Abstract
Background/Objectives
Neuroimaging investigations of brain pathways involved in reward and motivation have primarily focused on adults. This study sought to identify brain responses to visual food cues and explore its relationships with adiposity and sex in pre-pubertal children.
Methods
Brain responses to palatable food vs. non-food cues were measured in 53 children (age: 8.18±.66 years; sex: 22 boys, 31 girls) after an overnight fast. Whole-brain analysis (cluster-correction Z>2.3, P<.05) was performed to examine brain food cue reactivity and its relationships with adiposity and sex.
Results
Greater brain activity in response to food vs. non-food cues was observed in regions implicated in reward (orbital frontal cortex (OFC), striatum), taste (insula, postcentral gyrus), appetite (hypothalamus), emotion (amygdala), memory (hippocampus), visual processing (occipital cortex) and attention (parietal cortex). A negative association was found between percent body fat and food cue reactivity in the medial prefrontal cortex and lateral OFC adjusting for age and sex. Boys compared with girls had increased brain food cue reactivity in right hippocampus and visual cortex.
Conclusions
These data suggest that body fat and sex are important moderators of brain food cue reactivity in children.
Keywords: food cue reactivity, fMRI, children, adiposity, sex
Introduction
In the United States, childhood obesity rate has more than tripled since 19701. In the context of the dramatic rise of childhood obesity, it is critical to investigate behavioral and neural mechanisms of pediatric obesity.
Food-related cues are ubiquitous in the current environment, and behavioral studies have shown that the sight of food can trigger food cravings and the motivation to eat2. A number of functional magnetic resonance imaging (fMRI) studies in adults have found that a constellation of brain areas implicated in reward and motivation (e.g., orbital frontal cortex (OFC), striatum), taste (e.g., insula), learning and memory (e.g., hippocampus, amygdala), and visual processing (e.g., occipital cortex, postcentral gyrus) are more responsive to visual food cues relative to non-food cues3,4. In addition, metabolic state (fasted vs. fed)4, energy content of food stimuli4, and habitual dietary intake5–8 have been shown to modulate neural food cue reactivity.
Compared with a large number of fMRI food cue studies in adults, fewer studies have examined brain food cue reactivity in children. A meta-analysis9 of fMRI food cue studies performed in children and adolescents identified a collection of brain regions with a heightened response to food vs. non-food cues, including the OFC, amygdala, insula, parietal cortex, anterior cingulate cortex (ACC), and visual processing regions.
Examining neural processing of food cues in children may provide novel insights into processes relevant to risk for obesity. Although studies in adults and adolescents have shown that individuals with obesity compared to lean individuals have greater responses to food cues in reward regions10–13, this data pattern was not seen in normal-weight adolescents at high-risk for obesity14, nor in children15,16. These data suggest that hypersensitivity to food cues in reward regions may be a consequence of obesity rather than antedate obesity. Interestingly, obesity-related effects were observed in prefrontal inhibition regions in response to food cues in pediatric cohorts. While some studies found positive relationships between obesity and brain responses to food cues in prefrontal regions17,18, others found negative correlations19,20. Of note, the prior two studies that observed positive obesity-brain relationships included both girls and boys with a wide age range spanning from childhood through adolescence (e.g., 9-18 years old in Davids et al., 2010; 10-17 years old in Bruce et al., 2010). Among the other studies that reported negative relationships, one was performed in girls and boys age between 10 and 14 years old19, and the other in adolescent girls during a food-related response inhibition task20. There are significant changes in body composition as children transition through puberty, and these changes occur differentially with sex. Thus, it is important to consider pubertal stages and sex when examining adiposity-related effects in brain food cue reactivity. Although some studies included both girls and boys, no prior studies have examined sex differences in brain food cue reactivity in children. However, sex differences in food cue reactivity have been reported in adults. Under fasting conditions, women compared to men had greater food cue reactivity in regions involved in visual attention, reward and cognitive processing21–23. It was suggested that these sex differences may be related to sex hormones, sex specific socialization, genetic/brain structural differences and behavioral traits such as dietary restraint .
