Abstract
Rationale:
Somatic overexpression in mice using adeno-associated virus (AAV) as gene transfer vectors has become a valuable tool to analyze the roles of specific genes in cardiac diseases. The lack of atrial-specific AAV vector has been a major obstacle for studies into the pathogenesis of atrial diseases. Moreover, gene therapy studies for atrial fibrillation would benefit from atrial-specific vectors. Atrial natriuretic factor (ANF) promoter drives gene expression specifically in atrial cardiomyocytes.
Objective:
To establish the platform of atrial specific in vivo gene delivery by AAV-ANF.
Methods and Results:
We constructed AAV vectors based on serotype 9 (AAV9) that are driven by the atrial-specific ANF promoter. Hearts from mice injected with AAV9-ANF-GFP exhibited strong and atrial-specific GFP expression without notable GFP in ventricular tissue. In contrast, similar vectors containing a cardiac troponin T promoter (AAV9-TNT4-GFP) showed GFP expression in all 4 chambers of the heart, while AAV9 with an enhanced chicken beta-actin promoter (AAV-enCB-GFP) caused ubiquitous GFP expression. Next, we used Rosa26mT/mG (mT/mG), a double-fluorescent Cre reporter mouse that expresses membrane-targeted tandem dimer Tomato (mT) prior to Cre-mediated excision, and membrane-targeted green fluorescent protein (mG) after excision. AAV9-ANF-Cre led to highly efficient LoxP recombination in mT/mG mice with high specificity for the atria. We measured the frequency of transduced cardiomyocytes in atria by detecting Cre-dependent GFP expression from the Rosa26mT/mG allele. AAV9 dose was positively correlated with the number of GFP-positive atrial cardiomyocytes. Finally, we assessed whether the AAV9-ANF-Cre vector could be used to induce atrial-specific gene knockdown in proof-of-principle experiments using conditional junctophilin-2 knockdown mice. Four weeks after AAV9-ANF-Cre injection, a strong reduction in atrial expression of JPH2 protein was observed. Furthermore, there was evidence for abnormal Ca2+ handling in atrial myocytes isolated from mice with atrial-restricted JPH2 deficiency.
Conclusions:
AAV9-ANF vectors produce efficient, dose-dependent, and atrial-specific gene expression following a single-dose systemic delivery in mice. This vector is a novel reagent for both mechanistic and gene therapy studies on atrial diseases.
Keywords: Animal Models of Human Disease, Arrhythmias, Gene Therapy, Genetically Altered And Transgenic Models, Adeno-associated virus, atrial natriuretic factor, Cre, atrial fibrillation, gene therapy, arrythmia, animal models of human disease remodeling, genetically altered mice
INTRODUCTION
Diseases of the atria can pose a serious threat to human health. Atrial fibrillation (AF), for example, is the most common sustained cardiac arrhythmia associated with high morbidity and mortality rates.1 Since current treatment modalities - both pharmacological therapies and cardiac ablation - remain ineffective in about 40% to 50% of AF patients,2 there is an urgent need for improved therapies. A better understanding of the molecular mechanisms underlying AF is a prerequisite to the development of new therapeutic approaches. While mouse models have been very helpful in this regard, a major limitation remains ventricular dysfunction arising in global or cardiomyocyte-specific overexpression and deletion models.3 The sarcolipin-Cre mouse model allows for atrial-specific gene manipulation,4 but this involves labor-intensive and time-consuming crosses. Moreover, the heterozygous sarcolipin-Cre mice are haplo-insufficient for sarcolipin and susceptible to pacing-induced AF induction (data not shown).
Adeno-associated virus (AAV) is widely used as a gene transfer vector in biomedical and gene therapy studies.5 AAV serotype 9 (AAV9) can efficiently transduce cardiac myocytes, among other cell and tissue types.6 AAV9 can package sequences up to 4.7 kb that may include a tissue-specific promoter that drives gene expression in the transduced cells.7 Atrial-specific gene delivery could therefore be achieved by careful selection of an atrial-specific promoter. Atrial natriuretic factor (ANF), a peptide hormone synthesized and stored in the atria, was originally identified as a major participant in the systemic regulation of extracellular fluid volume and electrolytes.8 Nppa, the gene encoding ANF, is initially expressed throughout the whole heart at the early stages of development, but its postnatal expression is restricted to the atrial chambers.9
Here we developed an atrial-specific gene transfer vector containing a shortened ANF promoter. AAV9 vectors containing a 653 bp segment of the mouse NPPA promoter10 were used to drive expression of either enhanced green fluorescent protein (GFP) or Cre recombinase. The AAV9-ANF-GFP vector caused highly-specific atrial expression of GFP in vivo in mice. Moreover, a single injection of AAV9-ANF-Cre resulted in efficient and highly specific Cre recombination in the atria, for both a fluorescent Cre-dependent reporter, as well as an endogenous gene. Together, these studies demonstrate that AAV9 vectors with the ANF promoter produce efficient, dose-dependent, and atrial-specific gene expression following a single systemic delivery in mice.
