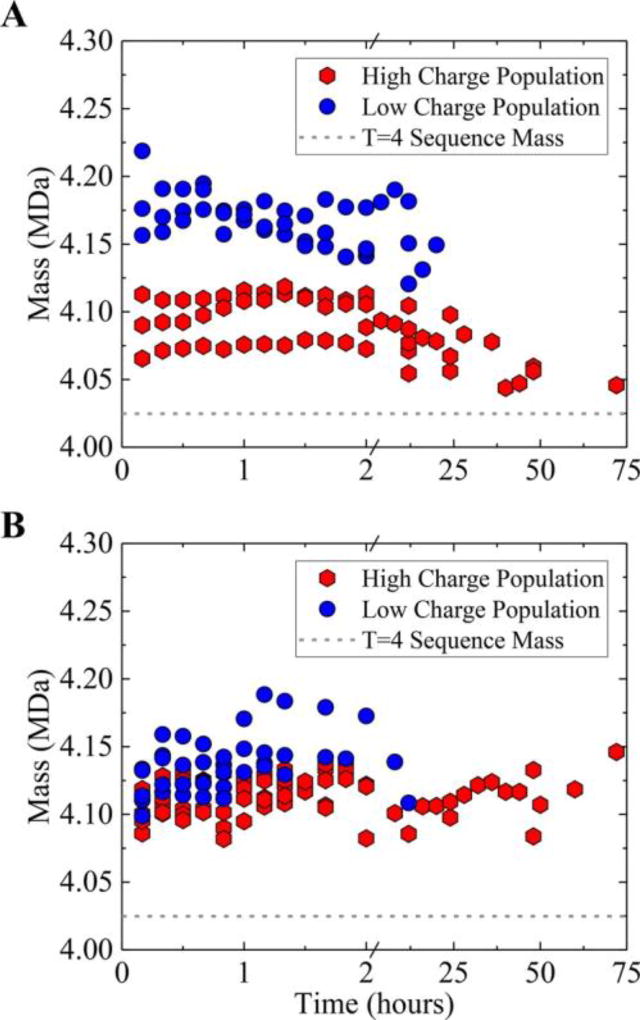
The peak in the mass distribution at 2 h (Figure 3) is broader and shifted to higher mass than expected mainly because it includes the low charge cluster, which has a broader mass distribution and a higher average mass than the high charge cluster. Figure 5A shows a plot of the center masses of each CDMS spectrum for a sample collected within the first 2 h of initiating assembly of 5 µM dimer in 210 mM ammonium acetate. The black trace is for the same sample 72 h after assembly was initiated. The gray shaded area shows the Gaussian peak shape expected for T = 4 capsid based on instrumental resolution (see text).
Figure 5.
Relaxation of high charge and low charge populations as a function of time. (A) Plot showing the center mass of each charge population (see text) plotted against reaction time for three independent experiments. The higher charge population (around 155e) is the red hexagons and the lower charge population (around 140 e) is the blue circles. The gray dashed line shows the sequence mass of the T = 4 capsid. The three experiments have slightly different amounts of overgrowth but show the same trend. (B) A similar plot recorded for assembly in 510 mM ammonium acetate. In the higher salt solution the overgrown capsids remain trapped on the time scale shown in the plot.
Conversely, the results at shorter times indicate that many of the capsids are overgrown (i.e., they contain more than the expected 120 dimers).
Figure 4A shows a scatter plot of charge versus mass for all ions recorded in the capsid region of the spectrum for first 120 min of charge component against time. Results are shown at 10 min intervals up to 120 min and at typically 4 h intervals for longer times. The center masses were determined by isolating the low and high charge clusters, binning the masses into a histogram, and then fitting the histogram with a Gaussian. The results of three different experiments are plotted, and the different experiments show some systematic variability in the amount of overgrowth. For the low charge cluster (blue circles) the average center mass is at around 4.17 MDa (around 0.15 MDa above the sequence mass). Results are only shown for times up to 20 h for the low charge cluster, after which the number of low charge ions is too small to reliably define a center mass. The average center mass for the high charge cluster (red hexagons) starts at around 4.08 MDa, drops to around 4.05 MDa, and remains there out to 72 h. Again, 4.05 MDa is in line with the sequence mass of 4.02 MDa plus <1% for residual solvent.
