This study demonstrated the feasibility of integrating cervical cancer screening into safer conception services in Johannesburg, South Africa. One in 5 human immunodeficiency virus–affected women attempting pregnancy had cervical pathology requiring colposcopy.
Abstract
Background
Sub-optimal cervical cancer screening in low- and middle-income countries contributes to preventable cervical cancer deaths, particularly among human immunodeficiency virus (HIV)-positive women. We assessed feasibility and outcomes of integrating cervical cancer screening into safer conception services for HIV-affected women.
Methods
At a safer conception service in Johannesburg, South Africa, HIV-affected women desiring pregnancy received a standard package of care designed to minimize HIV transmission risks while optimizing prepregnancy health. All eligible women were offered Papanicolaou smear, and those with significant pathology were referred for colposcopy before attempting pregnancy. Multivariable analyses identified associations between patient characteristics and abnormal pathology.
Results
In total, 454 women were enrolled between June 2015 and April 2017. At enrolment, 91% were HIV-positive, 92% were on antiretroviral therapy (ART) and 82% virally suppressed. Eighty-three percent (376 of 454) of clients were eligible for cervical cancer screening and 85% (321 of 376) of these completed screening. More than half had abnormal cervical pathology (185 of 321) and 20% required colposcopy for possible high-grade or persistently atypical lesions (64 of 321). Compared with HIV-negative women, abnormal pathology was more likely among HIV-positive women, both those on ART <2 years (adjusted prevalence ratio, 2.5; 95% confidence interval, 1.2–5.0) and those on ART 2 years or longer (adjusted prevalence ratio, 2.1; 95% confidence interval, 1.0–4.2).
Conclusions
Integrating cervical cancer screening into safer conception care was feasible with high coverage, including for HIV-positive women. Significant pathology, requiring colposcopy, was common, even among healthy women on ART. Safer conception services present an opportunity for integration of cervical cancer screening to avert preventable cancer-related deaths among HIV-affected women planning pregnancy.
Each year over 250,000 women die of cervical cancer, a preventable condition.1 In high-income countries, population-level cervical cancer screening programs have effectively reduced cervical cancer associated morbidity and mortality.2 Unfortunately, in the most affected low- and middle-income countries (LMICs), screening coverage remains low.3 In South Africa, for example, poor program coverage contributes to over 4000 cervical cancer deaths each year, making cervical cancer the leading cause of cancer deaths in women of reproductive age.4 Integration of cervical cancer screening into human immunodeficiency virus (HIV) programming in LMICs, such as South Africa, is particularly important as this cancer disproportionately affects HIV-positive women,5 even once they commence antiretroviral therapy (ART).6 Screening opportunities for HIV-infected women are, however, frequently missed due to low awareness and poorly integrated services.3,7 Considering the dual burden of HIV and cervical cancer seen in many LMICs, integrating cervical cancer screening, and other sexual and reproductive health (SRH) services into established HIV programs is clearly a priority.8
Built on a framework of reproductive rights, comprehensive safer conception services support HIV-affected couples to safely achieve their fertility goals while minimizing risks of horizontal or vertical HIV transmission, and optimizing the couples' overall health status before a pregnancy.9 In South Africa, over half of women accessing ART are of reproductive age and many express a desire to have children now or in the future.10 Recognizing this overlap between HIV and fertility desires, safer conception services are recommended as part of the South African National Contraceptive and Fertility Policy released in 2012.11 Such services create a valuable opportunity for HIV and SRH integration, including for cervical cancer screening.12
This study aimed to investigate the feasibility of integrating cervical cancer screening into safer conception services as one component of a comprehensive package of care. The study, in Johannesburg, South Africa, was set within one of the first public sector safer conception services in the country. We also sought to assess the outcomes of cervical cancer screening.
METHODS
Study Context
In April 2015, a safer conception clinic was established in a busy, community health care center in inner-city Johannesburg. The facility offers general services and has an ART clinic, attended by over 21,000 HIV-positive clients. Although national policies have supported the provision for safer conception services since 2012,11 no standardized, integrated approach or public sector service delivery model has been developed. In particular, prior to introducing the safer conception service at the facility, screening for fertility intentions was ad hoc, and the provision of SRH services, including family planning and cervical cancer screening for HIV-positive women, took place in a separate building from the ART clinic, staffed by different providers. Patients requesting these services were referred to the relevant providers.
