Supplemental Digital Content is available in the text
Keywords: LMR, meta-analysis, prognosis, urologic cancer
Abstract
Background:
The prognostic value of pretreatment lymphocyte to monocyte ratio (LMR) in patients with urologic tumors remains controversial. Therefore, we herein conducted a meta-analysis to systematically assess the prognostic value of LMR in patients with urologic tumors.
Methods:
We comprehensively searched PubMed, EMBASE and Web of Science to identify eligible studies. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were used to assess the prognostic value of LMR in patients with urologic tumors. This meta-analysis was registered in PROSPERO (CRD42018108959).
Results:
A total of 20 studies were included in this meta-analysis. Our synthesized analysis showed that low LMR was significantly correlated with poor overall survival (OS) and progression-free survival (PFS) in patients with upper tract urothelial cancer (UTUC). We also found that renal cell cancer (RCC) patients with low LMR had poor OS, PFS and cancer-specific survival (CSS). Besides, it was observed that low LMR predicted poor OS, RFS and CSS in patients with bladder cancer (BC).
Conclusion:
This meta-analysis demonstrated that pretreatment LMR is associated with survival, and may be a useful prognostic parameter in urologic tumors. Nevertheless, more prospective and heterogeneous studies with large samples are required to further confirm our findings before it is applied for daily clinical decision making.
1. Introduction
Cancer remains one of the major causes of mortality and a huge economic burden worldwide.[1] Urologic tumor, including upper tract urothelial cancer (UTUC), renal cell cancer (RCC), bladder cancer (BC) and prostate cancer, is a common type of malignancy. Although the comprehensively therapeutic strategy integrating surgery, immunotherapy, and molecular-targeted therapy has been improved largely in recent years, a subset of patients with urologic tumors still have unfavorable prognosis due to ineffective drug response, local relapse, and distant metastasis.[1–3] A lack of effectively prognostic biomarkers might partly account for the poor prognosis in patients with urologic tumors. Hence, effective and reliable biomarkers that could provide additional prognostic information are imminently needed.[4]
Numerous studies have shown that systemic inflammatory response plays key roles in tumor initiation and progression.[5,6] Several systemic inflammatory biomarkers such as platelet-to-lymphocyte ratio, albumin-to-globulin ratio, neutrophil-to-lymphocyte ratio and C-reactive protein-to-albumin ratio, have been reported to have potential as prognostic biomarkers in a wide variety of tumors.[7,8] Recently, many studies indicated that a lower peripheral lymphocyte-to-monocyte ratio (LMR) was closely associated with worse prognosis in patients with various cancers and might be an easily available and reliable prognostic biomarker.[9–19] In addition, because of the limitation of small sample from individual studies and the presence of conflicting conclusions, several meta-analyses have been performed to further validate the prognostic value of LMR in patients with various tumors.[20] Nevertheless, to date there were no meta-analyses that specifically focus on investigating the association between LMR and patients with urologic tumors. Therefore, it is very imperative to perform a meta-analysis to thoroughly assess the prognostic value of LMR in patients with urologic tumors.
2. Methods
This meta-analysis was undertaken according to preferred reporting items for systematic reviews and meta-analyses statement[21] and it was registered in PROSPERO (CRD42018108959). All analyses were based on previously published studies; thus, no patient consent and ethical approval are required.
2.1. Search strategy
A comprehensive search was performed in PubMed, EMBASE, and Web of Science for eligible studies that explored the prognostic role of LMR in patients with urologic tumors from inception to November, 2018. The search terms included: “lymphocyte and “monocyte or leukomonocyte” and ratio” and “upper tract urothelial carcinoma or UTUC or renal or kidney or bladder or prostate or urinary or urologic or urothelial or transitional”, and “tumor or cancer or carcinoma or adenocarcinoma or malignancy or malignant or neoplasm or neoplastic”. The detailed search strategy in PubMed database was presented in supplement.
2.2. Selection criteria
Study that met the following issues were included:
-
1.
Cohort study or observational study;
-
2.
Patients with urologic tumors were histopathologically confirmed
-
3.
Hazard ratios (HR) and corresponding 95% confidence intervals (CIs) that assessed association between LMR and prognosis of patients with urologic tumor, including overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), disease-free survival (DFS), and progression-free survival (PFS), could be extracted;
-
4.
Full text should be accessible for extracting relevant data.
OS was defined as the interval from the date of surgery on the primary tumor to death. CSS was defined as the interval from the date of surgery on the primary tumor to death for urologic tumors. DFS was defined as the interval from the date of surgery on the primary tumor to local, regional, or distant recurrence or death from any cause. RFS was defined as the interval from the date of surgery on the primary tumor to local, regional, or distant recurrence. PFS was defined as the interval from the date of surgery on the primary tumor until disease progression (including local recurrence or distant metastasis) or death.
Exclusion criteria excluded:
-
1)
The studies were editorials, letters, review articles, meeting abstracts or case reports;
-
2)
The studies included overlapped patients;
-
3)
The studies focused on investigating the relationship between LMR and non-urological tumors.
Only studies in English were considered in this meta-analysis.