Figure 4.
Charge versus mass scatter plots for capsid ions at different times. (A) 2 h after initiating assembly of 5 µM dimer in 210 mM ammonium acetate a bimodal charge distribution of charge is observed. The charge is expected to reflect that size of the ion, thus the lower charge cluster has more compact particles (i.e., particles with a smaller average external radius). (B) 24 h after initiating assembly the T = 4-sized ions show almost a single distribution of charge and mass. Each point in the plot represents a single ion measured by CDMS.
Figure 5B shows a plot of the center masses of both charge components against time for assembly in 510 mM ammonium acetate. With the higher salt concentration the assembly reaction occurs much more quickly and appears to be complete within a few minutes. At the higher salt concentration the lower charge cluster initially contains a larger proportion of the capsid ions (60% versus 30% at lower salt). However, in high salt the distinction in charge state is much shorter lived, essentially disappearing just before or slightly after the 2 h time point. Another difference is that at the higher salt concentration the masses do not drift down to approach the sequence mass within the 72 h time scale shown in Figure 5. They do eventually start to relax back, but clearly the capsids remain overgrown for longer at the higher salt concentration.
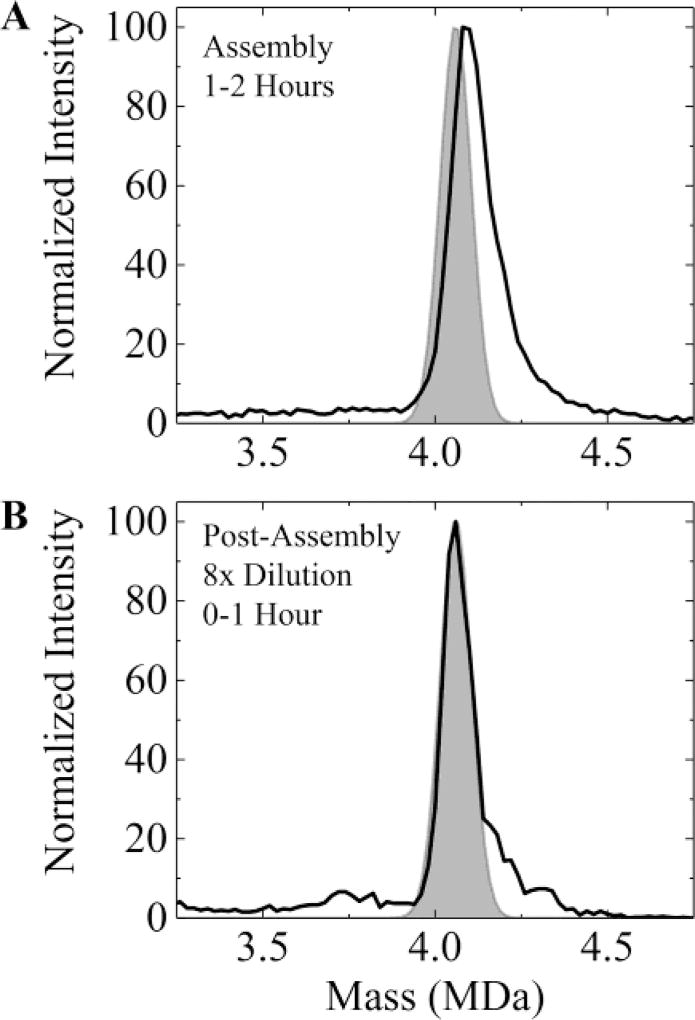
Because lower ionic strength results in weaker protein– protein interactions with Cp149, we examined whether the self- correction of overgrown particles could be accelerated by lowering the ammonium acetate concentration after the initial assembly reaction had occurred. The solid line in Figure 6A shows the mass spectrum measured in the 1–2 h time window after initiating assembly with 10 µM dimer in 210 mM ammonium acetate. The shaded area shows the expected position and peak width for a T = 4 capsid with 120 dimers (taking into account the expected ~0.6% shift due to the solvent). The measured peak is broader, shifted to higher mass, and has a high mass tail. Two hours after initiating the assembly reaction (i.e., after the initial assembly reaction was complete and after the spectrum in Figure 6A was recorded) the solution was diluted by a factor of 8 to give an ammonium acetate concentration of 26 mM. Figure 6B shows the spectrum measured in the 0–1 h time window after dilution. The capsid peak has shifted back to the expected position for a T = 4 capsid, though some of the high mass tail remains. A higher dimer concentration (10 µM) was used for the initial assembly reaction in these experiments to partly compensate for the subsequent dilution. With the higher dimer concentration the initial assembly reaction occurs faster but otherwise the results are similar to those obtained with a dimer concentration of 5 µM.