Recognizing the lack of service integration, a safer conception clinic was introduced as a dedicated service which operated within the same building as the ART clinic. The service was rendered by primary healthcare nurses who were supervised by a doctor and had received training in safer conception care. An integrated package of care was provided prepregnancy during the baseline visit and subsequent monthly to three monthly follow-up visits. The package included risk reduction (safer conception) counseling, support for HIV status disclosure, partner HIV testing, ART for women and their partners, preexposure prophylaxis for HIV-negative partners, screening and management of sexually transmitted infections (STIs), care for comorbid opportunistic infections or noncommunicable diseases for both members of the couple, and cervical cancer screening for women with referral for further management where indicated.
Services were delivered according to national guidelines. In the case of cervical cancer screening, South Africa's recommendations differ somewhat from international norms,1 with screening for HIV-negative women being conducted once in their third, fourth and fifth decades of life, and HIV-positive women being screened as soon as they are diagnosed with HIV infection and then every 1 to 3 years thereafter.
Donor funding supported the safer conception clinic staffing, including clinical, administrative and research staff, job aides and distribution of communication materials to inform patients attending the clinic of this new service. Routine clinical procedures, such as ART supply and Pap smears were funded through the Department of Health and the project was implemented at a Department of Health public sector facility. The only nonintegrated component was medical male circumcision; male partners who requested this intervention were referred to the medical male circumcision clinic within the same facility. Additionally, donor-supported, colposcopy services, which had previously only been available at a nearby tertiary hospital, had recently been established at the study facility.13 No services for infertility were provided at the clinic. If women did not conceive within 6 to 9 months of trying they were offered referral to a low-cost fertility service in Pretoria, a city about 50 km away.
Enrolment into the demonstration project was completed in April 2017; however, efforts have been undertaken to adapt the model and integrate services into nine primary healthcare facilities in other parts of inner-city Johannesburg.
Study Population
All women of reproductive age who were in an HIV-affected relationship (HIV seroconcordant, serodiscordant, or where one partner had unknown HIV status) who currently desired pregnancy were eligible to attend the service, with or without their partner. Men and women self-referred after hearing about the safer conception service during health talks presented in clinic waiting areas, from leaflets and posters within the facility, or by word of mouth. In addition, facility staff referred clients whom they screened as currently desiring pregnancy.
Cervical Cancer Screening Process
Clients were provided with a standard package of safer conception care with the aim of optimizing their health status before pregnancy and minimizing HIV transmission risks, including cervical cancer screening in the form of a Pap smear which was conducted according to national guidelines.14 The HIV–positive women, regardless of age, were eligible for a Pap smear if they had never been screened before, or they reported having been screened, but no result could be traced, their last abnormal Pap smear was more than 12 months ago, or they had a normal result more than 24 months previously. The HIV–negative women were offered cervical cancer screening if they were older than 30 years and had not had a normal Pap smear result in the past 10 years. Screening tests for human papillomavirus (HPV) were unavailable within public sector facilities at the time of this study.
Women who were eligible for a Pap smear were offered the service on the same day as their baseline visit, unless they were menstruating, in which case it was conducted at their next visit. Most smears were performed by the safer conception service nurses in a one-stop shop approach, although some were done by a postnatal clinic nurse situated in a nearby room during periods of high patient load.
Pap smear results, which have a reported sensitivity and specificity of 75.8% and 83.4%, respectively,15 were available within 4 to 6 weeks. If required, women were referred to the onsite colposcopy service and advised to delay pregnancy attempts until their colposcopy results indicated no need for further management. This approach was taken given the risks of having untreated abnormal cervical pathology during pregnancy including pregnancy loss and premature labor and delivery.16 The rationale for delaying pregnancy attempts was explained to each woman, and her partner if present. Care was taken to minimize the anxiety associated with receipt of abnormal Pap smear results while supporting the couple to delay pregnancy until any abnormal pathology had been addressed.
Data Collection and Analysis
Data on patient demographics and health status were captured on case report forms, medical history was extracted from patient medical records and clinical data were collected during procedures performed as part of the service. Results of Pap smears and colposcopy were obtained by reviewing women's clinical file notes or searching the database of the National Health Laboratory Service, where all the samples were assessed. Data were entered into a REDCap database17 and analyzed in STATA 14 (College Station, TX).
Coverage (percentage of clients offered screening) and uptake (percentage of clients offered screening who had a Pap smear done) were assessed. Characteristics of women who had a Pap smear were compared with those who did not using χ2 tests or nonparametric equality of medians tests, as appropriate.