In this study, we investigated patterns of brain food cue responsivity in a cohort of healthy, typically developing pre-pubertal children between the ages of 7 and 11 years. We hypothesized that viewing food vs. non-food cues would be associated with increased brain activity in regions involved in reward, taste, conditioned responses to food, and visual and attention processing. Given mixed results in previous pediatric studies, we conducted whole-brain analysis to examine relationships between adiposity and neural food cue reactivity, and hypothesized differential engagement of brain regions involved in processing food cues between children with greater adiposity vs. smaller adiposity. Although sex-based differences in brain food cue reactivity remain an open question in children, on the basis of adult studies, we hypothesized that girls (relative to boys) may exhibit greater brain food cue reactivity.
Methods
Participants
Sixty-three healthy children between the ages of 7 to 11 years old were recruited from Kaiser Permanente Southern California (KPSC) and participated in this study. Participants’ parents gave written informed consent and children provided informed assent, and all experimental procedures were approved by the Institutional Review Board of the University of Southern California (USC) and KPSC. Participants were healthy, free from any psychological and neurological disorders, all right-handed and had normal or corrected to normal vision.
Ten participants were excluded from final analysis: eight participants due to excessive motion (larger than 2mm or 2˚ in any direction), one due to a technical error during scanning, and one due to incidental brain findings. Fifty-three pre-pubertal children were included in the final data analysis. There were no significant differences in age, sex, body mass index (BMI), or percent body fat (%BF) between children excluded and those in the final dataset.
Anthropometric measurements
Height was measured to the nearest 0.1 cm using a portable stadiometer and weight to the nearest 0.1 kg using a portable scale. BMI(kg/m2), BMI percentiles (age and sex specific) and BMI z-scores (age and sex-specific standard deviation score) were determined based on Center for Disease Control standards. % BF was measured by bioelectrical impendence analysis (BIA) using Tanita Body Composition Analyzer SC 331S, which measures body composition using a constant current source with a high frequency current (50kHz, 90μA) and has been FDA approved for assessments of body composition in children age 5 to 17 years. The equations used to estimate %BF are proprietary (Tanita Cooperation), but the algorithm includes sex, age and height alongside weight and impedance data. A recent systematic review on body composition estimation in children showed excellent reproducibility for %BF assessments by BIA. The fat mass and fat-free mass estimated by BIA were strongly correlated with the reference methods in both sexes, however BIA underestimated the fat mass in both sexes24.
Physical Exam
Tanner stage was assessed by physical exam25,26 and/or by a validated sex-specific assessment questionnaire for children and parents27. Forty-seven participants opted for both physical exam and questionnaire, and 16 participants opted for questionnaire only. Only pre-pubertal children with Tanner Stage <2 were included.
Food Cue Task
All scans were conducted in the morning between 8 and 10 am after a 12-h overnight fast, a time when food cue reactivity is robust. Therefore, the time since last meal intake was standardized across participants. First, we familiarized participants to the MRI scanner by utilizing a Mock Scanner, which helps to reduce anxiety. Prior to scanning, children were required to rate their hunger from 1 to 5 (1= very hungry, 2 = quite hungry, 3 = just right, 4 = quite full, 5 = not hungry at all) using a picture questionnaire validated in primary school children28. In the MRI scanner participants completed the food cue task. In this randomized block design, participants were presented with a total of 12 visual food cue and non-food cue blocks using Matlab (The MathWorks, Inc., Natick, Massachusetts, United States) and Psychtoolbox on a Mac laptop. There were 3 colorful photographs presented in a random order in each block, and each photograph was presented for 4 seconds with 1 second waiting time between photographs, resulting in a total of 3 minutes and 16 seconds running time. Participants were instructed to watch these pictures attentively during the scan. Food cues consisted of palatable food items such as cupcakes and French fries. The control stimuli consisted of non-food neutral pictures such as books and rulers. Details on our study stimuli were presented in the Supplemental Materials (STable 1). Pictures were selected after pilot testing in studies conducted in children within the same age range. Only food pictures that were rated as “appealing” and “familiar”, and only the non-food pictures that were rated as “familiar” were included in the fMRI studies. These pictures were selected from International Affective Picture System29 as well as internet sources such as food blogs. All stimuli had the same resolution (1024 pixels × 768 pixels), but food and non-food pictures were not matched for visual characteristics, such as saliency, color, shape and complexity.
