ABSTRACT
Purpose
The treatment of partial-thickness rotator cuff tears (PTRCT) remains controversial. Few studies have focused on the conservative and new measurements of small to medium PTRCT. The use of sodium hyaluronate (SH) or platelet-rich plasma (PRP) as a method for rotator cuff repair requires further investigation. The aim of this study was to evaluate the combined use of SH and PRP in the treatment of small to medium PTRCT.
Study Design
A double-blinded randomized trial was used in this study.
Methods
Individuals with PTRCT detected by clinical examination and magnetic resonance imaging (MRI) were included in this study. The patients were randomly assigned to receive subacromial injections of normal saline, SH, PRP, or SH + PRP once a week for 4 wk. The primary outcome measure was the Constant score, and the secondary outcomes included the American Shoulder and Elbow Surgeons (ASES) and the visual analog scale scores. All of the clinical outcomes were assessed at pretreatment and 1, 3, 6, and 12 months posttreatment. MRI was used to evaluate the evolution of the cuff defect after 1 yr.
Results
The PRP group and the SH + PRP group showed a significantly higher Constant score and ASES score after the treatments. There were significant differences between the SH + PRP group and the SH or PRP group at 12 months in the Constant, visual analog scale, and ASES scores. MRI results showed that the tear size significantly decreased in both the PRP and the SH + PRP groups, especially in the SH + PRP group.
Conclusion
Our study provided evidence of the efficacy of PRP injection in the healing of small to medium PTRCT. Moreover, the combined injection of SH and PRP yielded a better clinical outcome than SH or PRP alone.
Key Words: PLATELET-RICH PLASMA, SODIUM HYALURONATE, ULTRASOUND-GUIDED INJECTION, PARTIAL-THICKNESS ROTATOR CUFF TEARS
Rotator cuff tears (RCT) are characterized by pain and activity limitation and accounts for approximately 20%–40% of shoulder joint diseases (1). On the basis of the tear size, RCT can be divided into full-thickness tears and partial-thickness tears (2). Although relatively few studies are available on the natural history and progression of partial-thickness rotator cuff tears (PTRCT), there is substantial clinical evidence to suggest that most partial tears lack self-healing ability (3). We reviewed several studies on full-thickness RCT, but limited clinical reports were found regarding PTRCT. Currently, most of the studies on PTRCT have focused on surgical techniques or outcomes, whereas few studies have focused on conservative therapy. Several conservative treatments are available to treat PTRCT, such as nonsteroidal anti-inflammatory drugs, corticosteroid injections, pain medications, and physical therapy (4). However, these treatments were required repeatedly, and they do not affect the progression of the disease. Recent reports also questioned the efficacy of such treatments and suggested that they can merely relieve clinical symptoms but cannot enhance healing of the injured rotator cuff (5,6). Therefore, further studies are needed to search for an effective treatment for PTRCT.
Sodium hyaluronate (SH) is present in the extracellular matrix of soft connective tissue and synovial fluid, exerting various physiological roles in tissues (7). Studies have shown that patients with rotator cuff injuries who are treated with SH had an obvious reduction in pain and improvement in range of motion and daily life activities (8–10). A review conducted by Osti et al. (11) also showed that injection of SH was significantly effective in treating RCT without severe adverse reactions. However, although SH viscosupplementation has a symptomatic effect, it does not act on the degenerative process of the rotator cuff, and its long-term effects remain unclear.
Recently, there has been a trend toward using blood derivatives to promote the relief of joint pain and the healing process in injured muscles and tendons. Platelet-rich plasma (PRP) is a platelet concentrate centrifuged from autologous whole blood with high concentrations of platelets that, once activated, can release growth factors that promote the regeneration of injured tissue. Clinical studies and animal experiments demonstrated the ability of PRP to enhance tendon repairs (12–14). In 2008, Randelli et al. (15) initially reported the effect of PRP in enhancing the recovery of arthroscopic rotator cuff repairs as evidenced by improved visual analog scale (VAS), University of California at Los Angeles, and Constant scores. Later studies tested the ability of PRP to promote the recovery of injured rotator cuffs (16–19). Recent systematic reviews have concluded that PRP use does not universally improve retear rates or affect clinical outcome scores at the time of arthroscopic rotator cuff repair (20,21). However, patients with small to medium RCT seem to show better outcomes with PRP than without it (19,22,23). Further studies of PRP efficacy and its effect on postoperative pain, functional outcome, and the structural integrity of the rotator cuff repair are therefore warranted.
