Abstract
Specific genetic variations in the gene for the selenium-containing anti-oxidant protein glutathione peroxidase 1 (GPX1) are associated with the risk of a variety of common diseases, including cancer, diabetes, and cardiovascular disorders. Two common variations have been focused upon, one resulting in leucine or proline at codon 198 and another resulting in 5, 6 or 7 alanine repeats were previously shown to affect the distribution of GPX1 between the cytoplasm and mitochondria. Human MCF7 cells engineered to exclusively express GPX1 with 5 alanine repeats at amino terminus and proline at codon 198 (A5P) and 7 alanine repeats at amino terminus and leucine at codon 198 (A7L), as well as derivatives targeted to the mitochondria by the addition of a mitochondrial localization sequence (mA5P and mA7L) were used to assess the consequences of the expression of these proteins on the cellular redox state and bioenergetics. Ectopic expression of A5P and A7L reduced the levels of reactive oxygen species and the mitochondrial targeted derivatives exhibited better activity in these assays. Bioenergetics and mitochondrial integrity were assessed by measuring mitochondrial membrane potential, oxygen consumption, ATP levels and the levels of lactate dehydrogenase. The results of these assays indicated distinctive and sometimes opposing patterns with regard to differences between the consequences of the expression of A5P, A7L, mA5P, and mA7L. These data provide new information on the consequences of differences in the primary structure and cellular location of GPX1 proteins and contribute to the understanding of how these effects might contribute to human disease.
Keywords: Glutathione peroxidase, gene polymorphisms, oxidative stress, mitochondria, respiration
Introduction
Glutathione peroxidases (GPXs) are antioxidant enzymes that detoxify hydrogen peroxide (H2O2) or lipid hydroperoxides using reducing equivalents obtained from glutathione [Brigelius-Flohe and Maiorino, 2013]. One member of this family is GPX1, a selenocysteine-containing enzyme expressed in most tissues and cell types, being present in both the cytoplasm and mitochondria [Lubos et al., 2011]. Lower levels of GPX1 have generally been associated with increased cancer risk although this association is not absolute (reviewed in (1). We have previously reported on a positive correlation between tissue GPX activity and Gleason score, a measure of prostate cancer aggressiveness [Jerome-Morais et al., 2012] and a recent manuscript indicated a possible tumor-promoting role of the protein in oral squamous cell carcinomas [Lee et al., 2017].
GPX1 has also been implicated in the risk of several diseases, including diabetes and cancer, due to the association of specific allelic variations and disease risk. The best studied of these is a single nucleotide polymorphism (SNP) in the coding sequence that results in either of proline or leucine at codon 198 and a trinucleotide variation resulting in a variable number of alanine residues in the amino terminus of the protein. The codon 198 polymorphism was previously shown to be functional, impacting the regulation of GPX1 by selenium [Hu and Diamond, 2003] and specific interactions with GPX1 alleles differing at both these variations with a functional polymorphism in the gene for another anti-oxidant protein, MnSOD, impacted the expression of several proteins associated with cancer etiology [Ekoue et al., 2017].
In order to study the molecular consequences of the allelic differences in GPX1, we examined the cellular distribution of GPX1 in MCF7 human breast carcinoma cells engineered to exclusively express GPX1 proteins with combinations of the codon 198 variation and either 5 or 7 alanines [Bera et al., 2014]. It was determined that GPX1 containing a leucine at codon 198 and 7 alanines distributed more to the cytoplasm than the other variants examined. The impact of subcellular localization was further analyzed by directing each combination to the mitochondria of MCF7 cells with the addition of a mitochondrial localization signal to the corresponding cDNA. The results obtained indicated both the primary GPX1 sequence and the cellular location had distinct effects on oxygen consumption and cell signaling [Bera et al., 2014]. Here, we used the same approach of comparing cytoplasmic- and mitochondria-directed GPX1 variants expressed in MCF7 cells to further investigate the impact on the generation and sensitivity to reactive oxygen species (ROS) and mitochondrial function, and ultimately gain new insight into the mechanism of impact of allelic variations in GPX1 on human disease.