MRI Imaging Parameters
MRI data were collected using 3T Siemens scanner. Participants laid supine on a scanner bed, viewing stimuli through a mirror mounted over the head coil. Blood-oxygen-level-dependent (BOLD) functional scans were acquired with a single-shot gradient echo planar imaging sequence. Thirty-two 4 mm thick slices that cover the whole brain were acquired using the following parameters: Repetition time (TR)=2,000 msec, echo time (TE)=25 msec, bandwidth=2520 Hz/pixel, flip angle=85°, field of view (FOV) = 220×220 mm, matrix=64×64. A high resolution 3D Magnetization Prepared Rapid Gradient Echo sequence (TR=2530 ms; TE=2.62 ms; bandwidth=240 Hz/pixel; flip angle= 9°; slice thickness=1mm; FOV=256×256 mm; voxel size=1mmx1mmx1mm) was used to acquire structural images for multi-subject registration.
fMRI Analysis
We used fMRI Expert Analysis Tool version 6.00, part of the Oxford University Centre for Functional MRI of the Brain Software Library (http://www.fmrib.ox.ac.uk/fsl) to process fMRI data. FMRI preprocessing steps included motion correction, high-pass filtering (100 s) and spatial smoothing with a Gaussian kernel of full-width at half-maximum=5mm. The functional data were first mapped to each participant’s anatomical image, and then registered into standard space using affine transformation with FLIRT. Based on prior studies conducted in similar age range as our participants15,30, we used the Montreal Neurological Institute (MNI) template for registration. Food and non-food events were added to the General Linear Model (GLM) after convolution with a canonical hemodynamic response function. The GLM model also includes standard motion parameters as regressors of no interest. For each subject, food vs. non-food cues contrast maps were created on the first-level analysis, and then submitted for random-effects group-level analysis using FLAME1 (FMRIB’s Local Analysis of Mixed Effects). We performed whole-brain regression analysis to examine relationships between adiposity and neural food cue reactivity. Log transformation was done on %BF due to skewed distribution. Sex and age were included in the body fat model to account for variances in body fat for boys and girls of different ages. Since BMI z-scores were used, age and sex were not included in the BMI model. Unpaired-t test was used to examine sex differences on brain responses to food vs. non-food cues. Whole brain analysis was carried out using cluster forming threshold Z>2.3 (equivalent to P<.01), a minimum cluster size for a FWE correction of P<.05 using the FLAME1 modeling procedure, which demonstrated a nominal 5% false positive rate31.
Results
Participants Characteristics (Table 1)
Table 1.
Participants Characteristics
| Mean (SD) | Range | |
|---|---|---|
| Age (years) | 8.18 (.66) | 7.27~11.21 |
| BMI (kg/m2) | 18.35 (3.69) | 13.79~30.09 |
| BMI Z-Score | 0.67 (1.02) | −1.65~2.59 |
| BMI Percentile | 67.58 (26.44) | 5.28~99.52 |
| Body fat (%) | 24.08(8.26) | 12.7~57.1 |
| Hunger | 2.04 (0.95) | 1~4 |
| N(%) | ||
| Sex | Male: 22 (41.5%) Female: 31 (58.5%) |
|
| Pubertal Tanner Stage | Tanner Stage 1: 53 (100%) | |
| Weight Status Category | Normal weight (BMI percentile ≥5th <85th: 34 (64.1%) Overweight (BMI percentile ≥ 85th <95th): 9 (17%) Obese (BMI percentile ≥ 95th): 10 (18.9%) |
|
Hunger scale from 1 to 5, 1 being most hungry, 5 being least hungry.
Fifty-three pre-pubertal children (age: 8.18 ± 0.66 years; sex: 22 boys, 31 girls) were included in the final neuroimaging data analysis. 64.1% of our participants were normal weight, 17% were overweight and 18.9% had obesity. Their % BF ranged from 12.7 % to 57.1%, and BMI z-scores ranged from −1.65 to 2.59. Children reported being “quite hungry” with average hunger scores of 2.04 ± 0.95. % BF was significantly correlated with BMI z-scores (r=0.82, P<.0001), but not significantly related with age or hunger (Ps>.05). There were no significant sex differences in age, %BF, BMI z-scores, or hunger (Ps>.05).
Whole-brain Analysis of Food vs. Non-food Cues
Viewing pictures of food vs. non-food cues was associated with increased BOLD signal change in the bilateral ventral striatum, putamen/pallidum, caudate, insula, amygdala, hippocampus, occipital cortex, parietal cortex, thalamus, hypothalamus, precentral gyrus, postcentral gyrus, OFC, ACC, posterior cingulate cortex, medial prefrontal cortex (mPFC), brain stem and left frontal pole (Figure 1, Table 2).