Clinically, PTRCT is a common diagnosis; however, the underlying mechanisms and optimized treatments for PTRCT are still being explored. To the best of our knowledge, few recent studies have investigated the potential effect of PRP in the treatment of small to medium PTRCT, with even fewer studies investigating the combination of PRP and other drugs. In this study, we focused on the effect of SH combined with PRP injection in the subacromial space guided by ultrasound (US) in PTRCT. We hypothesize that the combination of SH and PRP injection is effective for bursal-sided PTRCT.
MATERIALS AND METHODS
Study design
Approval for this trial was obtained from the affiliated Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, and informed consent was obtained before the study. From January 2014 to May 2016, 184 patients with PTRCT diagnosed by clinical examination and magnetic resonance imaging (MRI) were included in this prospective, randomized, controlled, double-blind trial. All patients were bursal-sided tears and met the inclusion and exclusion criteria (Fig. 1). We measured the AP tear size with scores 1 and 2 by 3.0 T MRI (score 0, no tear; score 1, <5 mm; score 2, 5–10 mm; score 3, >10 mm) (2).
FIGURE 1.
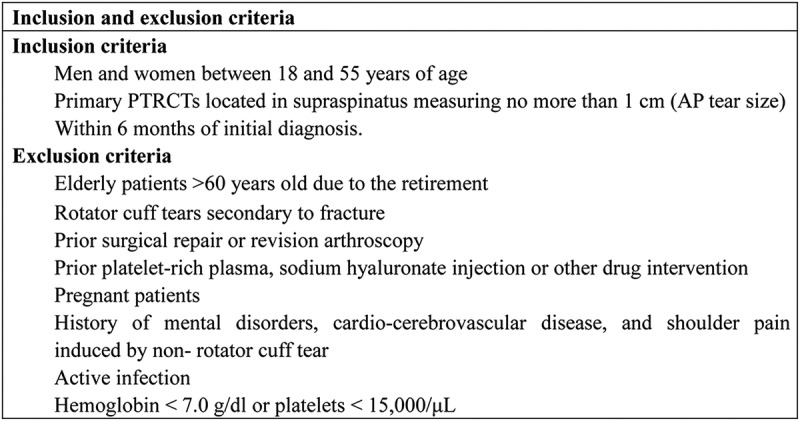
Inclusion and exclusion criteria. PTRCT, partial-thickness RCT. AP, anterior and posterior position.
The patients were randomly divided into the normal saline (NS) group, SH group, PRP group, and SH + PRP group by simple randomization method, using computer-generated simple random tables. The subacromial injection was administered consecutively once a week for 4 wk. All injections were performed under US guidance with a high-frequency (5–12 MHz) linear-array probe device (PHILIPS IE22, Amsterdam, Netherlands). The NS group received 4 mL of NS. The SH group received 4 mL of SH (Haohai Biological Technology, Shanghai, China). The PRP group received 4 mL of PRP, and the SH + PRP group was treated with 2 mL of PRP and 2 mL of SH. The patients and outcome assessor were all blinded throughout the study.
Outcome measures
The primary outcome was evaluated using the Constant score. The secondary outcome measures were the American Shoulder and Elbow Surgeons (ASES) and the VAS scores. The VAS, which ranges from 0 for no pain to 10 for severe pain, was used to evaluate pain during motion. Pretreatment evaluation was performed to establish baseline scores. Repeated questionnaires were also administered at 1, 3, 6, and 12 months posttreatment.
