Abstract
Chronic pain is often difficult to treat, requiring a comprehensive multidisciplinary therapeutic intervention and a high level of management expertise.
This is particularly true for patients who are unresponsive to standard treatments for chronic pain, for which Scrambler Therapy (ST) is indicated. The aim of the present study was to evaluate the impact of ST on patient-reported moderate to severe chronic pain.
This was a prospective trial on 219 patients affected by chronic pain from April 2010 to March 2016. The study consisted of 2 consecutive weeks of treatment with ST (one 30-min daily session, 5 days a week) (T0, T1, T2) and a 2-week follow-up (T3, T4). Patients were asked to describe the pain using the Numeric Rating Scale (NRS) immediately prior to and after the treatment.
Two hundred nineteen patients were treated for chronic pain of different nature with mean values of 6.44 (± 2.11) at T0, 3.22 (± 2.20) at T2, and 3.19 (± 2.34) at T4. A reduction in the symptomatology from T0 to T2 was maintained throughout T4 (P value < .0001). Of the 219 patients treated with ST, 83 (37.9%) had cancer pain and 136 (62.1%) had non-cancer pain. No adverse events were reported.
Future research should focus on individual response, retreatment, and maintenance therapy. The data showed a statistically significant impact of ST, which was maintained during follow-up, on patients suffering from chronic pain of different nature.
Keywords: calmare therapy, cancer pain, chronic pain, neuropathic pain, non-cancer pain, scrambler therapy
1. Introduction
Chronic pain is not only the temporal extent of acute pain but also an ill-adapted response to the pain. It ranks among one of the most common, costly, and incapacitating conditions in life. The pathological condition causing the pain is usually known but not attacked, and is persistent over time. Its continued presence establishes a vicious cycle of depression, anxiety, and other emotional stimuli, becoming an independent syndrome with a major repercussion on the social life and the psycho-social aspects of the person.[1] It is estimated that approximately 1.5 billion people in the world suffer from chronic pain with a remarkable impact on society and public health care.[1] In the United States, 20% of the population (approximately 42 million people) suffer from chronic pain, with a yearly expenditure of at least 560 to 635 billion dollars for health care, and 297 to 336 billion dollars for loss of productivity.[2]
Pain is a subjective experience, combining the sensory component with the experiential and emotional components that equally modulate the pain stimulus perceived by the patient.[3] In fact, the generated pain signal is modulated at different levels, corresponding to different clinical interpretations. This explains how the pain is the result of a complex system of interactions, where the severity and nature of the pain are modulated by a variety of factors.[4]
Chronic pain is the long-lasting pain that accompanies a chronic disease, and is often determined by the persistence of a harmful stimulus and/or phenomena of self-maintenance, which perpetuate the nociceptive stimulation despite the fact that the initial cause has been limited. Chronic pain is very difficult to treat: it requires comprehensive and frequent multidisciplinary therapeutic interventions, managed with high level of expertise and specialization.[5]
In 1985, Bonica estimated a 50% prevalence of oncologic pain worldwide. This value rose to 71% in advanced patients.[6] In 2007, van den Beuken-vanErdingen, carried out a systematic review with a metanalysis of the studies published in last the 40 years.[7] Older studies report higher rates of prevalence (52%–77%) than more recent ones (24%–60% for patients on pharmacological treatment and 62%–86% for advanced patients).[8]
Scrambler Therapy (ST) is indicated in the advanced setting, acting through the neuromodulation by electrocutaneous stimulation in a non-invasive way through C fibers surface receptors, combing no-pain information in the nerve fibers.[9] This system represents an efficient and safe alternative for several types of refractory chronic pain, with a very rare possibility of adverse events. However, few data is currently available on the efficacy of ST in cancer pain induced by skeletal and visceral metastases.[10] The active principle of ST is not to inhibit the transmission of pain, but to replace the pain with summary information of no pain. For this reason ST can have the immediate effect of zeroing the pain during treatment, followed by the progressive re-modulation of the pain system after repeated treatments that statistically represent the number of cycles necessary to achieve a successful follow-up.[11]
The aim of the present study was to evaluate the impact of ST on patient-reported moderate to severe chronic pain.