Figure 1.
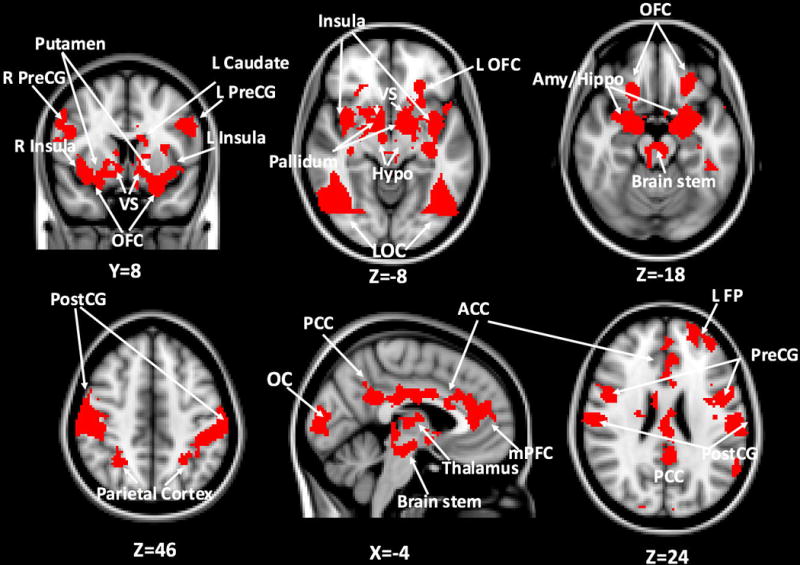
Whole-brain analysis of food vs. non-food contrast: red areas indicate brain regions responding more to food vs. non-food cues (cluster corrected Z>2.3, p<.05 for multiple comparisons). OFC: orbitofrontal cortex; Hypo: hypothalamus; LOC: lateral occipital cortex; Amy: amygdala; Hippo: hippocampus; PreCG: precentral gyrus; PostCG: postcentral gyrus; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; mPFC: medial prefrontal cortex; FP: frontal pole
Table 2.
Whole-brain analysis results of food vs. non-food contrast, whole-brain regression analysis relating individual differences in percent body fat to food vs. non-food contrast adjusting for age and sex, and unpaired- t test results of boys relative to girls for food vs. non-food contrast (cluster level correction, Z>2.3, P<.05).
| Region | Peak Voxel Coordinates (mm) | Max Z | |
|---|---|---|---|
| Food vs. non-food contrast | L Orbital Frontal Cortex | −24,32,−12 | 5.66 |
| R Orbital Frontal Cortex | 22,28,−14 | 4.20 | |
| L Ventral Striatum | −10, 10, −6 | 2.97 | |
| R Ventral Striatum | 8,12,−6 | 3.65 | |
| L Putamen/Pallidum | −14,10,−10 | 3.24 | |
| R Putamen/Pallidum | 20,10,−8 | 3.54 | |
| L Caudate | −14,10,18 | 4.01 | |
| R Caudate | 10,10,8 | 2.99 | |
| L Insula | −36,−8,12 | 5.18 | |
| R Insula | 36,−8,14 | 6.00 | |
| L Amygdala | −18,−8,−18 | 4.90 | |
| R Amygdala | 18,−4,−18 | 6.12 | |
| L Hippocampus | −22,−12,−16 | 4.15 | |
| R Hippocampus | 16,−8,−20 | 3.85 | |
| L Occipital Cortex | −36,−84,2 | 4.77 | |
| R Occipital Cortex | 40,−78,2 | 4.21 | |
| L Parietal Cortex | −26,−56,52 | 3.67 | |
| R Parietal Cortex | 30,−52,52 | 4.01 | |
| L Thalamus | −2,−10,12 | 3.43 | |
| R Thalamus | 6,−6,12 | 3.83 | |
| L Hypothalamus | −8,−2,−8 | 3.91 | |
| R Hypothalamus | 10,−2,−8 | 2.88 | |
| L Precentral Gyrus | −52,2,24 | 4.28 | |
| R Precentral Gyrus | 46,4,24 | 4.08 | |
| L Postcentral Gyrus | −60,−20,24 | 4.07 | |
| R Postcentral Gyrus | 58,−18,24 | 4.26 | |
| Anterior Cingulate Cortex/Medial Prefrontal Cortex | −6,34,22 | 3.15 | |
| Posterior Cingulate Cortex | |||
| Brain Stem | 8,−28,−14 | 3.43 | |
| Left Frontal Pole | −26,54,30 | 3.57 | |
| Percent Body fat | Medial Prefrontal Cortex | 10,40,−10 | 3.62 |
| R Lateral Orbital Frontal Cortex | 14,62,−10 | 3.21 | |
| Boys > Girls | R Hippocampus | 24,−44,−14 | 3.68 |
| R Temporal Occipital Fusiform Cortex | 26,−24,−14 | 3.21 |
Relationship between Child Adiposity and Neural Food Responsiveness
There was a significant negative relationship between %BF and mPFC/right lateral OFC response to food vs. non-food cues adjusting for age and sex (Figure 2A, Table 2). A scatterplot of this relationship was presented in the Supplemental Materials (SFigure 1). BMI z-score was not significantly associated with neural food cue reactivity.