MRI
PTRCT was detected by MRI (3 T), which was conducted by an experienced musculoskeletal radiologist. MRI was performed at pretreatment and 12 months posttreatment. The same radiologist read both the pre- and the posttreatment MRIs. Approximately 3-mm cuts were obtained with a 0.3-mm gap between successive cuts. T1- and proton density-weighted fat-saturated images (axial, sagittal, and coronal) and T2- and proton density-weighted fat-saturated images (axial, sagittal, and coronal) were obtained. AP tear size is measured on sagittal T2-weighted images at anterior and posterior position (2).
PRP preparation
The PRP was prepared by reference to Spaková et al. (24). An autologous venous blood sample (20 mL) collected from each patient was injected into an anticoagulant tube (sterile sodium citrated tubes). The total blood was first centrifuged at 4°C for 10 min at 1500 rpm. Then all plasma and the upper one-third of red blood were transferred to a new sterile tube. The second centrifugation was implemented at 4°C for 10 min at 2500 rpm, and the supernatant (5–6 mL) without deposition was collected for the following experiments. The number of platelets in the PRP reached 1 × 1012 L−1, and PRP was a low-WBC concentration. All procedures were followed using aseptic techniques.
Subacromial injection
Patients were taken to an injection room and were informed of the necessary side effects. The injection processes were based on standardized sterile techniques. After standardized sterile preparation and local anesthesia administration (1% lidocaine to numb the skin), a standard double needle (a 25-gauge spinal needle through a 20-gauge introducer needle) was injected into the subacromial space by US guidance. Then a new sterile syringe containing the treatment was injected into the subacromial space through the introducer needle. Finally, the area was disinfected, and a compression bandage was placed. An ice pack was recommended to avoid local discomfort for the first 24 h posttreatment.
Follow-up
After the final injection, patients were followed-up at 1, 3, 6, and 12 months, and follow-up questionnaires were conducted by an independent observer. Nine patients were not included in this study (intolerance, two cases; loss to follow-up, seven cases).
Data analysis
The statistician was blinded to the study, and statistical analyses were completed using SPSS 21.0. Baseline differences between groups were analyzed by ANOVA for continuous data and by the χ2 test for categorical data. Continuous data are expressed as the mean ± SD, and the normality of distribution was tested by the Q–Q plot. Data were analyzed using two-way ANOVA, and a value of P < 0.05 was considered statistically significant.
The study sample size was calculated assuming a type 1 error of 0.05 and a type 2 error of 20%; With use of our previous data, a sample size of 50 patients per group was determined to be sufficient to detect a 20% difference in Constant score at final follow-up.
RESULTS
Flow chart
A total of 262 patients were screened from January 2014 to May 2016, and 62 patients were excluded because they declined to participate in the study (Fig. 2). Sixteen patients (8%) were lost to follow-up (Fig. 2). Of these patients, 20.7% (38 patients) had score 1 tears, and 79.3% (146 patients) had score 2 tears. The baseline clinical demographics and characteristics were comparable among the four groups (Table 1).
FIGURE 2.
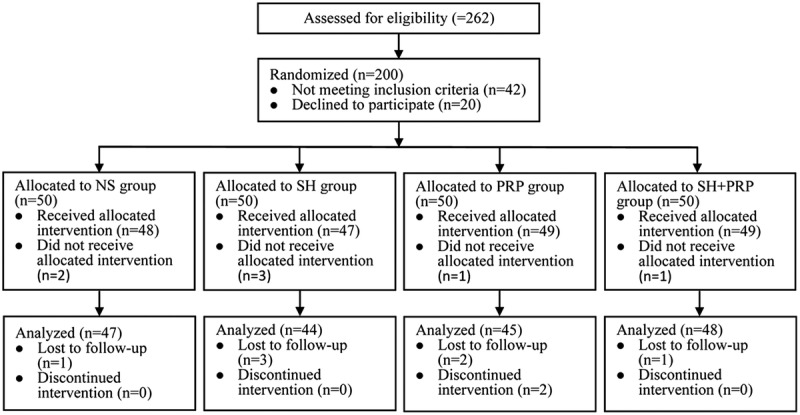
Flow chart of study participants.
TABLE 1.
Patient data.