2.3. Data extraction and quality evaluation
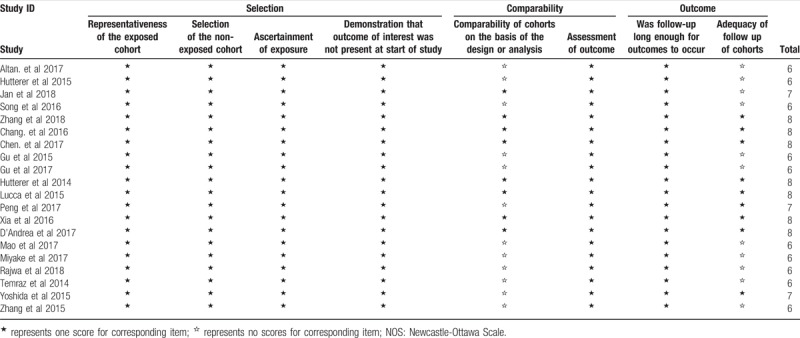
Two investigators extracted relevant data from the included studies independently (Jialin Li and Yusheng Cheng) and divergences in data extraction were resolved by the corresponding author. The extracted information included: first author, publication year, country, tumor type, tumor stage, case number, primary therapy, neoadjuvant therapy, sexual ratio, median age, median follow-up, cut off value for a low LMR, analysis type, and HRs (95% CIs) assessing the association between LMR and prognosis, including OS, DFS, RFS, PFS, and CSS. If the data form both univariable and multivariable analysis were available in the articles, the data from multivariable analysis was extracted for pooling analysis. If the survival data was reported as Kaplan-Meier curves, we used the Engauge Digitizer (version 4.1) and the Tierney methods to extract the HR and its 95% CIs.[22] The quality of the included studies was assessed according to the Newcastle-Ottawa Scale (NOS),[23] and studies with 6 or more were considered as high-quality ones.
2.4. Statistical analysis
Statistical analysis was performed using STATA version 12.0 (Stata Corporation, College Station, TX). HRs with 95% CIs were used to assess the association between LMR and prognosis. The concurrence of with the 95% CI not crossing 1, and HR > 1 indicates that patients with low LMR had a worse prognosis compared to a high LMR. In addition, the link between LMR and clinicopathological features of urologic tumors was assessed using ORs (odds ratios) with 95% CIs. Chi-square-based Q and I2 tests were conducted to assess the heterogeneity among the included studies. Random effects model was applied to combine data if there was significant heterogeneity among the included studies (P < .01 or I2 > 50%), otherwise fixed effects model was used. Sensitivity analysis was performed to further explore the potential sources of heterogeneity and meanwhile test the robustness of our pooled results by sequentially omitting individual studies step by step. Begg test and the Egger test were used to assess the publication bias.[24,25] Duval nonparametric trim-and-fill method was used to evaluate the potential effect of publication bias.[26]
3. Results
3.1. Study search
A of total 163 studies were identified through literature search with 42 from PubMed, 74 from EMBASE, and 47 from Web of Science. After excluding 82 duplicated records, 81 studies were left for title and abstract screening and then 30 studies were excluded for irrelevant topics. The remaining 51 studies were further screened by full text, in which 31 studies were excluded for reviews, conference abstracts, lack of relevant data and overlapped patients. Finally, a total of 20 studies were included in our meta-analysis.[10,13,27–44] The flow diagram of identifying eligible studies was presented in Figure 1.
Figure 1.
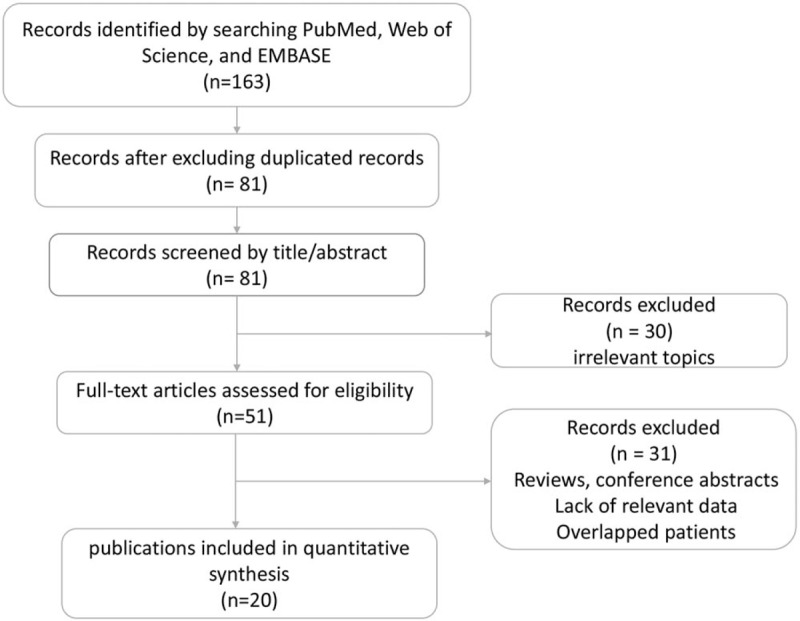
Flow diagram of identifying eligible studies.
