Abstract
Osteoporosis and bone fractures occur at higher frequency in patients with inflammatory bowel disease (IBD), and decreased bone mass is observed in animal models of colitis. Another consistent feature of colitis is increased serotonin (5-HT) availability in the intestinal mucosa. Since gut-derived 5-HT can decrease bone mass, via activation of 5-HT1B receptors on pre-osteoblasts, we tested the hypothesis that 5-HT contributes to bone loss in colitis. Colitis was chronically induced in mice by adding dextran sodium sulfate (DSS) to their drinking water for 21 days. At day 21, circulating 5-HT levels were elevated in DSS-inflamed mice. Micro-computed tomography of femurs showed a decrease in trabecular bone volume fraction, formation, and surface area, due largely to decreased trabecular numbers in DSS-treated mice. The colitis-induced loss of trabecular bone was significantly suppressed in mice treated with the 5-HT synthesis inhibitor, p-chloro-DL-phenylalanine (PCPA; 300mg/kg/day IP daily), and in mice treated with the 5-HT1B receptor antagonist GR55562 (1mg/Kg/day SC daily). The 5-HT reuptake transporter (SERT) is critical for moving 5-HT from the interstitial space into enterocytes and from serum into platelets. Mice lacking SERT exhibited significant deficits in trabecular bone mass that are similar to those observed in DSS-inflamed mice, and these deficits were not extensively worsened by DSS-induced colitis in the SERT−/− mice. Taken together, findings from both the DSS and SERT−/− mouse models support a contributing role for 5-HT as a significant factor in bone loss induced by colitis.
Keywords: Peripheral 5-HT, 5-HT1B, bone loss, DSS-induced colitis
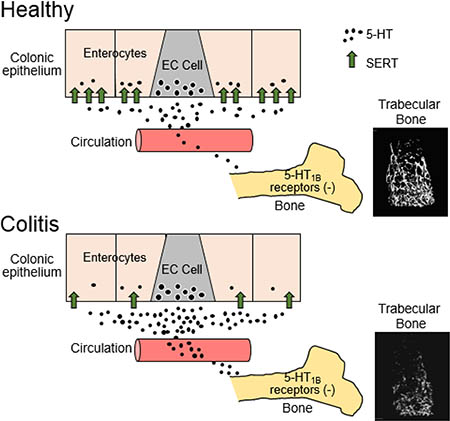
GRAPHICAL ABSTRACT
1. INTRODUCTION
Bone metabolism is a highly dynamic process, involving a constant balance between the anabolic activity of the osteoblasts and the catabolic activity of the osteoclasts, that is essential for the development and maintenance of healthy bone (1). Both intrinsic and extrinsic signaling factors such as hormones, growth factors and cytokines modulate the activity of osteoblasts and osteoclasts, and therefore affect the balance between bone formation and resorption. Pathological conditions, including gut dysfunction, as well as drug treatments can lead to impaired balance of bone formation by affecting activity of the signaling factors exchanged among intramedullary bone cell populations and received from other organs and tissues (2, 3). Osteoporosis is common in patients with gastrointestinal inflammatory disorders. Inflammatory bowel disease (IBD), an umbrella term used clinically to describe the two forms of chronic inflammatory disorders of the gastrointestinal tract, Crohn’s Disease and Ulcerative Colitis, affects an estimated 3 million adults (1.3% of the US population) according to the Center for Disease Control. In patients with IBD increased activity of proinflammatory cytokines has been associated with decreased bone density (4). Additionally, antiinflammatory drugs, such as corticosteroids, have a negative impact on bone formation, as well as leading to bone degradation, particularly in patients with IBD (5, 6).
In order to differentiate between the direct contribution of inflammatory cytokines in chronic IBD and the role of anti-inflammatory drugs, including steroids, on changes in bone properties, animal models of colitis have been used (7–9). In adult mice treated chronically for 2 weeks with dextran sulfate sodium (DSS), a stimulant of intestinal inflammation (7). Colitis leads to a decrease in trabecular bone volume that results from decreased bone formation and increased bone resorption, while no changes are detected in the femur cortical zone. Specific inflammatory cytokines have been shown to contribute to decreased trabecular and cortical bone in DSS-treated juvenile mice during the inflammation phase (8). Following recovery from colitis, however, bone measurements return to control levels, as serum levels of pro-inflammatory cytokines subside to normal levels.
In addition to steroids and pro-inflammatory cytokines, another signaling factor that could contribute to colitis-associated bone loss is gut-derived serotonin (5-hydroxytryptamine; 5-HT) (10). Serotonin produced by, and released from, enterochromaffin (EC) cells in the epithelial layer of the gastrointestinal tract activates local 5-HT receptors to stimulate intrinsic and extrinsic enteric reflexes (11). Following activation of 5-HT receptors, most of the 5-HT is rapidly taken up by epithelial cells of the gut, which express the serotonin selective reuptake transporter (SERT), to terminate signaling by 5-HT from the interstitial space (11). During this process, some 5-HT enters the circulation and is sequestered by platelets, which also express SERT. Findings from a number of investigations of human tissue samples as well as animal models of colitis have demonstrated that epithelial SERT expression is decreased in inflammatory conditions (12). A decrease in expression of SERT in the epithelium likely leads to an increase in the amount of 5-HT entering the bloodstream. EC cell-derived 5-HT has been reported to be a negative regulator of bone density specifically by inhibiting osteoblast formation via activation of pre-osteoblast 5-HT1B receptors (13).
The current study was undertaken to test the hypothesis that increased 5-HT available in mice treated chronically with DSS contributes to the colitis-associated bone defects. To accomplish this, we used micro-computed tomography (μCT) imaging to evaluate changes in the properties of bone tissue in mice with chronic DSS colitis. Bone is a dynamic organ where trabecular bone in the medullary cavity is continuously being resorbed by osteoclasts to maintain serum calcium and reformed by osteoblasts, while the cortical bone has different properties to support bone strength. In trabecular bone the balance between osteoblast and osteoclast activities are critical in maintaining trabecular bone mass. The μCT data provide quantitative information on the bone forming and resorbing parameters for both trabecular and cortical bones. Several approaches were used to assess the effects of altered 5-HT signaling on bone mass, including evaluation of femurs of SERT −/− mice, mice treated with the systemic 5-HT synthesis inhibitor, p-chloro-DL-phenylalanine (PCPA) and mice treated with the 5-HT1B receptor antagonist, GR55562. Previous studies have examined the relationship between inflammation and bone loss in colitis (7, 8), but the possibility that 5-HT plays a contributing role has not been previously examined. Thus, this investigation addresses the compelling question as to the specific and direct influence of 5-HT on bone loss in inflammatory colitis. The results of these experiments support a contributing role of gut-derived peripheral 5-HT acting on 5-HT1B receptors, in addition to previously described action of pro-inflammatory circulating cytokines, in colitis-mediated bone deficits.
2. MATERIAL and METHODS:
2.1. Animal Preparations
Experiments conducted for this study were approved by the Institutional Animal Care and Use Committee of the University of Vermont. Mice were euthanized by isoflurane overdose and exsanguination or by carbon dioxide inhalation and cervical dislocation.