Figure 6.
Relaxation of high mass populations as a function of ionic strength. (A) The solid line shows the mass spectrum measured 1–2 h after initiation of assembly of 10 µM dimer in 210 mM ammonium acetate. The shaded area shows the expected peak position and width for a T = 4 capsid with 120 dimers (taking into account the ~0.6% shift due to solvent). (B) The solid line shows the spectrum measured 0–1 h after dilution of the sample in (A) by a factor of 8 to give a final ammonium acetate concentration of 26 µM. The dilution was performed 2 h after initiation of the assembly reaction. In (A) the capsid peak is broader and at a higher mass than expected for the T = 4 capsid. In (B) the peak is at the expected position for the T = 4 capsid though some of the high mass tail persists.
DISCUSSION
By examining mass as well as the charge we have obtained important information about capsid assembly that is obscured in low resolution and ensemble measurements. The apparent “errors” in assembly, overgrowth and changes in charge, suggest a much richer variation in assembly path than previously suspected from experimental and computational studies.
Changes in charge state suggest conformational changes. Near-spherical, MDa-sized ions generated by electrospray are believed to be formed by the charge residue mechanism42 where larger objects should carry more charge. The higher charge cluster of ions that persist in the charge versus mass scatter plot (Figure 4) are presumably icosahedral T = 4 (or almost icosahedral T = 4) capsids. The high charge is consistent with a hollow spherical cage geometry.43
The low charge clusters in Figure 5 presumably result from a geometry that is slightly more compact (i.e., a smaller average external radius) than the icosahedral T = 4 capsid. A similar low charge cluster was observed in the charge versus mass scatter plots for woodchuck hepatitis virus (WHV).44 It was attributed to capsids that had collapsed slightly as they transition into the gas phase. However, this explanation is inconsistent with the low charge cluster disappearing over time. For HBV at least, we hypothesize that the low charge cluster consists of defective capsids; one way to realize this more compact geometry would be to have the capsid adopt a cowry shell geometry, where some dimers are internalized at a seam. Indeed, a small fraction of slightly oblong particles have been observed in cryomicrographs of woodchuck hepatitis virus.44 In an analogous reaction, seams have been observed in HIV-1 capsids in some experiments.45
The lower charge cluster (see Figure 4) initially contains around 60% of the ions for the higher salt concentration but only 30% for the lower. In both cases, the average masses of the lower charge clusters are larger than expected for a perfect T = 4 capsid by around 3–5 dimers. The higher charged cluster is overgrown by 1–2 dimers on average with the lower salt concentration. With the higher salt concentration the higher charged cluster is more overgrown and relaxes back on a longer time scale. The larger overgrowth and slower relaxation at the higher salt concentration is consistent with the stronger dimer–dimer interactions found at higher ionic strength. These results also suggest differences in the geometry of the intersubunit interaction. While the majority of the particles are initially overgrown, they have a relatively narrow size distribution and the overgrowth is strictly limited.