2. Materials and methods
2.1. Study design
In this prospective study, from April 2010 to March 2016 we enrolled 219 patients with chronic pain with different etiology to start treatment with ST. The study was approved by the Ethics Committee of our center (Comitato Etico della Romagna) and all patients provided written informed consent before study treatment.
2.2. Patient characteristics
Patients needed to have the following inclusion criteria:
clinical evidence of moderate-severe chronic pain;
performance status (ECOG) 0 to 3;
ability to give written informed consent and to participate in the treatment;
ability to undergo a follow-up and to communicate their feelings about the modification of the pain over time;
ability to participate in a clinical study for a period longer than 3 months;
age ≥ 18.
All newly referred patients were considered for participation in the study.
Exclusion criteria were:
use of a pacemaker;
presence of automatic defibrillator, aneurysm clips, vena cava clips, skull plates, neurolytic blocks or neurolesive pain control treatment;
epilepsy.
2.3. Methods
ST was administered by application of a disposable surface electrodes on the skin areas corresponding to the pain. The study consisted of 2 consecutive weeks of treatment of ST (1 daily 30-min session, 5 days a week) and 2 weeks of follow-up. Patients were asked to describe the level of pain using the Numeric Rating Scale (NRS) immediately before the initiation (T0), after 1 week of treatment (T1), at the end of the treatment (T2) and after each week of follow up (T3,T4). This scale consists of 11 scores for grading pain as perceived by the patient, going from absence of pain (score 0) to highly severe pain (score 10). This simple, widely used test could be easily repeated and understood by the majority of patients. Subgroup analyses were performed, considering patients with moderate-severe (NRS ≥ 4) and severe pain (NRS ≥ 7) before treatment. Another subgroup analysis considered patients with neuropatic pain and who had undergone multiple courses. All analyses considered patients at first treatment; patients with multiple courses were analyzed separately. The efficacy, the duration and the side effects of ST were also evaluated during treatment and follow up.
2.4. Statistical analysis
The sample size was estimated from the clinical practice. We analyzed a consecutive case series that met the inclusion criteria and underwent standard treatment according to the original protocol. Frequency was calculated for categorical variables. Age at diagnosis and data on pain were shown using mean and standard deviation (±SD). Plots of mean value of pain score and relative SD were plotted for each time points. Normality distribution of pain score was evaluated with the Shapiro-Wilk test. The non-parametric Friedman test was used for detecting differences in NRS between the time points T0, T2, T4 because the normality assumption was not satisfied. Corrections for multiple testing were not performed. Missing data were always shown. P values < .05 were considered statistically significant. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC).
3. Results
Of the 219 people treated with ST, 100 (45.7%) patients were male and 119 (54.3%) were female. The mean age of the patients was 64.7 years (±13.4). Performance status was ECOG 0 for 118 patients, (54.4%), ECOG 1 for 89 patients (41.0%), and ECOG ≥2 for 10 patients (4.6%). Eighty-three patients (37.8%) were affected by cancer pain due to primary cancer, metastasis or anticancer therapies, while the remaining 136 (62.2%) were affected by non-cancer pain of diverse origin. Baseline characteristics of enrolled patients are shown in Table 1. Among cancer patients the cause of treatment was pain from cancer in 62 patients, post-surgical neuropathy in 7 patients, post chemotherapy neuropathy in 12 patients, and post radiotherapy neuropathy in 2 patients. Non-cancer patients suffered from neuropathic pain syndromes, sciatic and lumbar and dorsal pain, postherpetic neuralgia (PHN), trigeminal neuralgia, post-surgery nerve lesion neuropathy, low back pain (LBP), other neuropathies, polyarthrosis, vertebral fractures, hernial lesions, contractures, tendinous lesions, vertebral discopathies, osteonecrosis, traumas, arthritis.
Table 1.
Patient characteristics.