We used several mouse strains and substrains (from different sources) in order to reproduce baseline results from previous studies. Mice used in this study: C57BL/6J (Jackson Labs, Bar Harbor, ME), C57BL/6NTac (Taconic Biosciences, Albany, New York), BALB/cJ (Jackson Labs), BALB/cAnNCrl (BALB/cCrl, Charles River Labs, St-Constant, Québec, Canada). SERT−/− mice (14) were transferred to the University of Vermont from Columbia University. Mice were obtained from specific pathogen free colonies and were housed in individually-ventilated caging (LabProducts™) with adlibitum access to food and water. Environmental parameters in the facility meet all recommendations of the Guide for the Care & Use of Laboratory Animals (NRC, 2011), with 12:12 light:dark cycle and temperatures of 72 F (+/− 2 degrees). The animal care program is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALACi).
2.2. Colitis model
Colitis was induced in 7–8 week old male mice by dissolving the DSS (MW 35-50 kDa; MP Biomedicals, LLC, Solon, OH, USA) in their drinking water for 21 days. To account for the different sensitivities to DSS in the different strains and substrains of mice, its initial concentration in the drinking water ranged from 3 to 5% between the groups (15, 16) Severity of colitis was monitored daily using the disease activity index (DAI) scale that grades weight loss, stool consistency, and presence of fecal blood (17). Hemoccult Single Slide testing slides (Beckman Coulter, Brea, CA) were used to detect fecal blood.
After five days, the DSS concentration was lowered to 1% and adjusted (from 0–1.5%) to maintain a moderate level of inflammation with disease activity index ranging between 4 and 10. Mice were euthanized 21 days post-DSS induction. All DSS-inflamed mice had a significantly higher disease activity index than mice in the associated control group at the time of euthanasia.
2.3. Whole Blood Serotonin
Blood was collected by cardiac puncture, mixed with 5mM EDTA and 10μM Prostaglandin E1 (Sigma-Aldrich, St-Louis MO), flash frozen and analyzed by high-performance liquid chromatography (HPLC) at the Vanderbilt Neurochemistry Core Lab (Vanderbilt University, Nashville TN). Whole blood was collected to ensure that 5-HT levels in platelets would be accounted for our analysis of bone defects.
2.4. Drug treatment
Concentration of gut-derived 5-HT was reduced in C57BL/6NTac mice by daily injection of PCPA (300mg/kg/day IP; Sigma-Aldrich, St-Louis MO), a compound that inhibits the activity of tryptophan hydroxylase (Tph), the rate-limiting enzyme for 5-HT synthesis (18). Injections began 5 days before DSS administration was initiated and continued throughout the 21 days of DSS treatment. Animals that were not treated with DSS, but which received saline vehicle injections served as a control group. Since 5-HT can act as a proinflammatory molecule in the colon (18–20), and decreasing 5-HT synthesis can reduce inflammation (18), mice were monitored daily and DSS concentration was adjusted to maintain comparable levels of inflammation, as measured daily by DAI scores, between all groups. Colonic 5-HT content was measured in distal colon (1cm) collected and homogenized in 0.1 M perchloric acid, buffered in 0.5 M borate buffer and analyzed using an enzyme immunoassay kit according to manufacturer’s protocol (Beckman Coulter, Brea, CA). Colonic 5-HT levels were decreased by over 30% in mice receiving daily PCPA injections in both DSS-inflamed and non-DSS-inflamed mice. As reported previously (21), 5-HT levels were increased in DSS-inflamed mice when compared to controls. In studies of the role of 5-HT1B receptors activation, BALB/cJ mice received the 5-HT1B receptor antagonist, GR55562 (1 mg/Kg/day SC, Tocris Minneapolis, MN) or saline vehicle by SC injection daily.
2.5. Micro-computed tomography (μCT)
Femurs were excised, fixed overnight in periodate-lysine-paraformaldehyde and scanned at a nominal resolution of 10 μm (Scanco μCT 40, MSK Imaging Core, UMASS Medical School, Worcester, MA) and images were reconstructed using Scanco software v5.0. Both trabecular and cortical analyses were performed. For the trabecular analysis, a 3.0 mm section of trabecular bone running proximally from the distal femoral growth plate was analyzed using a threshold range 220–1000 and a density range superior to 500mg of HA/cm3. For the cortical analysis, a 5.0 mm section of cortical bone in the center diaphysis of the femur was analyzed using a threshold range 260–1000 and a density range superior to 600mg of HA/cm3.
2.6. Dual calcein labeling
For dynamic measurements of bone formation, dual calcein labeling was carried out. Mice were given two intraperitoneal injections of 2.5 mg/ml calcein (Sigma-Aldrich, St-Louis MO) four days apart. Fixed bones were dehydated in 70% ethanol and processed for plastic sections using JB4 embedding reagents (Polysciences, Warrington, PA). Images were captured on an Olympus AX70 fluorescence microscope using an Optronics MagnaFire digital camera and software. Distances (in microns) between 2 calcein labels were measured along the periosteal and endosteal surfaces of the cortical bone using ImageJ software (22).
2.7. Statistical analysis
Statistical analyses were performed by unpaired 2-tailed student’s t-test or one-way ANOVA with Bonferroni’s multiple comparisons test, as appropriate, and shown as mean ± SEM. Statistical analyses were performed using the GraphPad Prism software application (version 6.0c, GraphPad Software, La Jolla, CA).
3. RESULTS
This study was performed using DSS colitis, a well-established model of intestinal inflammation in mice (16). As previously described (15) sensitivity to DSS varied amongst the strains that were studied. Therefore, we adjusted the initial concentrations of DSS within in a range of 3–5% (see Table 1) in order to achieve a moderate level of colitis among all strains of mice used in our study. Inflammation was assessed by daily determination of their disease activity index, which was higher (≥ 4) than that of untreated mice at the time of euthanasia.
Table 1.
