Abstract
There are limited data on the role of neck ultrasound (US) in the surveillance of patients with follicular thyroid cancer (FTC). Here, we analyze the likelihood of US to find structural disease in patients with FTC and evaluate if initial American Thyroid Association (ATA) risk stratification and the response to therapy categories [the latter based on thyroglobulin (Tg) levels] modify that likelihood. We conducted a retrospective cohort study of 32 patients with FTC in our institution. We included all patients with well-differentiated FTC who underwent total thyroidectomy and radioactive iodine (RAI) treatment without neck structural disease at the time of RAI and with Tg and US at least 6 months after RAI. After a median follow-up of 4.3 years, two patients (6.3%) had structural disease by US. None of the 18 patients with initial ATA low-risk disease had structural disease by US in contrast to higher, but not significant, frequency of 18.2% (2/11) in patients with initial ATA high-risk disease (p = 0.14). Based on Tg levels, 24/32 patients had excellent response to therapy and 8/32 had biochemical incomplete/indeterminate response. None of the patients with excellent response had structural disease by US versus 2/8 (25%) patients with biochemical incomplete/indeterminate response all of whom had other sites of structural disease (p = 0.054). Our findings suggest that neck US in FTC is unlikely to find structural disease with initial low-risk ATA or excellent response to therapy but can detect structural disease in some patients with initial ATA high-risk or incomplete/indeterminate responses to therapy.
Keywords: Follicular thyroid cancer, Ultrasound, Structural disease
Introduction
Follicular thyroid carcinoma (FTC) is the second most common thyroid malignancy after papillary thyroid carcinoma (PTC) with a rate estimated at 5–15% of all thyroid cancer specimens [1, 2]. There has been a reported decrease in the overall incidence of FTC in the past decades in the USA, which has been attributed to changes in histological diagnoses over time (detection of the follicular variant of PTC, exclusion of atypical follicular adenoma) and institution of iodine supplementation programs [3].
While both PTC and FTC are classified as subtypes of differentiated thyroid cancer, the two subtypes have biological differences. At the molecular level, there are differences in the somatic driver genetic alterations found in each of these two different types of DTC. In PTC, tumor-driving genetic alterations can be identified in more than 80% of tumors, including point mutations or rearrangement of the genes encoding BRAF (35–70% of PTCs), RAS (10%), TRK (< 5%), ALK (< 5%), and RET/PTC rearrangements (20%) [4]. In FTC on the other hand, only ~ 50% of tumors have identifiable driver alterations, most frequently mutations in the RAS family genes or PAX8-PPARγ, loss of PTEN expression, or mutations in the genes encoding phosphatidyl (OH) 3 kinase (PI3K) subunits [4].
In terms of the clinical behavior, PTC commonly metastasizes to locoregional lymph nodes, and when aggressive, the tumors often invade into extrathyroidal tissues and blood vessels [1, 5]. In contrast, lymph node involvement in FTC is uncommon at 0–10% at different studies [2, 3, 6, 7] and it more typically metastasizes to distant organs such as the lungs or bone (10–15% at the time of presentation) [2, 3]. Compared to PTC, lung metastases in FTC are twice more common, and bone metastases are 10 times more common on a per case basis [5, 8]. Brain metastases are less common overall in both histological types of tumors, but are more frequent in FTC [5].