METHODS
The authors declare that all supporting data including complete vector sequences are available within the article and its Online Supplementary Material. Detailed experimental protocols are provided in the Online Supplement Material. All experiments were performed by operators who were blinded to the genotype/experimental group the animal or sample belonged to.
AAV9 production and delivery.
Detailed plasmid information and sequences are available in the Online Supplemental Material. Plasmids will be publicly shared through Addgene upon publication.
Animal studies and in vivo vector delivery.
All studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996). The mT/mG mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). A transgenic mouse expressing shRNA targeting JPH2 (shJPH2) was previously generated.5 ShJPH2 mice were crossed to transgenic mice expressing Cre recombinase driven by the αMHC promoter (αMHC)-MerCreMer (MCM) to achieve cardiac-specific knockdown of JPH2. MCM-shJPH2 mice were intraperitoneal injected (30 mg/kg) with daily 100 μl tamoxifen (Sigma-Aldrich Co., St. Louis, MO) for 5 consecutive days. Both male and female mice were used and randomly assigned to treatment groups. AAV vectors were injected retro-orbitally into the venous sinus at doses ranging from 5×1011 to 5×1012 genome copies (GC)/mouse diluted in 100μL of saline.11
Confocal imaging.
Sections were imaged using a Zeiss LSM 880 confocal imaging system. Images were collected using a Zeiss Axiocam 503 mono camera. Images were captured with Zen Software.
Atrial myocyte Ca2+ imaging.
Detailed methods are provided in the Online Supplemental Material.
Western blot analysis.
Tissues were snap-frozen in liquid nitrogen. Western blotting was performed as detailed in the Online Supplemental Material.12
Statistical analysis.
Results are expressed as mean ± SEM. Continuous variables were evaluated using SPSS or Prism using an unpaired Student t test or ANOVA, after performing the D’Agostino-Pearson normality test for normal distribution of the data. Categorical variables were evaluated with Fisher’s exact test. The generalized estimating equation approach was performed by the use of the binomial distribution to study the dichotomous spontaneous SR Ca2+ release event. For other multiple group comparison, one-way ANOVA followed by Tukey’s post-test was used. P<0.05 was considered statistically significant.
RESULTS
AAV9-ANF-GFP induces atrial myocyte-specific GFP expression.
To assess the specificity and potency of the ANF promoter fragment within an AAV9 vector, we generated an AAV9-ANF-GFP virus for in vivo studies with a GFP reporter gene (Figure 1A). GFP expression patterns were compared to those induced by the AAV9-TNT4-GFP vector with a predicted pan-cardiomyocyte specific expression pattern, and AAV9-enCB-GFP for ubiquitous expression. AAV9-ANF-GFP was delivered to adult C57BL/6J mice at 3 different doses (5×1011, 1×1012, 5×1012 genome copies [GC]) by retro-orbital injection. GFP expression in atrial tissue collected after 3-weeks correlated positively with the AAV9 dose (Figure 1B). Mice injected with AAV9-ANF-GFP exhibited robust and specific expression of GFP in the atria (Figure 1B), without notable GFP in ventricles or other extracardiac tissues (Figure 1C, 1D, Online Figure I). Mice treated with AAV9-TNT4-GFP showed GFP expression in both atria and ventricles (Figure 1C, 1E). In contrast, AAV9-enCB-GFP produced ubiquitous expression in the entire heart and other tissues (Figure 1C, Online Figure I).
Figure 1. AAV9-ANF-GFP induces atrial myocyte-specific GFP expression.