Materials and methods
Reagents:
Lipofectamine-3000 was obtained from Invitrogen Inc, MitoSox and DCFH-DA were obtained from Molecular Probes, USA, JC-1 and GPx1 assay kits were obtained from the Cayman Chemical Company, USA. ATP-Lite luminescence assay kit was obtained from Perkin-Elmer, USA. Modified Eagles Medium (MEM), OptiMEM, Fetal Bovine Serum, penicillin-streptomycin solution, G418, and Trypsin were obtained from Himedia Lab, India. MTT was obtained from SRL, India. Other reagents were analytical grades and were obtained from local suppliers.
Cell Culture:
MCF7 cells were transfected, with previously generated constructs containing either the codon for leucine or proline at position 198 and 5 or 7 alanine repeats [Bera et al., 2014] or the pLNCX vector transiently or stable transfectants were isolated following selection in Modified Eagles medium (MEM) supplemented with 10%FBS, 1% penicillin-streptomycin and 200μg/ml of G418 (geneticin) at 370C in a humidified CO2 incubator. Cells were maintained in the G418-free medium for 2 days prior to the beginning of experiments. Cellular proliferation was determined by seeding 2 X 104 cells/ml into 96 well plates and treated with the indicated doses of menadione or H2O2 the following day and the viable cells were quantified using MTT.
Sub-cellular fractionation:
Cells were cultured and harvested by trypsinization, suspended in ice-cold mitochondria isolation buffer (250 mM sucrose, 10 mM Tris-Cl, pH 7.4, 1 mM EDTA), and homogenized in a Dounce homogenizer. The lysate was centrifuged at 700 X g for 10 minutes at 40C and the supernatant was again centrifuged at 14,000 X g for 10 minutes at 40C and the pellet was suspended in the isolation buffer. The resulting final supernatant was also stored and used as a mitochondria-free cytosolic extract.
GPx activity assay:
GPx enzyme activity was determined on whole cell lysates as well as the mitochondrial and cytosolic fractions using a coupled spectrophotometric assay kit that measures the GPX dependent consumption of NADPH (Cayman Chemical Company, USA). Enzyme activity was expressed as nmole NADPH oxidized per minute per milligram of total protein.
ROS Quantification:
Mitochondrial superoxide was determined using MitoSOX and total cellular ROS was quantified using dichloro-dihydro-fluorescein diacetate (DCFH-DA). 2 X 104 cells/ml were seeded into 96 well black plates for 24 hrs and were treated with either 5μM MitoSOX for estimation of mitochondrial superoxide or 100nM DCFH-DA for estimation cellular ROS level. The plates were incubated at 370C for 30 mins in dark. The excess unbound dyes were washed off with PBS and the plates were read at Ex/Em=510/580nm for MitoSOX and Ex/Em= 485/595nm for DCFH-DA. Cells were incubated with 5μM MitoSOX or 100nM DCFH-DA for 30 mins in the dark, excess unbound dye was removed by washing the cells with sterile PBS and readings were taken at 510/580nm for MitoSOX or 485/595nm for DCFH.
Mitochondrial Membrane potential assay:
Alterations in the mitochondrial membrane potential were measured using the JC-1 dye (Invitrogen). 2 X 104 cells were seeded into 96 well black plates for 24hrs with or without the addition of 3mM glucose. After the incubation period, 200nM of JC-1 was added to each well and incubated further at 37°C for 30 minutes in the dark. Excess dye was removed by washing with sterile PBS and fluorescence readings were taken at Ex/Em = 535/595 for J aggregate (red fluorescence) and Ex/Em = 485/535nm for J monomers (green fluorescence). The data were expressed as the ratio of red/green fluorescence with a higher ratio indicating metabolically active mitochondria.
Cellular ATP content:
ATP concentrations were quantified using a Perkin Elmer ATP-Lite kit which is based on the luminescence following a luciferin-luciferase reaction. Briefly, 2 X 104 cells were seeded into 96 well white plates for 24 hrs. 3mM glucose was added to each well and incubated for 2hr at 370C to stimulate ATP formation, which was then determined following the manufacturer’s instructions.