Effects of DSS Colitis on Features of Trabecular Bone in Different Strains and Substrains of Mice
| C57BL/6 | Jackson | Taconic | |||||||||
| Vehicle (n=5) | 3% DSS (n=5) | Vehicle (n=10) | 4% DSS (n=7) | ||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | Mean | ± SEM | Mean | ± SEM | Diff. | ||
| Bone Volume Fraction (BV/TV; %) | 6.44 | ± 0.12 | 3.97 | ± 0.14 | **** | 3.56 | ± 0.14 | 1.17 | ± 0.07 | **** | |
| Trabecular Thickness (μm) | 40.7 | ± 1.8 | 36.3 | ± 1.1 | ns | 41.4 | ± 1.5 | 34.6 | ± 1.8 | ** | |
| Trabecular Number (1/mm) | 3.76 | ± 0.18 | 3.19 | ± 0.08 | * | 2.19 | ± 0.13 | 1.62 | ± 0.14 | * | |
| Trabecular Space (μm) | 267.7 | ± 13.7 | 313.3 | ± 7.6 | * | 477.5 | ± 28.5 | 656.5 | ± 65.2 | * | |
| Connective Density (1/mm3) | 51.5 | ± 1.5 | 26.7 | ± 1.3 | **** | 26.5 | ± 0.7 | 4.6 | ± 0.2 | **** | |
| Bone Density (mg HA/cm3) | 806.8 | ± 3.0 | 778.9 | ± 4.955 | ** | 787.2 | ± 6.2 | 760.0 | ± 11.4 | * | |
| Total Volume (TV; mm3) | 4.714 | ± 0.127 | 5.084 | ± 0.165 | ns | 6.189 | ± 0.175 | 6.719 | ± 0.307 | ns | |
| Bone Volume (BV; mm3) | 0.303 | ± 0.011 | 0.201 | ± 0.009 | **** | 0.220 | ± 0.010 | 0.080 | ± 0.007 | **** | |
| SMI | 2.87 | ± 0.09 | 3.05 | ± 0.01 | ns | 2.89 | ± 0.07 | 3.30 | ± 0.05 | *** | |
| Bone Surface (BS; mm2) | 19.44 | ± 0.46 | 13.95 | ± 0.23 | **** | 13.80 | ± 0.70 | 5.68 | ± 0.63 | **** | |
| BS/BV (1/mm) | 6.44 | ± 0.19 | 3.97 | ± 0.14 | ns | 69.10 | ± 2.70 | 83.30 | ± 3.28 | ** | |
| BALB/c | Jackson | Charles River | |||||||||
| Vehicle (n=5) | 5% DSS (n=5) | Vehicle (n=5) | 5% DSS (n=5) | ||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | Mean | ± SEM | Mean | ± SEM | Diff. | ||
| Bone Volume Fraction (BV/TV; %) | 8.50 | ± 0.29 | 4.32 | ± 0.19 | **** | 7.44 | ± 0.17 | 5.19 | ± 0.30 | *** | |
| Trabecular Thickness (μm) | 41.1 | ± 0.8 | 37.9 | ± 0.7 | * | 41.4 | ± 0.32 | 40.48 | ± 1.4 | ns | |
| Trabecular Number (1/mm) | 3.66 | ± 0.10 | 2.48 | ± 0.07 | **** | 3.30 | ± 0.09 | 2.58 | ± 0.10 | *** | |
| Trabecular Space (μm) | 275.1 | ± 7.8 | 409.3 | ± 12.1 | **** | 305.7 | ± 9.2 | 392.9 | ± 14.5 | *** | |
| Connective Density (1/mm3) | 114.1 | ± 4.4 | 41.0 | ± 2.4 | **** | 90.1 | ± 2.6 | 52.3 | ± 3.3 | **** | |
| Bone Density (mg HA/cm3) | 817.5 | ± 5.3 | 810.0 | ± 2.4 | ns | 819.9 | ± 4.0 | 798.4 | ± 4.7 | ** | |
| Total Volume (TV; mm3) | 3.250 | ± 0.070 | 3.440 | ± 0.070 | ns | 3.080 | ± 0.060 | 3.39 | ± 0.080 | * | |
| Bone Volume (BV; mm3) | 0.276 | ± 0.009 | 0.149 | ± 0.007 | **** | 0.227 | ± 0.004 | 0.175 | ± 0.007 | *** | |
| SMI | 2.46 | ± 0.02 | 2.79 | ± 0.04 | *** | 2.54 | ± 0.05 | 2.72 | ± 0.08 | ns | |
| Bone Surface (BS; mm2) | 17.04 | ± 0.49 | 9.67 | ± 0.32 | **** | 13.80 | ± 0.14 | 10.71 | ± 0.19 | **** | |
| BS/BV (1/mm) | 66.49 | ± 1.32 | 72.45 | ± 1.72 | * | 65.58 | ± 0.87 | 67.65 | ± 2.97 | ns | |
| SERT-/- on a C57BL/6 background | |||||||||||
| Vehicle (n=4) | 3% DSS (n=4) | ||||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | |||||||
| Bone Volume Fraction (BV/TV; %) | 2.48 | ± 0.14 | 2.06 | ± 0.02 | * | ||||||
| Trabecular Thickness (μm) | 36.1 | ± 2.2 | 32.7 | ± 1.3 | ns | ||||||
| Trabecular Number (1/mm) | 2.39 | ± 0.22 | 2.47 | ± 0.15 | ns | ||||||
| Trabecular Space (μm) | 432.3 | ± 38.2 | 409.6 | ± 24.5 | ns | ||||||
| Connective Density (1/mm3) | 13.73 | ± 1.07 | 8.78 | ± 0.67 | ** | ||||||
| Bone Density (mg HA/cm3) | 790.1 | ± 9.2 | 760 | ± 4.69 | * | ||||||
| Total Volume (TV; mm3) | 4.05 | ± 0.17 | 4.93 | ± 0.26 | * | ||||||
| Bone Volume (BV; mm3) | 0.100 | ±0.005 | 0.101 | ± 0.005 | ns | ||||||
| SMI | 3.24 | ±0.03 | 3.19 | ± 0.02 | ns | ||||||
| Bone Surface (BS; mm2) | 6.93 | ± 0.68 | 7.59 | ± 0.39 | ns | ||||||
| BS/BV (1/mm) | 79.32 | ± 5.45 | 87.13 | ± 4.41 | ns | ||||||
Trabecular bone μCT measurements of femurs of DSS-treated mice (initial concentration differed between strains) compared to controls (vehicle) with unpaired 2-tailed student’s t-test
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
ns non-significantly different
3.1. Circulating serotonin is increased in DSS colitis
The serotonin transporter, SERT is expressed by all enterocytes in the mucosa of the colon to terminate mucosal 5-HT signaling by removing it from the interstitial space (11). This rapid action is important as it prevents prolonged exposure and sensitization of the 5-HT receptors located on cells near the site of 5-HT release. Previous studies using animal models of colitis have shown a decrease in colonic SERT expression resulting in an increased availability of 5-HT in mucosa of the colon (21, 23, 24). Whole blood samples from DSS-treated BALB/cJ mice and controls were examined for levels of 5-HT. We found that the average level of 5-HT from DSS-treated mice was 24.5±1.7 μmol/L a 36% increase compared to the level observed in control mice (15.7 ± 4.4 μmol/L) (p<0.03).