The overall 5- and 10-year survival rates in patients with FTC are lower than those with PTC: 91 and 85% for patients with FTC versus 97 and 93% for patients with PTC [4]. Bone and brain metastases are particularly associated with poor outcomes [5]. In addition, the extent of tumor invasion [1–3, 5, 6, 8, 9], the degree of vascular invasion [6, 8, 9], and the presence of identifiable distant metastases [2, 6, 8, 9] at presentation are poor prognostic factors in FTC. Absence of RAI uptake has also been identified as a poor prognostic factor for FTC [7, 8]. These data have led to the categorization of FTCs as minimally invasive FTC (MI-FTC), when there is limited capsular and/or vascular invasion and widely invasive FTC (WI-FTC), when there is wide infiltration of adjacent thyroid tissue and/or blood vessels. WI-FTC corresponds to 9–39% of patients with FTC [6]. A more recent classification by the World Health Organization (WHO) in 2017 further classified these tumors into minimally invasive FTC where there is no vascular invasion, encapsulated angioinvasive FTC where there is minimal vascular invasion and widely invasive FTC where there is more significant vascular invasion [10]. Generally speaking, the less invasive FTC carries a much better prognosis compared to widely invasive FTC. The 2015 American Thyroid Association (ATA) guidelines consider absence of vascular invasion or presence of a minimal invasion of < 4 vessels as low-risk disease and presence of vascular invasion of at least 4 vessels as high-risk disease [11].
The overall management of both FTC and PTC includes primary surgery, with selective use of 131I [3]. Lymph node dissection in patients with FTC is usually performed only in unusual cases when there is suspicion for node involvement on preoperative imaging [3, 9]. RAI use in both PTC and FTC is individualized and primarily focused on patients with greatest risk of having residual or recurrent disease. In either case, once initial treatment is completed, surveillance ensues using primarily thyroglobulin and neck ultrasound or other cross-sectional imaging as indicated based on the sites of initial disease detection.
Neck ultrasound (US) is a sensitive method for detecting disease in patients with PTC in which nodal metastases are common; however, its role in FTC seems less certain and the published data are limited and to the best of our knowledge, only one study by Baek et al. [12] was specifically designed to investigate the role of neck ultrasound in monitoring patients with FTC [13–16]. Because of the paucity of data, guidelines do not clearly specify the optimal imaging modalities for monitoring patients with FTC and therefore there is not clear guidance to clinicians regarding surveillance of patients with FTC as compared to PTC. The primary aim of the study is to begin to address this knowledge gap to better inform clinical care by evaluating the likelihood of detection of structural disease by neck ultrasound in patients with FTC. The secondary aims are to determine if the likelihood of detection of structural disease by neck US differs based on either initial risk stratification or response to therapy categories according to on ATA classification to enable personalized approaches.
Patients and Methods
Patients and Data Collection
This is a retrospective cohort study conducted at The Ohio State University (OSU) Wexner Medical Center and the Ohio State Comprehensive Cancer Center. The study was approved by the Ohio State University Institutional Review Board. Patients were identified through a query of the Ohio State University Endocrine Neoplasia Repository. This repository was approved on 8/5/2006; enrollment is offered to all thyroid cancer patients seen subsequently in the OSU thyroid cancer clinic. All patients enrolled in ENR who had total thyroidectomy and RAI therapy prior to 12/31/2014 were eligible for the study. Data analysis was completed on 10/19/2016. We included all patients with well-differentiated FTC who underwent total thyroidectomy and RAI without evidence of structural disease in the neck at the time of RAI (however, we accepted patients, n = 1, who had distant structural disease at the time of RAI). All patients had a post-treatment whole body scan after their post-thyroidectomy RAI. Preparation was either with recombinant TSH or thyroid hormone withdrawal. All patients were placed on a low iodine diet for 2 weeks. The median I-131 dose was 101 mCi (range 29–692). All patients had a thyroglobulin (Tg) measurement and an ultrasound at least 6 months after RAI. Patients were excluded if they had Hurthle cell thyroid carcinoma (HCTC) given reported unique characteristics that are different from FTC [17], anaplastic or poorly differentiated thyroid cancer, if they underwent lobectomy instead of total thyroidectomy, or if they did not receive RAI.