LDH assay:
2 X 106 cells were seeded into 6 well plates for 24 hrs and then 3mM glucose were added to each well, incubated for 2hr at 370C, collected and lysed in RIPA lysis buffer in the cold and centrifuged to discard the cell debris. The resulting supernatant was used in the LDH assay. The reaction was initiated by adding 1mM β-NADH to an assay mixture containing 200mM Tris-Cl buffer (pH 7.4), 30mM sodium pyruvate and 20μl cell lysate. The decrease in β-NADH absorbance at 340nm was monitored for 5 mins. Enzyme activities were expressed as nmole β-NADH oxidized per minute per milligram of total protein.
Oxygen consumption rate (OCR) measurement:
Cellular oxygen consumption was measured using an Oxygraph Plus system (Hansatech, UK) according to a published protocol [Barrientos et al., 2009]. MCF7 cells expressing the GPx1 allelic isoforms were grown for 24hrs, suspended in respiration buffer and added to the oxygraph chamber. NaCN sensitive oxygen consumption was measured in the presence or in absence of 3mM glucose. Briefly, cells were trypsinized, washed in ice-cold Dulbecco’s Phosphate Buffer Saline (DPBS) and immediately re-suspended in respiration buffer (300 mM mannitol, 10 mM KCl, 5 mM MgCl2, and 10 mM K2PO4, pH 7.4). The cell suspension was added to the polarographic chamber for intact cell-coupled endogenous cell respiration. 700μM sodium cyanide (NaCN), a mitochondrial complex IV inhibitor, was added to block respiration. Data were calculated using O2view software (Version 2.06) and expressed as nmol O2/minute/107 cells.
Statistical analysis:
The experimental data are presented as means ± standard deviation for three independent repeats (n = 3). The statistical significance of the experimental data was determined using the Student’s two-tailed unpaired t-test. Values of p ≥ 0.05 were considered statistically significant.
Results:
Ectopic expression of GPX1 isozymes reduces ROS levels.
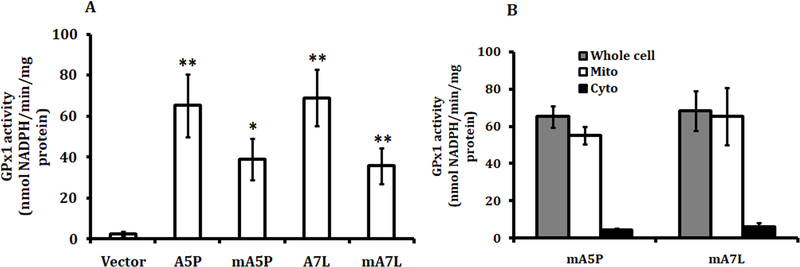
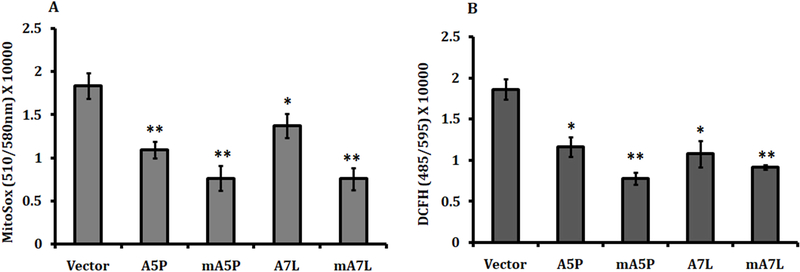
MCF7 cells do not produce detectable levels of GPX1 and were used to exclusively express GPX1 with 5 alanines and a proline198 (A5P) or 7 alanines and a leucine198 (A7L) or the corresponding proteins targeted to the mitochondria (mA5P and mA7L), selected for study based on our previous analysis of GPX1 proteins expressing different combinations of these variations (Bera et al. Cancer Res 2014). Stable transfectants expressing similar total cellular GPX activity were selected for further study (Figure 1A). Fractionation of cytoplasm and mitochondria from the transfectants confirmed the successful targeting of GPX1 to the mitochondria (Figure 1B), as previously reported (Bera et al. Cancer Res 2104). The levels of superoxide produced in the mitochondria and the total ROS in transfectants was evaluated using MitoSox and DCFH-DA, respectively (Figure 2A and 2B). While the expression of all of the GPX1 isoforms resulted in reduced levels of both mitochondrial superoxide and total ROS, localizing either GPX1 isoform to the mitochondria significantly enhanced the enzymes anti-oxidant efficacy, perhaps due to the greater proximity to the principal site of ROS generation, the mitochondria.