3.2. Characteristics of the included studies
A total of 20 studies were included in this meta-analysis. These studies were published between 2014 and 2018, 11 of which from China,[10,13,28,29,31,35,37,39,41–43] 4 from Austria,[30,32–34] 2 from Japan,[36,40] 1 of each study from Turkey,[27] Lebanon,[38] and Poland.[44] A total of 5 studies exploring the prognostic value of LMR in patients with UTUC were included in this meta-analysis.[27,32,37,42,43] Among these studies, 3 studies with 706 patients referred to OS.[32,42,43] Three studies involving 353 patients reported about DFS.[27,37,42] Three studies with 677 patients referred to PFS.[27,37,43] A total of 8 studies exploring the prognostic value of LMR in patients with RCC were included in this meta-analysis.[10,13,28,29,31,33,34,39] Among these studies, 7 studies with 3608 patients referred to OS.[13,27,29,30,32,37,41] Two studies involving 1505 patients reported about PFS.[10,13] Two studies with 1094 patients referred to CSS.[29,33] A total of 7 studies exploring the prognostic value of LMR in patients with BC were included in this meta-analysis.[30,35,36,38,40,41,44] Among these studies, 6 studies with 4969 patients referred to OS.[28,36,38,40,42,43] Two studies involving 324 patients reported about PFS.[35,36] Two studies with 4479 patients referred to CSS.[30,44] Two studies with 4542 patients reported about RFS.[30,35] Across all included studies, the ratio of female patients ranged from 11.8% to 40.3%; The cut-off values for a low LMR were inconsistent, ranging from 2 to 4.44. More information about the main characteristics were summarized in Tables 1 and 2. The scores of quality of all the included studies ranged from 6 to 8, suggesting that all the included studies were eligible for synthesized analysis in this meta-analysis (Table 3).
Table 1.
The main characteristics of the included studies.
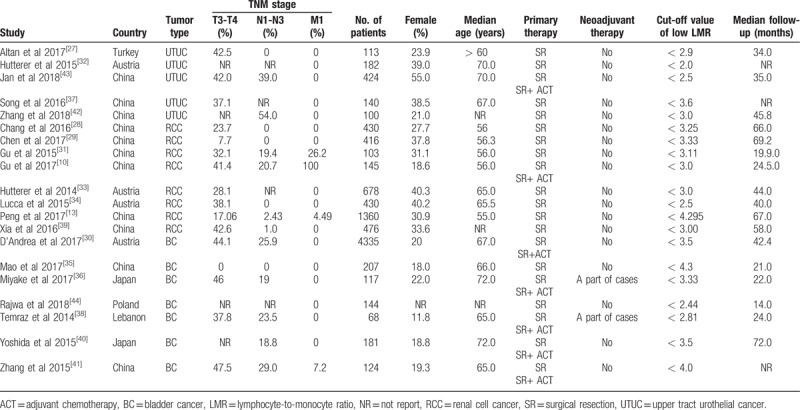
Table 2.
HRs (95% CIs) assessing the association between LMR and prognosis in patients with urologic tumor.
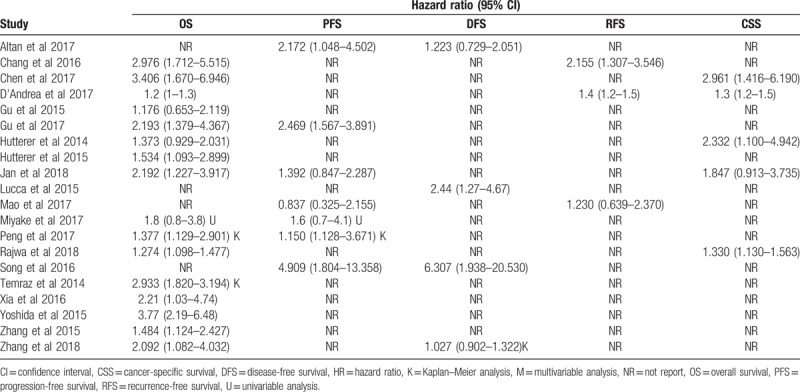
Table 3.
The Newcastle-Ottawa Scale (NOS) quality assessment of the included studies.
3.3. The prognostic value of pretreatment LMR in patients with UTUC
A total of 5 studies exploring the prognostic value of LMR in patients with UTUC were included in this meta-analysis. Among these studies, 3 studies with 706 patients referred to OS. Three studies involving 353 patients reported about DFS. Three studies with 677 patients referred to PFS. The synthesized analyses showed that low LMR was significantly correlated with poor OS (fix-effect model, HR: 1.85, 95% CI: 1.34–2.56, P < .05) (Fig. 2A) and PFS (fix-effect model, HR: 2.20, 95% CI: 1.13–4.26, P < .05) (Fig. 2B), but not with DFS (random-effect model, HR: 1.53, 95% CI: 0.80–2.94, P > .05) (Fig. 2C) in patients with UTUC. Considering that the number of eligible studies was only limited, we did not perform sensitivity analysis and publication bias assessment for the pooling results about the prognostic value of LMR in patients with UTUC.
Figure 2.
