Abstract
Rationale:
Pulmonary vascular resistance (PVR) fails to decrease appropriately during exercise in patients with heart failure with preserved ejection fraction (HFpEF). Interventions that enhance pulmonary vasodilation might be beneficial in this cohort, but could also worsen left atrial hypertension, exacerbating lung congestion. Intravenous β-agonists reduce PVR, but are not suitable for chronic use.
Objective:
We hypothesized that the inhaled β-adrenergic agonist albuterol would improve pulmonary vasodilation during exercise in patients with HFpEF, without increasing left heart filling pressures.
Methods and Results:
We performed a randomized, double blind, placebo-controlled trial testing the effects of inhaled albuterol on resting and exercise hemodynamics in subjects with HFpEF using high fidelity micromanometer catheters and expired-gas analysis. The primary end point was PVR during exercise. Subjects with HFpEF (n=30) underwent resting and exercise hemodynamic assessment and were then randomized 1:1 to inhaled, nebulized albuterol or placebo. Rest and exercise hemodynamic testing was then repeated. Albuterol improved the primary endpoint of exercise PVR as compared to placebo (−0.6±0.5 vs +0.1±0.7 Wood units, p=0.003). Albuterol enhanced cardiac output reserve and right ventricular-pulmonary artery coupling, reduced right atrial and pulmonary artery pressures, improved pulmonary artery compliance, and enhanced left ventricular transmural distending pressure (all p<0.01), with no increase in pulmonary capillary hydrostatic pressures.
Conclusions:
Albuterol improves pulmonary vascular reserve in patients with HFpEF without worsening left heart congestion. Further study is warranted to evaluate the chronic efficacy of β-agonists in HFpEF as well as other forms of pulmonary hypertension.
Keywords: Pulmonary vascular disease, pulmonary hypertension, exercise, heart failure, HFpEF, beta agonist, albuterol, hemodynamics, treatment, Heart Failure, Hemodynamics, Pathophysiology, Pulmonary Hypertension, Treatment
INTRODUCTION
Pulmonary hypertension is common and associated with increased mortality in patients with heart failure with preserved ejection fraction (HFpEF).1–8 The initial increase in pulmonary artery (PA) pressures in HFpEF is passive, caused by high left heart pressures, but many patients also develop vascular disease within the lungs. This ranges in severity from mild limitations PA dilation with exercise to impairments in alveolar capillary gas exchange to structural remodeling in advanced stages.9–14
Pulmonary vascular disease in HFpEF eventually leads to the development of right ventricular (RV) dysfunction.15–17 Patients with this phenotype display underfilling of the left ventricle (LV) during exertion due to inadequate RV ejection of blood through the lungs, limiting cardiac output reserve due to failure of Frank-Starling mechanism.18 However, these phenomena are not restricted to patients with advanced HFpEF, because similar (though less severe) abnormalities in RV-PA interaction are also observed during exercise in patients with early stage HFpEF.9 While interventions that enhance pulmonary vascular reserve with exercise hold promise in HFpEF,5, 6 they might also worsen lung congestion as venous return to the left atrium increases in the setting of LV diastolic dysfunction.19–21
We recently observed that the intravenous β-agonist dobutamine elicits robust PA vasodilator effects at rest in patients with HFpEF, without increasing left atrial pressures.22 This led us to hypothesize that similar benefits could be achieved during exercise with inhaled β-agonists. To test this hypothesis, we conducted this randomized controlled trial to determine whether the inhaled β-agonist albuterol would improve pulmonary vascular function during exercise in patients with HFpEF.
METHODS
The data, analytical methods, and study materials will not be made available to other researchers.
This double-blind, randomized, placebo-controlled, parallel-group trial was designed to study the effects of inhaled, nebulized albuterol on pulmonary vascular hemodynamics and cardiac function at rest and during exercise in subjects with HFpEF. Patients referred to the Mayo Clinic cardiac catheterization laboratory for invasive hemodynamic exercise testing in the evaluation of unexplained dyspnea were enrolled. Written informed consent was obtained from all patients. The study was approved by the Mayo Clinic Institutional Review Board and the trial was registered (NCT02885636; Inhaled Beta-adrenergic agonists to Treat Pulmonary Vascular Disease in Heart Failure with Preserved Ejection Fraction: BEAT HFpEF).
Study population.
Subjects were consented prior to catheterization and qualified for randomization if they demonstrated hemodynamic evidence of HFpEF by resting end-expiratory pulmonary capillary wedge pressure (PCWP, ≥15 mmHg) and/or an exercise PCWP (≥25 mmHg) at a 20 Watt workload.23, 24 Patients with significant left-sided valvular heart disease (>mild stenosis, >moderate regurgitation), coronary disease requiring revascularization, infiltrative, restrictive or hypertrophic cardiomyopathies, constrictive pericarditis, significant obstructive or restrictive pulmonary disease, high output HF, albuterol use within 48 hours, prior EF<50%, pulmonary embolism, and right ventricular myopathies were excluded.
Overall design.
All studies were performed in the supine position on chronic medications in the fasted state using the invasive hemodynamic exercise-testing protocol as previously described.25, 26 Following assessment of baseline (resting) hemodynamics, subjects underwent supine cycle ergometry exercise with simultaneous expired gas analysis at a fixed workload of 20 W (pedal speed 60 rpm) for 5 minutes, with repeat assessment of hemodynamics. Following exercise, a period of ≥5 minutes was allowed for recovery to re-establish baseline vital signs. Subjects then received study drug in a randomized, double-blind fashion, with repeat assessment of resting and exercise hemodynamics in the exact same manner as above.25, 26 Randomization was performed using a random number generator in SAS (SAS Institute Inc., Cary, North Carolina) in permuted blocks of 10.
Hemodynamic assessment.