Figure 2.
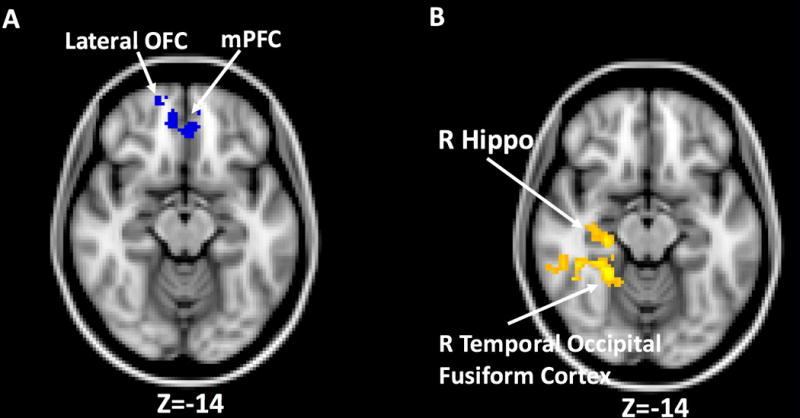
Relationships between percent body fat, sex and brain food cue reactivity. A) blue areas indicate a negative relationship between percent body fat and food cue reactivity in the medial prefrontal cortex (mPFC) and right lateral orbital frontal cortex (OFC) from whole-brain analysis (cluster corrected Z>2.3, p<.05 for multiple comparisons). B) yellow areas indicate boys relative to girls showed greater brain responses to food vs. non-food cues in right hippocampus (Hippo) and temporal occipital fusiform cortex (cluster corrected Z>2.3, p<.05 for multiple comparisons).
Sex Differences in Neural Food Responsiveness
Boys vs. girls showed greater neural food cue reactivity in right posterior hippocampus and temporal occipital fusiform cortex (Figure 2B, Table 2). No significant clusters were observed for the reversed comparison. There were no significant sex differences in age, %BF, BMI z-scores, or hunger. Thus, we did not include these as covariates in our analysis.
Discussion
In this study, we examined neural correlates of food cue exposure in a cohort of healthy, typically developing pre-pubertal children. We assessed the statistical relationship between brain responses to food cues and both adiposity and sex. We reasoned that doing so might offer clues regarding factors that contribute to divergence in obesity trajectories later in adolescence, which could be considered in subsequent longitudinal research.
Consistent with prior work in pediatric and adult populations3,4,9, we found that brain regions involved in reward and motivation (e.g., OFC, ventral and dorsal striatum), metabolic signaling (e.g., hypothalamus), emotion (e.g., amygdala), learning and memory (e.g., hippocampus), taste (e.g., insula and postcentral gyrus), attention and visual processing (e.g., occipital cortex and parietal cortex) were recruited more during viewing of food vs. non-food cues in healthy pre-pubertal children. Our sample size (N=53) was considerably larger than most previous work in children9, and our data support the notion that the neural appetitive responses to food cues may be a basic function that develops early in life9.