Figure 1. Ectopic GPx 1 expression in MCF7 cells.
GPx1 allelic variants and the mitochondrially-directed isoforms were expressed in MCF7 and analyzed for (A) GPx activity in the whole cell lysates and (B) in the sub-cellular fractions to confirm the mitochondrial targeting of the proteins. The statistical significances in (A) were calculated from the similarly treated vector-only transfected control and the values are the means ± sd, n = 3. *P < 0.05; ** P < 0.001
Figure 2. Estimation of cellular ROS levels.
Transfected cells were treated with either 5μM MitoSOX to determine mitochondrial superoxide levels (A) or 100nM DCFH-DA for estimation total cellular ROS levels (B). The statistical significances were calculated from the vector-transfected control and the values are the means ± sd, n = 3. *P < 0.05; ** P < 0.001.
Both cytoplasmic and mitochondrial-located GPX1 protect cells against ROS toxicity.
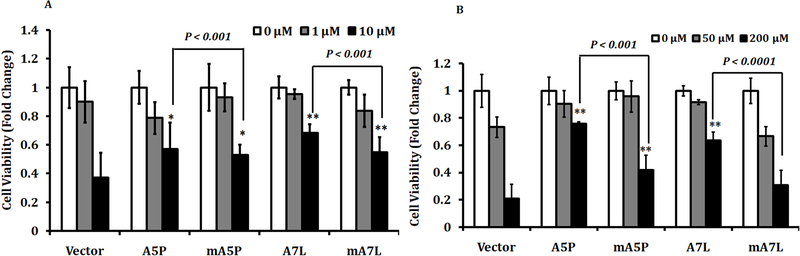
The data presented above indicated that the steady-state levels of ROS were affected by both the genotype and cellular location of GPX1. In order to evaluate if these same variables altered the cellular response to challenge with ROS, transfectants were exposed to menadione, a superoxide generator, and H2O2 followed by the determination of cell viability after 24hrs. All the transfectants expressing mitochondrially targeted or non-targeted GPX1 responded similarly to menadione treatment showing marginal but statistically significant protection against the 10 μM dose (Figure 3A). In contrast, cell viability following challenge with H2O2 improved when each of the GPX1 isoforms was expressed, but the protection offered by GPX1 expression was less for the mitochondrially-targeted isozymes than those retained in the cytoplasm (Figure 3B).
Figure 3. Cellular proliferation under oxidative stress.
Transfected were treated with menadione (A) or hydrogen peroxide (B) for 48hrs and the MTT assay was performed post-treatment. The statistical significances were calculated from the similarly treated vector transfected control and from the non-targeted isoforms, the values are the means ± sd, n = 3. *P < 0.05; ** P < 0.001.
Differential effects of GPX1 isozymes on mitochondrial integrity and function.