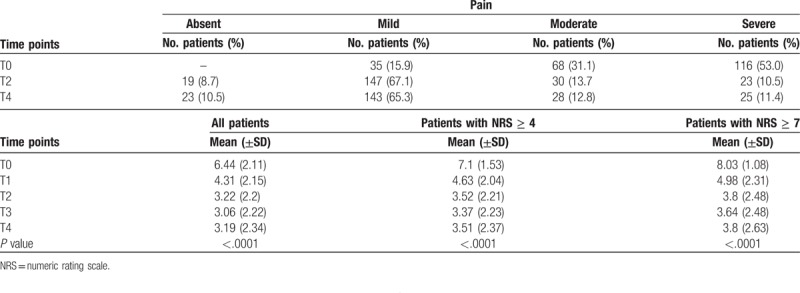
Globally the mean scores of the patient-reported pain were 6.44 (± 2.11) at T0, 3.22 (± 2.20) at T2 and 3.19 (± 2.34) at T4 (Fig. 1 and Table 2). A reduction in the symptomatology is highlighted from T0 to T2 and maintained throughout T4 (P value < .0001). Similar patterns were reported for the subgroup of patients with NRS ≥4 and NRS ≥7 at T0. At T0 15.9% of patients reported mild pain, 31.1% moderate, and 53.0% severe. At T2 the pain appeared mild in 67.1% of patients, moderate in 13.7%, severe in 10.5%, and absent in the remaining 8.7%. At T4, 65.3% of patients maintained mild pain, 12.8% moderate, 11.4% severe, while 10.5% reported no pain (Table 2).
Figure 1.
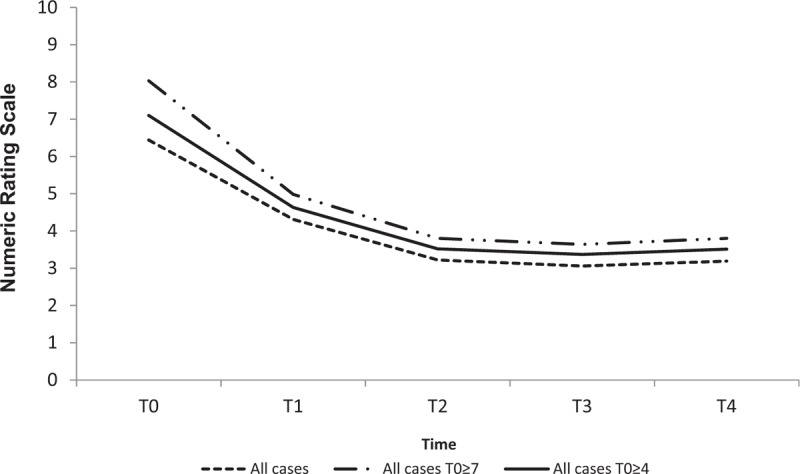
Pain trend over time for overall patients.
Table 2.
Variation in the number and percentage of patients with pain over time.
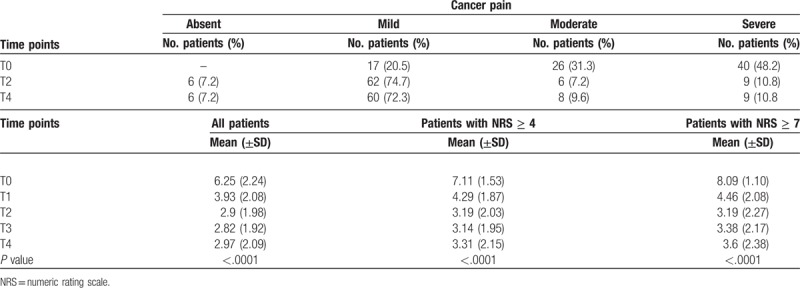
3.1. Cancer pain
The mean scores of cancer pain were 6.25 (±2.24) with a reduction to a mean value of 2.90 (± 1.98) at T2, and 2.97 (±2.09) at T4, and with an absolute reduction of 3.35 from T0 to T2 (P value < .0001). At treatment initiation the number of patients affected by severe pain was 40 (48.2%). It reduced to 9 (10.8%) at the end of treatment. At T4 72.3% of patients had mild pain (P value < .0001). Considering the 66 patients with moderate-severe pain (NRS ≥4) before treatment, a mean NRS score of 7.11 (±1.53) was observed with an absolute reduction of 3.92 at T2 and a mean NRS score of 3.19 (±2.03) was maintained throughout T4 with an NRS of 3.31 (±2.15) (P value < .0001). The 40 patients with NRS ≥7 and a mean NRS score of 8.09 (±1.10) at T0 had a mean NRS score of 3.19 (±2.27) at T02 with an absolute reduction of 4.9 and a mean NRS score of 3.6 (±2.38) at T4 (P value < .0001). (Fig. 2 and Table 3). No adverse events were reported.