A, Schematic representation of the AAV transgenes used for these studies. ITR, I-terminal repeat; GFP, green fluorescent protein. B, Western blots showing GFP expression in atria of mice receiving different doses of AAV9-ANF-Cre (low: 5×1011 GC; mid: 1×1012 GC; high: 5×1012 GC), or saline (Ctrl). C. Images of cardiac sections showing atrial-restricted GFP expression in mice treated with AAV9-ANF-GFP (1×1012 GC). Mice receiving AAV9-TNT4-GFP and AAV9-enCB-GFP exhibited GFP expression in both atria and ventricles. D, Western blots showing GFP expression only in atrial tissue but not in ventricle or extra-cardiac tissues. E, Images of cardiac sections incubated with GFP antibody confirming atrial GFP expression in mice treated with AAV9-ANF-GFP, while both atria and ventricles expressed GFP in AAV9-TNT4-GFP treated mice. Scale bar, 100 μm.
AAV9-ANF-Cre provides efficient LoxP recombination in atrial myocytes of mT/mG mice.
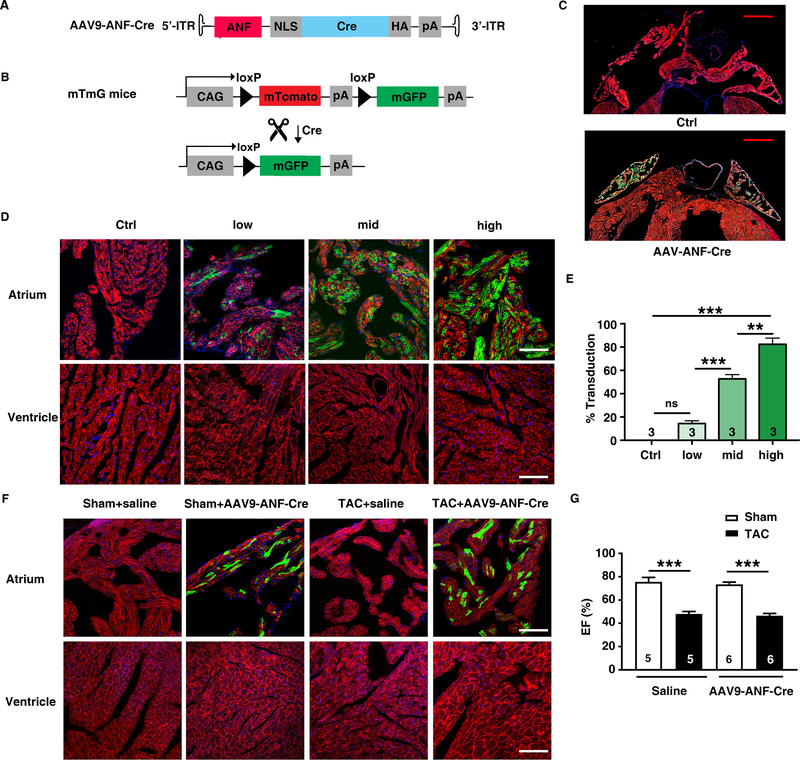
To determine whether the ANF promoter fragment within the AAV9 vector could be used for inducible atrial-specific excision of floxed alleles, we generated an AAV9-ANF-Cre virus for in vivo studies (Figure 2A). We used mT/mG, a double-fluorescent Cre reporter mouse that expresses membrane-targeted tandem dimer Tomato (mT) prior to Cre-mediated excision, and membrane-targeted green fluorescent protein (mG) after excision (Figure 2B). AAV9-ANF-Cre induced highly efficient LoxP recombination in mT/mG mice only within atria, without notable GFP-positive cells in ventricle or other extracardiac tissues (Figure 2C, Online Figure II). To determine the effect of AAV9 dosage on LoxP recombination in mT/mG mice, we treated mice with 3 different doses of AAV9-ANF-CRE: 5×1011, 1×1012, or 5×1012 GC/mouse (Figure 2D). The number of transduced cardiomyocytes (CMs) in atria was detected by Cre-dependent GFP expression from the Rosa26mT/mG allele. AAV dose was positively correlated with the number of the GFP-positive atrial CMs (Figure 2D, 2E). The data indicate that a systemic dose of 1×1012 GC injected at the age of 2–3 months produces highly efficient Cre recombination in the majority of atrial cardiomyocytes.
Figure 2. AAV9-ANF-Cre provides efficient LoxP recombination in atrial myocytes of mT/mG mice.