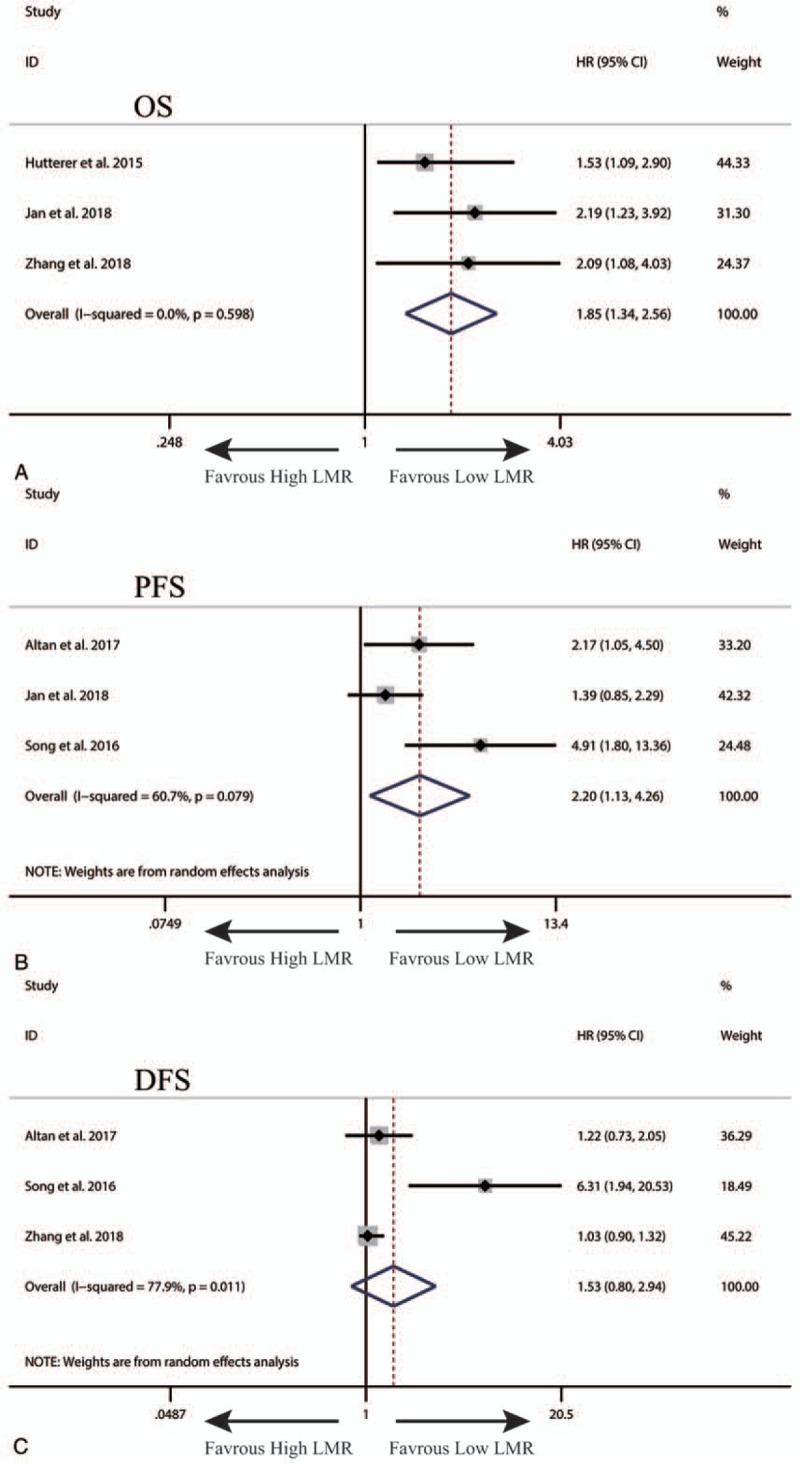
The synthesized HR assessing the prognostic value of pretreatment LMR for OS (A), PFS (B) and DFS (C) in upper tract urothelial cancer. DFS = disease-free survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival, PFS = progression-free survival.
3.4. The prognostic value of pretreatment LMR in patients with RCC
A total of 8 studies exploring the prognostic value of LMR in patients with RCC were included in this meta-analysis. Among these studies, 7 studies with 3608 patients referred to OS. Two studies involving 1505 patients reported about PFS. Two studies with 1094 patients referred to CSS. The synthesized analyses showed that RCC patients low LMR had poorer OS (fix-effect model, HR: 1.81, 95% CI: 1.37–2.40, P < .05) (Fig. 3A) and CSS (fix-effect model, HR: 2.63, 95% CI: 1.56–4.46, P < .05) (Fig. 3B), but equal PFS (random-effect model, HR: 1.75, 95% CI: 0.97–3.17, P < .05) (Fig. 3C). Additionally, we performed sensitivity analysis by sequentially omitting single study and publication bias assessment to further explore the stability and reliability of the pooled result about OS. From the results, we found that the pooled HR about OS was not significantly altered in sensitivity analysis (Fig. 4A), the funnel plot of assessing publication bias was symmetric (Fig. 4B) and the P values of Begg and Egger tests were more than .05, all of which suggested that our pooled result about OS were robust and dependable.
Figure 3.