Figure 2.
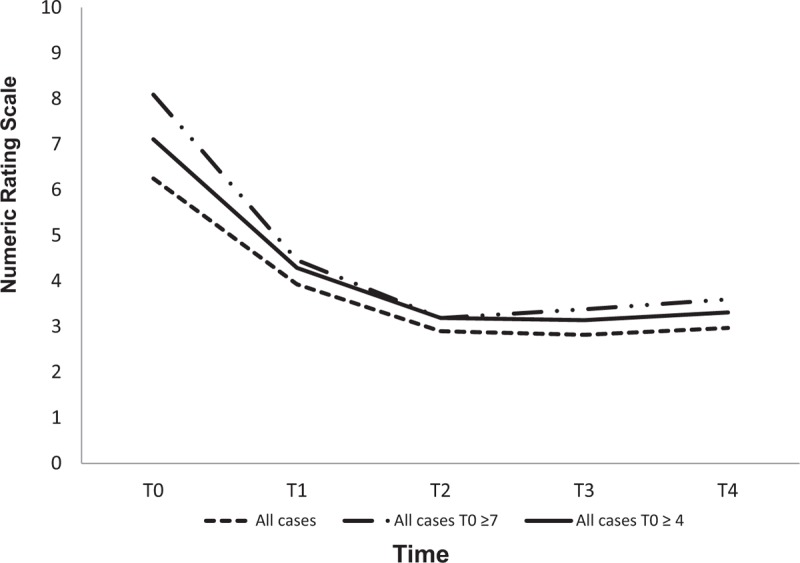
Cancer pain trend.
Table 3.
Variation in the number and percentage of patients with cancer pain over time.
3.2. Non-cancer pain
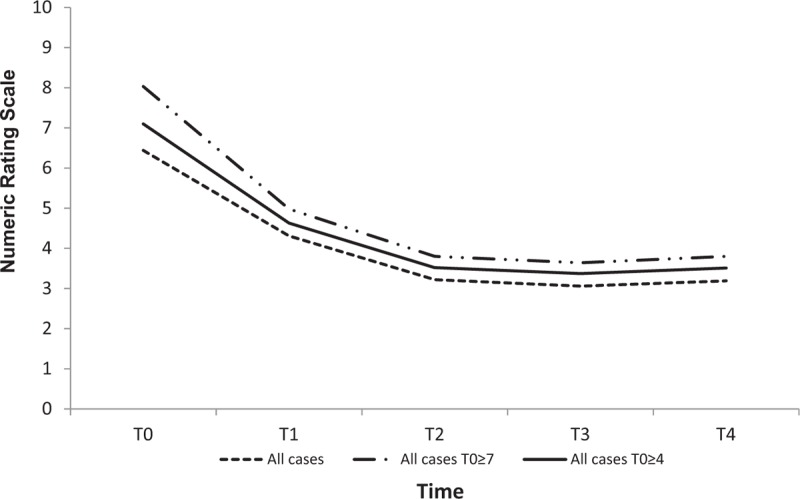
We analyzed 136 patients with non-cancer pain with a mean NRS of 6.55 (±2.03) at T0, 3.42 (± 2.31) at T2, and an absolute reduction of 3.13 (P < .0001) from T0 to T2.
The 118 patients with moderate-severe pain and a mean NRS of 7.09 (±1.54) at T0 reduced to 3.70 (±2.29) at T2 and to 3.63 (±2.48) at T4 (absolute reduction of 3.39) with a P value < .0001. The 76 patients with severe pain and a mean NRS of 8.01 (±1.07) at T0 reduced mean NRS to 4.12 (±2.54) at T2 and to 3.90 (±2.78) at T4 with a P value < .0001 and an absolute reduction of 3.89 at T2 (Table 4 and Fig. 3). The observation of non-oncologic pain at various time points showed that the number of patients with severe pain reduced from 76 (55.9%) at T0 to 16 at T4 (11.8%). No adverse events were reported.
Table 4.
Variation in the number and percentage of patients with non-cancer pain.