Neck Ultrasound
Neck US was performed by thyroid cancer specialty endocrinologists at our institution except that early in their follow-up, six of these patients also had additional US evaluations performed by technician and supervised by attending radiologists either at our institution, or at other institutions. When performed at our institution, neck US was initially performed using a GE Logiq 200 Alpha with a 7.5-MHz linear probe, which was replaced in March 2005 by the Esoate Picus Pro with the LA523 13–4 MHz probe. In June 2007, the Biosound Esoate My Lab 25 with the same probe was added. After May 2010, US was performed utilizing the Logiq P5 ultrasound system manufactured by General Electric Medical Systems with the (12 L) linear array probe which has a frequency range of 5–15 Mhz and a field of view of 192 mm and the 8C probe which has a frequency range of 4–11 MHz and a field of view of 22 × 12 mm. US examinations routinely inspected the superior mediastinum, the bilateral central, and the bilateral lateral neck compartments. Through chart review, US findings were classified as suspicious, indeterminate, or negative. Additional imaging tests were performed at the physician’s discretion during clinical follow-up.
Laboratory Values
Over the course of the time of patient care since initiation of the repository, several different Tg (Tg IMA) and Tg antibody (anti-TgAb) assays were utilized. Prior to 2006, various assays for Tg and Tg antibodies were utilized as previously described [18]. After 2006, Tg was measured using the following assays and for the following time periods: (1) 2006–2013: Immulite 2000 XPi Thyroglobulin assay (Siemens Inc., Deerfield, IL, Tg-I) which has a functional sensitivity with a CV < 20% of 0.9 ng/mL. (2) 2013–2016: Beckman Access Tg (Beckman Coulter, Tg-B) with a functional sensitivity of 0.1 ng/mL. For anti-TgAb, the following assays were utilized (1) 2006–2013: Immulite 2000 XPi anti-Thyroglobulin Antibody assay (Siemens Inc., Deerfield IL; catalog no. L2KTG2; anti-Tg Abs-I) with an analytical functional sensitivity of 20 IU/mL or (2) 2013–2015: Roche assay (Anti-Tg Abs-R) with functional sensitivity of 20 IU/mL and (3) 2015–2016: Access Thyroglobulin Antibody II assay (Beckman Coulter, Anti Tg Abs-B.) with a functional sensitivity of 1.8 IU/mL. For the statistical analysis, a detectable level, as defined with the cut-points above for each assay, was considered to be a positive result that was utilized. In some cases, it was not possible to ascertain the precise assay used for some of the measurements (n = 6), as these measurements were performed at outside labs.
Clinical Follow-Up
The patients were usually seen every 3–12 months, and Tg was usually measured at the same intervals, at the clinician’s discretion. For each patient, the data collection ended on 10/19/2016. For patients with no evidence of structural disease at any point during their follow-up, we used their Tg level at the final follow-up to classify them as Tg excellent response versus Tg indeterminate/incomplete response according to the ATA guidelines [11] while for patients with evidence of structural disease at some point during their follow-up, we used the Tg level at the time of disease detection for that classification. For patients (n = 1) with evidence of structural disease at diagnosis, the first Tg level at the 6 months post-initial treatment was utilized.
Statistical Analysis
Patient demographics were summarized by frequency and percent for categorical variables and median with interquartile range (IQR) and range for continuous variables. Characteristics of patients with structural disease are reported at the individual patient level. Ultrasound results (positive or negative for structural disease) were cross-tabulated by the initial ATA pathology risk (low, high, unknown) and then by the Tg response (excellent or incomplete/indeterminate) to therapy according to the ATA guidelines. Fisher’s exact tests were used to compare the proportion of positive ultrasound results for low versus high ATA risk and excellent versus incomplete/indeterminate Tg responses.
Results
Patient Characteristics
Thirty-two patients met the inclusion criteria and had at least one sonographic evaluation. Most patients were female (68.8%), with a median age at diagnosis of 46 years. The average tumor size was 3.6 cm and was unifocal in 28/32 patients. The characterization of low-risk versus high-risk FTC was based either on the extent of vascular or extrathyroidal extension, when available, or on the report by the pathologist when the extent of vascular or extrathyroidal extension was not available in the record. Fifty percent of patients did not have pathologically confirmed lymph node disease around the time of thyroidectomy and 50% did not have any lymph node dissection at the time of surgery. At the time of diagnosis, distant metastases were identified in 1/32 (3.1%) patients. According to the American Thyroid Association (ATA) clinical practice guidelines [11], 56.3% of the patients were considered low risk and 34.4% of the patients were considered high risk (Table 1).
