Abstract
Objectives
Aim of present study was designed to investigate the soporific effect of fennel among menopausal women.
Methods
The present double-blinded and placebo-controlled trial examined the fennel effect on Pittsburgh Sleep Quality Index (PSQI). Total score and relevant 7 components, including sleep duration, sleep latency, use of sleeping medication, subjective sleep quality, sleep disturbances, daytime dysfunction and habitual sleep efficiency among 50 menopausal women compared to control group within a 12-week follow-up.
Results
The patients in both groups reported no certain side effects and all subjects completed the study. The mean actual sleep duration was 5 hours and 66 minutes. Intergroup comparison revealed no statistically significant differences in the mean total PSQI score (P = 0.439), subjective sleep quality (P = 0.826), habitual sleep efficiency (P = 0.127), sleep disturbances (P = 0.130), use of sleeping medication (P = 0.52) and daytime dysfunction (P = 0.439). A tendency toward significant between 2 groups was seen concerning the sleep duration (P = 0.059). Intergroup comparison showed significantly borderline levels (P = 0.059).
Conclusions
The treatment of 12 weeks with fennel caused a slight effect that did not reach to significant. These findings should be considered cautiously because of small sample size, short-term follow-up and subjective measure of sleep quality.
Keywords: Foeniculum, Menopause, Sleep, Sleep wake disorders
Introduction
Sleep is so integral to daily growth and development throughout our lives.1 Reportedly, about 30% of global populations are suffering from the sleep disorders, especially menopausal women.2,3 Complained of sleep disturbances are more common among women compared to men in the same age range.1 A study in the US (2007) indicated that women aged 40 to 54 years (46%) and 55 to 64 years (48%) reported sleep disorders.2
Menopause is defined as the stope of menstrual period permanently as a result of ovarian estrogen deficiency.4 The sleep problems were seen among 70% of healthy menopause women in Tehran, Iran.1 Several adverse outcomes have been reported associated with the sleep disturbances, such as daytime dysfunction, fatigue, low quality of life (QOL) and huge healthcare utilization, among which insomnia accounts for major depressive disorder.
The quality of sleep in individuals is affected by multifactorial etiology.2,5 For example, the prevalence of sleep-related issues can be due to aging.6 As menopausal women are exposed to various parameters regarding their quality of sleep, such as loss of ovaries function and thus reduction in hormones, physiological change (hot flash, frequent nocturia and incontinence and osteoporosis), physical illness (backache, stomach discomfort, arthritis and headaches), and psychological status (feeling of despair, failure, uselessness and depressant life style).2
The treatment of insomnia can currently occur using both benzodiazepine hypnotics like flurazepam and triazolam and nonbenzodiazipine hypnotics like zolpidem and zopiclone.2 Hormone therapy is one of the used approaches to attenuate the sleeping disorders and to improve the quality of sleep during menopause.5
Nevertheless, there are various complications following the administration of benzodiazepine7 and hormone therapy.8 According to the results obtained from the national research, the daily use of dietary supplements, including herbal medications, are common among over 50% of middle-aged to elderly adults. More than 1.5 million American adults exploit the released new findings on the use of complementary and alternative medicine (CAM) to resolve their sleep-related disturbances.9 Phytoestrogens have been reportedly proven to be useful in reducing the sleep problems.2 Fennel is one of the effective phytoestrogens in this regard.10 The effectiveness of the concurrent use of fennel and officinalis (Melissa) to manage the sleep disorder has been evaluated in a study whose results showed soporific effect of fennel on the sleep disorder, though the details of this conclusion are ambiguous in relation to the combined or individual effects of these medicinal herbs.11 The present study was designed to investigate the soporific effect of fennel among menopausal women.
Materials and Methods
1. Methods
The current research has been adapted from a randomized, double-blinded, placebo-controlled clinical trial evaluating the fennel effect on improving the quality of sleep in Iranian postmenopausal women from January 2015 to June 2016.
The Ethics Committee of Mashhad University of Medical Science approved the study protocol (Ethics code, IR.MUMS. REC. 1393.42). The signed informed consent was obtained from all research units who were free to leave the study at any time.
The study inclusion criteria were healthy postmenopausal women (women aged range of 45–65 years with no vaginal bleeding at least for a year), no history of systemic or tropical estrogen taking during the last 6 months and a normal mammogram in the last year. The study statistical populations were menopausal women referring to health centers of Mashhad city, the subjects were chosen using a cluster sampling method. Subsequently, the city was divided into 4 districts in which 10 centers were randomly selected. Sampling in each center were convenience. A list of menopausal women was provided those had a file in these centers. All women on list were contacted on phone and invited to the gynecology clinic of Game Hospital. Sampling was continued until 50 patients who met the inclusion criteria to complete a questionnaire.