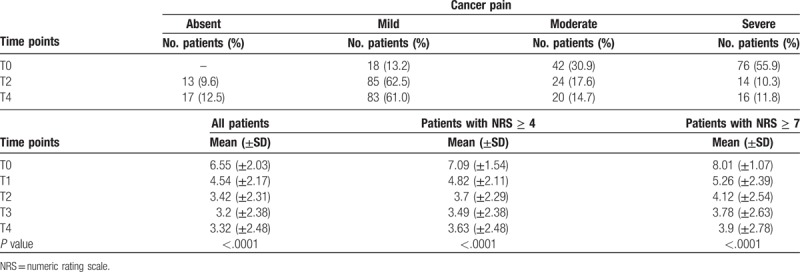
Figure 3.
Trend of Numeric Rating Scale over time for non-cancer pain patients.
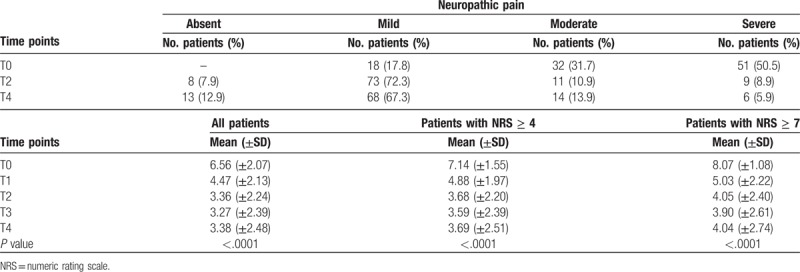
3.3. Neuropathic pain
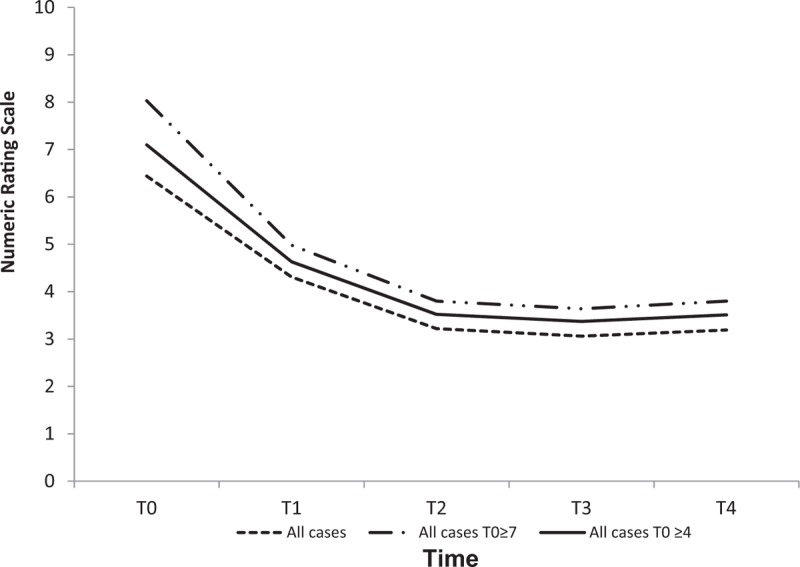
We also analyzed a subgroup of patients (n = 118, 53.9%) suffering from neuropathic pain by dividing it into 3 categories like the previous subgroups. This subgroup of patients had a mean NRS score of 6.56 (±2.07) at T0, which reduced to 3.36 (±2.24) at T2 and 3.38 (±2.48) at T4 (P value < .0001). Absolute reduction from T0 to T2 was 3.2. As shown in Figure 4 and Table 5, there were 53 neuropathic patients with NRS ≥4, with a mean NRS score of 4.71 (±1.37) before treatment, which reduced to a mean NRS of 2.51 (±1.69) at T2 and 2.57 (±1.85) at T4 (P value < .0001) with an absolute reduction of 2.2. Patients with severe neuropatic pain (NRS ≥7) before treatment were 65, with a mean NRS score of 8.07 (±1.08) at T0. After ST, their mean NRS score fell to 4.05 (±2.4) at T2 and stabilized at 4.04 (±2.74) at T4. These results were always statistically significant with an absolute reduction of 4.02 from T0 to T2. After evaluating patients suffering from neuropathic pain at various time points, we observed that the number of patients with severe pain dropped from 50.5% at T0 to only 5.9% at T4. Conversely, the number of patients with mild pain raised from 16 (17.8%) at T0 to 68 (67.3%) at T4.