Table 1.
Demographics (based on 8th edition of AJCC [28]
| Characteristic | Patients (n = 32) |
|---|---|
| Age at diagnosis, median (IQR; range) | 46 (28–55; 16–83) |
| Sex | |
| Female | 22 (68.8%) |
| Male | 10 (31.3%) |
| T stage | |
| 1a | 0 (0%) |
| 1b | 9 (28.1%) |
| 2 | 11 (34.4%) |
| 3a | 11 (34.4%) |
| 3× | 0 (0%) |
| 4b | 1 (3.1%) |
| N stage | |
| N0 | 16 (50.0%) |
| N1 | 0 (0%) |
| Nx | 16 (50.0%) |
| M stage | |
| 0 | 31 (96.9%) |
| 1* | 1 (3.1%) |
| Size, median (IQR; range) | 3.6 (1.8–5.1; 1.2–8.1) |
| Multifocal | |
| Yes | 2 (6.3%) |
| No | 28 (87.5%) |
| Unknown | 2 (6.3%) |
| Vascular invasion | |
| Yes, < 4 blood vessels | 4 (12.5%) |
| Yes, ≥ 4 blood vessels | 8 (25.0%) |
| Yes, unknown number of blood vessels | 5 (15.6%) |
| No vascular Invasion | 14 (43.8%) |
| Unknown | 1 (3.1%) |
| Extrathyroidal extension (microscopic) | |
| Yes | 0 (0%) |
| No | 31 (96.9%) |
| Unknown | 1 (3.1%) |
| I131 cumulative dose, median (IQR; range) | 100 (50–135; 29–692) |
| ATA risk | |
| Low | 18 (56.3%) |
| Intermediate | 0 (0%) |
| High | 11 (34.4%) |
| Unknown | 3 (9.4%) |
| EBRT | |
| No | 30 (93.8%) |
| Neck | 2 (6.3%) |
| Bone | 0 (0%) |
| Systemic Rx-kinase inhibitors | 1 (3.1%) |
| TgAB | |
| Positive | 1 (3.1%) |
| Positive then negative | 8 (25.0%) |
| Duration of follow-up (IQR; range) | 4.3 (2.1–7.9; 0.5–19.9) |
IQR interquartile range (25th to 75th percentiles), range min to max, *M1 patients with metastatic disease at the time of initial therapy
Ultrasound Results
Thirty-two patients had at least one neck US evaluation 6 months after the RAI or later. Overall, each patient underwent 1–8 ultrasound evaluations during the period of the clinical follow-up (median follow-up of 4.3 years). Other imaging modalities were performed at the discretion of the treating physician. Ultrasound was positive in 2/32 patients (both pathology proven) (6.3%), both with distant metastases. There were two additional patients without structural disease in neck who had distant metastatic disease (total number of patients with structural disease: 4, described in Table 2). In none of the two patients with neck structural disease was neck ultrasound the only study to identify the structural disease in the neck: the first had soft tissue nodules versus lymph nodes initially seen by CT and then by US, and this finding led to neck debulking surgery and the second patient had a large paratracheal mass initially identified by US, and, as a result, underwent surgical removal of the mass.
Table 2.