Analysis focused on 2 outcomes of interest: (1) women with any abnormal Pap smear and (2) women with significant pathology requiring colposcopy. Significant pathology included: high-grade squamous intraepithelial lesions (HSIL); persistent low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of unknown significance; or any smear in which HSIL or cervical intraepithelial neoplasia (CIN) I or II could not be excluded.
Pap smear results are presented and modified robust Poisson regression was used to separately assess correlates of having an abnormal Pap smear and having a result requiring colposcopy. Robust Poisson regression was used given that the prevalence of the outcome was greater than 10% and the log binomial models failed to converge consistently.18 Bivariate analyses identified associations between demographic and clinical characteristics and the two outcomes of interest (abnormal Pap smear and significant pathology requiring colposcopy). The final, multivariable models included age, based on face validity and a priori hypotheses, and variables associated with the outcome in the bivariate analysis at P value less than 0.10.
Ethics
Ethical approval was secured from the Human Research Ethics Committee of the University of the Witwatersrand (M150146). The study was conducted according to good clinical practice guidelines and all enrolled clients completed written, informed consent.
RESULTS
Overall, 454 women attended the safer conception service. Of these, 91% (n = 413 of 454) were HIV-positive, having had a positive HIV test a median of 5 years ago (interquartile range, 3–9). The vast majority (92%, 382 of 413) were already on ART, with 19% (n = 71 of 382) having initiated in the past year; 51% had a CD4 cell count 500 cells/mm3 or greater and only 7% had a CD4 cell count 200 cells/mm3 or less. Overall, 75% (n = 311 of 413) of HIV-positive women had an undetectable viral load (<50 copies/mm3) at their baseline visit, and viral suppression was 82% among those taking ART (311 of 382).
Of all women, 83% (n = 376 of 454) were eligible for a Pap smear (Fig. 1). Of these, 18 were never offered a Pap smear by the provider, representing missed screening opportunities due to provider oversight (n = 358 of 376 or 95% screening coverage). A further 30 women were offered screening, but did not take it up (92% uptake). No differences were detected in demographic or clinical characteristics between the 15% (n = 70 of 454, Fig. 1) of women who recently had a Pap smear and those who did not. Overall, a Pap smear was performed for 87% (n = 328 of 376) of eligible women, 93% (n = 306) of whom were HIV-positive. Women who were eligible for a Pap smear, but did not receive one, were more likely to be lost to follow-up after their baseline safer conception visit, precluding an opportunity to conduct the Pap smear during follow-up (31% compared with 8% among women who did receive a Pap smear, P < 0.01).
Figure 1.
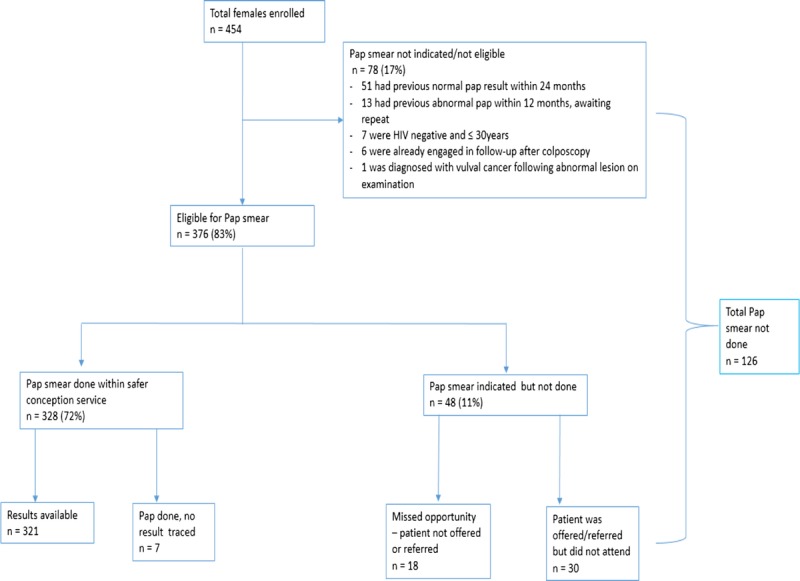
Flow diagram of females enrolled and accessing cervical cancer screening via safer conception service.