Figure 4.
Neuropathic pain trend.
Table 5.
Variation in the number and percentage of patients with neuropathic pain.
3.4. Multiple courses
Out of 219 total patients, 44 repeated treatment due to its effectiveness and lack of side effects. We analyzed this subgroup of patients to assess the pain trend over subsequent cycles. A mean NRS score of 6.27 (±2.25) at T0, 2.83 (±1.7) at T2, and 2.94 (±2.24) at T4 (P value < .0001) was recorded, with an absolute reduction of 3.44 between T0 and T2. Notably, the subgroup of 22 patients with NRS ≥7 and a mean NRS score of 8.11 (±1.21) at T0 reduced their pain NRS score to 3.23 (±2.0) at T2 and 3.68 (±2.50) at T4.
Of the 13 patients suffering from cancer pain, 11 had moderate-severe pain (NRS ≥4), and 9 severe pain at T0 (NRS ≥7). The 13 patients with cancer pain that repeated ST reduced their mean NRS score from 6.58 (±2.06) at T0 to 2.85 (±2.08) at T2 and to 2.92 (±2.14) at T4 with an absolute reduction of 3.73 (P value < .0001). Patients with severe cancer pain (NRS 7.67; ± 1.00) at T0 had a mean NRS score reduction of over 50% by T4. The 11 patients with moderate-severe cancer pain had a reduction in pain ranging from 7.23 (±1.37) at T0 to 3.18 (±2.14) between T0-T4.
The 31 patients with non-cancer pain who underwent a second treatment of ST reduced their mean NRS score from 6.15 (±2.35) at T0 to 2.82 (±1.55) at T2 and to 2.95 (±2.31) at T4. The non-cancer severe pain category (NRS ≥7) included 13 patients with a mean NRS score of 8.42 (±1.29) at T0, 3.08 (±1.99) at T2, and 3.77 (±2.77) at T4, with an absolute reduction of 5.34 between T0 and T2 (P value < .0001).
An evaluation of the time elapsed between the first and second cycle of ST showed that 31 patients repeated the treatment within 2 to 5 months of the end of the first cycle; in detail 7 patients repeated treatment after 1 month, 12 within 2 to 3 months, 8 after 4 months, and 3 after 5 months with no significant difference in the initial and final mean NRS score between first and second course of treatment (data not shown). Thirteen patients underwent second ST after 6 months, without any significant difference in the initial and final mean NRS score between first and second course of treatment (data not shown). Twenty-one patients (67.7%) repeated the second ST on the same site as the first. No side effects were observed in any of the 219 patients treated.
4. Discussion
Patients suffering from chronic pain are usually difficult to treat with conventional therapy. In the last years, these patients have been tested for the effect of nerve stimulation, in particular ST, on which 37 studies are currently available. Our study is the second prospective study to have combined clinical experience, prospective single-arm clinical trials, a randomized open-label controlled trial and 2 blinded, randomized placebo-controlled trials.
Marineo first developed ST and reported successful results in 11 cancer patients in 2003.[12] In 2012, Ricci et al published a trial on 82 (73 evaluable) prospectively treated patients, about half of whom had cancer-related pain. Mean NRS score reduced from 6.2 to 1.6 before and after treatment, respectively, and was 2.9 one month after treatment had finished. Similar results were seen in patients with and without cancer. When patients were asked whether they would repeat this treatment, 97% (71/73) said they would.[13]
Other prospective trials on cancer patients with chemotherapy-induced peripheral neuropathy reported positive results similar to our study with a long-lasting pain reduction by about 50%.[14] The pain was long reduced throughout follow-up and up to a period of 3 months.[15–20] Marineo published a randomized trial on 52 patients with neuropathic pain comparing ST to standard pharmacologic recommendations, obtaining statistically significant results for the ST group.[21] Other studies have attempted to reproduce the same findings in neuropathic pain obtaining results from 28% pain reduction (due to lack of experience, intractable pain, concomitant therapy) to over 50% up to 3 months of follow-up.[22–26] As for our study, the total data show a statistically significant reduction in pain from T0 to T4 in all patients, confirming the literature results. A good response was also reached by both categories of patients with and without cancer pain from T0 to T2, with a maintenance response at T4. Better results were reached by cancer patients with severe pain.