Four patients with structural disease with details
| Patient ID | ATA risk at diagnosis | Tg (ng/mL)a | Neck US | Location of metastases | Treatments since initial therapy | Final clinical outcome |
|---|---|---|---|---|---|---|
| 14 | High | 0.7 | (−) | L | Surveillance | Structural incomplete |
| 22 | High | 617.7 | (+) | N, M, L | Neck debulking surgery, I-131, systemic therapy | Structural incomplete |
| 30 | High | 21.6 | (+) | N, L, Bo | I-131, neck dissection, EBRT, systemic therapy | Deceased |
| 31 | High | 47 | (−) | L, Bo | EBRT | Structural incomplete |
aAll patients had negative anti-thyroglobulin antibodies at the time of disease detection. The Tg level in table reflects either the level at the time of finding structural disease if that occurred during follow-up or 6 months after initial therapy if structural disease was found at time of diagnosis (n = 1: patient 31)
Tg unstimulated thyroglobulin, US ultrasound, (+) = positive, (−) = negative, N neck, M mediastinum, L lung, Bo bone
There were three patients with indeterminate findings on the neck ultrasound: two of them had undetectable Tg, and long-term US follow-up was consistent with residual benign thyroid tissue and the 3rd patient had positive Tg Abs that remained stable, with surgical excision of the indeterminate lesion showing benign fibroadipose tissue. Neck US was negative in all patients considered to be in excellent response state based on Tg level. It was positive in 2/8 (25%) patients with Tg level in the biochemical indeterminate response to therapy state.
There were four patients who had Tg/anti-Tg Abs values within the biochemical incomplete/indeterminate response range who did not have identifiable structural disease. The characteristics of these four patients and the imaging modalities utilized are described in Table 3.
Table 3.
Four patients with Tg biochemical incomplete/indeterminate disease and no structural disease
| Patient ID | ATA risk at diagnosis | Unstimulated Tg at final follow-up (ng/mL) | Tg antibody status at final follow-up (IU/mL) | Imaging performed during follow-up |
|---|---|---|---|---|
| 4 | Low | 0.8 | negative | I-131 post-Rx scan, US |
| 5 | Unknowna | < 0.1 | 10.1 | I-131 post-Rx scan, I-123 scan, US, CT neck and chest |
| 6 | Low | 0.2 | negative | I-131 post-Rx scan, US, CT chest, abdomen and pelvis |
| 32 | High | 1.9 | negative | I-131 post-Rx scan, I-123 scan, US, CT neck and chest, FDG-PET/CT, MRI brain, MRI bone |
aThere is vascular invasion, but exact number of blood vessels is unknown
Likelihood of Finding Structural Disease in Neck by US According to Initial ATA Risk Stratification and Response to Therapy:
None of the patients with initial ATA low-risk disease had structural disease in neck or at distant locations in contrast to 36.3% (4/11) (p = 0.01) of patients with initial ATA high-risk disease. In evaluating neck US in detecting structural disease, 18.2% (2/11) of the patients with ATA high-risk disease had structural disease (Fig. 1, p = 0.14 compared to ATA low-risk). Overall, based on the 2015 ATA clinical practice guideline criteria regarding Tg levels, 24 patients had excellent response to therapy, and 8 patients had biochemical incomplete or indeterminate response to therapy. None of the patients with excellent response to therapy based on the Tg value (Tg < 0.2 ng/mL or stimulated Tg < 1.0 ng/mL) had evidence of disease (neck or distant location) versus 4/8 (50%) of patients with indeterminate/incomplete response (p = 0.002). In evaluating neck US in detecting structural disease, 2/8 (25%) patients in the indeterminate/incomplete categories had structural disease in neck detected by US (Fig. 2, p = 0.057 compared to Tg excellent response category).
Fig. 1.