Constant and ASES scores
The Constant and ASES scores in the NS group were not significant at any time point (Table 2). In the SH group, the Constant score gradually improved after pretreatment, whereas the ASES score increased significantly at 3 months, followed by a gradual decline (Table 2). In the PRP and the SH + PRP groups, the Constant and ASES scores had a similar upward trend after the final injection (Table 2). At 1 and 3 months, the Constant and ASES scores in the SH and SH + PRP groups, especially in the SH + PRP group, showed obvious improvement compared with those in the NS group (Fig. 3A and B). Interestingly, the Constant and ASES scores were higher in the PRP and SH + PRP groups compared with the SH and NS groups at 6 months, and there was a similar consistent trend after 6 months (Fig. 3A and B).
TABLE 2.
ASES, Constant, and VAS scores.
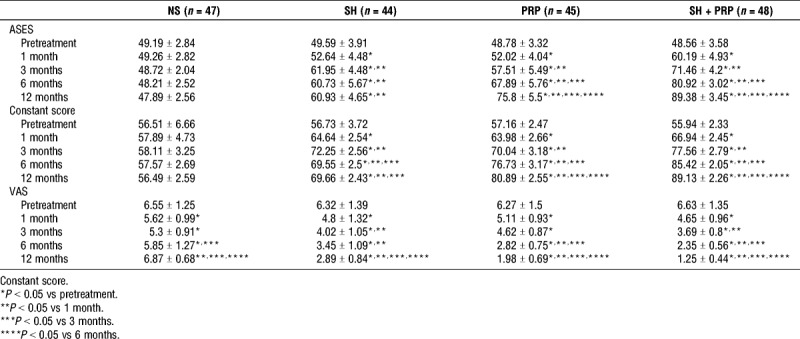
FIGURE 3.
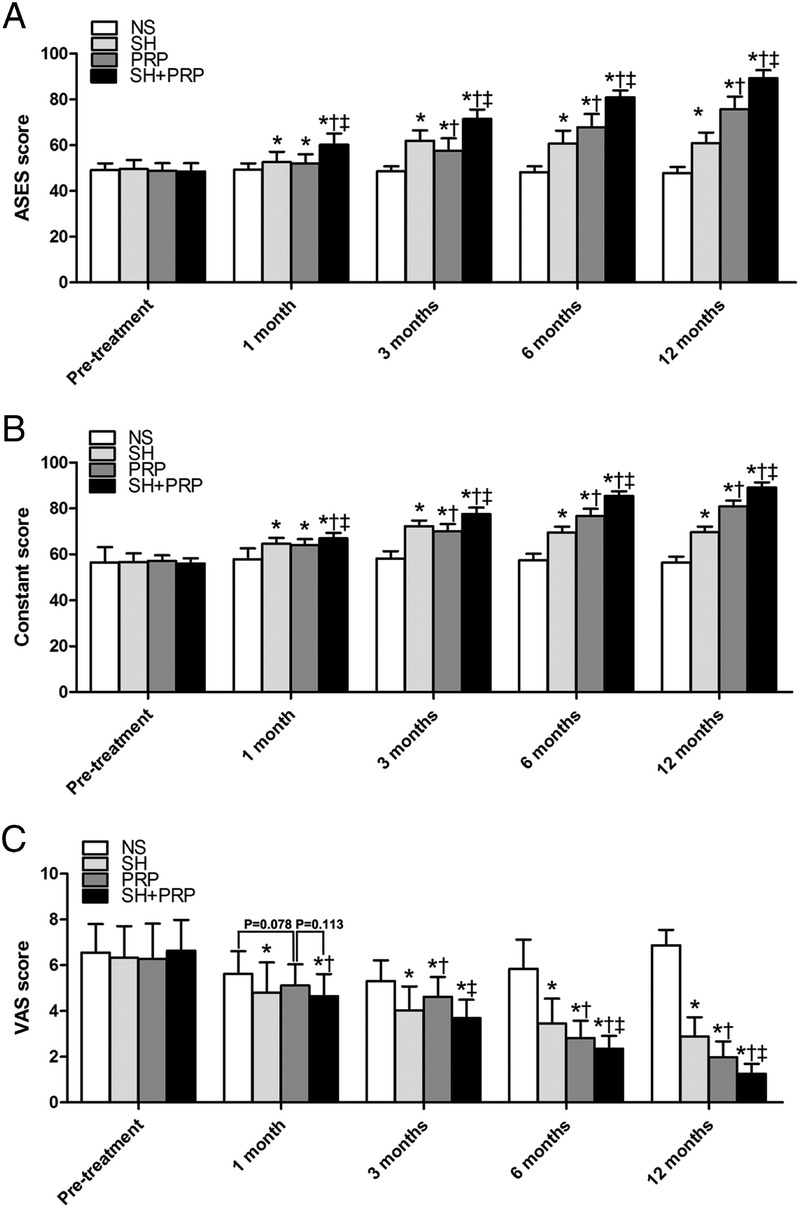
A, B, and C, The mean ASES scores, Constant scores, and VAS scores of the four groups, respectively. *P < 0.01 vs NS. †P < 0.01 vs SH. ‡P < 0.01 vs PRP.