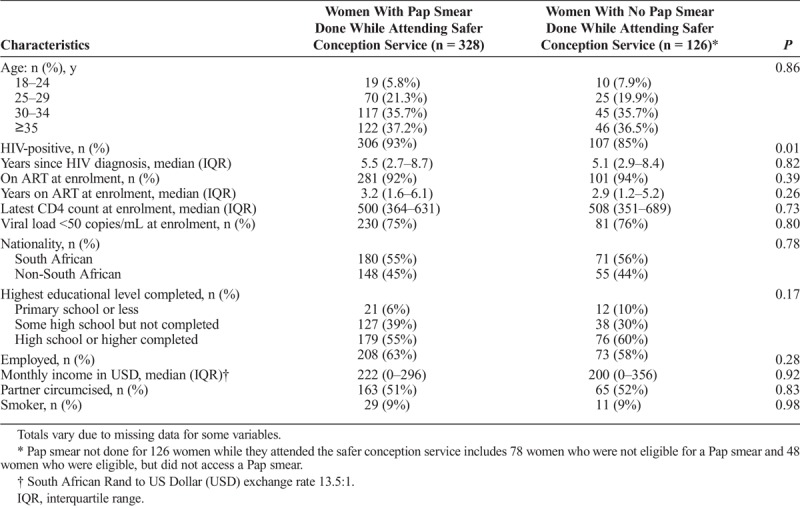
Across all demographic and clinical variables, the only difference detected between those accessing Pap smears through the safer conception service was HIV status, with those receiving a Pap smear being more likely to be HIV-positive (Table 1).
TABLE 1.
Demographics of Female Clients Attending a Safer Conception Service (N = 454)
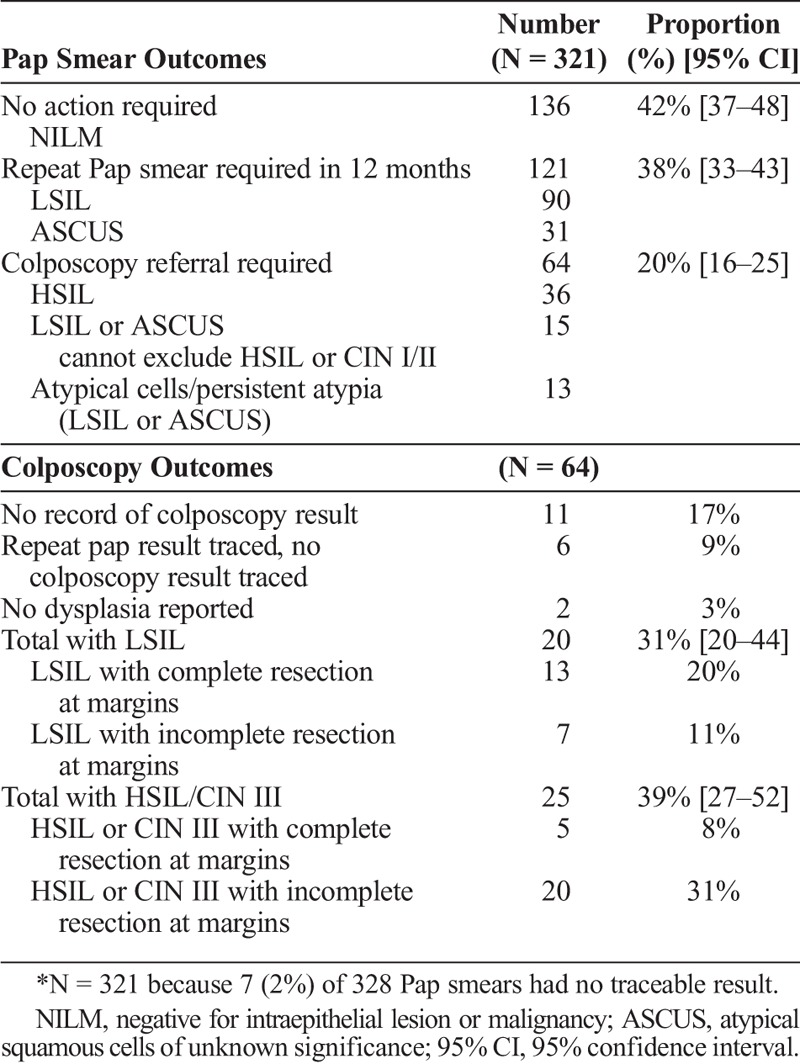
Of the 321 results available for analysis (7 results were untraceable), 58% (n = 185 of 321) had abnormal cytology, equating to 41% (185 of 454) of all women attending the clinic (Table 2). In total, 20% of those who had an abnormal Pap smear result (n = 64 of 321) required colposcopy prior to beginning pregnancy attempts, which translates into 14% (64 of 454) of all women attending the clinic. Among women requiring colposcopy, 31% (n = 20 of 64) were younger than 30 years. Additionally, bacterial vaginosis was detected in 38% (n = 122 of 321) of Pap smears and Trichomonas vaginalis (TV) in 3.4% (n = 11 of 321).