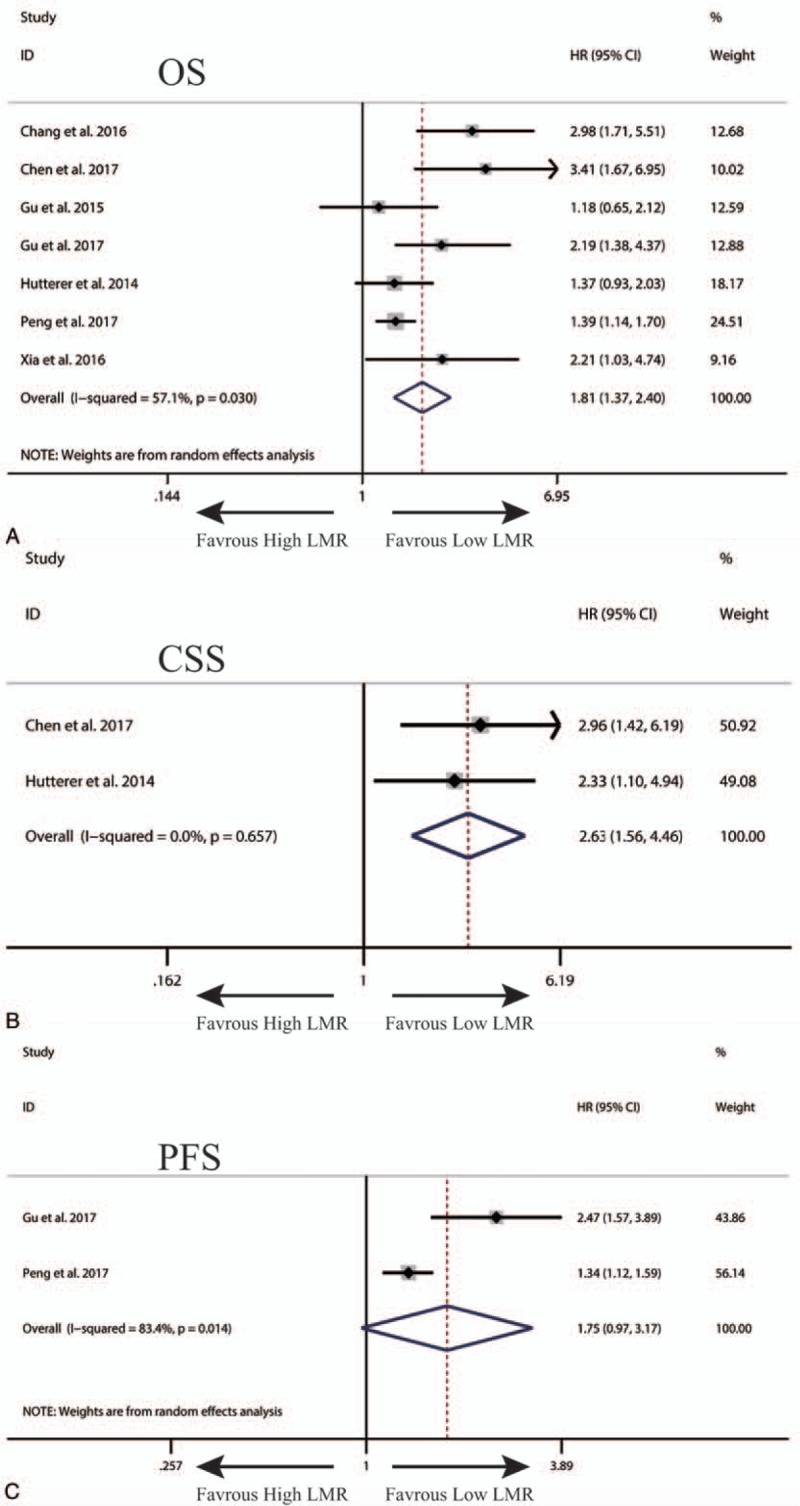
The synthesized HR assessing the prognostic value of pretreatment LMR for OS (A), CSS (B) and PFS (C) and in renal cell cancer. CSS = cancer-specific survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival, PFS = progression-free survival.
Figure 4.
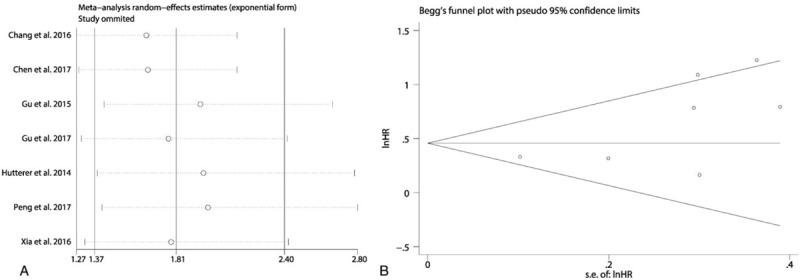
The sensitivity analysis of the synthesized HR assessing the prognostic value of pretreatment LMR for OS in renal cell cancer (A). The funnel plot of Begg test for the publication bias assessment of the synthesized HR assessing the prognostic value of pretreatment LMR for OS in renal cell cancer (B). HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival.
3.5. The prognostic value of pretreatment LMR in patients with BC
A total of 7 studies exploring the prognostic value of LMR in patients with BC were included in this meta-analysis. Among these studies, 6 studies with 4969 patients referred to OS. Two studies involving 324 patients reported about PFS. Two studies with 4479 patients referred to CSS. Two studies with 4542 patients reported about RFS. The synthesized analyses showed that low LMR was related to poor OS (random-effect model, HR: 1.58, 95% CI: 1.24–2.03, P < .05) (Fig. 5), RFS (fix-effect model, HR: 1.39, 95% CI: 1.25–1.56, P < .05) (Fig. 6A) and CSS (fix-effect model, HR: 1.31, 95% CI: 1.19–1.44, P < .05) (Fig. 6B), but not to PFS (fix-effect model, HR: 1.18, 95% CI: 0.62–2.26, P > .05) (Fig. 6C) in patients with BC. Additionally, we performed sensitivity analysis by sequentially omitting single study and publication bias assessment to further explore the stability and reliability of the pooled result about OS of BC patients. From the results, we found that the pooled HR about OS was not significantly altered in sensitivity analysis (Fig. 7). The funnel plot of assessing publication bias was asymmetric (Fig. 8A) and the P values of Begg and Egger tests was less than .05, which indicated that there was significant publication bias for the pooled result about OS of BC patients. Thus, we performed trim-and-fill analysis determine whether the publication bias significantly affected the reliability of the pooled result about OS of BC patients. The result of trim-and-fill analysis showed that the adjusted HR for OS was still more than 1 (random -effect model, HR: 1.25, 95% CI: 1.14–1.36, P < .05), and the adjusted funnel plot of publication bias assessment became symmetric (Fig. 8B), suggesting that the publication bias did not significantly affect the reliability of the pooled result about OS of BC patients.
Figure 5.