Pain
The VAS scores were similar among the four groups at pretreatment and had similar downward trends throughout the follow-up period in the SH, PRP, and SH + PRP groups (Table 2). In the NS group, the VAS score showed a downward trend at 1 and 3 months, but no reduction in pain was observed after 3 months (Table 2). In the SH, PRP, and SH + PRP groups, the VAS scores were significantly lower at the final injection compared with pretreatment (Table 2). The analysis revealed that the VAS scores in the SH and SH + PRP groups were significantly lower than those in the NS and PRP groups at 1 month, but no significant difference in scores was found between the SH and the SH + PRP groups (Fig. 3C). At 3 months, the VAS score in the NS group was higher than that in the other groups (Fig. 3C). The SH + PRP group had the greatest VAS score improvement compared with the other groups at 6 and 12 months (Fig. 3C).
AP tear size measured by MRI
We determined the healing degree by calculating the difference in AP tear size between pretreatment and 12 months using MRI (2). A positive value indicates improvement, whereas a negative value indicates deterioration. After 1 yr, the healing degree in the SH + PRP group was more significantly improved compared with that in the NS, SH, and PRP groups (Table 3). The healing degree in the PRP group tended to be better than that in the NS and SH groups (Table 3).
TABLE 3.
AP tear size.

DISCUSSION
Our study demonstrated the following outcomes: 1) PRP enhanced the recovery of small to medium bursal-sided PTRCT by alleviating pain and improving the Constant and ASES scores, and 2) SH in combination with PRP had a better effect compared with PRP injection alone.
Results and comparison with previous studies
Clinical evidence suggests that injections of SH are effective in shoulder pain relief and joint function improvement (25). Shibata et al. (26) and Chou et al. (27) found that SH was effective and well tolerated for the treatment of rotator cuff injury with or without complete tears. Recent studies also demonstrated that the subacromial injection of SH guided by US was effective in treating rotator cuff disease (11,28,29). However, the above studies had the limitations of no randomization, a short follow-up time, and a relatively small sample size. Very few studies have included longer follow-up periods in patients with PTRCT who underwent subacromial SH injection. In our study, the injection of NS into the subacromial space may have relieved shoulder pain, but the results were transient. The SH group showed better clinical outcomes at the 12-month follow-up visit. However, the Constant and ASES scores in the SH group did not show a long-term improvement after 3 months postinjection. The optimal injection medications and their possible combination with current therapies need to be investigated to provide long-term benefits.
A retrospective cohort comparison conducted by Jiménez-Martin et al. (30) showed that PRP had a role in improving the pain score and reducing rehabilitation time in patients who underwent arthroscopic subacromial surgery. The following studies also demonstrated that PRP could augment tissue healing in arthroscopic rotator cuff repair, which was consistent with our findings and supports a possible effect of PRP on the reduction of joint pain (16–19). However, Castricini et al. (31) and Jo et al. (32) reported no differences in pain scores and functional scores between the PRP and the control groups in patients undergoing rotator cuff surgery. The above studies did not use the US-guided injection method and instead used a mixture placed on the surface of the injured tissue, which may limit comparisons with the present study. The intervention was implemented during the surgery, and researchers did not observe the continuous long-term effect postoperation. Holtby et al. (22) and Hak et al. (33) used a PRP injection for the repair of rotator cuffs postoperation and analyzed clinical outcomes. They found that there was no significant difference in outcome measures between the PRP and the control groups in patients with arthroscopically repaired RCT. Some limitations existed in this study, including a short follow-up period and patients with tear sizes above the 3-cm limit. In view of these findings, the application of PRP injections in PTRCT guided by US had clinical value and should be further explored.