A, Schematic diagram of the AAV9-ANF-Cre vector used in the mT/mG mice. B, Schematic diagram of the mT/mG allele before and after Cre-mediated recombination. C, Representative images of hearts from mT/mG mice treated with AAV9-ANF-Cre and saline. Scale bar, 1.0 mm. D, Representative images of heart sections from mT/mG mice that were injected with different doses of AAV9-ANF-Cre (low: 5×1011 GC; mid: 1×1012 GC; high: 5×1012 GC), or saline (Ctrl). Scale bar, 100 μm. E, Bar graph showing average percentages of GFP-positive cardiomyocytes. F, Representative images of heart sections from TAC mice pretreated with AAV9-ANF-Cre (1×1012 GC) showing efficient Cre recombination in atrial myocytes resulting in GFP expression, while no GFP-positive cells were observed in ventricles. Scale bar, 100 μm. G, Echocardiography revealed reduced ejection fraction (EF) in mice 6 weeks after TAC. **p < 0.01; ***p < 0.001.
ANF expression has been reported in ventricular tissue under pathological conditions such as heart failure.13 To determine whether pathological conditions induce extra-atrial Cre expression driven by the ANF promoter fragment within the AAV9 vector, we performed transverse aortic constriction (TAC) on mTmG mice treated with AAV9-ANF-Cre 1×1012 GC/mouse 1 week before surgery. Ventricular function of these mice was assessed using echocardiography at 6 weeks after TAC, demonstrating a decline in EF and dilatation of the left ventricle (Figure 2G, Online Table I). Cardiac sections from TAC mice injected with AAV9-ANF-Cre exhibited efficient Cre recombination in the atria, without notable GFP-positive cells in ventricle (Figure 2F). Thus, these data demonstrate that the ANF promoter fragment can be used for atrial-specific gene expression even under pathological conditions such as pressure overload.
AAV9-ANF-CRE mediated gene knockdown in atrial myocytes of shJPH2 floxed mice.
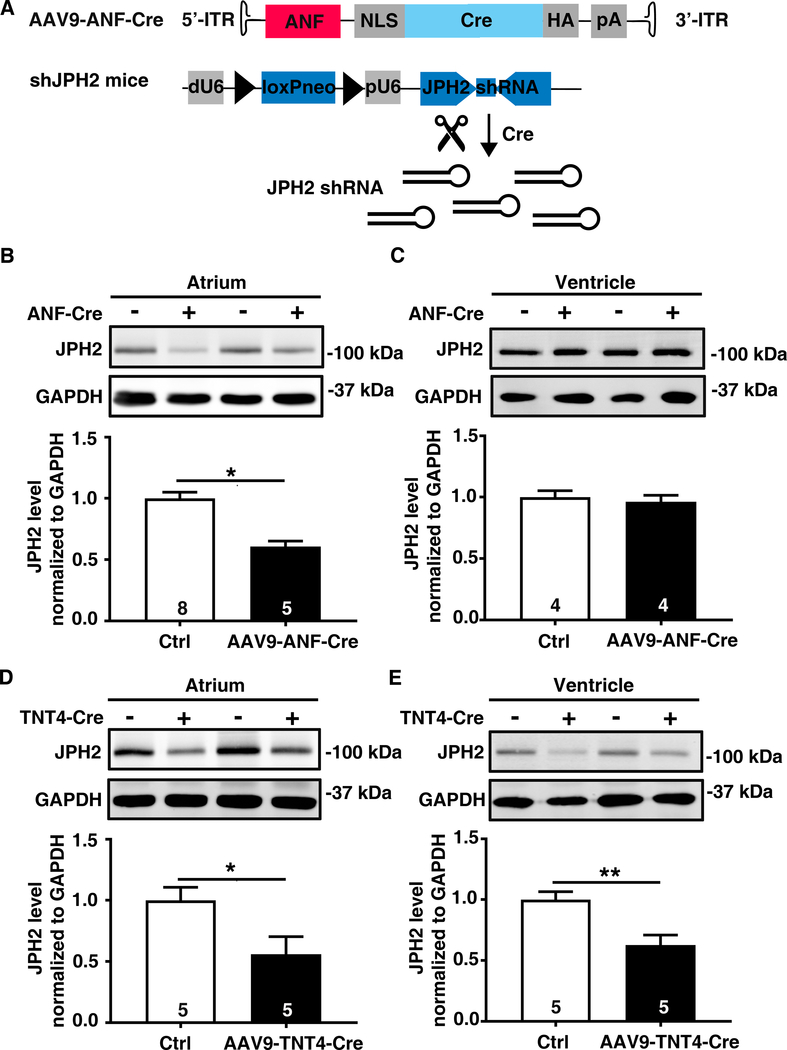
Next, we tested whether the AAV9-ANF-Cre vector could be used to selectively knock down a gene within atrial myocytes. AAV9-ANF-Cre was administered to adult transgenic mice overexpressing shRNA targeting junctophilin-2 (shJPH2) upon Cre-mediated removal of a loxP-flanked STOP cassette (Figure 3A).14 Four weeks following retro-orbital injection of AAV9-ANF-CRE (1×1012 GC/mouse), a strong reduction of JPH2 protein expression was observed in atrial samples, while JPH2 levels were unaltered in ventricular tissue, liver, kidney, spleen, and lung (Figure 3B-C). In contrast, JPH2 protein levels were reduced in both atrial and ventricular tissues from shJPH2 mice injected with AAV9-TNT4-Cre (Figure 3D-E), and MCM-shJPH2 mice injected with tamoxifen (Online Figure III). These data demonstrate the utility of AAV9-ANF-Cre for inducible atrial-specific gene knock down.