Patients suffering from non-cancer pain obtained positive results, with almost up to 50% pain reduction maintained throughout follow-up. Patients with neuropathic pain, especially severe pain, who had had almost up to 50% pain reduction, maintained the pain reduction over time, confirming the data from the literature. For patients that had undergone a second cycle of ST, the good response to treatment was confirmed with over 50% response maintained over time and greater absolute reduction during follow up. In particular, if patients started the second ST within 2 to 5 months, they would report an initial lower and a final higher median NRS score than in the first treatment. It is noteworthy that several patients repeated the treatment within 2 to 5 months with statistically significant response over time. Patients who repeated ST after 6 months of the end of the first treatment had a higher median NRS score than in the first cycle also at the end of the follow-up. Our data confirmed the effectiveness of ST on chronic pain, especially in diseases that are difficult to treat, such as arthritis.
Cancer pain is more complex to treat with ST due to concomitant therapies such, as radiation therapy and chemotherapy. In fact, ST must often be discontinued or suspended due to chemotherapy-related side effects or other cancer-related complications. All the operators received specific training to deal with these critical issues.
We suggest that all new prospective studies perform a standardization of the patient selection. ST, however, showed many undoubted advantages, including the results obtained with repeated treatments, the patient optimal compliance, the reduction of the use of opioids, which led to fewer severe adverse events. Future research should focus on the factors related to response and repetition of treatment, the placebo effect, and maintenance therapy.
5. Conclusions
The data collected and analyzed showed a statistically significant impact of the treatment with ST maintained throughout follow-up in patients suffering from chronic pain of multiple nature. ST represents a complementary perspective for analgesic control thanks to its safety and non-invasiveness. The success of this technique greatly depends on the patient selection and the level of expertise of the operator. The literature data, however, are heterogeneous and not exhaustive on the long-term effectiveness of the treatment. For this reason, further research is needed to promote prospective studies on response maintenance over time.
Acknowledgments
The authors would like to thank Veronica Zanoni and Gráinne Tierney and for editorial assistance.
Author contributions
All authors contributed equally to this paper, read, and approved the final version of the manuscript for submission.
Conceptualization: Marianna Ricci, Marco Maltoni.
Data curation: Marianna Ricci, Laura Fabbri, Sara Pirotti, Nicola Ruffilli, Flavia Foca.
Formal analysis: Marianna Ricci, Laura Fabbri, Nicola Ruffilli, Flavia Foca, Marco Maltoni.
Investigation: Marianna Ricci, Laura Fabbri, Sara Pirotti, Nicola Ruffilli, Marco Maltoni.
Methodology: Marianna Ricci, Laura Fabbri, Flavia Foca, Marco Maltoni.
Resources: Marianna Ricci, Sara Pirotti, Nicola Ruffilli, Flavia Foca, Marco Maltoni.
Supervision: Flavia Foca, Marco Maltoni.
Validation: Marianna Ricci, Marco Maltoni.
Visualization: Marianna Ricci, Laura Fabbri, Sara Pirotti, Nicola Ruffilli, Flavia Foca, Marco Maltoni.
Writing – original draft: Marianna Ricci, Laura Fabbri, Flavia Foca, Marco Maltoni.
Writing – review & editing: Marianna Ricci, Marco Maltoni.
Footnotes
Abbreviations: LBP = low back pain, NRS = numeric rating scale, PHN = postherpetic neuralgia, ST = scrambler therapy.
The authors have no conflicts of interest to disclose.