These controversies inspired us to assess the effect of PRP injections on PTRCT at 1 and 3 months to investigate the early phases of cuff healing, 6 months to study the middle phase of cuff healing, and 12 months to study the long-term outcome. The Constant and ASES scores in the PRP group were significantly improved compared with those in the NS group as early as the first month. One month later, the scores showed different curves over time between the NS and the PRP groups. The Constant and ASES scores in the PRP group showed continuous improvement, whereas little change was observed in the NS group. A lower VAS score was obtained in the PRP group at 1 and 3 months posttreatment, but the medium- to long-term follow-up demonstrated that the VAS score in the PRP group decreased significantly compared with that in the NS group. Our results are consistent with those of a recent study conducted by Zafarani et al. (19), which showed that PRP injection had a positive effect on improved pain, function, and shoulder joint range of motion in PTRCT.
In the existing reports, no randomized clinical trial investigated the results in patients experiencing small to medium PTRCT after injection with SH + PRP. Meanwhile, there is a lack of knowledge regarding the mechanism by which PRP exerts its role. Under US guidance, SH in combination with PRP was injected into the subacromial and helped repair the injured rotator cuff. Compared with previous studies, this study showed that a minimally invasive injection can prevent the influence of surgical trauma and the stress response on the assessment of the therapeutic effect of PRP. On the basis of our results, we believe that SH + PRP could significantly improve the function of the shoulder, as demonstrated by the Constant and ASES scores, and relieve pain, as demonstrated by the VAS score, as these scores were better in the SH + PRP group compared with the SH and PRP groups, as shown previously.
Potential mechanisms
In our study, the PRP volume used for the PRP-only group was almost twofold greater than that used for the SH + PRP group (4 vs 2 mL). Interestingly, SH + PRP showed better results than PRP in patients with PTRCT. On the basis of this observation, we may infer that the mechanisms exerted by SH and PRP may be additive when both products are injected together without altering their original relevant characteristics. The potential mechanisms are as follows:
1) These positive clinical results can be related to the ability of both SH and PRP to regulate various healing mechanisms in the tendon and to present similar mechanisms of biological action without immunogenicity, enhancing tendon healing and reducing inflammatory activities and pain mediators (34–36). 2) The compound provides a closed system and a cell-friendly SH network that can increase the residence time of the growth factors and facilitates their release to the injured rotator cuff. The molecular diffusion and presentation of the proteins to their receptors located in the cytoplasmic membrane of the target competent cell could therefore be facilitated.
CONCLUSION
To date, no clinical study has investigated the treatment of PTRCT using the SH and PRP combination, and the potential mechanisms of SH/PRP on PTRCT remain unclear. However, excellent results of the SH + PRP combination have been reported in clinical trials (34,37,38). Our findings showed the positive utility of PRP for the treatment of rotator cuff injury and the cumulative effect of repeated injection, which were consistent with the outcomes of previous studies. In addition, we first performed the application of SH in combination with PRP during the PTRCT healing process, which indicated that the SH + PRP combination was better than SH or PRP alone. We conclude that this method is safe, reliable, and effective, with good clinical outcomes, for the treatment of PTRCT. With the advancement of research, future long-term studies need to be performed to corroborate the findings of this study before this treatment becomes widely accepted.
Limitations of the study
The limitations of this study include the unknown optimal concentration and dosage of PRP, the unknown optimal administration time, and the time points at which data were collected. Furthermore, more cases and larger multicenter studies are warranted to verify the effects of SH in combination with PRP in PTRCT. Currently, there is no widely accepted classification system for PTRCT, making comparison of different studies difficult because partial tears can vary widely in size and involve the articular, bursal, or both sides of the rotator cuff tendon. Longer-term studies evaluating the treatment of partial RCT, using a standardized classification system, are clearly needed before any treatment algorithm can be fully validated.