Right heart catheterization was performed through a 9 Fr sheath via the right internal jugular vein as previously described.9, 18, 25, 26 Mean right atrial (RA) pressure, PA pressures, and PCWP were measured using a high fidelity micromanometer (Aeris, St Jude Medical) advanced through the lumen of a 7 Fr fluid-filled catheter (Balloon wedge, Arrow). PCWP position was confirmed by appearance on fluoroscopy, characteristic pressure waveforms, and oximetry (saturation≥94%). All pressures were taken as the average across multiple respiratory cycles over a 15 second period, reflecting the mean of 750–2000 cardiac cycles. Pressure tracings were recorded, digitized (240 Hz), and stored for offline analysis by one investigator experienced in exercise hemodynamic assessment (BAB).
Breath-by-breath oxygen consumption (VO2) was measured continuously throughout each phase of the study (MedGraphics, St. Paul, MN). A 4–6 Fr radial arterial cannula was used to measure arterial blood pressure and allow sampling of arterial blood gases. Simultaneous PA blood samples were obtained to measure systemic and mixed venous O2 contents (=saturation*hemoglobin*1.34). Arterial-venous O2 content difference (AVO2 diff) was calculated from systemic arterial and PA O2 contents. Cardiac output (QC) was calculated by the direct Fick method (= VO2÷AVO2 diff). Stroke volume (SV) calculated as the quotient of QC and heart rate. Pulmonary vascular resistance (PVR) was calculated as (mean PA-PCWP)/QC, PA compliance by the ratio of SV/PA pulse pressure, and pulmonary elastance by the ratio of PA systolic pressure/SV.9, 25, 26 Systemic vascular resistance (SVR) was calculated by [mean BP-RAP]/ QC.
While PCWP reflects the hydrostatic pressure distending the lung capillaries, it does not adequately reflect the net distending pressure that determines LV preload, which is quantified by LV transmural pressure.18, 27–29 The total pressure measured in the LV during diastole (estimated by PCWP) is equal to the sum of transmural pressure and the external pressure applied to the LV from the pericardium and right heart. As shown by Tyberg et al., this external pressure is best estimated by RA pressure, so LV transmural pressure is calculated as PCWP minus RAP.18, 27–29
Assessment of RV function and RV-PA coupling.
Echocardiography was performed simultaneously at rest and during exercise as previously described to assess RV function.9, 24,30 Tricuspid Annular Plane Systolic Excursion (TAPSE), RV systolic tissue velocity at the lateral tricuspid annulus (RV s’) and Fractional area change (FAC) were measured at each stage. RV dysfunction was defined according to the American Society of Echocardiography recommendations as either RV FAC<35% or RV s’ <10 cm/s (or both).30 Right ventricular-PA coupling was assessed by the ratio of TAPSE to PA pressure as in prior studies.31, 32 Ratios of FAC and RV s’ to PA pressure were also examined as complementary indices of RV-PA coupling analogous to TAPSE/PA pressure ratio.
Study drug intervention.
After the first exercise phase was completed and hemodynamics returned to baseline, subjects were randomized 1:1 to either inhaled placebo (normal saline solution) or inhaled albuterol sulfate (2.5 mg) administered through a high-efficiency nebulizer (Solo-Idehaler device, Aerogen Galway, Ireland/DTF Saint-Etienne, France).25 The albuterol/placebo solutions were identical in appearance and prepared by research pharmacy, ensuring blinding of both participant and investigators.
Study endpoints and sample size considerations.
The primary endpoint of the trial was PVR during exercise. Exercise PVR was chosen rather than rest PVR because hemodynamic perturbations are known to be greater during exercise in HFpEF (where patients are symptomatic) as compared to rest,6 and based upon our prior observation of abnormal PVR during exercise but not rest in HFpEF.9 Secondary endpoints included resting PVR, as well as rest and exercise PCWP, PA compliance, RA pressure, PA pressure, QC, SV, RV systolic function, and RV-PA coupling
The sample size of 30 patients (15 in each arm) was determined based on power calculations from our prior data testing the effects of dobutamine, where PVR was reduced by 0.8 WU in subjects with HFpEF.22 Assuming a standard deviation of 0.5 WU in exercise PVR based upon our prior data,25, 26 this sample size was estimated to provide 98% power (α=0.05) to detect a reduction in exercise PVR of this magnitude or greater with albuterol as compared to placebo.
Statistical analysis.
Data are presented as mean (standard deviation), median (interquartile range) or number (%). Between-group differences were compared by unpaired t test, Wilcoxon rank sum test, χ2 or Fisher’s exact test as appropriate. Changes between rest and exercise hemodynamics before study drug were compared by paired t test. Analysis of covariance (ANCOVA) was used to determine the effect of albuterol on exercise PVR using the initial exercise PVR (prior to study drug) as the covariate.25, 26 Linear regression analyses were used to assess relationships between changes in variables of interest. Analysis was performed using JMP 13.0.0 (SAS). A p value of <0.05 was considered statistically significant.
RESULTS
Study population.
Of 40 subjects enrolled, 10 did not meet eligibility criteria and were therefore not randomized (6 had normal rest and exercise hemodynamics, 1 left-to-right shunt, 1 high output HF, 1 found to have coronary disease necessitating revascularization, 1 Group 1 PH). The remaining 30 subjects qualified and were randomized. Subjects were older aged, obese and hypertensive, representing a typical HFpEF population. There were no differences in baseline characteristics in subjects randomized to placebo or albuterol (Table 1).