2. Sample Size
The sample size primary outcome (QOL) was estimated at 20 in each fennel and control (placebo) groups in according to previous study12 to attain a statistical power of 80%. Considering the attrition, final sample size was determined to be totally 50 (25 in each group).
3. Measurements
The 19-item Pittsburgh sleep quality index (PSQI) is self-reported questionnaire to assess the sleep quality and disturbances during previous months. The questionnaire consists of 7 components, including sleep duration, sleep latency, use of sleeping medication, subjective sleep quality, sleep disturbances, daytime dysfunction and habitual sleep efficiency. Each subscale is ranged from 0 to 3, and total PSQI score from 0 to 21, higher score indicating worse sleep quality. The total PSQI score over 5 defined as poor quality of sleep.
4. Randomization and blinding
A computerized random number generator was used to allocate the patients in the 2 treatments and control groups. The fennel and placebo capsules had the same color (yellow), shape and weight (100-mg capsules; Barij Essence Company, Tehran, Iran) to blind both patients and researchers; and they all contained sunflower oil, as well as high-density polyethylene and labeled with “A” and “B”. Assistant researchers who were unaware of the study prescribed all drugs. The identity of the capsule was not recognized until the end of the study.
5. Intervention, adherence, and adverse event measures
All subjects for a 3-month follow-up were administered the capsules 3 times a day, morning, noon and night. The sunflower oil was added to 100 mg of soft capsules containing 30% fennel (standardized to 21–27 mg anethole) in accordance with instructions on http://www.barijessence.com. The patients were requested to bring the unused capsules themselves at each follow-up to check the adherence to medication. The patient's self-report was the retrospective criterion to assess the side effects.
6. Data analysis
The obtained data were analyzed in SPSS version 11 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the normal distribution of data. The differences between the 2 groups were evaluated by χ2 (for categorical data) and Student's t-test (for continual data). Mann-Whitney U test was used for non-normal data. The paired t-test was applied for normal data and Wilcoxon signed rank test for non-normal data to compare pre- and post-treatment results.
Results
The patients reported no side effects in both groups and all subjects completed the study. The 2 groups were same at baseline regarding age (56 ± 4.2 and 55 ± 4.7; P = 0.414), number of children (5.2 ± 2.3 and 5.1 ± 1.7; P = 0.496), educational level (P = 0.836), and number of years since menopause (6.2 ± 3.8 and 5.2 ± 4.2; P = 0.346), body weight (68.4 ± 16.25 and 70.92 ± 12.40 P = 0.541), history of using hormone therapy (8% and 8%; P = 0.695) and CAM (P = 0.417).
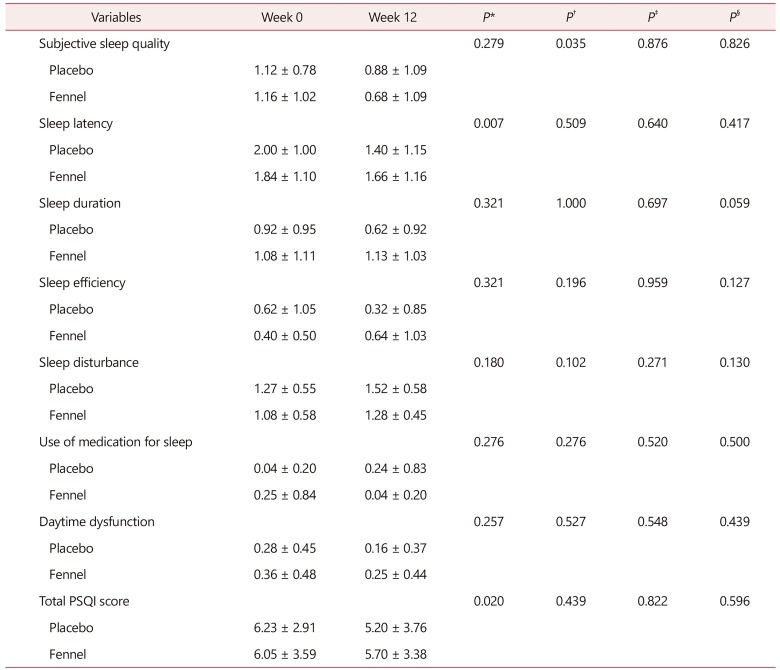
The mean sleep duration was 5 hours and 66 minutes. Severity of all component of PSQI was comparable between 2 groups at baseline. Intergroup comparison showed no statistically significant difference in terms of the mean total PSQI score (P = 0.439), subjective sleep quality (P = 0.826), sleep efficiency (P = 0.127), sleep disturbance (P = 0.130), use of sleeping medication (P = 0.52) and daytime dysfunction (P = 0.439). The sleep duration was increased by 4% while the habitual sleep efficiency was worse in the placebo group (Table 1). A tendency toward significant between the 2 groups was seen concerning the sleep duration (P = 0.059). Surprisingly, the sleep latency and total PSQI were significantly higher in the placebo group. After the administration of the fennel, a statistically significant decrease was seen at the severity of subjective sleep quality (P = 0.035) (Table 1). The mean total PSQI score was more than 5, suggesting the poor quality of sleep, which was seen in 68% of menopausal women. However, no more effects were appeared in the patients with total PSQI score greater than 5 (fennel vs. placebo; P = 0.840 data not shown).