The selection of nonoperation measurements and surgical indications regarding articular tears and bursal-sided tears are different. Whether effective clinical outcomes existed in both articular tears and bursal-sided tears remains unclear. Finally, PRP was mainly classified into leukocyte PRP and pure PRP. The choice of different PRP preparations for the treatment of PTRCT should be further determined.
Acknowledgments
The authors thank Jinyun Pu, Department of Pediatrics, Tongji Hospital, Huazhong University of Science and Technology, and Ruoyu Zhao, Department of Anesthesiology, University of Mississippi Medical Center, for their advice on the article. Fundings were received from the National Natural Science Foundation of Wuhan (grant no. WX18Q21 to Y. C.), the National Natural Science Foundation of Hubei (grant no. 2018CFB568 to Z. X. S.), and the National Natural Science Foundation of China (grant no. 81701716 to Z. X. S.).
The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and the results of the study do not constitute endorsement by the American College of Sports Medicine. The authors declare no competing interests. Trial Registration: Chinese Clinical Trial Registry ChiCTR-IPR-17013172.
Y. C. and P. F. Z. conceived and designed the experiments. Y. C., B. K. L., Z. Q. S., and Z. X. S. performed the experiments. T. X. analyzed the data. Y. C. and P. F. Z. wrote the article.
Footnotes
Yu Cai, Zhenxing Sun, and Bokai Liao contributed equally to this work.
REFERENCES
- 1.Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer S, Wang A, Butler R, et al. Reliability of a 3 T MRI protocol for objective grading of supraspinatus tendonosis and partial thickness tears. J Orthop Surg Res. 2014;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff AB, Sethi P, Sutton KM, et al. Partial-thickness rotator cuff tears. J Am Acad Orthop Surg. 2006;14:715–25. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi F, Papalia R, Del Buono A, et al. Repair of partial tears of the rotator cuff. Sports Med Arthrosc. 2011;19(4):401–8. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez CM, Litchfield R, Jackowski D, et al. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis. Am J Sports Med. 2005;33(2):255–62. [DOI] [PubMed] [Google Scholar]
- 6.Lee WH, Do HK, Lee JH, et al. Clinical outcomes of conservative treatment and arthroscopic repair of rotator cuff tears: a retrospective observational study. Ann Rehabil Med. 2016;40(2): 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaine T, Moskowitz R, Udell J, et al. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial. A multicenter study. J Bone Joint Surg Am. 2008;90:970–9. [DOI] [PubMed] [Google Scholar]
- 8.Costantino C, Olvirri S. Rehabilitative and infiltrative treatment with hyaluronic acid in elderly patients with rotator cuff tears. Acta Biomed. 2009;80(3):225–9. [PubMed] [Google Scholar]
- 9.Honda H, Gotoh M, Kanazawa T, et al. Hyaluronic acid accelerates tendon-to-bone healing after rotator cuff repair. Am J Sports Med. 2017;45:3322–30. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Park JY, Lee CS, et al. Does hyaluronate injection work in shoulder disease in early stage? A multicenter, randomized, single blind and open comparative clinical study. J Shoulder Elbow Surg. 2012;21(6):722–7. [DOI] [PubMed] [Google Scholar]
- 11.Osti L, Buda M, Buono AD, et al. Clinical evidence in the treatment of rotator cuff tears with hyaluronic acid. Muscles Ligaments Tendons J. 2016;5(4):270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Jones IA, Park C, et al. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46:2020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasterlain AS, Braun HJ, Harris AH, et al. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41(1):186–93. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Al-Ani MK, Sun Y, et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2017;11(4):1173–84. [DOI] [PubMed] [Google Scholar]
- 15.Randelli PS, Arrigoni P, Cabitza P, et al. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20–22):1584–9. [DOI] [PubMed] [Google Scholar]