Table 1:
Baseline characteristics
| Placebo (n=15) | Albuterol (n=15) | P value | |
|---|---|---|---|
| Age, years | 64 ± 17 | 68 ± 8 | 0.4 |
| Female, n (%) | 8 (53) | 6 (40) | 0.5 |
| Body mass index, kg/m2 | 35.6 ± 9.2 | 33.7 ± 7.0 | 0.5 |
| Comorbidities | |||
| Coronary disease, n (%) | 4 (27) | 5 (33) | 0.7 |
| Diabetes Mellitus, n (%) | 4 (27) | 3 (20) | 0.7 |
| Hypertension, n (%) | 14 (93) | 15 (100) | 1.0 |
| Atrial fibrillation, n (%) | 1 (7) | 4 (27) | 0.3 |
| Medications | |||
| ACEI or ARB, n (%) | 7 (47) | 9 (60) | 0.5 |
| Beta blocker, n (%) | 4 (27) | 6 (40) | 0.5 |
| Calcium channel blocker, n (%) | 1 (7) | 4 (27) | 0.3 |
| Loop diuretic, n (%) | 10 (71) | 9 (60) | 0.7 |
| Laboratories | |||
| Creatinine, mg/dl | 1.1 ± 0.4 | 1.3 ± 0.7 | 0.5 |
| Hemoglobin, g/dl | 13.4 ± 1.2 | 12.9 ± 1.1 | 0.2 |
| NT-proBNP, pg/ml | 185 [42–1517] | 106 [56–689] | 0.5 |
| Echocardiography | |||
| LV Ejection Fraction, % | 61 ± 6 | 61 ± 7 | 0.8 |
| LV mass index, g/m2 | 93 ± 14 | 105 ± 30 | 0.2 |
| LA volume index, ml/m2 | 32 ± 15 | 36 ± 10 | 0.4 |
| LV e’ velocity, cm/s | 7 ± 2 | 7 ± 2 | 0.5 |
| LV E/e’ ratio | 12 ± 6 | 14 ± 6 | 0.5 |
| RV dysfunction*, n (%) | 10 (67) | 6 (40) | 0.3 |
Values represent mean ± standard deviation, or median [interquartile range]. There were no significant differences between the placebo and albuterol groups in baseline characteristics.
RV dysfunction was defined by American Society of Echocardiography recommendations as either RV FAC<35% or RV s’ <10 cm/s ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; NT-proBNP- N-terminal pro Brain Natriuretic Peptide; E/e’; ratio of early diastolic transmitral filling velocity (E) and early diastolic mitral annular tissue velocity (e’); LV, left ventricular; LA, left atrial, RV, right ventricular
Baseline hemodynamics and right heart structure before randomization.
As expected, mean PA pressure and biventricular filling pressures were elevated at rest and became markedly elevated with exercise, with mildly elevated PVR at rest that failed to decline during exercise (Table 2). There was a slightly lower exercise PCWP in the HFpEF subjects randomized to albuterol, but no other group differences in rest or exercise hemodynamics (Table 2).
Table 2:
Rest-Exercise Hemodynamics prior to Study Drug
| Rest | Exercise | |||
|---|---|---|---|---|
| Placebo (n=15) | Albuterol (n=15) | Placebo (n=15) | Albuterol (n=15) | |
| Heart Rate, bpm | 71±10 | 71±13 | 94±14* | 96±18* |
| Mean Arterial BP, mmHg | 105±12 | 103±10 | 114±13^ | 116±12^ |
| Systolic Arterial BP, mmHg | 158±23 | 154±17 | 181±26^ | 176±22^ |
| Diastolic Arterial BP, mmHg | 73±8 | 71±8 | 81±8^ | 79±9^ |
| SVR, WU | 16.4±3.4 | 18.2±8.7 | 10.5±2.8* | 11.6±4.0^ |
| Ventricular Filling pressures | ||||
| RA pressure, mmHg | 11±5 | 9±4 | 19±6* | 16±5* |
| PCWP, mmHg | 17±5 | 15±4 | 28±5* | 24±5* |
| LV transmural pressure, mmHg | 6 ± 3 | 6 ± 3 | 9 ± 5^ | 8 ± 5 |
| Pulmonary Vascular Function | ||||
| PA systolic pressure, mmHg | 41±14 | 38±9 | 61±13* | 56±10* |
| PA mean pressure, mmHg | 27±9 | 25±5 | 43±8* | 39±7* |
| PVR, Wood units | 1.9±1.1 | 2.0±0.6 | 1.8±1.0 | 1.9±0.7 |
| PA compliance, ml/mmHg | 4.0±1.3 | 4.1±2.3 | 3.7±1.4 | 3.3±1.6^ |
| PA Ea, mmHg/ml | 0.5±0.2 | 0.5±0.2 | 0.7±0.2^ | 0.7±0.2^ |
| Right Ventricular Function | ||||
| RV s’, cm/s | 9±2 | 11±3 | 10±2 | 11±3 |
| TAPSE, mm | 19±3 | 19±5 | 21±4^ | 20±4^ |
| RV FAC, % | 49±8 | 53±6 | 52±10^ | 57±5^ |
| RV-PA Coupling | ||||
| RV s’/PA mean, cm.s−1/mmHg | 0.38±0.16 | 0.45±0.19 | 0.24±0.07^ | 0.30±0.10^ |
| TAPSE/PA mean, mm/mmHg | 0.76±0.24 | 0.78±0.24 | 0.49±0.11^ | 0.53±0.13* |
| FAC/PA mean, %/mmHg | 2.02±0.72 | 2.23±0.54 | 1.29±0.38^ | 1.52±0.33* |
| Integrated Indices | ||||
| Stroke volume, ml | 80±21 | 80±36 | 98±26^ | 94±41^ |
| Cardiac output, L/min | 5.6±1.3 | 5.6±2.2 | 9.2±2.5* | 8.7±3.4* |
p<0.0001 versus baseline within subject change,
<p<0.05 versus baseline within subject change
BP, Blood pressure; RA, Right atrial; PCWP, Pulmonary Capillary Wedge Pressure; LV, Left ventricular; SVR, Systemic Vascular Resistance; TAC, Total Arterial Compliance; Ea, Elastance; PA, Pulmonary Artery; PVR, Pulmonary Vascular Resistance; RV-Right Ventricular; TAPSE, Tricuspid Annular Plane Systolic Excursion; s’, systolic tissue Doppler velocity at the lateral tricuspid annulus; FAC, Fractional Area Change
Following the initial exercise period, all hemodynamics returned to baseline values, with the exception of heart rate, which was slightly elevated at re-baseline assessment as compared to the initial baseline (74±13 vs 71±11 bpm, p<0.001, Online Table I). However, importantly there were no differences in re-baseline heart rate or other hemodynamics between subjects randomized to albuterol and placebo (Online Table II).