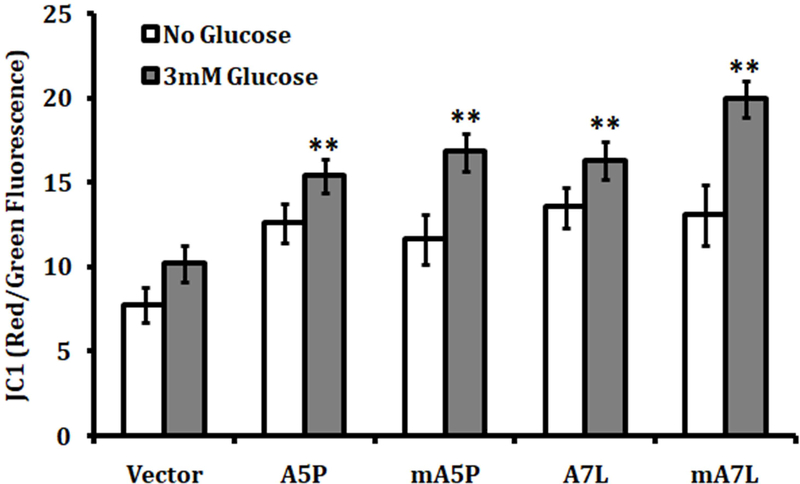
The impact of GPX1 allelic identity and cellular location on mitochondrial integrity was investigated. An adequate mitochondrial membrane potential is required for efficient oxidative phosphorylation whereas a decline in potential could be indicative of a damaged or inefficient organelle. Ectopic expression each of the GPX1 isoforms enhanced the mitochondrial membrane potential of transfected cells to a similar degree (Figure 4). Moreover, adding glucose to culture media to stimulate glycolysis and respiration resulted in an increase in mitochondrial membrane potential in all of the transfectants, although the greatest enhancement following glucose supplementation occurred when the cells were expressing mA7L (Figure 4).
Figure 4. The effect of GPX1 isoforms on mitochondrial membrane potential.
The mitochondrial membrane potential of transfected cells was determined using JC-1 fluorescence detection described in the Methods. The data is the ratio of red fluorescence (595 nm) which is proportional to the membrane potential and the green fluorescence (535 nm) which is emitted in necrotic or apoptotic cells. The statistical significances were calculated from the similarly treated vector transfected control and the values are the means ± sd, n = 3. ** P < 0.001.
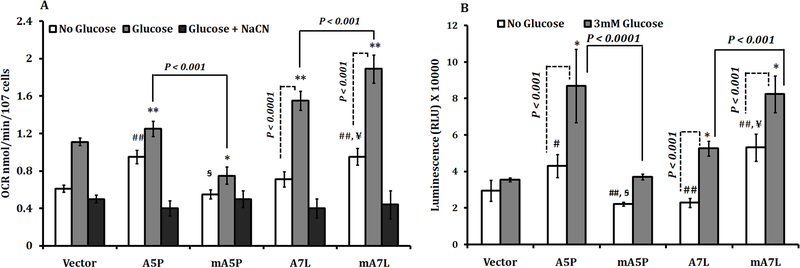
As an additional measure of the impact of GPX1 isozymes on mitochondrial function, the oxygen consumption rate (OCR) of each of the transfectants was analyzed. The expression of the native A5P, but not the native A7L, increased the OCR above that seen in vector-only transfected cells (Figure 5A), whereas targeting these same proteins to the mitochondria has opposite effects, mA5P decreased OCR and mA7L increased OCR compared to their native counterparts. These effects on OCR were most likely due to effects on the mitochondria as examining mitochondrial-independent oxygen consumption achieved by treating the cells with sodium cyanide, indicated in the figure as the third bar, was unchanged among all of the transfectants examined. Given that oxygen consumption is tightly linked to ATP synthesis in the mitochondria, we examined ATP levels in the transfectants. Mirroring the data obtained on oxygen consumption, ATP levels were higher in the cells expressing the native GPX1 isozymes, with lower ATP levels in the mA5P and higher ATP levels in the mA7L as compared to their cytoplasmic counterparts (Figure 5B).
Figure 5. Oxygen consumption and ATP levels in MCF7 cells expressing different GPX1 isoforms.
(A) NaCN sensitive oxygen consumption was measured in the presence of reduced levels (Figure 5) in absence of 3mM glucose as described in the Methods. The statistical significances were calculated from the similarly treated vector transfected control and the values are the means ± sd, n = 3. ## P < 0.001 versus non-treated vector, * P<0.05, **P<0.001 versus glucose treated vector, P<0.05 versus non-treated A5P, P<0.001 versus non-treated A7L. (B) ATP levels were estimated in the presence or in absence of 3mM glucose. The statistical significances were calculated from the similarly treated vector transfected control and the values are the means ± sd, n = 3. # P < 0.05 versus non-treated vector ## P < 0.001 versus non-treated vector, * P<0.001 versus glucose treated vector, #P<0.001 versus non-treated A5P, P<0.001 versus non-treated A7L.