In contrast, substantial overgrowth has been observed in the assembly of woodchuck hepatitis virus (WHV) capsids where a prominent feature with 150 dimers was attributed to T = 4 capsids elongated along their 5-fold axis by the addition of an extra ring of hexamers.44 However, in this case the overgrown capsids were not found to evolve with time, though we did not investigate the consequences of lowering the salt concentration after the initial assembly reaction.
The assembly of icosahedral virus capsids has previously been described by a three step process: initiation by formation of a critical nucleus; elongation by addition of capsid building blocks to the growing nucleus; and completion where the last building block is incorporated to complete the icosahedral shell. Little was known about the last step because of the lack of appropriate tools to examine it. Incorporation of the last dimer was thought to be a slow process due to steric effects (i.e., the difficulty of locating the open site).8 Thus, intermediates with less than the expected number of dimers were anticipated.
For HBV capsids, we find the reverse. At what appears to be the end of the assembly reaction according to low resolution techniques such as light scattering and SEC, many of the particles are in fact defective and overgrown. The lower charge cluster, attributed to particles with a seam, anneals away first, and then the overgrown capsids relax back to the expected mass of a perfect T = 4 capsid. These results indicate that the completion step is annealing and relaxation, and it occurs on a time scale that is much longer than the time scale of the initial assembly reaction. The spontaneous self-assembly of molecular components is usually thought to occur through free energy minimization. However, a stable ordered structure can only result (on a reasonable time scale) for a narrow range of component binding energies. If the binding energies are too strong, assembly errors are locked-in and a disordered structure is formed. If the binding energies are too weak, the assembly is unstable. For component binding energies in the optimum range, proof-reading (error correction) can occur. Misplaced components are less strongly bound, and during the assembly reaction they may rearrange in situ or dissociate. The results presented here indicate that not all errors are corrected during the initial assembly reaction for HBV. A small number of defects are locked-in, and may propagate to form a seam. However, the trapped defects anneal away over a longer time scale. This secondary proof-reading process is presumably driven by the decrease in free energy associated with correctly locating all components with icosahedral symmetry.
If assembly in vivo follows the same pathway as in vitro assembly, HBV must have evolved methods for mitigating the barriers to completion. While a space filling cargo, such as nucleic acid, may help to scaffold capsid formation, most of the HBV capsids formed in vivo are empty. Note that our measurements do not conflict with other studies that indicate that HBV forms perfect icosahedral capsids,21 as the defective and overgrown capsids we observe self-correct over time.
In model studies with rigid building blocks, the rate of subunit addition decreases as the capsid approaches completion because it becomes more difficult to locate an empty site. However, beside this steric issue, inserting the last rigid building block into a rigid structure is not expected to be more difficult than inserting any other. The soft and deformable nature of real capsid building blocks may contribute to the accumulation of defects during capsid growth. The addition of oligomers of dimers to the growing capsid rather than single dimers may also contribute to the defects and overgrowth. The relatively weak intersubunit association energy of Cp149 dimers must facilitate the relaxation of the overgrown particles.2
CONCLUSIONS
CDMS measurements show that capsid completion is the slow step in capsid assembly. The initial assembly reaction is not completely error free, yielding defective and overgrown particles with a few more subunits than expected for a perfect icosahedron. The defective particles anneal and then the overgrown particles relax back to the mass expected for a perfect T = 4 capsid. As a capsid with imperfections is expected to be more labile than a perfect capsid,46,47 accumulation of imperfections may be a proof-reading mechanism. While the results presented here are for HBV, a well-studied model for capsid assembly, we anticipate that the assembly errors will be observed in other viruses when probed with higher resolution techniques; indeed, defects have been observed in Ross River virus capsids and seams and gaps in HIV.45,47,48
MATERIALS AND METHODS
Preparation and Characterization of Core Protein Cp149
The truncated form of the core protein containing only the assembly domain (Cp149) was expressed in E. coli and purified as previously described.49 A reassembly and dissociation step was included to remove inactive protein. The assembly competent protein was dialyzed into 20 mM ammonium acetate at pH 7.5. Assembly was initiated by raising the ammonium acetate concentration to increase the ionic strength. A series of light scattering and size exclusion chromatography (SEC) measurements were performed to characterize HBV assembly in ammonium acetate.