3.2. Influence of colitis on bone mass
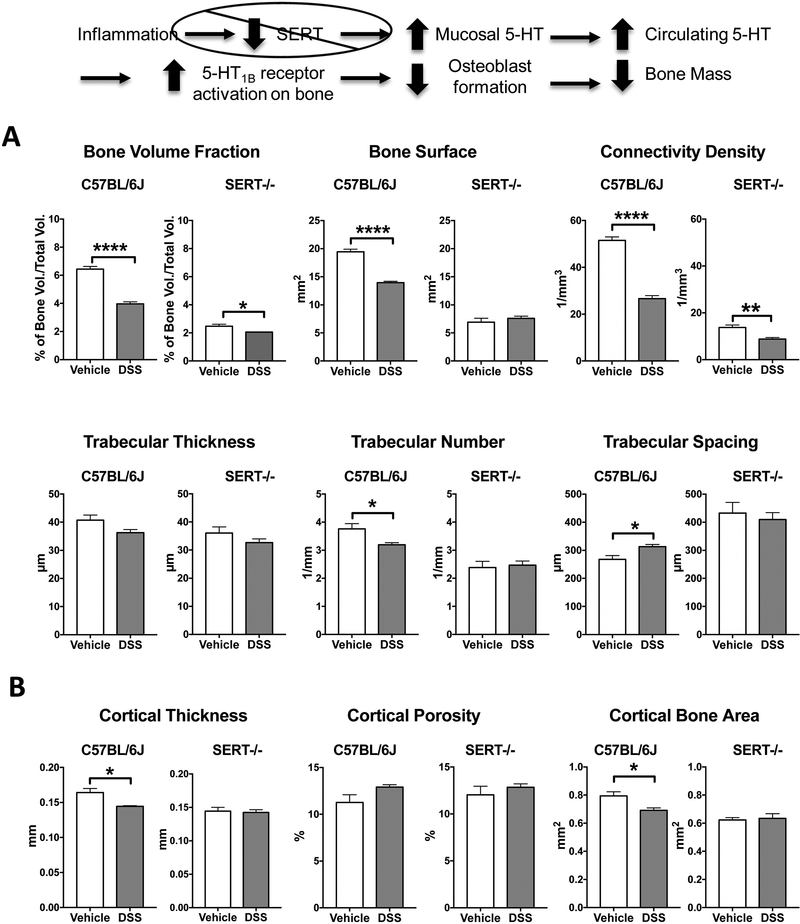
Colitis has been previously linked to decreased bone mineral density (mineral content/mm3) in patients (4). To measure the effect of inflammatory colitis on changes in bone structure, we performed μCT imaging of femoral bone in substrains of controls mice and in mice following 21 days of DSS treatment (Table 1). Analysis of the trabecular bone of DSS-treated mice, showed extensive reductions in the bone volume fraction (bone volume/total volume). Interestingly, compared to controls the C57BL6/NTac and BALB/cJ had the greatest DSS colitis-induced decreases in trabecular bone volume fraction at 66.6% and 49.2%%, respectively, whereas the C57BL/6J and BALB/cCrl strains exhibited decreases of 38.3% and 32.2%. These strain-related differences were consistent for all other parameters measured (Table 1). For example, the C57BL/6NTac and BALB/cJ had greater decreases in connectivity density and bone surface, and increases in trabecular space, which are indicators of extensive bone loss (Fig. 1).
Figure 1:
Effects of DSS colitis on features of trabecular (A) and cortical (B) bone in C57BL6/J and SERT-/- mice. Morphometric analysis of the femurs of DSS-inflamed C57BL6/J mice showed deficits in trabecular bone. Only trabecular thickness remained unchanged after chronic DSS treatment when compared to control. SERT-/- mice drinking water had lower bone volume fraction, bone surface and connective density as well as reduced trabecular number and increased trabecular spacing when compared to control C57BL6/J mice. Of these trabecular bone measurements, only bone volume fraction and connective density were further decreased following DSS-induced inflammation. Of the cortical bone measurements, only cortical thickness was affected by colitis. C57BL6/J+Vehicle, n=5; C57BL6/J+DSS, n=5; SERT-/- +Vehicle, n= 4; SERT-/- + DSS, n=4. * P < 0.05, ** P < 0.01, **** P < 0.0001; unpaired 2-tailed student’s t-test.
With respect to the cortical bone of DSS-inflamed mice, both cortical thickness and bone mineral density were reduced in all mouse strains (Table 2). However, decreased cortical bone area and bone volume were observed only in C57BL/6-inflamed mice (either substrains), while BALB/c-and C57BL/6NTac-inflamed mice additionally showed a substantial increase of bone porosity (Table 2). These effects on cortical bone are mediated by the ability of osteocytes, each cell embedded in their own lacunae, to respond to signaling molecules in the vasculature such as inflammatory cytokines (25).
Table 2.
Effects of DSS Colitis on Features of Cortical Bone in Different Strains and Substrains of Mice
| C57BL/6 | Jackson | Taconic | |||||||||
| Vehicle (n=5) | 3% DSS (n=5) | Vehicle (n-10) | 4% DSS (n=7) | ||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | Mean | ± SEM | Mean | ± SEM | Diff. | ||
| Bone Surface (mm2) | 4.764 | ± 0.070 | 4.727 | ± 0.088 | ns | 4.433 | ± 0.087 | 4.377 | ± 0.067 | ns | |
| Bone Volume (BV; mm3) | 0.397 | ± 0.015 | 0.346 | ± 0.009 | * | 0.362 | ± 0.014 | 0.300 | ± 0.011 | ** | |
| Cortical Thickness (mm) | 0.164 | ± 0.006 | 0.144 | ± 0.001 | ** | 0.161 | ± 0.004 | 0.135 | ± 0.004 | *** | |
| Cortical Porosity % | 11.260 | ± 0.830 | 12.900 | ± 0.267 | ns | 12.570 | ± 0.472 | 15.540 | ± 0.289 | *** | |
| Cortical Bone Area (mm2) | 0.794 | ± 0.029 | 0.692 | ± 0.018 | * | 0.724 | ± 0.028 | 0.599 | ± 0.023 | ** | |
| Bone Density (mg HA/cm3) | 995.7 | ± 5.5 | 976.1 | ± 3.0 | * | 980.7 | ± 5.4 | 958.5 | ± 1.3 | ** | |
| Bone Volume Fraction (BV/TV; %) | 88.730 | ± 0.831 | 87.120 | ± 0.272 | ns | 87.430 | ± 0.476 | 84.480 | ± 0.290 | *** | |
| BALB/c | Jackson | Charles RIver | |||||||||
| Vehicle (n=5) | 5% DSS (n=5) | Vehicle (n=5) | 5% DSS (n=5) | ||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | Mean | ± SEM | Mean | ± SEM | Diff. | ||
| Bone Surface (mm2) | 3.753 | ± 0.052 | 3.857 | ± 0.093 | ns | 3.744 | ± 0.058 | 3.673 | ± 0.037 | ns | |
| Bone Volume (BV; mm3) | 0.404 | ± 0.004 | 0.381 | ± 0.013 | ns | 0.381 | ± 0.008 | 0.353 | ± 0.010 | ns | |
| Cortical Thickness (mm) | 0.212 | ± 0.003 | 0.194 | ± 0.003 | ** | 0.200 | ± 0.004 | 0.189 | ± 0.006 | ns | |
| Cortical Porosity % | 5.280 | ± 0.222 | 6.480 | ± 0.188 | ** | 7.200 | ± 0.311 | 8.020 | ± 0.464 | ns | |
| Cortical Bone Area (mm2) | 0.808 | ± 0.008 | 0.761 | ± 0.025 | ns | 0.762 | ± 0.016 | 0.706 | ± 0.021 | ns | |
| Bone Density (mg HA/cm3) | 1140.0 | ± 5.1 | 1099.0 | ± 4.1 | *** | 1080.0 | ± 8.6 | 1061.0 | ± 8.1 | ns | |
| Bone Volume Fraction (BV/TV; %) | 94.720 | ± 0.237 | 93.530 | ± 0.190 | ** | 92.800 | ± 0.313 | 91.980 | ± 0.463 | ns | |
| SERT-/- on a C57BL/6 background | |||||||||||
| Vehicle (n=4) | 3% DSS (n=4) | ||||||||||
| Mean | ± SEM | Mean | ± SEM | Diff. | |||||||
| Bone Surface (mm2) | 4.288 | ± 0.245 | 4.396 | ± 0.129 | ns | ||||||
| Bone Volume (mm3) | 0.311 | ± 0.009 | 0.317 | ± 0.016 | ns | ||||||
| Cortical Thickness (mm) | 0.144 | ± 0.006 | 0.142 | ± 0.004 | ns | ||||||
| Cortical Porosity % | 12.030 | ± 0.932 | 12.830 | ± 0.382 | ns | ||||||
| Cortical Bone Area (mm2) | 0.623 | ± 0.018 | 0.635 | ± 0.033 | ns | ||||||
| Bone Density (mg HA/cm3) | 991.0 | ± 18.5 | 982.9 | ± 1.7 | ns | ||||||
| Bone Volume Fraction (BV/TV; %) | 87.990 | ± 0.931 | 87.200 | ± 0.368 | ns | ||||||
Cortical bone μCT measurements of femurs of DSS-treated mice (initial concentration differed between strains) compared to controls (vehicle) with unpaired 2-tailed student’s t-test
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
ns non-significantly different.