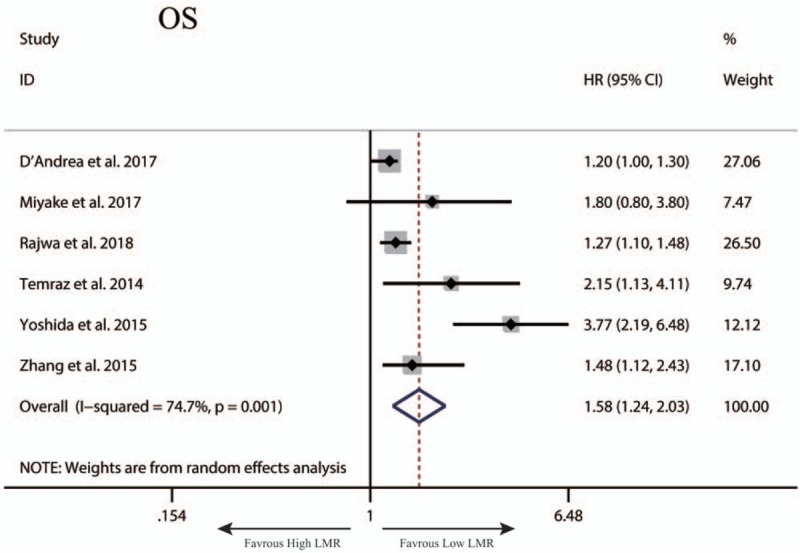
The synthesized HR assessing the prognostic value of pretreatment LMR for OS in bladder cancer. LMR = lymphocyte-to-monocyte ratio, OS = overall survival.
Figure 6.
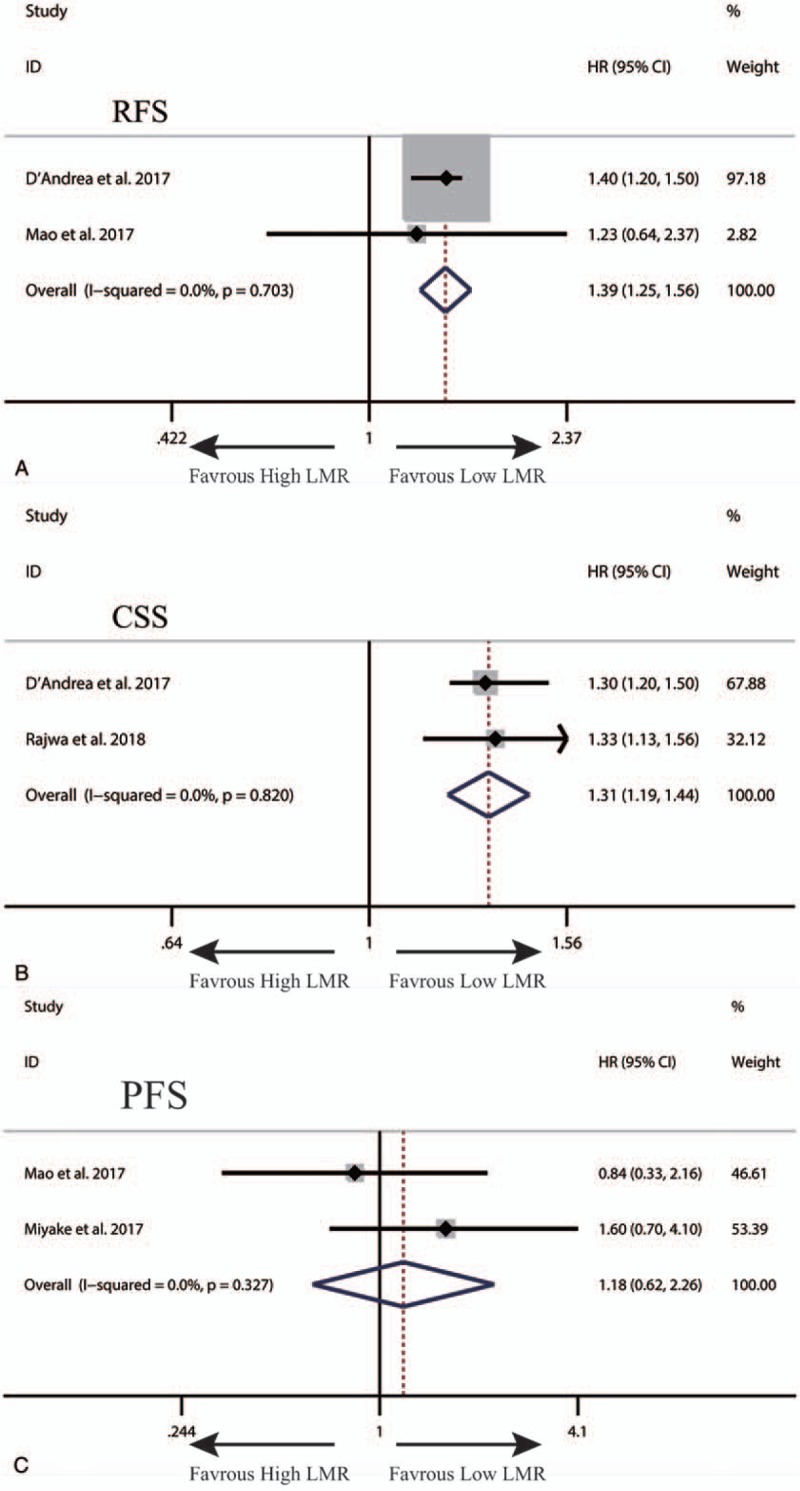
The synthesized HR assessing the prognostic value of pretreatment LMR for RFS (A), CSS (B) and PFS (C) in bladder cancer. CSS = cancer-specific survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
Figure 7.