The expression of GPX1 isoforms differentially affects glycolysis.
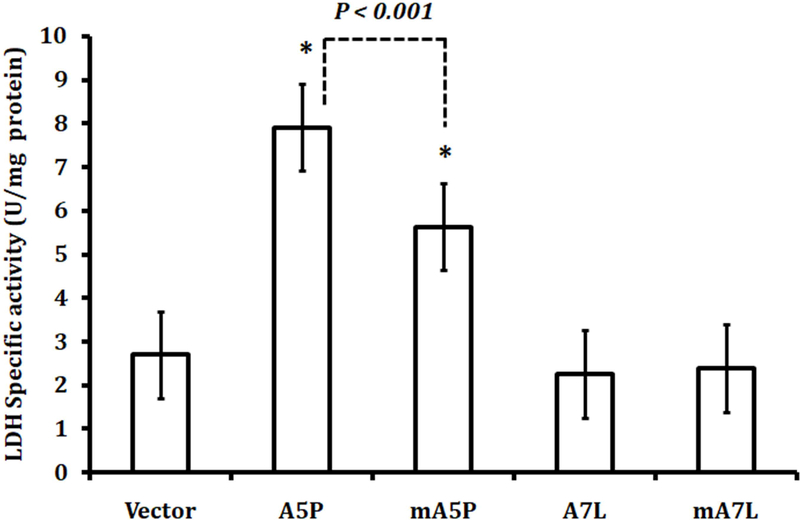
In addition to oxidative phosphorylation, glycolysis represents an alternative but the less efficient mechanism of energy metabolism. Lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate, the final product of glycolysis, to lactate and converts NAD+ to NADH. The levels of LDH in the transfectants were examined (Figure 6). The expression of either A5P or mA5P resulted in increased levels of LDH, with LDH levels being lower in cells expressing mA5P. In contrast, cells expressing either A7L or mA7L did not exhibit elevated LDH.
Figure 6. GPx1 affects LDH activity.
LDH was assayed using lysates as described in the Methods section. The statistical significances were calculated compared to the vector-only transfected controls and the values are the means ± sd, n = 3. * P<0.001.
Discussion
GPX1 has been implicated in a broad range of diseases due to the association of distinct alleles, such as those expressing an SNP at codon 198 or a variable number of alanine repeats, with risk [Lubos et al., 2011; Rayman, 2009]. In order to investigate the biological consequences of these variations, we took advantage of the observation that MCF7 cells do not produce detectable levels of GPX1 and expression of specific GPX1 alleles in these cells revealed that GPX1 proteins expressed from these alleles differentially partitioned between the cytoplasm and mitochondria [Bera et al., 2014]. To explore the consequences of differences in the amino acid sequence and cellular location of GPX1, the proteins expressed from two different GPX1 alleles were engineered to be targeted to the mitochondria, and GPX1 sequence and cellular location were both factors in determining the resulting phenotypes [Bera et al., 2014]. These results were extended herein by assessing several parameters associated with energy metabolism and oxidative stress. A potential weakness of these studies is the experimental design of targeting GPX1 isoforms to the mitochondria using a mitochondria localization sequence although the native GPX1 does have such a localization sequence. While this difference may impact the consequences of GPX1 enzyme activity in the mitochondria, the use of transfectants producing similar enzyme activity and exclusively expressing GPX1 in either the cytoplasm or mitochondria provides new information about the potential impact of GPX1 in each compartment. Ectopic expression of either the A5P or A7L alleles resulted in lower levels of mitochondrial superoxide and total ROS levels and targeting either protein to the mitochondria resulted in even lower levels (Figure 2). In addition, expression of both A5P and A7L also increased the mitochondrial membrane potential of transfected cells, used as a measure of mitochondrial integrity which was improved upon by targeting the respective proteins to the mitochondria (Figure 4). These results are consistent with the anticipated benefits of the expression of an antioxidant enzyme and their targeting to the mitochondria, a principal source of ROS, in protecting biomolecules from oxidative damage. A more complicated pattern emerged when other parameters, such as oxygen consumption rates and ATP levels (Figure 5) or glycolysis as determined by measuring LDH levels (Figure 6). These results are likely to reflect the impact of GPX1 expression and location on signaling pathways that are responsive to ROS. For example, GPX1 over-expression has been shown to alter EGF signaling by reducing the levels of mitochondrially produced ROS [Handy et al., 2009]. In contrast to our data presented above, these authors reported that over-expression of GPX1 decreased mitochondrial membrane potential as well as ATP synthesis [Handy et al., 2009]. Differences between the previously published data and that presented herein may reflect the different cell lines used or the consequences of over-expressing GPX1 in cells with significant endogenous levels of GPX1 as was done by Handy et al. vs. expressing GPX1 in cells that are initially null, as was done here. Elevated levels of GPX1 above endogenous levels can have profound effects on cellular metabolism, as evidenced by studies where it was demonstrated that mice engineered to over-express GPX1 developed insulin resistance [McClung et al., 2004; Wang et al., 2008].