3.3. Bone changes in SERT−/− mice treated with DSS
The serotonin transporter, which is expressed by enterocytes and platelets, removes free 5-HT from the mucosal interstitial space and serum. Therefore, in the absence of SERT (SERT−/− mice), with concomitant increased extracellular 5-HT, we could assess the influence of increased circulating 5-HT on features of bone structure in non-inflamed and DSS-inflamed mice. Consistent with the findings of Warden and colleagues (26) who compared bone features in non-inflamed SERT−/− and SERT+/+ mice, we observed a decrease in trabecular bone in SERT−/− mice as compared to control mice (Fig. 1; Table 1). Treatment of the SERT−/− mice with DSS resulted in a less extensive response in bone loss as compared to the response to DSS in SERT intact mice (Fig. 1; Table 1). It is worth noting that disease activity index of the untreated SERT−/− mice reached inflammatory levels that were not significantly different than the disease activity index of DSS-inflamed mice at the end of the chronic treatment period. Therefore, in the DSS-treated SERT−/− mice, little or no further reductions in trabecular and cortical features of bone mass were observed (Fig. 1; Tables 1 and 2). These results support the concept that 5-HT could contribute to bone deficits related to colitis.
3.4. Effect of decreased 5-HT synthesis on bone changes in DSS-inflamed mice
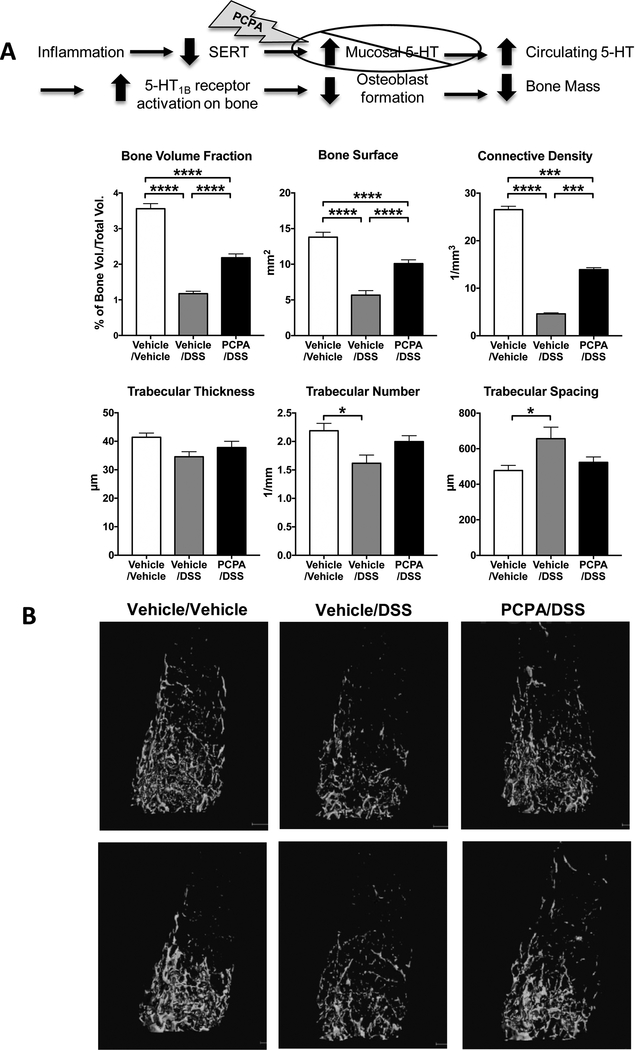
Tryptophan hydroxylase is the rate-limiting enzyme for 5-HT synthesis. Our data and those of others (13, 27) support a link between higher levels of gut-derived 5-HT and bone deficits. We therefore tested the hypothesis that inhibition of 5-HT synthesis could attenuate the detrimental effects of DSS inflammation on bone formation. Mice received daily injections of PCPA (300mg/kg/day IP), a compound that decreased colonic 5-HT content by 30% (see Materials and Methods).
While no difference was noted in bone measurements in non-inflamed mice treated with PCPA (PCPA/vehicle mice; data not shown) compared to vehicle-treated mice, a protective effect by reducing 5-HT production was observed in the trabecular bone of DSS-treated mice (Fig. 2). Trabecular number and spacing for PCPA/DSS mice were statistically comparable to those of non-inflamed control mice. Additionally, while trabecular bone volume fraction, bone surface and connective density in PCPA/DSS mice were still lower than those of control mice, their levels were significantly higher than those of vehicle/DSS mice (Fig 2A). These quantitative changes are consistent with the trabecular bone images in 3 experimental groups (Fig 2B). The vehicle/DSS mice exhibit strikingly fewer trabeculae that are not connected to each other, as compared to control mice. In DSS-treated mice, the PCPA prevented the extensive bone loss and bone volume and connectivity parameters were reaching control levels (Fig 2B). Colitis-induced deficits in cortical bone were not affected by PCPA treatment (data not shown). Taken together these data support the involvement of gut 5-HT in colitis-mediated bone loss.
Figure 2.
Deficits in trabecular bone of DSS-inflamed mice were protected in mice with attenuated 5-HT synthesis. A. Trabecular thickness and spacing in DSS-inflamed mice receiving PCPA (300mg/kg/day IP, daily) were similar to those observed in control mice, showing a protective effect of this treatment on bone mass. Bone volume fraction, bone surface and connective density were also significantly increased in the PCPA-treated DSS mice compared to DSS mice, but did not reach the levels measured in control mice. B. Representative images from μCT scans of the femoral trabecular bone from two mice per group. Vehicle/Vehicle, n=10; Vehicle/DSS, n=7; PCPA/DSS, n=14. * P < 0.05, *** P < 0.001, ****P < 0.0001; 1-way ANOVA with Bonferroni’s correction.