- 16.Chung SW, Song BW, Kim YH, et al. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med. 2013;41(12):2909–18. [DOI] [PubMed] [Google Scholar]
- 17.Pandey V, Bandi A, Madi S, et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25(8):1312–22. [DOI] [PubMed] [Google Scholar]
- 18.Randelli P, Arrigoni P, Ragone V, et al. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518–28. [DOI] [PubMed] [Google Scholar]
- 19.Zafarani Z, Mirzaee F, Guity M, et al. Clinical results of platelet-rich plasma for partial thickness rotator cuff tears: a case series. Arch Bone Jt Surg. 2017;5(5):328–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906–18. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Sun Y, Xu P, Cheng B. Can patients get better clinical outcomes by using PRP in rotator cuff repair: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. 2016;56(11):1359–67. [PubMed] [Google Scholar]
- 22.Holtby R, Christakis M, Maman E, et al. Impact of platelet-rich plasma on arthroscopic repair of small- to medium-sized rotator cuff tears: a randomized controlled trial. Orthop J Sports Med. 2016;4(9):2325967116665595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43:3071–6. [DOI] [PubMed] [Google Scholar]
- 24.Spaková T, Rosocha J, Lacko M, et al. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–7. [DOI] [PubMed] [Google Scholar]
- 25.Padua R, Alviti F, Padua L. Meta-analysis of hyaluronate injections for shoulder pain: comment on the article by Saito et al. Arthritis Care Res (Hoboken). 2010;62(11):1673; author reply 1673–4. [DOI] [PubMed] [Google Scholar]
- 26.Shibata Y, Midorikawa K, Emoto G, et al. Clinical evaluation of sodium hyaluronate for the treatment of patients with rotator cuff tear. J Shoulder Elbow Surg. 2001;10(3):209–16. [DOI] [PubMed] [Google Scholar]
- 27.Chou WY, Ko JY, Wang FS, et al. Effect of sodium hyaluronate treatment on rotator cuff lesions without complete tears: a randomized, double-blind, placebo-controlled study. J Shoulder Elbow Surg. 2010;19(4):557–63. [DOI] [PubMed] [Google Scholar]
- 28.Merolla G, Bianchi P, Porcellini G. Ultrasound-guided subacromial injections of sodium hyaluronate for the management of rotator cuff tendinopathy: a prospective comparative study with rehabilitation therapy. Musculoskelet Surg. 2013;97(1 Suppl):49–56. [DOI] [PubMed] [Google Scholar]
- 29.Moghtaderi A, Sajadiyeh S, Khosrawi S, et al. Effect of subacromial sodium hyaluronate injection on rotator cuff disease: a double-blind placebo-controlled clinical trial. Adv Biomed Res. 2013;2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez-Martin A, Angulo-Gutiérrez J, González-Herranz J, et al. Surgery of subacromial syndrome with application of plasma rich in growth factors. Int J Shoulder Surg. 2009;3(2):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–65. [DOI] [PubMed] [Google Scholar]
- 32.Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082–90. [DOI] [PubMed] [Google Scholar]
- 33.Hak A, Rajaratnam K, Ayeni OR, et al. A double-blinded placebo randomized controlled trial evaluating short-term efficacy of platelet-rich plasma in reducing postoperative pain after arthroscopic rotator cuff repair: a pilot study. Sports Health. 2015; 7(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WH, Lin CM, Huang CF, et al. Functional recovery in osteoarthritic chondrocytes through hyaluronic acid and platelet-rich plasma-inhibited infrapatellar fat pad adipocytes. Am J Sports Med. 2016;44:2696–705. [DOI] [PubMed] [Google Scholar]
- 35.Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339–46. [DOI] [PubMed] [Google Scholar]
- 36.Mitsui Y, Gotoh M, Nakama K, et al. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26(7):1032–7. [DOI] [PubMed] [Google Scholar]
- 37.Abate M, Verna S, Schiavone C, et al. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur J Orthop Surg Traumatol. 2015;25:1321–6. [DOI] [PubMed] [Google Scholar]
- 38.Andia I, Abate M. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? Expert Opin Biol Ther. 2014;14:635–49. [DOI] [PubMed] [Google Scholar]


