Introduction
Menopausal hormone therapy (MHT) is typically initiated to control climacteric symptoms during early postmenopause. MHT improves quality of life and prevents osteoporotic fracture.1 However, concerns about risks, including cancers and cardiovascular disease, are still highly prevalent. All-cause mortality (ACM) would be one definite endpoint to use in assessing a complex balance of benefits and risks with MHT. A Cochrane Database of Systematic Reviews (CDSR) study conducted by Boardman et al.2 in 2015 concluded that administering MHT at an age less than 10 years after menopause onset had a reduction of ACM (hazard ratio [HR], 0.70; 95% confidence interval [CI], 0.52–0.95) through a systematic review of 5 trials: the Danish Osteoporosis Prevention Study (DOPS; 2012),3 Estonian Postmenopausal Hormone Therapy (EPHT; 2006),4 Estrogen Replacement Therapy (ERT) II (1979),5 Women's Health Initiative (WHI) I (2002),6 and WHI II (2004).7
The U.S. Preventive Services Task Force (USPSTF)8 reported in 2017 that the HR of ACM with estrogen therapy (ET) during the intervention phase was 0.70 (95% CI, 0.46–1.09) among women aged 50 to 59 years but cited only 1 article from the WHI trials.9 In addition, ACM with estrogen plus progestogen therapy (EPT) declined similarly without statistical significance (HR, 0.67; 95% CI, 0.43–1.04). However, the USPSTF recommends against MHT in early postmenopausal women.10
The purpose of this study was to propose a scientific and valid position for the conflicting recommendations of the CDSR (2015)2 and the USPSTF (2017),10 to rearrange and update the selected articles to include newer publications, and to conduct subgroup analyses by MHT regimen.
Methods and Results
As though adapting an old clinical practice guideline, an adaptive meta-analysis was performed in order to add current evidences and then conduct additional meta-analysis.11 Using citation discovery tools provided by PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), lists of recent articles ‘citing’ the articles selected by previous meta-analyses were made. Accordingly, lists of the cited sources in 6 articles by the CDSR2 and the USPSTF10 were created and appraised. As a consequence, the WHI (2012)12 was used to update the WHI I (2002),6 WHI II (2004),7 and WHI (2008).9 In addition, 2 trials conducted in women with a concurrent medical disorder were added to the sensitivity analyses: EStrogen in the Prevention of ReInfarction Trial (ESPRIT; 2014)13 in patients who survived a first myocardial infarction and ERT II (1979)5 in patients hospitalized for chronic disease. During appraisal of the citation lists, articles related to Heart and Estrogen/progestin Replacement Study (HERS) I (1998),14 HERS II (2002),15,16 and EPHT (2006)4 were removed because they did not contain results for women less than 60 years old.
From the final selection of 4 trials,3,5,12,13 the authors extracted the HRs and their 95% CIs based on the MHT medication prescribed for postmenopausal women less than 60 years old. After calculating the standard error of log HR,17 we used the Stata/SE Version 14 statistical program (Stata-Corp, College Station, TX, USA) to estimate the summary HR (sHR) and to generate the forest plots.18
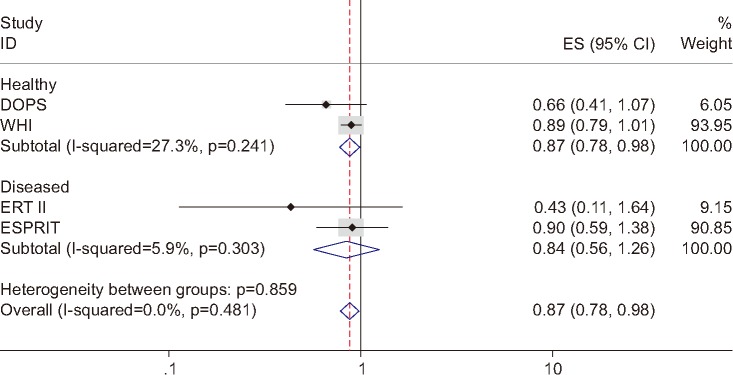
Figure 1 displays a forest plot for the 4 trials with all types of MHT. For the 2 trials conducted in healthy women,3,12 the sHR calculated using a random effect model was 0.87 (95% CI, 0.78–0.98) with no heterogeneity (I-squared, 27.3%). Even after including 2 trials of women with certain health conditions,5,13 the protective effects remained statistically significant (sHR, 0.87; 95% CI, 0.78–0.98).
Fig. 1. Forest plot of the 4 selected trials. ID: identification, ES: effect size, CI: confidence interval, DOPS: Danish Osteoporosis Prevention Study, WHI: Women's Health Initiative, ERT: estrogen replacement therapy, ESPRIT: EStrogen in the Prevention of ReInfarction Trial.