Effect of albuterol on resting hemodynamics.
Albuterol decreased RA pressure and systemic BP at rest with a trend towards higher LV transmural pressure (p=0.07), but had no effect on HR, SV, SVR or QC (Table 3, Online Table III). Mean PA pressure (p=0.08) and PA elastance (p=0.09) tended to decrease at rest with albuterol, with no significant change in resting PVR or PA compliance. RV-PA coupling improved at rest, as reflected by increases in the ratios of RV s’ and FAC to mean PA pressure, though the TAPSE/PA pressure ratio did not change at rest following albuterol treatment (Table 3).
Table 3:
Effect of albuterol on resting hemodynamics
| Placebo (n=15) | Albuterol (n=15) | p value | |
|---|---|---|---|
| Heart Rate, bpm | +1 ± 2 | +3 ± 7 | 0.3 |
| Mean Arterial BP, mmHg | +4 ± 5 | −7 ± 8 | 0.0002 |
| Systolic Arterial BP, mmHg | +3 ± 7 | −7 ± 10 | 0.005 |
| Diastolic Arterial BP, mmHg | +3 ± 4 | −5 ± 6 | 0.0003 |
| SVR, WU | +0.7 ± 3.6 | −3.0 ± 7.4 | 0.1 |
| Ventricular Filling pressures | |||
| RA pressure, mmHg | −1 ± 2 | −3 ± 2 | 0.03 |
| PCWP, mmHg | −2 ± 3 | −2 ± 2 | 0.9 |
| LV transmural pressure, mmHg | −1 ± 3 | +1 ± 3 | 0.07 |
| Pulmonary Vascular Function | |||
| PA systolic pressure, mmHg | −5 ± 4 | −7 ± 7 | 0.3 |
| PA mean pressure, mmHg | −3 ± 3 | −5 ± 3 | 0.08 |
| PVR, Wood units | −0.3 ± 0.8 | −0.6 ± 0.5 | 0.3 |
| PA compliance, ml/mmHg | +0.7 ± 1.0 | +1.2 ± 1.0 | 0.2 |
| PA Ea, mmHg/ml | −0.03 ± 0.13 | −0.11 ± 0.13 | 0.09 |
| Right Ventricular Function | |||
| RV s’, cm/s | −0.1 ± 0.8 | +0.5 ± 1.2 | 0.1 |
| TAPSE, mm | +0.8 ± 2.0 | +0.0 ± 0.6 | 0.2 |
| RV FAC, % | −1 ± 4 | +2 ± 5 | 0.1 |
| RV-PA Coupling | |||
| RV s’/PA mean, cm.s−1/mmHg | +0.05 ± 0.06 | +0.15 ± 0.10 | 0.005 |
| TAPSE/PA mean, mm/mmHg | +0.15 ± 0.16 | +0.22 ± 0.14 | 0.2 |
| FAC/PA mean, %/mmHg | +0.24 ± 0.34 | +0.70 ± 0.47 | 0.009 |
| Integrated Indices | |||
| Stroke volume, ml | +4 ± 15 | +6 ± 15 | 0.7 |
| Cardiac output, L/min | +0.4 ± 1.2 | +0.6 ± 1.2 | 0.7 |
Values are mean ± SD. Table shows baseline corrected values (resting values after receiving study drug minus resting values prior to study drug) Abbreviations as in table 2
Effect of albuterol on exercise hemodynamics.
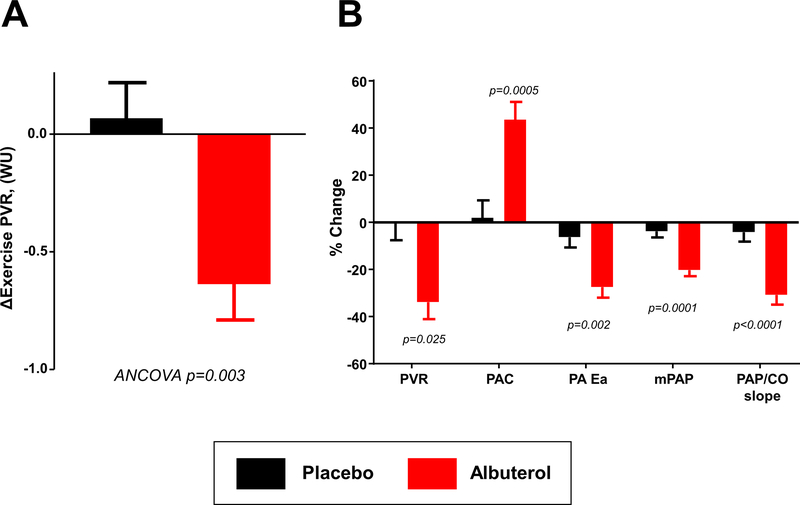
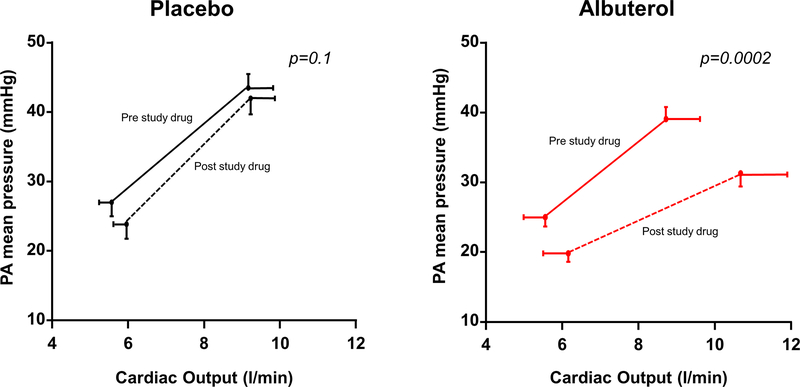
The primary endpoint of exercise PVR was significantly improved with albuterol, with a 35% reduction in exercise PVR (−0.6 ± 0.5 vs +0.1 ± 0.7 WU, p=0.003 by T test, p=0.003 by ANCOVA, Table 4, Figure 1). As compared to placebo, albuterol improved PA compliance and arterial elastance, enhanced RV-PA coupling, improved cardiac output and stroke volume reserve, and lowered RA pressure with exercise (Table 4, Figure 1, and Online Table III). Mean PA pressure decreased despite an increase in blood flow through the lungs, with a significant reduction in the slope of the PA pressure-flow relationship (change in ΔPA/ΔQC: −1.4 ± 0.8 vs −0.2 ± 0.6; p=0.0002 by T test, p=0.005 by ANCOVA, Figure 2).