Light scattering measurements were performed using a stopped-flow spectrometer (KinTek SF-300×). Scattered 320 nm light was detected at 90° from incident. SEC measurements were performed with a 10/300 Superose 6 column (GE Healthcare) on an HPLC system (Shimadzu) using UV absorbance at 280 nm. Equal volumes of dimer in 20 mM ammonium acetate and ammonium acetate were mixed, and the reactions were incubated at room temperature for 24 h, at which point the reaction mixture was analyzed by SEC. The chromatograms showed two peaks attributed to dimer and capsid.
Charge Detection Mass Spectrometry
In ion trap CDMS, single ions are trapped in a linear electrostatic ion trap where they oscillate back and forth through a conducting cylinder. When the ion is in the cylinder it induces a charge which is detected by a charge sensitive preamplifier. The time domain signal from the oscillating ion is analyzed to provide the m/z and charge, which are combined to provide the mass of the ion.
A detailed description of the charge detection mass spectrometer is given elsewhere.28–32 The ions are generated by electrospray, enter the instrument through a heated capillary and separated from the ambient gas flow. A narrow band of ion energies is selected by a dual hemispherical deflection energy analyzer (HDA) and focused into a modified cone trap that contains the charge detection cylinder. Potentials are applied to the end-cap electrodes to trap ions. A trapping period of 100 ms was employed, after which both end-caps were set to ground and the trapping cycle repeated. The signal is optimized to maximize the number of single ion trapping events. Empty, partial, and multiple ion trapping events are discarded during data analysis.
The ion oscillating back and forth through the detection cylinder induces a periodic signal which is amplified, digitized, and then analyzed by a Fortran program using fast Fourier transforms. The m/z is derived from the fundamental frequency and the charge is derived from the magnitudes of the fundamental and first harmonic. The methods used to calibrate the charge and m/z measurements have been described elsewhere.32 The probability an ion is trapped is inversely proportional to its velocity. To correct for this variation, the ion intensities are weighted by m/z−1/2, and then binned into a histogram to generate a mass spectrum. The uncertainty in the m/z is around 1%, and the uncertainty in the charge (which depends mainly on the trapping time) is around 1.2 e.41
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the NIH through Award Number 1R01AI118933.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b09932. Further evidence for negligible concentration of T = 3 capsid from assembly in ammonium acetate; Analysis of nonspecific aggregation during electrospray (PDF)
The authors declare the following competing financial interest(s): The authors, except AZ, declare no competing financial interests. AZ is associated with a company that is developing antiviral compounds.
References
- 1.Fischlechner M, Donath E. Angew. Chem., Int. Ed. 2007;46:3184–3193. doi: 10.1002/anie.200603445. [DOI] [PubMed] [Google Scholar]
- 2.Ceres P, Zlotnick A. Biochemistry. 2002;41:11525–11531. doi: 10.1021/bi0261645. [DOI] [PubMed] [Google Scholar]