3.5. Contribution of the 5-HT1B receptor to colitis-induced bone deficits
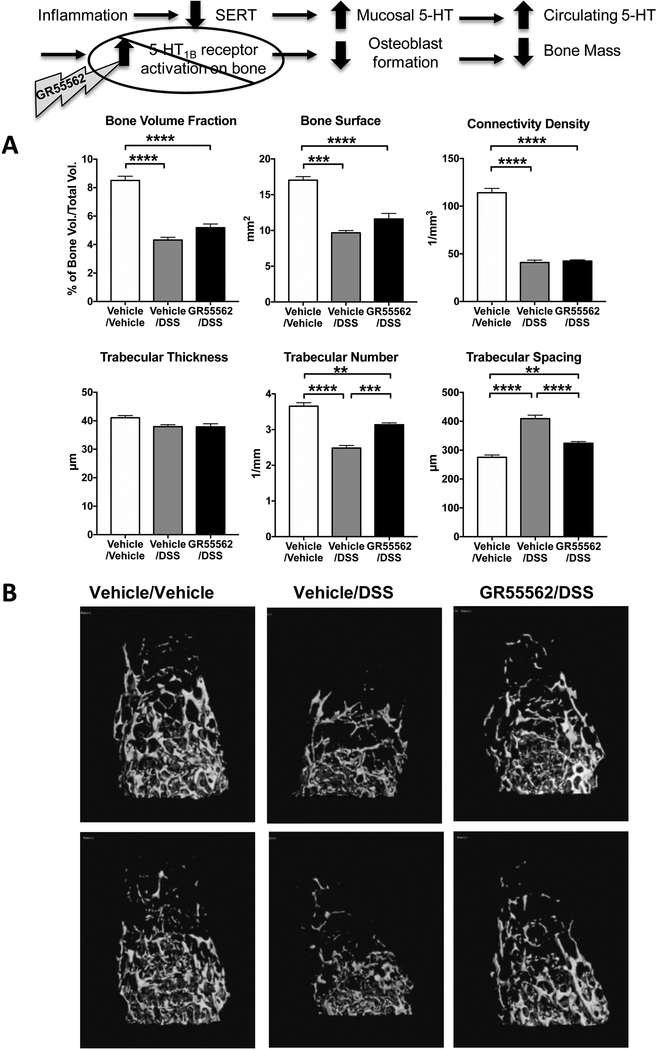
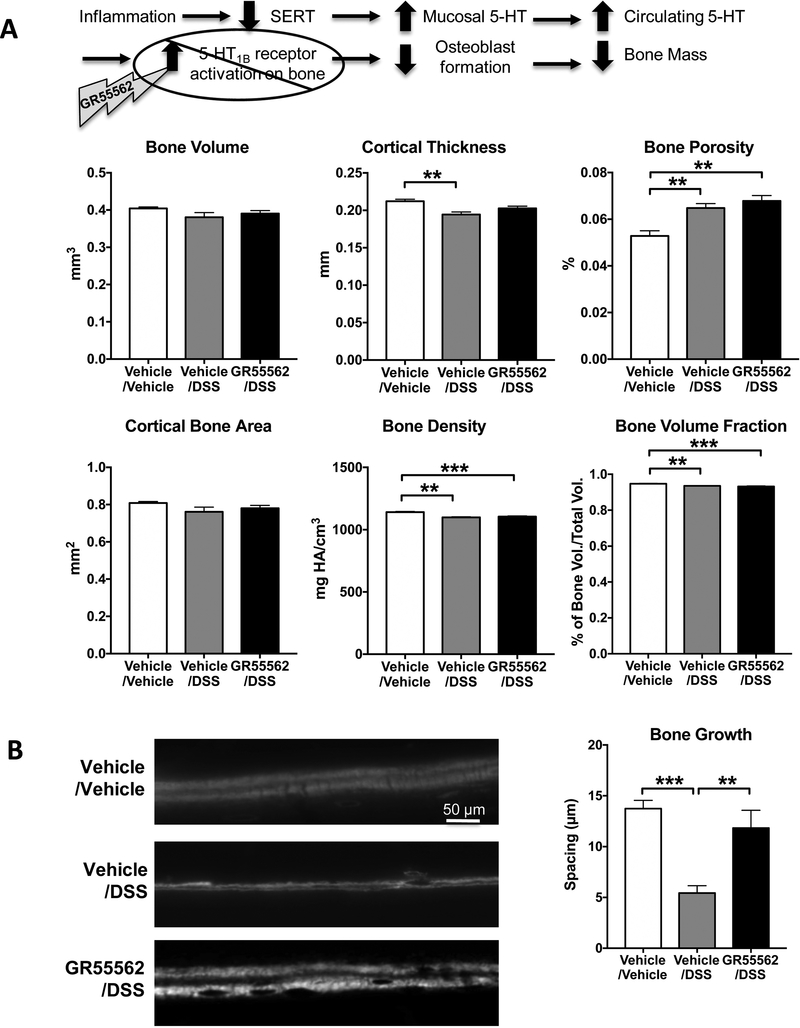
The negative impact of gut-derived 5-HT on bone mass involves activation of 5-HT1B receptors expressed by pre-osteoblasts (13). To further test our hypothesis that 5-HT contributes to bone loss in colitis and that this process results from activation of the 5-HT1B receptor, we evaluated whether administration of the 5-HT1B receptor antagonist, GR55562 could ameliorate the bone loss associated with DSS colitis. Inhibition of the 5-HT1B receptors appeared to have a protective effect on colitisinduced bone changes because, when compared to vehicle/DSS mice, GR55562/DSS mice had increased trabeculae number and decreased trabecular spacing (Fig. 3; p<0.001). This result indicates that the resorptive activity of trabecular bone was diminished in absence of a 5-HT-mediated signal that results in decreased osteoblast formation. The finding that GR55562 did not increase trabecular bone volume and bone surface demonstrates that 5-HT1B receptor inhibition could not completely rescue the bone loss induced by DSS. The 5-HT1B receptor antagonist also protected cortical bone. Micro-CT imaging demonstrated that cortical thickness was significantly improved in GR55562/DSS mice as compared to vehicle/DSS mice (Fig. 4A). Furthermore, additional information provided by dynamic calcein labeling for bone formation rate on the cortical bone surfaces showed that cortical bone formation was decreased in vehicle/DSS mice, but comparable to controls in GR55562/DSS animals (Fig 4B).
Figure 3.
Inhibition of the 5-HT1B receptor has a protective effect on trabecular bone of DSS-inflamed mice. A. Measurement of trabecular number and trabecular spacing in DSS-inflamed mice receiving the 5-HT1B receptor antagonist GR55562 (1 mg/Kg/day SC, daily) were significantly improved compared to DSS-inflamed mice. They did not, however, reached control levels. B. Representative images from μCT scans of the femoral trabecular bone from two mice per group. Vehicle/Vehicle, n=5; Vehicle/DSS, n=5; GR55562/DSS, n=5. ** P < 0.01, *** P < 0.001, **** P < 0.0001; 1-way ANOVA with Bonferroni’s correction.
Figure 4.
Changes in cortical thickness and bone growth in DSS-inflamed mice were partially protected by inhibition of the 5-HT1B receptor. Cortical thickness was protected by inhibition of the 5HT1B receptor with GR55562 (1 mg/Kg/day SC, daily). A. Parameters of cortical bone growth/homeostasis that were decreased in femurs from DSS-inflamed mice included cortical thickness, bone porosity, bone density and bone volume fraction. B. Dual injection of fluorescent calcein was used to measure dynamic bone growth. Decreased spacing of the bands growth in DSS mice correlates with decrease level of bone growth activity. Bone formation in inflamed animals was protected by daily treatment with the 5-HT1B antagonist. Vehicle/Vehicle, n=5; Vehicle/DSS, n=5; GR55562/DSS, n=5. ** P < 0.01, *** P < 0.001; 1-way ANOVA with Bonferroni’s correction.