Table 4:
Effect of albuterol on exercise hemodynamics
| Placebo (n=15) | Albuterol (n=15) | p value | |
|---|---|---|---|
| Heart Rate, bpm | −2 ± 4 | +3 ± 9 | 0.07 |
| Mean Arterial BP, mmHg | +3 ± 12 | −7 ± 9 | 0.01 |
| Systolic Arterial BP, mmHg | +3 ± 11 | −6 ± 13 | 0.03 |
| Diastolic Arterial BP, mmHg | −1 ± 7 | −5 ± 6 | 0.07 |
| SVR, WU | +0.5 ± 1.9 | −1.7 ± 2.0 | 0.005 |
| Ventricular Filling pressures | |||
| RA pressure, mmHg | −1 ± 2 | −5 ± 3 | 0.0007 |
| PCWP, mmHg | −3 ± 3 | −2 ± 6 | 0.7 |
| LV Transmural pressure, mmHg | −1 ± 2 | +3 ± 5 | 0.006 |
| Pulmonary Vascular Function | |||
| PA systolic pressure, mmHg | −3 ± 6 | −10 ± 5 | 0.002 |
| PA mean pressure, mmHg | −2 ± 4 | −8 ± 4 | 0.0002 |
| PVR, Wood units | +0.1 ± 0.7 | −0.6 ± 0.5 | 0.003 |
| PA compliance, ml/mmHg | +0.0 ± 0.7 | +1.6 ± 1.6 | 0.001 |
| PA Ea, mmHg/ml | −0.05 ± 0.11 | −0.17 ± 0.11 | 0.004 |
| Right Ventricular Function | |||
| RV s’, cm/s | +0.4 ± 1.1 | +1.1 ± 1.6 | 0.2 |
| TAPSE, mm | +0.5 ± 1.3 | +0.6 ± 0.8 | 0.8 |
| RV FAC, % | −1 ± 3 | +1 ± 3 | 0.3 |
| RV-PA Coupling | |||
| RV s’/PA mean, cm.s−1/mmHg | +0.02 ± 0.05 | +0.13 ± 0.12 | 0.003 |
| TAPSE/PA mean, mm/mmHg | +0.04 ± 0.07 | +0.17 ± 0.12 | 0.002 |
| FAC/PA mean, %/mmHg | +0.09 ± 0.17 | +0.44 ± 0.31 | 0.006 |
| Integrated Indices | |||
| Stroke volume, ml | −0.3 ± 2.1 | +1.6 ± 2.8 | 0.04 |
| Cardiac output, L/min | +0.1 ± 1.0 | +2.0 ± 2.1 | 0.004 |
Values are mean ± SD. Table shows baseline corrected values (exercise values after receiving study drug minus exercise values prior to study drug) Abbreviations as in Table 2.
Figure 1:
[A] Albuterol improved the primary endpoint of exercise pulmonary vascular resistance (PVR) as compared to placebo. [B] In addition, there were improvements in in key secondary endpoints including pulmonary artery compliance (PAC), pulmonary artery elastance (Ea), mean PA pressure (mPAP) and the mean PAP/cardiac output (CO) slope. Error bars indicate SE.
Figure 2:
Albuterol improved the pulmonary (PA) pressure-flow relationship with a lesser increase in PA pressure relative to the increase in cardiac output with exertion. Error bars indicate SE. P values represent the difference in slopes by t test before and after study drug.
Albuterol improved cardiac output reserve with exercise by 25% (Figure 2), with an increase in QC reserve relative to whole-body O2 consumption (ΔQC/ΔVO2 slope: +1.6 ± 2.8 vs −0.3 ± 2.1ml/ml, p=0.04). The improvement in exercise cardiac output with albuterol was predominantly related to an increase in stroke volume (R2=0.8, p<0.0001) rather than change in heart rate (R2=0.2, p=0.09). Albuterol also reduced exercise blood pressure and SVR, and enhanced RV-PA coupling during exercise (Table 4).