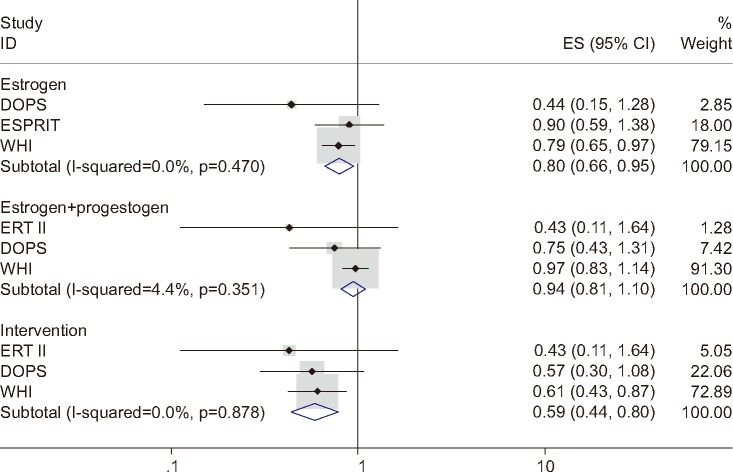
Figure 2 shows the results of the subgroup analyses. The sHRs were 0.80 (95% CI, 0.66–0.95) and 0.94 (95% CI, 0.81–1.10) in ET and EPT, respectively. In the follow-up results of the intervention periods, the sHR was 0.59 (95% CI, 0.44–0.80).
Fig. 2. A forest plot generated from subgroup analyses by menopausal hormone therapy regimen and also during the intervention period. ID: identification, ES: effect size, CI: confidence interval, DOPS: Danish Osteoporosis Prevention Study, ESPRIT: EStrogen in the Prevention of ReInfarction Trial, WHI: Women's Health Initiative, ERT: estrogen replacement therapy.
Discussion
Based on these results, MHT may reduce the ACM for postmenopausal women younger than 60 years. In our analysis, we first analyzed pooled data of the MHT combining ET and EPT, while the USPSTF showed separate results. A recent WHI analysis12 regarding long-term ACM showed no heterogeneity between ET and EPT and reported the pooled results of 2 trials (HR, 0.69; 95% CI, 0.51–0.94). Further, cumulative data, when available, were used, including intervention and post-intervention follow-ups in DOPS, WHI, and ESPRIT, which could provide more robust evidence. The USPSTF used WHI data during the intervention phase only. Interestingly, the protective effect of MHT seemed to be stronger during intervention periods.
ET decreased the ACM significantly in postmenopausal women younger than 60 years. However, EPT did not show statistical significance, even though sHR showed a protective tendency. In the EPT subgroup, the weight of the WHI trial was very high (91.3%). Medroxyprogesterone acetate was used in the WHI trial, and the reduction in ACM seemed to be quite attenuated in the cumulative follow-up compared with the intervention phase.12 Of note, the norethindrone acetate used in the DOPS trial showed a similar HR during both the intervention phase and in the cumulative data.3 Further research is warranted to determine better progestogen to produce a less adverse impact on estrogen in a cumulative follow-up study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boardman HM, Hartley L, Eisinga A, Main C, Roqué i, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229. doi: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 4.Veerus P, Hovi SL, Fischer K, Rahu M, Hakama M, Hemminki E. Results from the estonian postmenopausal hormone therapy trial [ISRCTN35338757] Maturitas. 2006;55:162–173. doi: 10.1016/j.maturitas.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979;54:74–79. doi: 10.1097/00006250-197907000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 8.Gartlehner G, Patel SV, Viswanathan M, Feltner C, Weber RP, Lee R, et al. Agency for Healthcare Research and Quality, editors. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: An evidence review for the U.S. preventive services task force. Rockville (MD): Agency for Healthcare Research and Quality; 2017. U.S. preventive services task force evidence syntheses, formerly systematic evidence reviews; p. 15-05227-EF-1. [PubMed] [Google Scholar]
- 9.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US preventive services task force recommendation statement. JAMA. 2017;318:2224–2233. doi: 10.1001/jama.2017.18261. [DOI] [PubMed] [Google Scholar]
- 11.Bae JM, Kim EH. Citation discovery tools for conducting adaptive meta-analyses to update systematic reviews. J Prev Med Public Health. 2016;49:129–133. doi: 10.3961/jpmph.15.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The women’s health initiative randomized trials. JAMA. 2017;318:927–938. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherry N, McNamee R, Heagerty A, Kitchener H, Hannaford P. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG. 2014;121:700–705. doi: 10.1111/1471-0528.12598. [DOI] [PubMed] [Google Scholar]
- 14.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 15.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, et al. Effect of hormone therapy on mortality rates among women with heart failure and coronary artery disease. Am J Cardiol. 2005;95:289–291. doi: 10.1016/j.amjcard.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008;11:23–32. doi: 10.1007/s10120-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 18.Shim SR, Shin IS, Bae JM. Intervention meta-analysis using STATA software. J Health Inform Stat. 2016;41:123–134. [Google Scholar]