Figure 3. AAV9-ANF-CRE mediated gene knockdown in atrial myocytes of NFATc1 floxed mice.
A, Schematic diagram of AAV9-ANF-Cre vector, and transgene in shJPH2 mice which contains a JPH2-silencing shRNA sequence downstream of a split U6 promoter, inactivate by a loxP-flanked neomycin stop element.14 B, AAV9-ANF-Cre administration to shJPH2 mice led to a reduction in atrial JPH2 protein levels assessed by western blotting, but C, while ventricular JPH2 levels remained unaltered. D, Western blots showing reduced atrial, and E, ventricular JPH2 protein levels in shJPH2 mice treated with AAV9-TNT4-Cre. N, number of mice. p<0.05, **p<0.01.
AAV9-ANF-Cre mediated JPH2 knockdown caused abnormal SR Ca2+ handling in atrial myocytes.
Prior studies revealed that JPH2 knockdown in ventricular myocytes led to profound alterations in intracellular Ca2+ handling.14 Confocal imaging revealed that atrial myocytes isolated from shJPH2 mice 4-weeks after AAV9-ANF-Cre administration exhibit a reduced amplitude of the Ca2+ transient at 1-Hz pacing (Figure 4A-B). The SR Ca2+ load assessed using a caffeine dump protocol was also significantly reduced due to JPH2 knockdown. To determine whether diastolic Ca2+ handling was also affected, spontaneous SR Ca2+ release events were measured in atrial myocytes (Figure 4D). Knockdown of JPH2 in shJPH2 mice injected with AAV9-ANF-Cre led to a significantly higher Ca2+ spark frequency (Figure 4E-F, Online Table II). Finally, echocardiography revealed normal ventricular function in these mice, demonstrating that the changes in atrial Ca2+ handling are not secondary to altered ventricular function (Online Table III).
Figure 4. Abnormal SR Ca2+ handling in atrial myocytes from shJPH2 mice injected with AAV9-ANF-Cre.
A, Representative tracings of Ca2+ transients after 1-Hz pacing in atrial myocytes from shJPH2 mice. Caffeine induced SR Ca2+ dump. B, Quantification of Ca2+ transient amplitude (CaT), and C, total SR Ca2+ content (SR load). D, Confocal microscopy line scans revealing Ca2+ sparks in atrial myocytes from shJPH2 mice. E, Bar graphs showing average Ca2+ spark frequency (CaSpF), and F, CaSpF normalized to SR Ca2+ load. *P<0.05; *** P<0.001.
DISCUSSION
AAV9 vectors are a commonly used tool for manipulating gene expression in mouse models of cardiac disease including atrial arrhythmias.3, 15 Time and tissue restricted alterations in gene expression can be achieved by combining Cre and loxP elements within mouse alleles and AAV9 vectors.15 However, it has been challenging to manipulate gene expression specifically in the atria. A sarcolipin-Cre knock-in mouse model has been described, which enables gene deletion in atrial myocytes.4 A limitation of this model is that Cre replaces one endogenous sarcolipin allele, thus rendering the mice haploinsufficient for this gene, which has important functions in the atria. In our hands, the heterozygous sarcolipin-CRE mice exhibit a high incidence of pacing-induced AF (not shown).