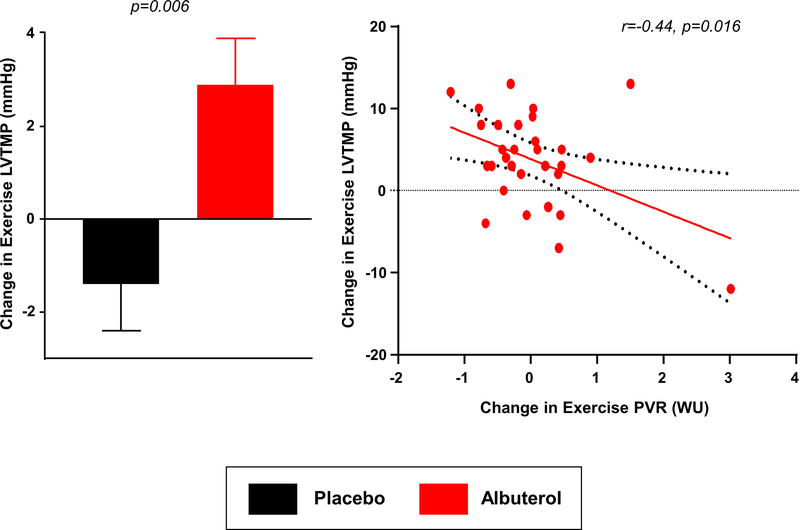
Despite a substantial increase in blood flow through the lungs, albuterol did not increase PCWP at rest or during exercise compared to placebo (Tables 3 and 4). However, there was an improvement in LV transmural pressure, which is more strongly related to LV chamber volume at end diastole (Table 4).27–29 Notably, the magnitude of decrease in exercise PVR was correlated with greater increase in LV transmural pressure during exercise (Figure 3). This suggests that pulmonary vasodilation improved the ability to augment LV filling and thus better utilize the Frank Starling mechanism, even as pulmonary venous pressure during exercise remained unchanged.18
Figure 3:
[A] Left ventricular (LV) Transmural pressure increased in response to albuterol as compared with placebo. [B] The degree of increase in LV transmural pressure was correlated with the reduction in exercise pulmonary vascular resistance (PVR).
DISCUSSION
In this randomized double blind placebo controlled trial we evaluated the effect of the inhaled β-adrenergic agonist albuterol, an inexpensive and widely-available drug, on pulmonary vascular function and cardiac hemodynamics at rest and during exercise in patients with HFpEF. We demonstrate that albuterol exerts favorable effects on pulmonary vascular load during exercise, even among patients without marked elevation in PVR, which is coupled with significant improvements in cardiac output reserve, right ventricular-pulmonary artery coupling, and left heart filling (transmural pressure), without increasing pulmonary capillary hydrostatic pressures. Further study is needed to evaluate the chronic efficacy of β-agonists in HFpEF, as well as other forms of pulmonary hypertension.
Pulmonary vascular disease as a therapeutic target in HFpEF.
Pulmonary hypertension is common and associated with adverse outcomes in HFpEF.1–8 Right ventricular dysfunction develops in HFpEF in response to chronic PH, and its presence identifies patients at very high risk of death.4, 15, 16 While trials in PH have generally moved toward requiring higher PVR for inclusion, recent data has revealed that even in the earliest stages of HFpEF, where PVR is normal at rest, there are impairments in PA vasodilation and RV functional reserve that become manifest during exercise, which are directly correlated with impairment in peak aerobic capacity.9 Indeed, failure to reduce PVR during exercise in early stage HFpEF is associated with increased risk of HF hospitalization during long-term follow up.33 Thus, there is a pressing need for therapies that improve pulmonary vascular function and therefore RV-PA coupling during exercise in HFpEF.5, 6
In healthy adults PVR decreases during exercise as pulmonary blood flow increases, due to the combination of vasodilation, pulmonary vascular recruitment and enhanced distensibility.34 Normal exercise-mediated reduction in PVR is lost in patients with HFpEF,9, 10, 33 but we demonstrate that this impaired vasodilation is partly reversible with albuterol. The potential role of pulmonary vascular-targeted therapy represents an area of intense interest in the field of HFpEF.20, 21, 25, 26, 35–42 One potential concern is that reduction in PVR in the setting of LV diastolic dysfunction may increase left atrial hypertension and cause worsening pulmonary congestion.19 This effect has been observed with direct NO administration,19 as well as with the phosphodiesterase 5 inhibitor sildenafil,20 which may account for its neutral effects in some trials,36, 37 and negative effect in another.21
Effects on the left heart.
An important observation from the current study was that albuterol did not increase PCWP. Despite this absent effect on pulmonary capillary hydrostatic pressure, there was an increase the transmural distending pressure that drives LV filling. Measured intravascular pressures reflect the sum of the transmural distending pressure and external pressure applied to the vessel (or chamber) where measurements are obtained. The external pressure on the left ventricle and atrium, mediated by the pericardium and right-sided chambers, is best approximated by RA pressure.27 Thus the LV transmural pressure can be calculated as the difference between intracavitary left heart filling pressure (PCWP) and external pressure (RA).27–29 The LV transmural pressure more accurately reflects LV end diastolic volume or true preload.28
We have recently reported that patients with HFpEF and advanced pulmonary vascular disease (elevated resting PVR) display a reduction in LV transmural pressure during exercise, which compromises cardiac output reserve through failure of the Frank-Starling mechanism, even as PCWP increases.18 The current data show that albuterol can target this pathophysiology even in patients without marked elevation in resting PVR, since transmural pressure was improved in relation to the degree of pulmonary vasodilation with exercise (Figure 3). The fact that PVR reduction from albuterol improved exercise cardiac output by 25% speaks to the relevance of pulmonary vascular disease as a viable target in patients with all stages of HFpEF, particularly since limitations in cardiac output reserve are known to be an important contributor to exercise intolerance in this cohort.9, 43, 44
Beta agonists in HFpEF.
Enhanced sympathetic drive plays a fundamental role in the pathophysiology of heart failure with reduced EF, and β-blockers represent a cornerstone in its management. However, similar salutary effects of β-blockers have not been observed in HFpEF.45 In mice, β-agonists elicit pulmonary vasodilation by enhancing nitric oxide signaling.46 We previously demonstrated in a non-placebo controlled trial that the nonspecific β-agonist dobutamine substantially improves PA vascular tone in HFpEF, to an extent that exceeded what was seen in subjects without HF.22 Because dobutamine is a parenterally-administered drug, and because of its potent β−1 mediated inotropic effects, it is not suitable for chronic administration in HFpEF.