Importantly, we observed that greater %BF was associated with decreased mPFC/lateral OFC response to food cues after controlling for age and sex. Although participants were only instructed to passively observe food stimuli, appetitive stimuli recruit automatic self regulatory responses32, which may instantiate in the mPFC and lateral OFC, regions implicated in cognitive control33. Our observation was directionally similar to two other studies, one in 10 to 14 years old children during passively viewing food vs. no-food logos19, and the other among adolescent girls in a task where they were required to inhibit prepotent responses to appetizing food20. The latter study paired a food cue paradigm with a response inhibition task, which further demonstrated specific functional roles of prefrontal regions (e.g., dorsolateral PFC (dlPFC), ventrolateral PFC (vlPFC), mPFC, OFC) during processing of food stimuli20. Taken together, these results suggested that hypo-responsivity to food cues in prefrontal inhibition regions may be a sensitive early marker of obesity. It is worth noting that two studies conducted in participants with an age range from childhood through adolescence, reported a positive relationship between obesity and prefrontal responses to food cues. Tanner stage was not assessed in both studies, and sex was neither matched between groups nor included as a covariate in Davids et al., 2010, which may impact their reported findings rather than obesity per se.
While BMI z-scores and %BF were highly correlated, we did not observe significant relationships between BMI z-scores and food cue reactivity. Although BMI is a metric widely used to classify obesity status, it cannot distinguish between lean and fat mass34. Adipose tissue sends signals to the brain via appetite regulating hormones such as leptin and adiponectin35, thus it is possible that body fat compared with BMI exhibited a more robust brain-adiposity relationship. In keeping with this notion, a prior study using whole-brain regression analysis revealed a more robust relationship between %BF and brain food cue reactivity than BMI among adolescents13. In comparison with BMI, measures more proximal to adiposity appear more relevant to understanding individual differences in brain responses to food cues.
Of great interest, we observed that boys had greater brain responses to food vs. non-food cues in right hippocampus and visual cortex than girls. This data pattern was opposite to our hypothesis, which was based on findings in adult populations21–23. Notably, our study only included pre-pubertal children. Changes in sex hormones that occur during puberty may contribute to sexual differentiation in the brain and could be an important factor related to the disparate patterns of sex differences observed in children compared to adults. There were also differences in study design between our study and adult studies, including duration of fasting, control of dietary intake prior to MRI scanning and BMI differences between sex. It is possible that sex differences on brain reactivity to food cues may be different between children and adults, but future studies are warranted to determine this possibility. It is also possible that other differences in study design may contribute to inconsistent findings.
We studied the neural processing of food cues among a well-characterized cohort of pre-pubertal children age 7 to 11 years old. The narrow age range among the child participants could reduce variability and improve reproducibility. Additionally, unlike prior pediatric studies which included discrete groups of healthy-weight children and children with obesity17–19, we included participants with a wide range of BMI and body fat which allowed us to examine how individual differences in BMI and body fat relate to brain food cue reactivity. Several weaknesses in this study should be noted. Our experimental stimuli were not matched for color, saliency, shape and complexity, which may have introduced bias in neural responses to food vs. non-food cues. Nevertheless, the patterns of brain food cue reactivity we observed were robust and consistent with prior studies. BIA estimates %BF, but cannot differentiate body fat distribution. Given the nature of our study design, we cannot determine the directionality of the relationship between %BF and the mPFC/lateral PFC response to food cues. A recent study reported significant relationships between brain structures and executive function in adolescents with obesity36. It is possible that there might be obesity-related brain structure differences in the prefrontal inhibition regions, which we did not investigate here.
Conclusions
We observed that children with greater %BF had lower mPFC/ lateral OFC response to food cues. Future longitudinal studies should examine if hypo-responsiveness to food cues in cortical regions predicts increases in adiposity over time. We also found that boys compared with girls exhibited greater food cue reactivity in right hippocampus and visual cortex. Future studies are warranted to determine whether there is a developmental shift in sex differences during observation of food vs. non-food stimuli.
Supplementary Material
Acknowledgments
We would like to thank the volunteers who participated in these studies. We would also like to thank Ana Romero and Christina Ramsey for assistance with study coordination, Mayra Martinez and Janet Mora-Marquez for recruitment; The Dana and David Dornsife Cognitive Neuroimaging Center at USC, especially Bosco Tjan and J.C. Zhuang for assistance with developing MRI protocols. The neuroimaging computation for the work described in this paper was supported by the University of Southern California’s Center for High-Performance Computing. A Research Electronic Data Capture, REDCap, database was used for this study, which is supported by the Southern California Clinical and Translational Science Institute (SC CTSI) through NIH UL1TR001855. This work was supported by an American Diabetes Association Accelerator Award (#1-14-ACE-36) (K.A.P) and by the National Institutes of Health, NIDDK R03DK103083 (K.A.P). SL and KP conceived experiments, SL and JA carried out experiments, SL analyzed data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Footnotes
Conflict of interest: authors do not have any conflict of interest.