In this study, we demonstrated that AAV9 with a NPPA proximal promoter fragment can be used for in vivo gene delivery to atrial myocytes in an efficient and highly specific manner. Mice injected with AAV9-ANF-GFP produced a strong and specific expression in atrial tissue, while both mice injected with AAV-TNT4-GFP and AAV-enCB-GFP showed a more universal expression in the whole heart. Atrial GFP expression levels were dependent on the AAV9 dose. We also demonstrated the utility of this vector for Cre-mediated recombination using the mT/mG reporter mouse.16 Although the efficiency of Cre recombination may vary between transgenes, our data suggest that a dose of 1×1012 GC injected at the age of 2–3 months allows for a highly efficient atrial-specific recombination. Our study also shows that AAV9-ANF-Cre is can efficiently knock down an endogenous protein (JPH2) in the atria of adult conditional transgenic mice that overexpress shRNA to silence JPH2 expression.17 Downregulation of JPH2 in atrial myocytes was associated with phenotypic changes in SR Ca2+ handling similar to those observed previously in ventricular myocytes from shJPH2 mice crossed with αMHC-mER-Cre-mER mice.14 A major advantage of our new vector is that genes can be overexpressed or downregulated in a time-restricted way within atrial myocytes only. Moreover, this platform provides the opportunity to study atrial phenotypes without confounding changes in ventricular gene expression or function, which may occur with pan-cardiomyocyte specific vectors.
The NPPA promoter fragment could also be used to develop transgenic mice.10 However, the ANF promoter is active in both atria and ventricles before birth, and is then repressed in the ventricle postnatally.10 Therefore, such mice would be expected overexpress the transgene during cardiac development, which might alter atrial or ventricular function. The AAV9-ANF approach in adult mice would be superior to ANF transgenic mice for studies into the role of genes within the atria without confounding ventricular phenotypes. Prior studies revealed that ANF can be reexpressed in ventricular tissue under pathological conditions such as heart failure.13 Our data, however, demonstrate that the ANF promoter fragment in our AAV9-ANF vectors can be used for atrial-specific gene expression even under pathological conditions such as pressure overload (Fig. 2F-G). Another advantage of the AAV9-ANF approach compared with conventional Cre transgenes is the reduction in time and effort needed to generate experimental cohorts. Breeding of double transgenic lines requires at least two mating rounds and potentially 5–10 rounds of backcrossing. In contrast AAV9-ANF-Cre can be applied directly in a homozygous mouse line using littermates as controls. AAV9-ANF vectors should provide investigators with a useful tool for atrial-specific overexpression or gene deletion in adult mice, accelerating research in atrial biology and disease.
Our atrial-specific AAV vectors have potential clinical implications for the future treatment of AF. Although AF is the most common arrhythmia currently affecting 6 million in the US, current pharmacologic treatments remain inadequate.18 Experimental gene therapy approaches are being developed, but rather than using an atrial-specific promoter these studies have relied on site-specific application, such as gene painting.19 AAV gene therapy may be a promising approach to directly target the molecular mechanisms of AF, improving long term efficacy while decreasing potential risks and side effects. We chose to use AAV9 because of the high transduction efficiency of this serotype for cardiomyocytes in mice.6, 20 AAV9 is also currently in human gene therapy trials, and has shown remarkably positive results for spinal muscular atrophy.21 A recent study found that very high doses of an AAV9 variant can cause severe liver and neuron damage in rhesus macaques and pigs,22 calling for caution with the use of this serotype. These doses likely far exceed what would be necessary to transduce the atria by intravenous delivery. It should also be noted that our ANF promoter based vector could be packaged into any naturally occurring or engineered AAV capsid serotype, and there are numerous other candidates for cardiac delivery.23 To our knowledge, atrial-specific gene therapy has not been attempted in humans with viral vectors. Atrial-specific AAV vectors will facilitate the identification of novel therapeutic targets as well as enable gene therapy for atrial diseases.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
Atrial fibrillation is the most common sustained cardiac arrhythmia associated with high morbidity and mortality rates.
Adeno-associated viruses (AAV) are widely used as gene transfer vectors in biomedical research and for gene therapy applications.
To date, no vector exists for atrial-specific gene transfer.
What New Information Does This Article Contribute?