In contrast, albuterol can be administered via metered dose inhaler or nebulizer, and is more β−2 selective than dobutamine, suggesting that this might be better option for chronic use. In this acute trial we observed favorable effects of albuterol on PA resistance and compliance during exercise, where vascular and cardiac abnormalities are most limiting in HFpEF.9 Vasodilation in the systemic circulation was also observed with albuterol. While the proportional reductions in systemic arterial pressure during exercise (3–6%) were far less than corresponding effects in the pulmonary circulation (18–20%), a systemic vasodilator effect might have also contributed to the salutary effects of albuterol on cardiac output reserve, which is limited in part by abnormal systemic vasodilation in HFpEF.9, 43
The mechanisms by which albuterol acts as a pulmonary vasodilator are unclear, but may relate to activation of β-receptors in vascular endothelium to increase NO, direct effects on vascular smooth muscle to reduce vasoconstriction, or indirect effects mediated by alterations in lung function and fluid homeostasis. Indeed, type I and II alveolar pneumocytes express β−2 receptors that regulate fluid transit across the alveolar-capillary interface, and acute treatment with albuterol was recently shown to facilitate fluid removal from the alveolar space.47 Since PVR is directly correlated with lung water content in patients with HFpEF,48 this effect may contribute in part to the salutary hemodynamic effects observed with albuterol in this trial. Activation of the β−2 adrenoreceptor in vascular smooth muscle reduces tone and thus arteriolar resistance, and β−2 receptors have also been localized to pulmonary artery endothelium, where agonist activity promotes vasorelaxation in an endothelial NO synthase-dependent manner.46 Finally, it is also possible that bronchodilator effects might alter ventilation-perfusion matching and secondarily impact pulmonary vascular tone.
Clinical implications.
Albuterol and other inhaled β-agonists are safe, inexpensive, and widely available. The current data call for the implementation of larger multicenter clinical trials to test the chronic effects of β-agonists in HFpEF, a disorder for which no proven treatment exists. While recent and ongoing trials have moved toward requiring elevated PVR for inclusion, the current study enrolled a cohort of subjects with earlier stage HFpEF, where PVR was not dramatically elevated at rest but failed to drop appropriately on exercise, and right ventricular function was not profoundly impaired.9, 10, 33 The current data serves as an important proof of concept that PA vascular targeted therapies may be beneficial even among HFpEF patients without advanced pulmonary vascular disease.5, 6 Further study is required in HFpEF patients with more abnormal PVR and RV dysfunction, to determine if β-agonists may also be effective in this cohort.
In addition, because elevation in PVR is common to virtually all forms of PH, it may be that β-agonists could improve PA vascular function and clinical status in other cohorts, including patients with isolated pulmonary vascular disease (Group 1), PH due to chronic lung disease (Group 3) or even thromboembolic disease (Group 4). The current data revealing favorable effects of albuterol also suggest that there could be deleterious effects on pulmonary and systemic vascular tone with beta-adrenergic antagonists, particularly non-selective antagonists.
Limitations.
This is a single center study from a tertiary referral center and as such has inherent limitations relating to both selection and referral bias. However, the use of invasive hemodynamics during exercise was necessary to the study design to obtain the primary and secondary endpoints, and the invasive data provides robust verification in the diagnosis of HFpEF. The sample size is modest; limiting subgroup analyses, but the trial was well-powered to test hypothesis proposed. Because the focus of this trial was on pulmonary vascular function and RV-PA coupling, evaluations of LV functional responses to albuterol were not performed. The effects of albuterol on pulmonary function tests and gas diffusion were not assessed. This was an acute, proof of concept study, and chronic effects of albuterol were not examined. Further studies testing more clinically meaningful endpoints, such as exercise capacity and symptoms are needed to determine whether the salutary hemodynamic effects of β-agonists in HFpEF will improve patient-centric outcomes.
Conclusions.
Albuterol improves pulmonary vasodilation during exercise in patients with HFpEF, leading to enhanced right ventricular-pulmonary artery coupling, improved cardiac output reserve, and more favorable left heart distension without untoward elevation in pulmonary venous pressures. Further study is needed to determine the chronic efficacy of β-agonists to improve clinical status in patients with HFpEF, as well as other causes of pulmonary hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
Pulmonary vascular resistance (PVR) fails to decrease appropriately during exercise in patients with heart failure with preserved ejection fraction (HFpEF) but no therapies are available that target this abnormality.
Enhancing pulmonary vasodilation at rest and during exercise might be beneficial in HFpEF, but could also worsen left atrial hypertension, exacerbating lung congestion.
What New Information Does This Article Contribute?
Albuterol, an inhaled β-adrenergic agonist, enhanced pulmonary vasodilation during exercise compared to placebo, reducing pulmonary artery pressures.
This was coupled with enhanced cardiac output reserve and reduced right heart congestion during exercise, without an increase in left sided filling pressures
This proof of concept study supports further investigation of interventions such as beta-agonists to target pulmonary vascular dysfunction in HFpEF.
In this double blind placebo controlled trial, we demonstrate that albuterol improves pulmonary vasodilation during exercise, effects that were coupled with enhanced forward cardiac output, reduced right atrial pressure, improved left heart distention, and enhanced right ventricular-pulmonary artery coupling in patients with HFpEF. This pulmonary vasodilation and increase in flow occurred without an untoward increase in left sided filling pressures. These data support future trials aimed at improving pulmonary vasodilation during stress with beta-agonists or other therapies.