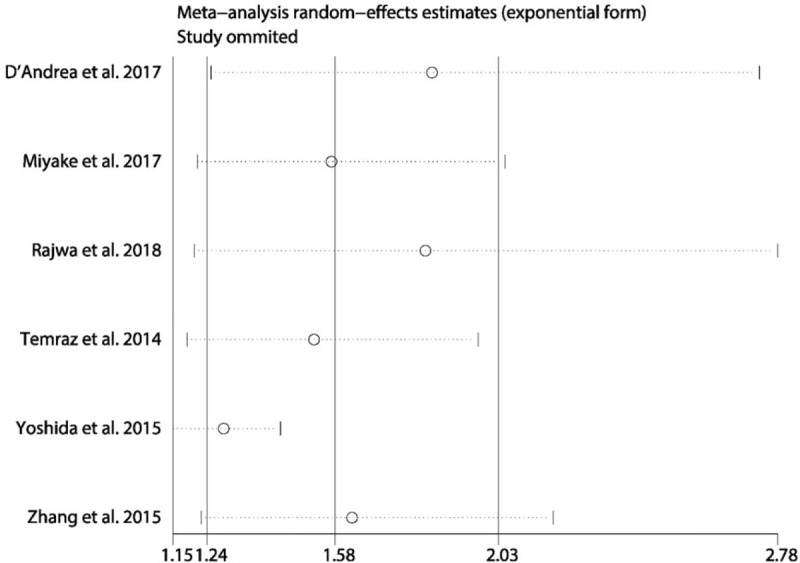
The sensitivity analysis of the synthesized HR assessing the prognostic value of pretreatment LMR for OS in bladder cancer. HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival.
Figure 8.
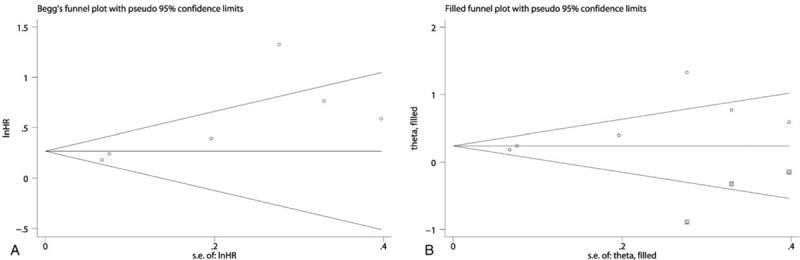
The funnel plot of Begg test for the publication bias assessment of the synthesized HR assessing the prognostic value of pretreatment LMR for OS in bladder cancer (A). The adjusted funnel plot of Begg test for the publication bias assessment of the synthesized HR assessing the prognostic value of pretreatment LMR for OS in bladder cancer (B). HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival.
4. Discussion
To date there were no meta-analyses that specifically focus on investigating the prognostic value of pretreatment LMR in patients with urologic tumors. Therefore, we herein conducted a meta-analysis to systematically assess the prognostic value of LMR in patients with urologic tumors. Our synthesized analysis showed that low LMR was significantly correlated with poor OS and PFS in patients with UTUC. We also found that RCC patients with low LMR had poor OS, PFS, and CSS. Besides, it was observed that low LMR predicted poor OS, RFS, and CSS in patients with BC. Thus, pretreatment LMR may serve as a promising parameter for predicting the prognosis of patients with urologic tumors.
The inflammatory cell's response resulted from tumors could cause the production and release of various cytokines and inflammatory mediators, ultimately promoting tumor invasion, migration, metastasis and progression.[5,45] Monocytes take up about 5% of the circulating leukocytes and play an essential role in innate immunity. Several studies reported that absolute monocyte count was related to survival in patients with colorectal cancer[46] and the infiltrated monocytes in tumor tissue could promote tumor invasion and cell growth in large B-cell lymphoma. However, the exact mechanisms underlying the role of monocytes in tumor progression have not yet been well elucidated. One of mechanisms is the link between monocytes and tumor-associated macrophages (TAMs). That is, macrophages derives from monocytes circulating in peripheral blood, thereby which suggest that the circulating level of monocytes may mirror a surrogate for formation or presence of TAMs. TAMs are sensitive to the chemotactic effect of the tumor microenvironment-secreted cytokines and chemokines, such as monocyte chemoattractant protein-1, tumor necrosis factor-α and others. Furthermore, the interaction between TAMs and cancer cell were capable of promoting tumor angiogenesis, migration, invasion, and depressing antitumor immunity, ultimately leading to tumor progression and poor prognosis of tumor patients.[47] Additionally, there is another hypothesis that may also explain the role of monocytes in tumor progression.[48] The infiltrative monocytes in tumor could release many soluble factors, such as interleukin (IL)-1, IL-6, IL-10 and tumor growth factor-α, and it has been well studied that these factors play an important role in promoting neo-angiogenesis, invasion and migration, and correlate with unfavorable prognosis in various malignant tumors.[49] Moreover, monocytes can restrain mitogen and antigen-induced lymphocytes proliferative response, which impair the lymphocyte-dependent anti-tumor defense, leading to suppression of anti-tumor immunity.[50] In other words, LMR, as the combination of lymphocytes and monocytes, reflects the relative proportion of the 2 types of inflammatory cells, which may be more accurate to reflect the status of tumor-stimulated inflammatory response and predict prognosis in tumor patients than lymphocytes or monocytes. However, the reason why LMR is altered in cancers has not been fully identified so far. One possibility is that under cancer background there may be an inflammatory-immune imbalance, in which the induction of the inflammatory-immune cells, such as lymphocytes or monocytes, is influenced by tumor-associated factors. In addition, as mentioned above, monocytes can restrain mitogen and antigen-induced lymphocytes proliferative response, which may also partly contributes to the alteration of LMR. In light of the content above, we may speculate that LMR could reflect the status of antitumor immunity and predict the prognosis of patients with urological cancers due to its indication of the relative proportion between lymphocytes and monocytes. Therefore, it may be possible to integrate this inflammatory marker into predictive models of prognosis. For example, Zhang et al established a new prognostic model for UTUC by integrating LMR, pathological stage, subsequent bladder tumor, multifocality, and tumor necrosis, and demonstrated that this model could successfully divide UTUC patients into high, intermediate and low risk 3 groups in terms of overall survival time.[42] Nonetheless, LMR has not been recommended in European Association of Urology (EAU) guidelines on BC,[51] UTUC[52] and RCC[53] yet, since most of the evidence supporting the prognostic value of LMR in these tumors came from retrospective studies with small sample size.