Adeno-associated virus serotype 9 (AAV9) containing a shortened atrial natriuretic factor (ANF) promoter provided highly specific atrial gene expression in vivo in mice.
A single injection of AAV9-ANF-Cre resulted in efficient and highly atrial-specific Cre recombinase expression in the atria.
Atrial-specific gene expression using AAV9-ANF vectors was not associated with extra-atrial transgene expression or off-target phenotypes.
The findings demonstrate that AAV9 vectors containing the shortened ANF promoter can be used to induce atrial-specific transgene expression or gene knockdown following a single systemic delivery in mice. Atrial-specific AAV vectors will facilitate the identification of novel therapeutic targets as well as enable gene therapy approaches for atrial diseases..
Acknowledgments
SOURCES OF FUNDING
This work is supported by National Natural Science Foundation of China (NSFC, No. 81470519) (L.N), NIH grants F30-HL140782 (H.M.C.), R56-HL131649, and R01-HL136389 (N.L.), R01-HL132840 (W.R.L.), R01-HL089598, R01-HL091947, and R01-HL117641 (X.H.T.W.), American Heart Association grants 17CPRE33660059 (H.M.C.), and 14SDG20080008 (N.L.), an Individual Investigator Research Grant Award from the RYR1 foundation (W.R.L.), and a grant from the Saving Tiny Hearts Foundation (X.H.T.W.).
Nonstandard Abbreviations and Acronyms:
- AAV9
adeno-associated virus serotype 9
- AF
atrial fibrillation
- ANF
atrial natriuretic factor
- GC
genome copies
- JPH2
junctophilin-2
- mT
membrane-targeted tandem dimer Tomato
- mG
membrane-targeted green fluorescent protein
- TAC
transverse aortic constriction
Footnotes
DISCLOSURES
XHTW is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders. Other authors have no conflicts.
REFERENCES
- 1.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: The rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, Santini M, Cobbe SM, Widdershoven JW, Baur LH, Levy S, Crijns HJ. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: Follow-up of the euro heart survey on atrial fibrillation. European heart journal. 2008;29:1181–1189. [DOI] [PubMed] [Google Scholar]
- 3.Dobrev D, Wehrens XHT. Mouse models of cardiac arrhythmias. Circulation Research. 2018:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimura D, Kusakari Y, Sasano T, Nakashima Y, Nakai G, Jiao Q, Jin M, Yokota T, Ishikawa Y, Nakano A, Goda N, Minamisawa S. Heterozygous deletion of sarcolipin maintains normal cardiac function. Am J Physiol Heart Circ Physiol. 2016;310:H92–103. [DOI] [PubMed] [Google Scholar]
- 5.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney HL. Adeno-associated virus (aav) serotype 9 provides global cardiac gene transfer superior to aav1, aav6, aav7, and aav8 in the mouse and rat. Hum Gene Ther. 2008;19:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Yang H, Colosi P. Effect of genome size on aav vector packaging. Mol Ther. 2010;18:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field LJ. Atrial natriuretic factor-sv40 t antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988;239:1029–1033. [DOI] [PubMed] [Google Scholar]
- 9.Small EM, Krieg PA. Transgenic analysis of the atrialnatriuretic factor (anf) promoter: Nkx2–5 and gata-4 binding sites are required for atrial specific expression of anf. Dev Biol. 2003;261:116–131. [DOI] [PubMed] [Google Scholar]
- 10.Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene nppa during development and disease. Cardiovasc Res. 2005;67:583–593. [DOI] [PubMed] [Google Scholar]
- 11.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY). 2011;40:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao C, Veleva T, Scott L Jr., Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Eissa NT, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte nlrp3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. [DOI] [PubMed] [Google Scholar]
- 17.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH. Nfatc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Donahue JK. The use of gene therapy for ablation of atrial fibrillation. Arrhythm Electrophysiol Rev. 2014;3:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264–270. [DOI] [PubMed] [Google Scholar]
- 20.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of aav serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. [DOI] [PubMed] [Google Scholar]
- 21.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, Kissel JT, Nagendran S, L’Italien J, Sproule DM, Wells C, Cardenas JA, Heitzer MD, Kaspar A, Corcoran S, Braun L, Likhite S, Miranda C, Meyer K, Foust KD, Burghes AHM, Kaspar BK. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. [DOI] [PubMed] [Google Scholar]
- 22.Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human smn. Hum Gene Ther. 2018;29:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: Advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 2015;108:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.