Because the 5-HT1B receptor antagonist treatment was effective in protecting cortical bone growth in DSS-inflamed mice, as measured by μCT scanning, cortical bone growth was further examined by obtaining dynamic measurements of bone formation using dual injection (separated by 4 days) of the fluorescent dye, calcein. In DSS-treated mice, the decreased spacing observed between the two fluorescent bands correlated with the decreased level of bone growth activity (Fig. 4B). Moreover, daily treatment of DSS-treated mice with GR55562 supports the concept that antagonism of the 5-HT1B receptor may protect bone formation in mice with colitis.
4. Discussion
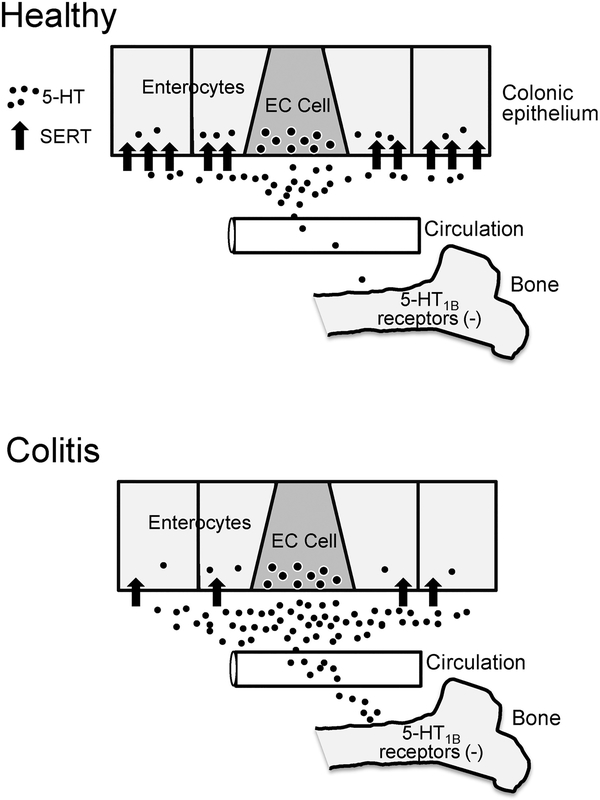
Osteoporosis and bone fractures are commonly associated with IBD, particularly in Crohn’s disease patients (4). In this study, we used an established chronic model of colitis in mice to investigate a potential link between increased gut-derived 5-HT and bone deficits associated with colonic inflammation. We report that DSS-inflamed mice have increased levels of circulating 5-HT and showed decreased trabecular and cortical bone parameters that were attenuated by lowering 5HT synthesis. Moreover, treatment with a 5-HT1B receptor antagonist reduced bone loss in DSSinflamed mice, indicating a possible site of action for the excess of gut-derived 5-HT in the blood. Collectively, these findings support the hypothesis that increased gut-derived 5-HT contributes to decreased bone formation in colitis, and its action involves stimulation of the 5-HT1B receptor (see Fig. 5).
Figure 5.
Schematic diagrams depicting the proposed relationship between 5-HT released from EC cells under healthy conditions and in colitis. In colitis, there is a reduction in SERT expression by enterocytes, which leads to more 5-HT entering the circulation and greater activation of 5-HT1B receptors in bone, which has a negative impact on bone mass.
The possibility that an interaction exists between gut-derived 5-HT and bone metabolism was recognized when links were made between low-density lipoprotein receptor-related protein 5 (Lrp5) and gut-derived 5-HT (28, 29). Lrp5 is an anabolic mediator of Wnt signaling in bone, and gain or loss of function mutations in the Lrp5 are associated with high bone mass syndrome or osteoporosis, respectively (30, 31). Lrp5 inhibits 5-HT synthesis in EC cells, and in turn, lowers circulating 5-HT levels, by decreasing Tph1 expression (13). Deletion of Lrp5 in mice leads to a marked increase in Tph1 expression, elevated circulating 5-HT levels, and decreased bone mass. The relationship between circulating 5-HT levels and bone metabolism is further supported by the finding that bone formation is increased when Tph1 is inhibited pharmacologically (32). However, it should be noted that other studies failed to detect evidence for a role for gut-specific Lrp5 action in bone metabolism and of a direct effect of the Lrp5 receptor on 5-HT level, and it was proposed that bone-specific Lrp5 is responsible for changes of function of the osteocyte population through the canonical Wnt pathway (33). In this model, 5-HT has a positive effect on bone mass by decreasing osteoclastogenesis. This controversy, based largely on findings in Tph1-/- mice, has led to an unresolved debate about the physiological role of Lrp5’s actions on EC cells and gut-derived 5-HT as it relates to bone loss (28, 34–37).
Regardless of the site(s) of Lrp’s physiological actions in influencing bone metabolism, our findings support the concept that gut-derived 5-HT can have a negative impact on bone growth, at least in pathological conditions. Our study design bypasses any involvement of Lrp5 and provides evidence that bone loss in association with colitis involves, at least in part, gut-derived 5-HT. As proposed, 5-HT released from EC cells and entering the blood stream has a negative impact on bone formation by inhibiting osteoblast formation. Our data demonstrate that lowering 5-HT levels with PCPA can also attenuate the deleterious effects of colitis on bone formation in the murine DSS colitis model. Furthermore, our data also support the concept that 5-HT negatively affects osteoblast proliferation via activation of 5-HT1B receptors because treatment with a 5-HT1B antagonist had a protective effect on bone mass.
Further evidence supporting the idea that 5-HT can negatively impact bone mass is found in studies, including those reported here, involving deletion or inhibition of serotonin transporter SERT. Deletion or inhibition of SERT results in increased 5-HT availability whenever the indole is released, as well as in the circulation. Consistent with a previous study involving SERT-/- mice (26), we found that bone mass was significantly reduced in SERT-/- mice. Furthermore, increased incidence of fractures and osteoporosis in adults, as well as decreased growth in youths, have been reported in patients who are prescribed SSRIs to treat depression (38–40).
The findings reported here are clinically relevant as they demonstrate a direct link between 5-HT and bone loss in IBD, and data from SERT suppression and knockout studies are relevant to the changes in 5-HT signaling in colitis. In studies of alterations in 5-HT signaling in intestinal inflammation, a change that has been consistently reported in IBD and a variety of animal models of colitis is a decrease in SERT expression (12).