TABLE 2.
Pap Smear and Colposcopy Outcomes for Safer Conception Service Clients (N = 321)*
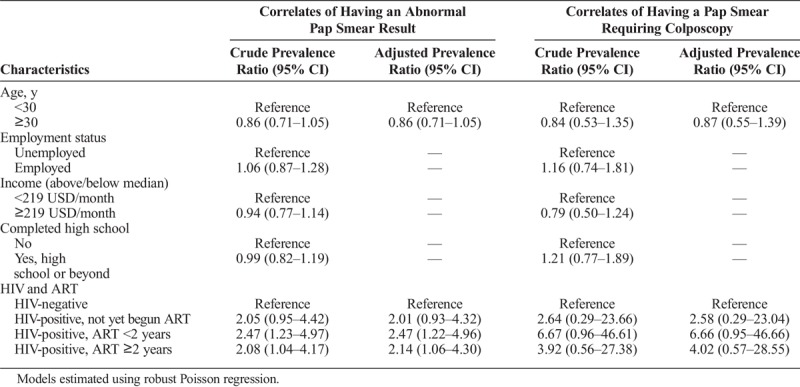
In the bivariate robust Poisson regression assessing correlates of having a Pap smear result of any abnormal cytology, only HIV status was associated with having an abnormal Pap smear (Table 3). These results were robust in the multivariable model, with only minor changes noted to point estimates. Being HIV-positive increased the magnitude of risk for an abnormal Pap smear about 2-fold, regardless of whether a woman had not begun ART, or had received ART for under 24 months or for over 24 months. Relationships were similar in the models assessing correlates of Pap smear results requiring colposcopy; the magnitude of the association between HIV and duration of ART use was stronger for the colposcopy outcome, though statistical significance was not reached.
TABLE 3.
Bivariate and Multivariable Correlates of Any Abnormal Pap Smear Result or Results Requiring Colposcopy (N = 321)
About three quarters of the 64 women who required colposcopy had a traceable histology result (n = 47, 73%) (Table 2). None of these women had invasive malignancy. Twenty-five (53%) women had evidence of HSIL or CIN II/III, with 20 of these having incomplete resection at the biopsy margins. These women required further follow-up, but no records of a repeat colposcopy could be located. Among the 64 women, 9 had a confirmed pregnancy, 2 of whom conceived before colposcopy was performed. No difference was detected in rates of retention in the safer conception service between women referred for colposcopy and women not requiring colposcopy (95% vs. 91%, P = 0.30).
DISCUSSION
Three key findings emerge from this study. First, integration of cervical cancer screening into safer conception services is feasible and is associated with high coverage and uptake. Most women, despite accessing routine HIV services, had not previously been screened for cervical cancer, illustrating the importance of integration initiatives, such as done in this study. Second, routine cervical cancer screening identifies high levels of significant pathology, particularly among HIV-positive women. Of all women attending the clinic, around 40% had abnormal pathology and one in seven required colposcopy. Third, significant pathology is common despite a large proportion of these women being on ART for several years. This reiterates the importance of cervical cancer screening, regardless of ART status.
Overall, the study supports increasing calls to improve integration of SRH services—including cervical cancer screening and family planning—with HIV services.8,19 The benefits of such integration have been demonstrated across numerous countries and service delivery models.20,21 In this study, the levels of coverage and uptake observed suggest a high level of client acceptability.
In South Africa, almost all colposcopy services are provided in tertiary-level facilities, with long waiting lists and limited capacity.13 For example, at the nearby tertiary hospital, only 250 to 300 women access colposcopy annually.13 Thus, even if current efforts to decentralize colposcopy services to district or primary level care are intensified, only a small portion of need would be met if rates of pathology were universally as high as seen in this study.22 This study therefore reinforces growing calls for South Africa to review their cervical cancer screening recommendations,14,23 particularly with a view to expanding the “see and treat” approaches to bypass weak referral pathways and reduce demand for colposcopy.15 This would also avoid the need for, and associated costs of, repeated follow-up visits by clients.