Table 1. Pittsburgh Sleep Quality Index total and its components at before and 12 weeks after interventions.
*P value between the placebo group and baseline
†P value between the fennel group and baseline
‡P value between the placebo and fennel at baseline (week 0)
§P value between the placebo and fennel at the end of study (week 12)
PSQI: Pittsburgh Sleep Quality Index
Discussion
Complained of sleep disturbances are more common among women compared to men in the same age range.1,3 Fennel showed beneficial effect in combine to other herbal medicine such as officinalis,11 lemon balm, and chamomile.13 Objective of current study is to assess the effect of alone fennel (mono-preparation) on quality of sleep.
The present study aimed to determine if the fennel could attenuate sleep disorder in the menopausal women. The fennel failed to reveal significant beneficial effect on all components such as sleep duration, sleep latency, use of sleeping medication, subjective sleep quality, sleep disturbances, and daytime dysfunction, as well as the total PSQI score. However, a tendency towards statistical significance was observed regarding the sleep duration
Literature review of national and international databases found only 2 relevant studies11,13 assessing the effect of concurrent use of fennel and officinalis (Melissa) on the sleep disorders. The first study was carried out by Shirazi et al.11, as 60 menopausal women diagnosed with sleep disturbances were divided into groups I to III. The Group I received the fennel (300 mg) combined with the officinalis (300 mg). In the group II, the treatment was started with 20 mg of citalopram followed by 30 mg after a week. The group III consumed the placebo. The Melissa was found to be more effective compared to other 2 groups.11 Another study suggested that the fennel combined with lemon balm, and chamomile in form of tea could significantly improve the sleep latency and duration and also total score of sleep quality.13
In contrast to above 2 studies, limited effects were found in the current study, probably due to several explains. First, the small sample size was evident in this current. Second, the significant effects may be related to a combined effect of both herbal and only officinalis.
Third, the present study investigated the effectiveness of fennel on menopausal women while 2 above trials evaluated the effect of fennel on all age groups. Fourth, the fennel is a phytoestrogen as a naturally found estrogen produced by plants.14 The effects of phytoestrogens depend on hormonal status.15 The fennel may have various impacts based on the estrogen status in women which menopausal women have less estrogen. Therefore, the effect of phytoestrogen like fennel dependent the level of estrogen. Fifth, 2 previous studies investigated the mixture fennel effects in the patient with sleep disorder10 and those with moderate stress level.13 Fennel may be more effective in women with sleep disorder and stress. However, in the present study, the treatment with fennel was not superior to placebo in subgroup of patients with poor sleeps quality.
The menopause symptoms can affect the quality of sleep in menopause women.2 Moreover, previous studies showed beneficial effects of fennel on depression, anxiety,16 vaginal atrophy,17 sexual satisfaction,18 physical symptoms and vasomotor.15 Therefore, slight effect appeared might be due to the indirect effect of the fennel on other menopause symptoms, such as vaginal atrophy, depression and anxiety.
The small sample size and short-term follow up was the main limitation in the current study. Future research with larger sample size and longer duration will be required to detect the effectiveness of fennel on the sleep quality. The present study assessed the fennel effect on menopausal women having lower estrogen levels compared to reproductive age. The findings of the study might not be generalized to reproductive age. Quality of sleep was measured by self-report. It should be measured by more objective measure.
Conclusion
The treatment of 12 weeks with fennel caused a slight effect that did not reach to significant. These findings should be considered cautiously because of small sample size, short-term follow-up and subjective measure of sleep quality.