References
- 1.Control C for D Prevention. Prevalence of Overweight and Obesity among Children and Adolescents: United States, 1963-1965 through 2011-2012. 2016 [Google Scholar]
- 2.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev. 2016;17(2):159–177. doi: 10.1111/obr.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol Behav. 2012;106(3):317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage. 2011;55(1):296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 5.Burger KS, Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr. 2013;97(6):1188–1194. doi: 10.3945/ajcn.112.055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream–based milkshake. Am J Clin Nutr. 2012;95(4):810–817. doi: 10.3945/ajcn.111.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger KS, Stice E. Neural responsivity during soft drink intake, anticipation, and advertisement exposure in habitually consuming youth. Obesity. 2014;22(2):441–450. doi: 10.1002/oby.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. NeuroImage. 2011;55(1):233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Meer F, van der Laan LN, Adan RAH, Viergever MA, Smeets PAM. What you see is what you eat: An ALE meta-analysis of the neural correlates of food viewing in children and adolescents. NeuroImage. 2015;104:35–43. doi: 10.1016/j.neuroimage.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 10.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapuano KM, Huckins JF, Sargent JD, Heatherton TF, Kelley WM. Individual Differences in Reward and Somatosensory-Motor Brain Regions Correlate with Adiposity in Adolescents. Cereb Cortex N Y N 1991. 2016;26(6):2602–2611. doi: 10.1093/cercor/bhv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at Risk for Obesity Show Greater Activation of Striatal and Somatosensory Regions to Food. J Neurosci. 2011;31(12):4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohon C. Brain response to taste in overweight children: A pilot feasibility study. PloS One. 2017;12(2):e0172604. doi: 10.1371/journal.pone.0172604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fearnbach SN, English LK, Lasschuijt M, et al. Brain response to images of food varying in energy density is associated with body composition in 7-to 10-year-old children: Results of an exploratory study. Physiol Behav. 2016;162:3–9. doi: 10.1016/j.physbeh.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Davids S, Lauffer H, Thoms K, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes. 2009;34(1):94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- 18.Bruce AS, Holsen LM, Chambers RJ, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34(10):1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce AS, Lepping RJ, Bruce JM, et al. Brain Responses to Food Logos in Obese and Healthy Weight Children. J Pediatr. 2013;162(4):759–764 e2. doi: 10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behav Brain Res. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-Based Differences in the Behavioral and Neuronal Responses to Food. Physiol Behav. 2010;99(4):538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killgore WDS, Yurgelun-Todd DA. Sex Differences in Cerebral Responses to Images of High vs Low Calorie Food. Neuroreport. 2010;21(5):354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Castro JAC, de Lima TR, DAS Silva. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J Bodyw Mov Ther. 2018;22(1):134–146. doi: 10.1016/j.jbmt.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, et al. Validity of Self-Assessment of Pubertal Maturation. PEDIATRICS. 2015;135(1):86–93. doi: 10.1542/peds.2014-0793. [DOI] [PubMed] [Google Scholar]
- 28.Bennett C, Blissett J. Measuring hunger and satiety in primary school children. Validation of a new picture rating scale. Appetite. 2014;78(Supplement C):40–48. doi: 10.1016/j.appet.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 29.LANG P. International affective picture system (IAPS) : affective ratings of pictures and instruction manual. Tech Rep. 2005 https://ci.nii.ac.jp/naid/20001061266/. Accessed March 7, 2018.
- 30.Boutelle KN, Wierenga CE, Bischoff-Grethe A, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes. 2015;39(4):620. doi: 10.1038/ijo.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fishbach A, Friedman RS, Kruglanski AW. Leading us not unto temptation: momentary allurements elicit overriding goal activation. J Pers Soc Psychol. 2003;84(2):296–309. [PubMed] [Google Scholar]
- 33.Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 35.Trayhurn P, Bing C, Wood IS. Adipose Tissue and Adipokines—Energy Regulation from the Human Perspective. J Nutr. 2006;136(7):1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 36.Groot CJ, Akker ELT, Rings EHHM, Delemarre-van de Waal HA, Grond J. Brain structure, executive function and appetitive traits in adolescent obesity. Pediatr Obes. 2016;12(4):e33–e36. doi: 10.1111/ijpo.12149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