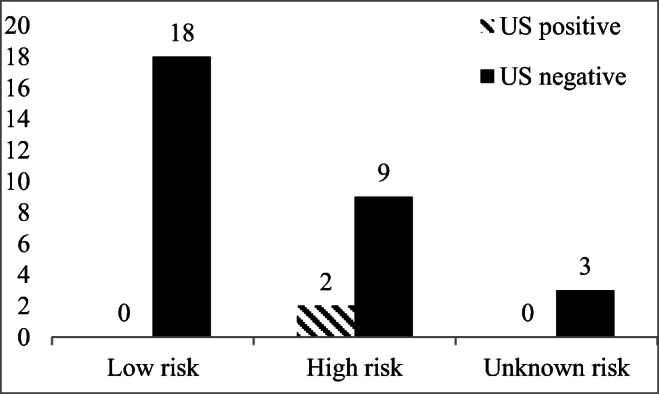
Relation between initial ATA Risk and finding structural disease on ultrasound (n = 32). Three patients in the US negative group had indeterminate US that was proven negative on follow up. Low risk versus high risk, p = 0.14, Fisher’s exact test
Fig. 2.
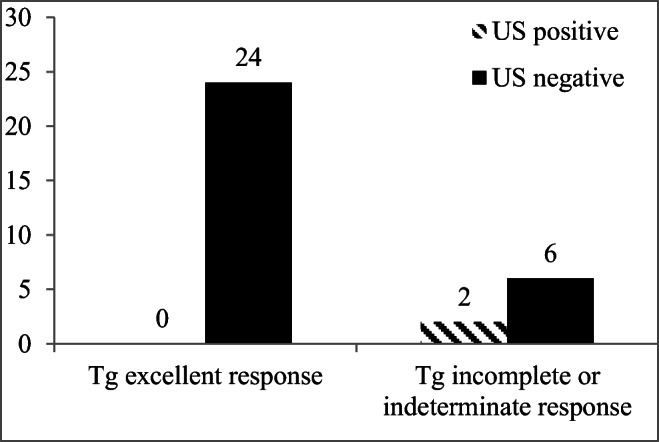
Relation between response to therapy categories based on Tg response (excellent or incomplete/indeterminate) and finding structural disease on ultrasound (n = 32). Patients in the US negative group had indeterminate US that was proven negative on follow-up. Tg excellent response versus Tg incomplete/indeterminate, p = 0.057, Fisher’s exact test
Discussion
There is limited information guiding the clinician regarding the appropriate imaging tests that should be used in their longitudinal clinical evaluation of FTC due to its low prevalence. While the ATA thyroid cancer guidelines have recommendations regarding the clinical follow-up of differentiated thyroid cancers, the vast majority of the data supporting these recommendations are derived from studies of patients with PTC [11]. Because of the differences between PTC and FTC, especially regarding the typical location of metastases, the utility of neck sonographic in patients with FTC is uncertain. Thus, we specifically aimed in this study to evaluate the likelihood that neck US would detect structural disease in patients with FTC. We studied the records of 32 patients with FTC who underwent total thyroidectomy and radioactive iodine treatment without evidence of structural disease in neck at time of RAI.
The overall neck disease prevalence in this population of patients with FTC was 6.3%. We identified that none of the ATA low-risk patients had structural disease, as compared to 18.2% (2/11) of the ATA high-risk patients (p = 0.14) and also none of the patients with Tg excellent response had structural disease, whereas 25% (2/8) patients with Tg biochemical/incomplete response had evidence of structural disease (p = 0.057). While the comparison between these groups was not statistically significant, this is probably related to low prevalence of structural disease in the neck and therefore need to have a higher number of patients. When comparing all structural disease however (neck or distant), there was a statistically significant higher likelihood of finding structural disease (neck and distant) in patients with initial ATA high risk compared to low risk and in Tg biochemical/indeterminate response to therapy compared to excellent response to therapy. While finding structural disease in neck in patients with proven distant metastases does not change the staging of thyroid cancer patients, it can be useful in identifying neck disease that can lead to increased morbidity related to locoregional invasion that may require therapeutic intervention in selected cases.