The impact of the expression of different GPX1 alleles on mitochondrial function indicates a plausible mechanism by which the encoded proteins can influence the risk of several diseases by affecting oxidative stress and alterations in ROS-responsive pathways. Some inconsistencies in the literature about the contribution of GPX1 to any particular disease may be due to modifying factors such as SNPs in other genes or the expression of other anti-oxidant or pro-oxidant enzymes, diet or environmental factors. Since GPX1 levels are responsive to the dietary intake of selenium, knowledge about the role played by GPX1 variations in disease risk may be useful in identifying individuals who should restrict or supplement their selenium intake to reduce risk, this being an area of research that merits additional study.
Acknowledgements:
This work was supported by research grants from the Science and Engineering Research Board, Department of Science and Technology, Govt. of India (Ref. SB/YS/LS-248/2013) to SB and (Ref. ECR/2016/000898) to AR, and from the Department of Biotechnology, Govt. of India (Ref. 6242-P5/RGCB/PMD/DBT/SMNB/2015) to SB and by grants from the National Institutes of Health (Grant # RO1CA127943, R21CA182103) to AMD.
Footnotes
Conflict of Interest: The authors wish to declare no conflict of interest.
References
- Barrientos A, Fontanesi F, Diaz F. 2009. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr Protoc Hum Genet Chapter 19:Unit19 3. 10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera S, Weinberg F, Ekoue DN, Ansenberger-Fricano K, Mao M, Bonini MG, Diamond AM. 2014. Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function. Cancer Res 74:5118–26. 10.1158/0008-5472.can-14-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Maiorino M. 2013. Glutathione peroxidaseseditor^editors. Biochim Biophys Acta. p 3289–303. [DOI] [PubMed] [Google Scholar]
- Ekoue DN, Bera S, Ansong E, Hart PC, Zaichick S, Domann FE, Bonini MG, Diamond AM. 2017. Allele-specific interaction between glutathione peroxidase 1 and manganese superoxide dismutase affects the levels of Bcl-2, Sirt3 and E-cadherin. Free Radic Res 51:582–590. 10.1080/10715762.2017.1339303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy DE, Lubos E, Yang Y, Galbraith JD, Kelly N, Zhang YY, Leopold JA, Loscalzo J. 2009. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem 284:11913–21. 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Diamond AM. 2003. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res 63:3347–51. [PubMed] [Google Scholar]
- Jerome-Morais A, Wright ME, Liu R, Yang W, Jackson MI, Combs GF, Jr., Diamond AM. 2012. Inverse association between glutathione peroxidase activity and both selenium-binding protein 1 levels and Gleason score in human prostate tissue. Prostate 72:1006–12. 10.1002/pros.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JR, Roh JL, Lee SM, Park Y, Cho KJ, Choi SH, Nam SY, Kim SY. 2017. Overexpression of glutathione peroxidase 1 predicts poor prognosis in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 10.1007/s00432-017-2466-7. [DOI] [PubMed] [Google Scholar]
- Lubos E, Loscalzo J, Handy DE. 2011. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957–97. 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. 2004. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A 101:8852–7. 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP. 2009. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta 1790:1533–40. 10.1016/j.bbagen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. 2008. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 51:1515–24. 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]