- 3.Caspar DLD, Klug A. Cold Spring Harbor Symp. Quant. Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 4.Endres D, Zlotnick A. Biophys. J. 2002;83:1217–1230. doi: 10.1016/S0006-3495(02)75245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zandi R, van der Schoot P, Reguera D, Kegel W, Reiss H. Biophys. J. 2006;90:1939–1948. doi: 10.1529/biophysj.105.072975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen HD, Reddy VS, Brooks CL. Nano Lett. 2007;7:338–344. doi: 10.1021/nl062449h. [DOI] [PubMed] [Google Scholar]
- 7.Hagan MF, Elrad OM. Biophys. J. 2010;98:1065–1074. doi: 10.1016/j.bpj.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan MF. Adv. Chem. Phys. 2014;155:1–67. doi: 10.1002/9781118755815.ch01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlotnick A, Johnson JM, Wingfield PW, Stahl SJ, Endres D. Biochemistry. 1999;38:14644–14652. doi: 10.1021/bi991611a. [DOI] [PubMed] [Google Scholar]
- 10.Prevelige PE, Thomas D, King J. Biophys. J. 1993;64:824–835. doi: 10.1016/S0006-3495(93)81443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casini GL, Graham D, Heine D, Garcea RL, Wu DT. Virology. 2004;325:320–327. doi: 10.1016/j.virol.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Kao CC, Dragnea B. J. Phys. Chem. A. 2008;112:9405–9412. doi: 10.1021/jp802498z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katen SP, Chirapu SR, Finn MG, Zlotnick A. ACS Chem. Biol. 2010;5:1125–1136. doi: 10.1021/cb100275b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kler S, Asor R, Li C, Ginsburg A, Harries D, Oppenheim A, Zlotnick A, Raviv U. J. Am. Chem. Soc. 2012;134:8823–8830. doi: 10.1021/ja2110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tresset G, Le Coeur C, Bryche J-F, Tatou M, Zeghal M, Charpilienne A, Poncet D, Constantin D, Bressanelli S. J. Am. Chem. Soc. 2013;135:15373–15381. doi: 10.1021/ja403550f. [DOI] [PubMed] [Google Scholar]
- 16.Tresset G, Decouche V, Bryche J-F, Charpilienne A, Le Coeur C, Barbier C, Squires G, Zeghal M, Poncet D, Bressanelli S. Arch. Biochem. Biophys. 2013;537:144–152. doi: 10.1016/j.abb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Comas-Garcia M, Garmann RF, Singaram SW, Ben-Shaul A, Knobler CM, Gelbart WM. J. Phys. Chem. B. 2014;118:7510–7519. doi: 10.1021/jp503050z. [DOI] [PubMed] [Google Scholar]
- 18.Roingeard P. Biol. Cell. 2008;100:491–501. doi: 10.1042/BC20070173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms ZD, Selzer L, Zlotnick A, Jacobson S. ACS Nano. 2015;9:9087–9096. doi: 10.1021/acsnano.5b03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton VL, Stockley PG, Stonehouse NJ, Ashcroft AE. Mass Spectrom. Rev. 2008;27:575–595. doi: 10.1002/mas.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uetrecht C, Versluis C, Watts NR, Roos WH, Wuite GLJ, Wingfield PT, Steven AC, Heck AJR. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9216–9220. doi: 10.1073/pnas.0800406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uetrecht C, Barbu IM, Shoemaker GK, van Duijn E, Heck AJR. Nat. Chem. 2011;3:126–132. doi: 10.1038/nchem.947. [DOI] [PubMed] [Google Scholar]
- 23.Holmes K, Shepherd DA, Ashcroft AE, Whelan M, Rowlands DJ, Stonehouse NJ. J. Biol. Chem. 2015;290:16238–16245. doi: 10.1074/jbc.M114.622035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelton H, Hendricks CD, Wuerker WF. J. Appl. Phys. 1960;31:1243–1246. [Google Scholar]
- 25.Fuerstenau SD, Benner WH. Rapid Commun. Mass Spectrom. 1995;9:1528–1538. doi: 10.1002/rcm.1290091513. [DOI] [PubMed] [Google Scholar]
- 26.Fuerstenau SD, Benner WH, Thomas JJ, Brugidou C, Bothner B, Siuzdak G. Angew. Chem., Int. Ed. 2001;40:541–544. [PubMed] [Google Scholar]