Various studies reviewed lymph node involvement and local recurrence in patients with FTC. Lymph node involvement at the time of surgery was reported in 2–10%, in patients with both MI-FTC and WI-FTC [19–24]. On the other hand, studies assessing the local recurrence in FTC are limited and report a more variable rate of 1–30% [9, 19, 25–27]. Therefore, overall, the expectation for local recurrence in the cervical lymph nodes or soft tissues is low, which affects the value of diagnostic US in these patients. Similar to our study, Baek et al. [12] retrospectively reviewed sonographic follow-up in patients with FTC and identified a 3.2% local recurrence rate at 37.5 ± 18.5 months after surgery. In our study, the rate of local recurrence detected by neck US was 2/32 (6.3%). The higher rate in our study is potentially due to a higher rate of patients with T3 and T4 tumors indicating a more aggressive disease. This is further supported by the frequent finding of more extensive disease using cross-sectional imaging in conjunction with ultrasound.
There are some limitations to our study. The sample size was small. In addition, this is a retrospective study and it was at the clinical discretion of the endocrinologist to order the imaging including neck US and standardization of choice of each imaging modality, number of re-evaluations using the same imaging modality, and intervals between the Tg measurements was not possible. Furthermore, our data represent a single center, which is a referral center for thyroid cancer, and a referral bias is likely. Another consideration is that our patients live in a region that is considered iodine sufficient, and the prevalence of FTC is known to increase in iodine-deficient areas [2, 3]. Finally, a possible limitation is the broad range of the years of diagnosis of FTC in our study (1992–2014). As the ultrasound imaging technology advanced, there may have been differences in the ability of detection of structural disease among patients diagnosed with FTC in the different time periods of this range.
In conclusion, our findings suggest that neck ultrasound in FTC as in PTC is unlikely to find structural disease in patients with low-risk initial ATA stratification and excellent response to initial treatment determined by Tg level. It also suggests that in patients with biochemical incomplete/indeterminate disease, neck US, even if positive, is not sufficient for identifying all structural disease in patients with FTC as distant metastases may be present but still may have utility for disease detection. Taken together, the data suggest that the use of neck ultrasound in patients with FTC should be limited to individuals with incomplete or indeterminate responses to initial therapy and performed in conjunction with other imaging modalities to assess for distant metastases. Future studies with larger number of patients are needed to confirm these findings.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Konstantinos Segkos, Phone: 801-396-0594.
Fadi A. Nabhan, Phone: 614-685-3343, Email: fadi.nabhan@osumc.edu
References
- 1.Aboelnaga EM, Ahmed RA. Difference between papillary and follicular thyroid carcinoma outcomes: an experience from Egyptian institution. Cancer Biol Med. 2015;12:53–59. doi: 10.7497/j.issn.2095-3941.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phitayakorn R, McHenry CR. Follicular and Hurthle cell carcinoma of the thyroid gland. Surg Oncol Clin N Am. 2006;15:603–623. doi: 10.1016/j.soc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.De Crea C, Raffaelli M. Actual incidence and clinical behaviour of follicular thyroid carcinoma: an institutional experience. Sci World J. 2014;2014:952095. doi: 10.1155/2014/952095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omur O, Baran Y. An update on molecular biology of thyroid cancers. Crit Rev Oncol Hematol. 2014;90:233–252. doi: 10.1016/j.critrevonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Chow SM, Law SC, Au SK, Leung TW, Chan PT, Mendenhall WM, Lau WH. Differentiated thyroid carcinoma: comparison between papillary and follicular carcinoma in a single institute. Head Neck. 2002;24:670–677. doi: 10.1002/hed.10080. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Sung JY, Oh YL, Kim JH, Son YI, Min YK, Kim SW, Chung JH. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck. 2014;36:1695–1700. doi: 10.1002/hed.23511. [DOI] [PubMed] [Google Scholar]