References
- [1].Merskey H, Bogduk N. International Association for the Study of Pain. Descriptions of chronic pain syndromes and definitions of pain terms. IASP Press, Classification of chronic pain. Seattle: 1994. [Google Scholar]
- [2].Greenwald HP, Bonica JJ, Bergner M. The prevalence of pain in four cancers. Cancer 1987;60:2563–9. [DOI] [PubMed] [Google Scholar]
- [3].Breivik H, Collett B, Ventafridda, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- [4].Loeser JD. Pain and suffering. Clin J Pain 2000;16:S2–6. [DOI] [PubMed] [Google Scholar]
- [5].Life Episteme Italia s.r.l. Manuale d’uso e manutenzione – CALMARE MC-5A. http://www.lifeepistemeitalia.it Accessed 26 September, 2018. [Google Scholar]
- [6].Marineo G. Untreatable pain resulting from abdominal cancer: new hope from biophysics. JOP 2003;4:1–0. [PubMed] [Google Scholar]
- [7].Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an openlabel study using MC5-A Calmare® device. Support Care Cancer 2012;20:405–12. [DOI] [PubMed] [Google Scholar]
- [8].Majithia N, Smith TJ, Coyne PJ, et al. Scrambler therapy for the management of chronic pain. Support Care Cancer 2016;24:2807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sparadeo F, Kaufman C, D’Amato S. Scrambler therapy: an innovative and effective treatment for chronic neuropathic pain. J Life Care Plan 2012;11:3–15. [Google Scholar]
- [10].Pachman DR, Weisbrod BL, Seisler DK, et al. Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer 2015;23:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Notaro P, Dell’Agnola CA, Dell’Agnola AJ, et al. Pilot evaluation of scrambler therapy for pain induced by bone and visceral metastases and refractory to standard therapies. Support Care Cancer 2016;24:1649–54. [DOI] [PubMed] [Google Scholar]
- [12].Lee SC, Park KS, Moon JY, et al. An exploratory study on the effectiveness of “Calmare therapy” in patients with cancer-related neuropathic pain: a pilot study. Eur J Oncol Nurs 2016;21:1–7. [DOI] [PubMed] [Google Scholar]
- [13].Sabato AF, Marineo G, Gatti A. Scrambler therapy. Minerva Anestesiol 2005;71:479–82. [PubMed] [Google Scholar]
- [14].Smith TJ, Coyne PJ, Parker GL, et al. Pilot trial of a patient-specific cutaneous electrostimulation device (MC5-A Calmare®) for chemotherapy-induced peripheral neuropathy. J Pain Symptom Manage 2010;40:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coyne PJ, Wan W, Dodson P, et al. A trial of scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother 2013;27:359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marineo G, Iorno V, Gandini C, et al. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage 2012;43:87–95. [DOI] [PubMed] [Google Scholar]
- [17].Abdi S, Lakkimsetty VM, Barrera J, et al. The use of “Scrambler Therapy” for failed back surgery syndrome. Pain Physician 2011;14: Abstr, E465. [Google Scholar]
- [18].Ghatak RK, Nandi SN, Bhakta A, et al. Prospective study of application of biological communication (cybernatics) in management of chronic low back pain a preliminary report. Nepal Medical Coll J 2011;13:257–60. [PubMed] [Google Scholar]
- [19].Smith TJ, Marineo G. Treatment of postherpetic pain with scrambler therapy, a patient-specific neurocutaneous electrical stimulation device. Am J Hosp Palliat Care 2018;35:812–3. [DOI] [PubMed] [Google Scholar]
- [20].Ko YK, Lee HY, Lee WY. Clinical experiences on the effect of scrambler therapy for patients with postherpetic neuralgia. Korean J Pain 2013;26:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sparadeo F, D’Amato S. Scrambler Therapy: effective use of artificial neurons for the treatment of neuropathic pain. J Nurse Life Care Plan 2014;14:19–30. [Google Scholar]
- [22].Starkweather AR, Coyne P, Lyon DE, et al. Decreased low back pain intensity and differential gene expression following Calmare®): results from a doubleblinded randomized sham-controlled study. Res Nurs Health 2015;38:29–38. [DOI] [PubMed] [Google Scholar]
- [23].Moon JY, Kurihara C, Beckles JP, et al. Predictive factors associated with success and failure for calmare (Scrambler) therapy: a multi-center analysis. Clin J Pain 2015;31:750–6. [DOI] [PubMed] [Google Scholar]
- [24].Compagnone C, Tagliaferri F. Chronic pain treatment and scrambler therapy: a multicenter retrospective analysis. Acta Biomed 2015;86:149–56. [PubMed] [Google Scholar]
- [25].Lesenskyj A, Maxwell C, Cruciani R. Neuropathic pain reduction with scrambler therapy. J Pain 2016;17:S73Abstr 393. [Google Scholar]
- [26].Joo SY, Cho YS, Cho SR, et al. Effects of pain Scrambler therapy for management of burn scar pruritus: a pilot study. Burns 2017;43:514–9. [DOI] [PubMed] [Google Scholar]