Acknowledgement
This work was supported by the Mashhad University of Medical Sciences, Mashhad, Iran [Grant number 921364].
Footnotes
Conflict of Interest: The Barij Essence Pharmaceutical Company supported this study by providing soft fennel capsules; however, the design of protocol, analysis, and research implementation were undertaken by the author.
References
- 1.Taavoni S, Ekbatani NN, Haghani H. Postmenopausal women's quality of sleep and its related factors. J Midlife Health. 2015;6:21–25. doi: 10.4103/0976-7800.153611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 3.Abdi F, Mobedi H, Roozbeh N. Hops for menopausal vasomotor symptoms: Mechanisms of action. J Menopausal Med. 2016;22:62–64. doi: 10.6118/jmm.2016.22.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zivdir P, Sohbet R. Effect of feelings of guilt and shame on life quality of women in menopause. J Menopausal Med. 2017;23:5–14. doi: 10.6118/jmm.2017.23.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarti CD, Chiantera A, Graziottin A, Ognisanti F, Sidoli C, Mincigrucci M, et al. Hormone therapy and sleep quality in women around menopause. Menopause. 2005;12:545–551. doi: 10.1097/01.gme.0000172270.70690.5e. [DOI] [PubMed] [Google Scholar]
- 6.Timur S, Sahin NH. Effects of sleep disturbance on the quality of life of Turkish menopausal women: a population-based study. Maturitas. 2009;64:177–181. doi: 10.1016/j.maturitas.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Listos J, Talarek S, Fidecka S. Adenosinergic system is involved in development of diazepam tolerance in mice. Pharmacol Biochem Behav. 2010;94:510–515. doi: 10.1016/j.pbb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Hattori A, Ando N, Hamaguchi K, Hussein MH, Fujimoto S, Ishikawa T, et al. Short-duration ACTH therapy for cryptogenic West syndrome with better outcome. Pediatr Neurol. 2006;35:415–418. doi: 10.1016/j.pediatrneurol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Taavoni S, Ekbatani N, Kashaniyan M, Haghani H. Effect of valerian on sleep quality in postmenopausal women: a randomized placebo-controlled clinical trial. Menopause. 2011;18:951–955. doi: 10.1097/gme.0b013e31820e9acf. [DOI] [PubMed] [Google Scholar]
- 10.Kooti W, Moradi M, Ali-Akbari S, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J Herb Med Pharmacol. 2015;4:1–9. [Google Scholar]
- 11.Shirazi M, Saedi N, Shariat M, Azadi F, Davari Tanha F. Comparison of melissa with citalopram and placebo in treatment of sleep disorders in menopausal women: Clinical trial. Tehran Univ Med J. 2016;74:562–568. [Google Scholar]
- 12.Asgari P, Zand S, Narenji F, Bahramnezhad F, Mahmoudi M. The effect of Glycyrriza glabra on quality of life in postmenopausal women. Complement Med J Fac Nurs Midwifery. 2015;5:1146–1154. [Google Scholar]
- 13.Talbott S, Perkins L, Young D. Effect of MonaVie Rest™ on sleep duration and sleep quality in moderately stressed subjects (647.23) FASEB J. 2014;28:647. [Google Scholar]
- 14.Fattah A. Effect of phytoestrogen on depression and anxiety in menopausal women: A systematic review. J Menopausal Med. 2017;23:160–165. doi: 10.6118/jmm.2017.23.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamison JR. Phytoestrogens: An HRT alternative? J Complement Med. 2002;1:62–65. [Google Scholar]
- 16.Ghazanfarpour M, Mohammadzadeh F, Shokrollahi P, Khadivzadeh T, Najaf Najafi M, Hajirezaee H, et al. Effect of Foeniculum vulgare (fennel) on symptoms of depression and anxiety in postmenopausal women: a double-blind randomised controlled trial. J Obstet Gynaecol. 2018;38:121–126. doi: 10.1080/01443615.2017.1342229. [DOI] [PubMed] [Google Scholar]
- 17.Yaralizadeh M, Abedi P, Najar S, Namjoyan F, Saki A. Effect of Foeniculum vulgare (fennel) vaginal cream on vaginal atrophy in postmenopausal women: A double-blind randomized placebo-controlled trial. Maturitas. 2016;84:75–80. doi: 10.1016/j.maturitas.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Najar S, Yaralizadeh M, Abedi P, Namjooyan F, Malehi O. Effect of fennel vaginal cream on dysparonia and sexual satisfaction among postmenopausal women: A double-blind randomized controlled trial. Iran J Obstet Gynecol Infertil. 2015;18:8–16. [Google Scholar]