- 27.Benner WH. Anal. Chem. 1997;69:4162–4168. [Google Scholar]
- 28.Contino NC, Jarrold MF. Int. J. Mass Spectrom. 2013;345– 347:153–159. [Google Scholar]
- 29.Contino NC, Pierson EE, Keifer DZ, Jarrold MF. J. Am. Soc. Mass Spectrom. 2013;24:101–108. doi: 10.1007/s13361-012-0525-5. [DOI] [PubMed] [Google Scholar]
- 30.Pierson EE, Keifer DZ, Contino NC, Jarrold MF. Int. J. Mass Spectrom. 2013;337:50–56. doi: 10.1007/s13361-012-0525-5. [DOI] [PubMed] [Google Scholar]
- 31.Pierson EE, Contino NC, Keifer DZ, Jarrold MF. J. Am. Soc. Mass Spectrom. 2015;26:1213–1220. doi: 10.1007/s13361-015-1126-x. [DOI] [PubMed] [Google Scholar]
- 32.Keifer DZ, Shinholt DL, Jarrold MF. Anal. Chem. 2015;87:10330–10337. doi: 10.1021/acs.analchem.5b02324. [DOI] [PubMed] [Google Scholar]
- 33.Pierson EE, Keifer DZ, Selzer L, Lee LS, Contino NC, Wang JC-Y, Zlotnick A, Jarrold MF. J. Am. Chem. Soc. 2014;136:3536–3541. doi: 10.1021/ja411460w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto Y, Yamada G, Mizuno M, Nishihara T, Kinoyama S, Kobayashi T, Takahashi T, Nagashima H. Lab. Invest. 1983;48:678–682. [PubMed] [Google Scholar]
- 35.Hu J, Liu K. Viruses. 2017;9:56. doi: 10.3390/v9030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selzer L, Zlotnick A. In: Hepatitis B and Delta Viruses. Seeger C, Locarnini S, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2014. pp. 91–108. [Google Scholar]
- 37.Crowther RA, Kiselev NA, Bottcher B, Berriman JA, Borisova GP, Ose V, Pumpens P. Cell. 1994;77:943–950. doi: 10.1016/0092-8674(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 38.Wingfield PT, Stahl SJ, Williams RW, Steven AC. Biochemistry. 1995;34:4919–4932. doi: 10.1021/bi00015a003. [DOI] [PubMed] [Google Scholar]
- 39.Selzer L, Katen SP, Zlotnick A. Biochemistry. 2014;53:5496–5504. doi: 10.1021/bi500732b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlotnick A, Cheng N, Conway JF, Booy FP, Steven AC, Stahl SJ, Wingfield PT. Biochemistry. 1996;35:7412–7421. doi: 10.1021/bi9604800. [DOI] [PubMed] [Google Scholar]
- 41.Pierson EE, Keifer DZ, Asokan A, Jarrold MF. Anal. Chem. 2016;88:6718–6725. doi: 10.1021/acs.analchem.6b00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez de la Mora J. Anal. Chim. Acta. 2000;406:93–104. [Google Scholar]
- 43.Keifer DZ, Motwani T, Teschke CM, Jarrold MF. J. Am. Soc. Mass Spectrom. 2016;27:1028–1036. doi: 10.1007/s13361-016-1362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierson EE, Keifer DZ, Kukreja AA, Wang JC-Y, Zlotnick A, Jarrold MF. J. Mol. Biol. 2015;428:292–300. doi: 10.1016/j.jmb.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Z, Dobro MJ, Woodward CL, Levandovsky A, Danielson CM, Sandrin V, Shi J, Aiken C, Zandi R, Hope TJ, Jensen GJ. J. Mol. Biol. 2013;425:112–123. doi: 10.1016/j.jmb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S, Zlotnick A. J. Biol. Chem. 2003;278:18249–18255. doi: 10.1074/jbc.M211408200. [DOI] [PubMed] [Google Scholar]
- 47.Wang JC-Y, Chen C, Rayaprolu CV, Mukhopadyay S, Zlotnick A. ACS Nano. 2015;9:8898–8906. doi: 10.1021/acsnano.5b02632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briggs JA, Riches JD, Glass B, Bartonova V, Zanetti G, Krausslich HG. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11090–11095. doi: 10.1073/pnas.0903535106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zlotnick A, Ceres P, Singh S, Johnson JM. J. Virol. 2002;76:4848–4854. doi: 10.1128/JVI.76.10.4848-4854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 ORCID
ORCID