Ideally, such a shift in approach would be accompanied by the introduction of HPV testing. This would improve the ability to triage women at highest risk of cervical cancer,24 enabling them to be prioritized for further management. In an evaluation of HPV testing, Pap smear, and visual inspection with ascetic acid (VIA), Firnhaber et al15 reported sensitivity/specificity rates of 92%/51.4% for HPV, 75.8/83.4% for Pap smear and 65.4/68.5% for VIA when used to screen HIV-infected women in South Africa. In light of the high sensitivity of HPV screening but lower specificity, a combined approach, using both HPV screening and VIA, or HPV screening, Pap smear, and colposcopy would help to optimize the potential benefits while reducing the potential harm of unnecessary interventions, such as colposcopy.24 This combined approach would help to avoid exposing women to unnecessary discomfort, significant anxiety and potential harm associated with colposcopy or other treatment modalities.24
Both bacterial vaginosis (BV) and TV are associated with increased HIV transmission and acquisition risks, as well as negative birth outcomes, including miscarriage and preterm labor and delivery.25 Detecting BV and TV at the time of cervical cancer screening enabled us to manage these conditions effectively. Furthermore, the relatively high rate of BV and TV seen in our cohort may suggest that we saw the “tip of the iceberg” of underlying STIs, as gonorrhea, chlamydia, and herpes simplex virus are not detected on Pap smear. In settings like South Africa, where clinicians rely on a syndromic approach to STI screening and management, many asymptomatic infections are missed.25 Samples collected during cervical cancer screening could also be used to conduct increasingly affordable point-of-care diagnostic tests for STIs. There is clearly an opportunity, within integrated services, to introduce molecular testing not only for HPV, but also for other common STIs. Assessment is needed of the costs of this approach, weighed against the potential benefits of reducing cervical cancer, mitigating HIV transmission risks, reducing poor obstetric outcomes and minimizing infertility related to STIs.26 Enhanced screening services could also incorporate screening and management of other conditions, such as noncommunicable diseases including diabetes, hypertension and obesity. Comprehensive, streamlined protocols that address multiple competing priorities are needed to ensure that no single intervention risks forcing other significant public health interventions off the providers' agenda.
The high rates of untreated pathology observed in this cohort may indicate numerous previously missed opportunities to provide cervical cancer screening, especially considering the majority of women had accessed routine HIV and ART care for several years. Such missed opportunities contribute to persistently high rates of preventable cervical cancer deaths in HIV-positive women across Africa.19,27 In light of increasing pressures to decongest overburdened health facilities and reduce clinic visits through the provision of decentralized, differentiated services for stable ART clients,28 it is essential that the need for regular cervical cancer screening among these women is not overlooked. Even though data on whether cervical cancer risk declines after ART are conflicting, a large number of women attending HIV care require cervical cancer screening, regardless of ART use.29–32 Within differentiated care models, including community-based adherence clubs, private pharmacy dispensing initiatives, and communication of results via mobile phone technology, women's need for screening for cervical cancer and other conditions needs to be promoted. Community awareness activities are needed to ensure women understand the importance of accessing and advocating for regular screening.33 Mobile phone technology could be used to send reminders to women accessing new community-based HIV services and to alert them to any results which require follow-up. Newer self-screening tests for HPV and other common STIs, would be ideal for use by women accessing community-based ART services.
In the long run, the HPV vaccination program, which began immunizing 9-year-olds in 2014, will substantially reduce the burden of HPV-related disease. The vaccine program should, however, not distract from the secondary prevention needs of older unvaccinated women, like those in this study.
The clinical management of abnormal Pap smears in women who are seeking care to achieve a healthy pregnancy poses important questions. Colposcopy procedures prepregnancy may diminish the risk of rapid progression of lesions during pregnancy,34,35 avoid the need for more aggressive treatment after childbirth, and reduce the possibility of poor pregnancy outcomes (miscarriage, premature labor and delivery) associated with untreated cervical pathology.16 Conversely, extensive excisional biopsy to treat cervical pathology has been associated with negative pregnancy outcomes, including mid-trimester pregnancy loss, and premature labor and delivery.36 These competing risks need to be balanced against each other.