In patients with IBD many confounding factors such as diet, nutrient malabsorption and corticosteroid exposure may affect bone metabolism in addition to inflammation(4). While the current study supports the concept that gut-derived 5-HT contributes to decreased bone formation in colitis, it is most likely just one of several factors that mediate the observed bone deficits. In the current study, neither lowering gut epithelial 5-HT levels with a non-absorbable Tph inhibitor nor administration of a 5-HT1B receptor antagonist completely restored bone mass in DSS-exposed mice. Moreover, there is strong evidence for a contribution of pro-inflammatory cytokines in colitis-associated bone deficits. For example, there is a direct correlation between the severity of colitis and bone loss in Helicobacter hepaticus-infected interleukin-10-deficient male mice (41, 42). In a chronic model of DSS colitis, bone mass measures were reported to be negatively correlated with intestinal disease score, as well as colon and bone TNF-α levels, and this relationship was independent of weight loss (41, 42). Further, in an acute model of DSS-induced mouse colitis, increased TNFα and decreased IGF-I levels correspond to bone deficits and simultaneously return to normal level during recovery (8)
In addition to the effect of gut-derived 5-HT on bone function, this study highlights the differences between mouse strains and substrains in bone metabolism. In control non-inflamed mice, differences in bone measurements were observed between C57BL/6 and BALB/c (see Table 1). Differences between C57BL/6J and C57BL/6NTac strains were even more significant. Differences in bone characteristics and sensitivity to DSS between strains have been reported previously (15). Our data also support the recent observations regarding bone characteristics in these two substrains by Sankara and colleagues (43) and stresses the fact that substrains should be carefully selected and even the same strain obtained from two different vendors are not necessarily interchangeable. Differences in mice of the same strain between vendors could involve genetic polymorphisms and/or differences in the gut microbiota of mice raised in different facilities.
In conclusion, the findings reported here strongly support the hypothesis that changes in mucosal 5-HT signaling in inflamed regions of the intestines can contribute to the bone deficits that are associated with colitis. Bone integrity was partially protected in our model of chronic colitis by inhibition of 5-HT synthesis and by antagonism of the 5-HT1B receptor. Furthermore, bone loss exhibited in SERT-/- was only mildly exacerbated by colitis. The findings reported here also support the concept that gut derived 5-HT can have a negative impact on bone growth, at least in pathological conditions.
Acknowledgements
The authors wish to acknowledge the assistance of Stacey Russell for assistance with the μCT scanning. KAS holds the Crohn’s and Colitis Canada Chair in IBD Research at the University of Calgary.
Supported by NIH grants DK62267 and R37DE012528.
Footnotes
Conflict of interest:
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen CJ, American Society for Bone and Mineral Research. Primer on the metabolic bone diseases and disorders of mineral metabolism 8th ed. Ames, Iowa: Wiley-Blackwell; 2013. xxvi, 1078 p. [Google Scholar]
- 2.Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell. 2016;164(6):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins FL, Schepper JD, Rios-Arce ND, Steury MD, Kang HJ, Mallin H, et al. Immunology of Gut-Bone Signaling. Adv Exp Med Biol. 2017;1033:59–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvester FA. Inflammatory Bowel Disease: Effects on Bone and Mechanisms. Adv Exp Med Biol. 2017;1033:133–50. [DOI] [PubMed] [Google Scholar]
- 5.Dubois-Camacho K, Ottum PA, Franco-Munoz D, De la Fuente M, Torres-Riquelme A, Diaz-Jimenez D, et al. Glucocorticosteroid therapy in inflammatory bowel diseases: From clinical practice to molecular biology. World J Gastroenterol. 2017;23(36):6628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briot K, Geusens P, Em Bultink I, Lems WF, Roux C. Inflammatory diseases and bone fragility. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017;28(12):3301–14. [DOI] [PubMed] [Google Scholar]
- 7.Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone. 2008;43(5):945–50. [DOI] [PubMed] [Google Scholar]
- 8.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CL, Moniz C, Chambers TJ, Chow JW. Colitis causes bone loss in rats through suppression of bone formation. Gastroenterology. 1996;111(5):1263–71. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie B, Lian JB, Mawe GM. Regulation of Bone Metabolism by Serotonin. Adv Exp Med Biol. 2017;1033:35–46. [DOI] [PubMed] [Google Scholar]
- 11.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature reviews Gastroenterology & hepatology. 2013;10(8):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coates MD, Tekin I, Vrana KE, Mawe GM. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46(6):569–80. [DOI] [PubMed] [Google Scholar]
- 13.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53(4):649–55. [DOI] [PubMed] [Google Scholar]
- 15.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12(7):1295–309. [DOI] [PubMed] [Google Scholar]
- 17.Spohn SN, Bianco F, Scott RB, Keenan CM, Linton AA, O’Neill CH, et al. Protective Actions of Epithelial 5-Hydroxytryptamine 4 Receptors in Normal and Inflamed Colon. Gastroenterology. 2016;151(5):933–44 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137(5):1649–60. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G685–95. [DOI] [PubMed] [Google Scholar]
- 20.Margolis KG, Pothoulakis C. Serotonin has a critical role in the pathogenesis of experimental colitis. Gastroenterology. 2009;137(5):1562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL, Lomax AE. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G446–55. [DOI] [PubMed] [Google Scholar]
- 22.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17(4):565–74. [DOI] [PubMed] [Google Scholar]
- 24.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G207–16. [DOI] [PubMed] [Google Scholar]
- 25.Bonewald LF. The amazing osteocyte. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(2):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–93. [DOI] [PubMed] [Google Scholar]
- 27.Inose H, Zhou B, Yadav VK, Guo XE, Karsenty G, Ducy P. Efficacy of serotonin inhibition in mouse models of bone loss. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(9):2002–11. [DOI] [PubMed] [Google Scholar]
- 28.Warden SJ, Robling AG, Haney EM, Turner CH, Bliziotes MM. The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of lowdensity lipoprotein receptor-related protein 5 (LRP5). Bone. 2010;46(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. The New England journal of medicine. 2002;346(20):1513–21. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptorrelated protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23. [DOI] [PubMed] [Google Scholar]
- 32.Blazevic S, Erjavec I, Brizic M, Vukicevic S, Hranilovic D. Molecular background and physiological consequences of altered peripheral serotonin homeostasis in adult rats perinatally treated with tranylcypromine. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2015;66(4):529–37. [PubMed] [Google Scholar]
- 33.Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, et al. Lrp5 functions in bone to regulate bone mass. Nature medicine. 2011;17(6):684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui Y, Niziolek PJ, MacDonald BT, Alenina N, Matthes S, Jacobsen CM, et al. Reply to Lrp5 regulation of bone mass and gut serotonin synthesis. Nature medicine. 2014;20(11):1229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kode A, Obri A, Paone R, Kousteni S, Ducy P, Karsenty G. Lrp5 regulation of bone mass and serotonin synthesis in the gut. Nature medicine. 2014;20(11):1228–9. [DOI] [PubMed] [Google Scholar]
- 36.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vernejoul MC, Collet C, Chabbi-Achengli Y. Serotonin: good or bad for bone. BoneKEy reports. 2012;1:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA. A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. The Journal of clinical psychiatry. 2010;71(3):338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Brand MW, Pouwels S, Samson MM, van Staa TP, Thio B, Cooper C, et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(10):1705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warden SJ, Fuchs RK. Do Selective Serotonin Reuptake Inhibitors (SSRIs) Cause Fractures? Current osteoporosis reports. 2016;14(5):211–8. [DOI] [PubMed] [Google Scholar]
- 41.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflammatory bowel diseases. 2013;19(8):1586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin R, Raehtz S, Parameswaran N, McCabe LR. Intestinal inflammation without weight loss decreases bone density and growth. American journal of physiology Regulatory, integrative and comparative physiology. 2016;311(6):R1149–r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sankaran JS, Varshney M, Judex S. Differences in bone structure and unloading-induced bone loss between C57BL/6N and C57BL/6J mice. Mamm Genome. 2017;28(11–12):476–86. [DOI] [PubMed] [Google Scholar]