Acknowledgments
SOURCES OF FUNDING
Dr. Borlaug is supported by the National Institutes of Health (R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262). Dr. Reddy is supported by T32 HL007111 from the NIH and a Fellow’s grant from the Heart Failure Society of America. Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Nonstandard Abbreviations and Acronyms:
- HFpEF
Heart Failure with preserved Ejection Fraction
- PA
Pulmonary Artery
- RV
Right Ventricle
- LV
Left Ventricle
- ANCOVA
Analysis of Covariance
- PCWP
Pulmonary Capillary Wedge Pressure
- PVR
Pulmonary Vascular Resistance
- TAPSE
Tricuspid Annular Plane Systolic Excursion
- FAC
Fractional Area Change
- EF
Ejection Fraction
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990 [DOI] [PubMed] [Google Scholar]
- 3.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Eur J Heart Fail. 2016;18:1472–1487 [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Lam CSP, Vachiery JL, Bauersachs J, Gerges C, Lang IM, Bonderman D, Olsson KM, Gibbs JSR, Dorfmuller P, Guazzi M, Galie N, Manes A, Handoko ML, Vonk Noordegraaf A, Lankeit M, Konstantinides S, Wachter R, Opitz C, Rosenkranz S. Pulmonary hypertension in heart failure with preserved ejection fraction: A plea for proper phenotyping and further research. Eur Heart J. 2017;38:2869–2873 [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J. 2017;38:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: Pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol. 2017;69:1718–1734 [DOI] [PubMed] [Google Scholar]
- 8.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: Implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:441–449 [DOI] [PubMed] [Google Scholar]
- 13.Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation. 2014;130:2310–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo-Leiro MG, Guazzi M, Harjola VP, Heymans S, Hill L, Lainscak M, Lam CSP, Lund LH, Lyon AR, Mebazaa A, Mueller C, Paulus WJ, Pieske B, Piepoli MF, Ruschitzka F, Rutten FH, Seferovic PM, Solomon SD, Shah SJ, Triposkiadis F, Wachter R, Tschope C, de Boer RA. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2018;20:16–37 [DOI] [PubMed] [Google Scholar]
- 18.Gorter TM, Obokata M, Reddy YN, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boilson BA, Schirger JA, Borlaug BA. Caveat medicus! Pulmonary hypertension in the elderly: A word of caution. Eur J Heart Fail. 2010;12:89–93 [DOI] [PubMed] [Google Scholar]
- 20.Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur Heart J. 2015;36:2565–2573 [DOI] [PubMed] [Google Scholar]
- 21.Bermejo J, Yotti R, Garcia-Orta R, Sanchez-Fernandez PL, Castano M, Segovia-Cubero J, Escribano-Subias P, San Roman JA, Borras X, Alonso-Gomez A, Botas J, Crespo-Leiro MG, Velasco S, Bayes-Genis A, Lopez A, Munoz-Aguilera R, de Teresa E, Gonzalez-Juanatey JR, Evangelista A, Mombiela T, Gonzalez-Mansilla A, Elizaga J, Martin-Moreiras J, Gonzalez-Santos JM, Moreno-Escobar E, Fernandez-Aviles F. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: A multicenter, double-blind, randomized clinical trial. Eur Heart J. 2017;39:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: Pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–550 [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: A simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682 [DOI] [PubMed] [Google Scholar]
- 27.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: An intraoperative study. Circulation. 1986;73:428–432 [DOI] [PubMed] [Google Scholar]
- 28.Moore TD, Frenneaux MP, Sas R, Atherton JJ, Morris-Thurgood JA, Smith ER, Tyberg JV, Belenkie I. Ventricular interaction and external constraint account for decreased stroke work during volume loading in chf. Am J Physiol Heart Circ Physiol. 2001;281:H2385–2391 [DOI] [PubMed] [Google Scholar]
- 29.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–688 [DOI] [PubMed] [Google Scholar]
- 31.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–1381 [DOI] [PubMed] [Google Scholar]
- 32.Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, Redfield MM. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: An ancillary analysis from the phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (relax) trial. Circ Heart Fail. 2016;9:e002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Oliveira RKF, Lei H, Systrom DM, Waxman AB. Pulmonary vascular resistance during exercise predicts long-term outcomes in heart failure with preserved ejection fraction. J Card Fail. 2017;24:169–176. [DOI] [PubMed] [Google Scholar]
- 34.Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479 [DOI] [PubMed] [Google Scholar]
- 35.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174 [DOI] [PubMed] [Google Scholar]
- 36.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, Lewinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB, Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CS, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (dilate-1): A randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the soluble guanylate cyclase stimulator in heart failure patients with preserved ef (socrates-preserved) study. Eur Heart J. 2017;38:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filippatos G, Maggioni AP, Lam CSP, Pieske-Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient-reported outcomes in the soluble guanylate cyclase stimulator in heart failure patients with preserved ejection fraction (socrates-preserved) study. Eur J Heart Fail. 2017;19:782–791 [DOI] [PubMed] [Google Scholar]
- 41.Simon MA, Vanderpool RR, Nouraie M, Bachman TN, White PM, Sugahara M, Gorcsan J 3rd, Parsley EL, Gladwin MT Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI insight. 2016;1:e89620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2:123–130 [DOI] [PubMed] [Google Scholar]
- 43.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy YN, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;epub ahead of press. DOI: 10.1016/j.jchf.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Bohm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Kotecha D. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: An individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leblais V, Delannoy E, Fresquet F, Begueret H, Bellance N, Banquet S, Allieres C, Leroux L, Desgranges C, Gadeau A, Muller B. Beta-adrenergic relaxation in pulmonary arteries: Preservation of the endothelial nitric oxide-dependent beta2 component in pulmonary hypertension. Cardiovasc Res. 2008;77:202–210 [DOI] [PubMed] [Google Scholar]
- 47.Taylor BJ, Snyder EM, Richert ML, Wheatley CM, Chase SC, Olson LJ, Johnson BD. Effect of beta2-adrenergic receptor stimulation on lung fluid in stable heart failure patients. J Heart Lung Transplant. 2017;36:418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: Haemodynamic, clinical, and prognostic implications. Eur J Heart Fail. 2015;17:1161–1171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.