We recognize this study has limitations. It was conducted at a single, urban site, characterized by a largely migrant population living in densely populated high-rise buildings.37 This, along with the small sample size, particularly of HIV-negative women and HIV-positive women not yet on ART, may limit the generalizability of our findings. Additionally, the study had limited power to assess the relationship between ART duration and the need for colposcopy, resulting in large confidence intervals around our effect estimates. To address this limitation, we also assessed the predictors of having any abnormal Pap smear result and found the results to be robust across the two models. Additionally, although we aimed for a fully integrated service with all Pap smears provided within the safer conception clinic, this was not always possible, perhaps contributing to missed opportunities. Going forward, complete service and provider-level integration would be recommended.
The dual epidemic of HIV and cervical cancer continues to take its toll on women across Africa.19 Even HIV-positive women who are otherwise well on ART continue to experience higher cervical cancer rates compared to their HIV-negative peers.6 Effective integration of cervical cancer screening into differentiated ART delivery models, including safer conception services, is critical if cervical cancer screening uptake is to be effectively supported and expanded in the era of decentralization of ART care and in the absence of large-scale national cervical cancer screening programs in LMICs. This project demonstrates that such integration is feasible and detects high levels of significant pathology. Our findings support others' calls for South Africa to update its cervical cancer screening policies, to shift to HPV testing with integrated STI screening via the use of point-of-care testing technologies, accompanied by an expansion of “see and treat” services to relieve the pressure on limited and overstretched colposcopy services.
Footnotes
Conflict of Interest: None declared.
Sources of Funding: This work was partially supported through USAID, Cooperative Agreement AID-674-A-12-00021 (Health System Strengthening ABF 393). USAID funding provided support for the costs of safer conception clinic staffing, training and development of job aides and the creation of information, education and communication materials to advertise the new service. The implementation took place at a Gauteng Department of Health (DOH) facility supported by DOH staff. The authors' views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government.
Acknowledgements: The authors are grateful to the study participants and to the study team for their time and dedication to the work. The authors thank the Hillbrow Community Health Centre, the City of Johannesburg and the Department of Health for their support of the project. The authors thank USAID and the United States Government for providing funding support.
REFERENCES
- 1.World Health Organization (WHO). Comprehensive cervical cancer control: a guide to essential practice. 2nd ed Geneva: WHO, 2014. [PubMed] [Google Scholar]
- 2.Tsu V, Jeronimo J. Saving the world's women from cervical cancer. N Engl J Med 2016; 374:2509–2511. [DOI] [PubMed] [Google Scholar]
- 3.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: Low average levels and large inequalities. PLoS Med 2008; 5:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruni L, Barrionuevo-Rosas L, Albero G, et al. Human papillomavirus and related diseases in South Africa: Summary Report South Africa, 2017. [Google Scholar]
- 5.Teeraananchai S, Kerr SJ, Amin J, et al. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med 2017; 18:256–266. [DOI] [PubMed] [Google Scholar]
- 6.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. The Lancet HIV 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert CC, Chandler R, McMillan S, et al. Pap test adherence, cervical cancer perceptions, and HPV knowledge among HIV-infected women in a community health setting. The Journal of the Association of Nurses in AIDS Care : JANAC 2015; 26:271–280. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS. HPV, HIV and cervical cancer: Leveraging synergies to save women's lives. Geneva: UNAIDS, 2016. [Google Scholar]
- 9.Matthews LT, Beyeza-Kashesya J, Cooke I, et al. Consensus statement: Supporting safer conception and pregnancy for men and women living with and affected by HIV. AIDS Behav 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence. In: Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press, 2014. [Google Scholar]
- 11.Department of Health, Republic of South Africa. National contraception and fertility planning policy and service delivery guidelines. Pretoria; 2012.