- 7.Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001;91:505–524. doi: 10.1002/1097-0142(20010201)91:3<505::AID-CNCR1029>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Chow SM, Law SC, Mendenhall WM, Au SK, Yau S, Yuen KT, Law CC, Lau WH. Follicular thyroid carcinoma: prognostic factors and the role of radioiodine. Cancer. 2002;95:488–498. doi: 10.1002/cncr.10683. [DOI] [PubMed] [Google Scholar]
- 9.Podda M, Saba A, Porru F, Reccia I, Pisanu A. Follicular thyroid carcinoma: differences in clinical relevance between minimally invasive and widely invasive tumors. World J Surg Oncol. 2015;13:193. doi: 10.1186/s12957-015-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam AK-y. Pathology of endocrine tumors update: World Health Organization new classification 2017—other thyroid tumors. Am J Surg Pathol: Reviews & Reports. 2017;22:209–216. [Google Scholar]
- 11.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek HJ, Kim DW, Lee S, Ryoo I, Lee CY, Choi YJ, Sung JY. Postoperative ultrasonography surveillance in patients with follicular thyroid carcinoma: a multicenter study. Radiol Med. 2017;122:530–537. doi: 10.1007/s11547-017-0753-7. [DOI] [PubMed] [Google Scholar]
- 13.Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, Lee JE, Evans DB. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–954. doi: 10.1016/S0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 14.Lepoutre-Lussey C, Maddah D, Golmard JL, Russ G, Tissier F, Tresallet C, Menegaux F, Aurengo A, Leenhardt L. Post-operative neck ultrasound and risk stratification in differentiated thyroid cancer patients with initial lymph node involvement. Eur J Endocrinol. 2014;170:837–846. doi: 10.1530/EJE-13-0888. [DOI] [PubMed] [Google Scholar]
- 15.Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, Lippi F, Taddei D, Grasso L, Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 16.Torlontano M, Crocetti U, Augello G, D'Aloiso L, Bonfitto N, Varraso A, Dicembrino F, Modoni S, Frusciante V, Di Giorgio A, Bruno R, Filetti S, Trischitta V. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–63. doi: 10.1210/jc.2005-1185. [DOI] [PubMed] [Google Scholar]
- 17.Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, Socci N, Kannan K, Mo Q, Fagin JA, Chan TA. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98:E962–E972. doi: 10.1210/jc.2012-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzaferri EL, Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002;87:1490–1498. doi: 10.1210/jcem.87.4.8338. [DOI] [PubMed] [Google Scholar]
- 19.D'Avanzo A, Treseler P, Ituarte PH, Wong M, Streja L, Greenspan FS, Siperstein AE, Duh QY, Clark OH. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100:1123–1129. doi: 10.1002/cncr.20081. [DOI] [PubMed] [Google Scholar]
- 20.Haigh PI, Urbach DR. The treatment and prognosis of Hurthle cell follicular thyroid carcinoma compared with its non-Hurthle cell counterpart. Surgery. 2005;138:1152–1157. doi: 10.1016/j.surg.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011;37:181–185. doi: 10.1016/j.ejso.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Kameyama K, Ito K. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid. 2011;21:751–757. doi: 10.1089/thy.2010.0353. [DOI] [PubMed] [Google Scholar]
- 23.Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Suzuki A, Masaki C, Ito K. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid. 2012;22:798–804. doi: 10.1089/thy.2012.0051. [DOI] [PubMed] [Google Scholar]
- 24.Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144:1070–1077. doi: 10.1016/j.surg.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Hirokawa M, Masuoka H, Yabuta T, Kihara M, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocr J. 2013;60:637–642. doi: 10.1507/endocrj.EJ12-0419. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 27.Witte J, Goretzki PE, Dieken J, Simon D, Roher HD. Importance of lymph node metastases in follicular thyroid cancer. World J Surg. 2002;26:1017–1022. doi: 10.1007/s00268-002-6668-y. [DOI] [PubMed] [Google Scholar]
- 28.Tuttle RM, Morris L, Haugen B, Shah J, Sosa J, Rohren E, Subramaniam R, Hunt J, Perrier N (2017) Thyroid-differentiated and anaplastic carcinoma. AJCC Cancer Staging Manual. New York, Springer, p. 873–890