- 12.Goggin K, Mindry D, Beyeza-Kashesyad J, et al. "Our hands are tied up": Current state of safer conception services suggests the need for an integrated care model. Health Care Women Int 2014; 35:990–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maimela G, Nene X, Sawry S, et al. Decentralising colposcopy services raises access to treatment of abnormal cervical smears: A pilot in an inner-city clinic in Johannesburg, South Africa. BMJ Open 2018; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botha MH, Dreyer G. Guidelines for cervical cancer screening in South Africa. Southern African Journal of Gynaecological Oncology 2017; 9(1):8–12. [Google Scholar]
- 15.Firnhaber C, Mayisela N, Mao L, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PloS one 2013; 8:e53494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royal College of Obstetricians and Gynaecologists. Reproductive outcomes after local treatment for preinvasive cervical disease: Scientific Impact Paper No 21. London: Royal College of Obstetricians and Gynaecologists, 2016. [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Information 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706. [DOI] [PubMed] [Google Scholar]
- 19.Aranda S, Berkley S, Cowal S, et al. Ending cervical cancer: A call to action. Int J Gynaecol Obstet 2017; 138(Suppl 1):4–6. [DOI] [PubMed] [Google Scholar]
- 20.Were E, Nyaberi Z, Buziba N. Integrating cervical cancer and genital tract infection screening into mother, child health and family planning clinics in Eldoret, Kenya. Afr Health Sci 2010; 10:58–65. [PMC free article] [PubMed] [Google Scholar]
- 21.Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population-level scale-up of cervical cancer prevention services in a low-resource setting: Development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PloS one 2015; 10:e0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanckenberg ND, Oettle CA, Conradie HH, et al. Impact of introduction of a colposcopy service in a rural South African sub-district on uptake of colposcopy. South African Journal of Obstetrics and Gynaecology. 2013; 19:81–85. Available at: http://www.sajog.org.za/index.php/SAJOG/article/view/388/410. Accessed October 10, 2018. [Google Scholar]
- 23.Campos NG, Lince-Deroche N, Chibwesha CJ, et al. Cost-effectiveness of cervical cancer screening in women living with HIV in South Africa: A mathematical modeling study. J Acquir Immune Defic Syndr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox JT, Castle PE, Behrens CM, et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: Results from the ATHENA HPV study. American journal of obstetrics and gynecology 2013; 208:184 e1–84 e11. [DOI] [PubMed] [Google Scholar]
- 25.Mudau M, Peters RP, De Vos L, et al. High prevalence of asymptomatic sexually transmitted infections among human immunodeficiency virus-infected pregnant women in a low-income South African community. Int J STD AIDS 2017; 956462417724908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low N, Broutet N, Adu-Sarkodie Y, et al. Global control of sexually transmitted infections. Lancet 2006; 368:2001–2016. [DOI] [PubMed] [Google Scholar]
- 27.Huchko MJ, Maloba M, Nakalembe M, et al. The time has come to make cervical cancer prevention an essential part of comprehensive sexual and reproductive health services for HIV-positive women in low-income countries. J Int AIDS Soc 2015; 18(Suppl 5):20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International AIDS Society. Differentiated care for HIV: A decision Framework for Differentiated Antiretroviral Therapy and Delviery for Children, Adolescents and Pregnant and Breastfeeding Women. Paris, France: International AIDS Society, 2017. [Google Scholar]
- 29.Soncini E, Zoncada A, Condemi V, et al. Reduction of the risk of cervical intraepithelial neoplasia in HIV-infected women treated with highly active antiretroviral therapy. Acta bio-medica : Atenei Parmensis 2007; 78:36–40. [PubMed] [Google Scholar]
- 30.Schuman P, Ohmit SE, Klein RS, et al. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis 2003; 188:128–136. [DOI] [PubMed] [Google Scholar]
- 31.Sirera G, Videla S, Lopez-Blazquez R, et al. Highly active antiretroviral therapy and incidence of cervical squamous intraepithelial lesions among HIV-infected women with normal cytology and CD4 counts above 350 cells/mm3. J Antimicrob Chemother 2008; 61:191–194. [DOI] [PubMed] [Google Scholar]
- 32.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis 2010; 201:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holme F, Kapambwe S, Nessa A, et al. Scaling up proven innovative cervical cancer screening strategies: Challenges and opportunities in implementation at the population level in low- and lower-middle-income countries. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2017; 138(Suppl 1):63–68. [DOI] [PubMed] [Google Scholar]
- 34.Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: A systematic global review. Int J STD AIDS 2014; 25:163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman-Wang T, Walker P. Colposcopy in special circumstances: Pregnancy, immunocompromise, including HIV and transplants, adolescence and menopause. Best practice & research Clin Obstet Gynaecol 2011; 25(5):653–665. [DOI] [PubMed] [Google Scholar]
- 36.Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: A systematic review and meta-analysis. BJOG 2011; 118:1031–1041. [DOI] [PubMed] [Google Scholar]
- 37.Rees H, Delany-Moretlwe S, Scorgie F, et al. At the Heart of the Problem: Health in Johannesburg's Inner-City. BMC Public Health 2017; 17(Suppl 3):554. [DOI] [PMC free article] [PubMed] [Google Scholar]