Although our meta-analysis overcame the limitation of small sample size in a degree and provided more strong evidence of the prognostic value of LMR in urologic tumors, several limitations still should be seriously considered in our meta-analysis. First, all the included studies were retrospectively designed, which may lead to bias. Second, although we performed synthesized analysis of previous studies, the total sample sizes were not large enough, which still could lead us to make a biased conclusion. Third, in some of the included studies, HRs and 95% CIs were calculated from the survival curves, which might cause some statistical errors more or less. Fourth, HRs and 95% CIs were from univariable analysis, in which confounding factors were not adjusted. Thus, combining these data may introduce a degree of bias and heterogeneity. Fifth, the cut-off values for low LMR were inconsistent across the included studies, which might introduce heterogeneity into our meta-analysis and limit its application in clinical decision making. Additionally, there were several other inconsistencies in aspects of sex ratio, age, tumor stages, and follow-up across the included studies, which may result in bias and heterogeneity as well. Sixth, although this study was a systematic review with a meta-analysis about the prognostic value of LMR in urologic tumors, none of studies focusing on the prognostic value of LMR in prostate cancer. Therefore, it may imperative and interesting to explore the prognostic value of LMR in prostate cancer.
5. Conclusion
This meta-analysis demonstrated that pretreatment LMR is associated with survival, and may be a useful prognostic parameter in urologic tumors. Nevertheless, more prospective and heterogeneous studies with large samples are required to further confirm our findings before it is applied for daily clinical decision making.
Author contributions
Conceptualization: Zhigang Ji.
Data curation: Jialin Li.
Formal analysis: Jialin Li, Yusheng Cheng.
Methodology: Yusheng Cheng.
Software: Jialin Li, Yusheng Cheng.
Visualization: Jialin Li.
Writing – original draft: Jialin Li.
Writing – review & editing: Yusheng Cheng.
Supplementary Material
Footnotes
Abbreviations: ACT = adjuvant chemotherapy, BC = bladder cancer, CI = confidence interval, CSS = cancer-specific survival, DFS = disease-free survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, NOS = Newcastle-Ottawa Scale, NR = not report, OS = overall survival, PFS = progression-free survival, RCC = renal cell cancer, RFS = recurrence-free survival, SR = surgical resection, UTUC = upper tract urothelial cancer.
All the authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- [3].Kimura T, Egawa S, Uemura H. Personalized peptide vaccines and their relation to other therapies in urological cancer. Nat Rev Urol 2017;14:501–10. [DOI] [PubMed] [Google Scholar]
- [4].Mathieu R, Vartolomei MD, Mbeutcha A, et al. Urothelial cancer of the upper urinary tract: emerging biomarkers and integrative models for risk stratification. Minerva Urol Nefrol 2016;68:381–95. [PubMed] [Google Scholar]
- [5].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [6].Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12:584–96. [DOI] [PubMed] [Google Scholar]
- [7].Vartolomei MD, Kimura S, Ferro M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol 2018;36:1019–29. [DOI] [PubMed] [Google Scholar]
- [8].Vartolomei MD, Porav-Hodade D, Ferro M, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol Oncol 2018;36:389–99. [DOI] [PubMed] [Google Scholar]
- [9].Failing JJ, Yan Y, Porrata LF, et al. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res 2017;27:596–600. [DOI] [PubMed] [Google Scholar]
- [10].Gu L, Ma X, Xie Y, et al. Pretreatment lymphocyte to monocyte ratio is an independent prognostic factor in metastatic clear cell renal cell carcinoma. Clin Genitourin Cancer 2017;15:e369–77. [DOI] [PubMed] [Google Scholar]
- [11].Kano S, Homma A, Hatakeyama H, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck 2017;39:247–53. [DOI] [PubMed] [Google Scholar]
- [12].Lieto E, Galizia G, Auricchio A, et al. Preoperative neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio are prognostic factors in gastric cancers undergoing surgery. J Gastrointest Surg 2017;21:1764–74. [DOI] [PubMed] [Google Scholar]
- [13].Peng D, He ZS, Li XS, et al. Prognostic value of inflammatory and nutritional scores in renal cell carcinoma after nephrectomy. Clin Genitourin Cancer 2017;15:582–90. [DOI] [PubMed] [Google Scholar]
- [14].Wang J, Gao K, Lei W, et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget 2017;8:5414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xia H, Sun Z, Deng L, et al. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with stage I non-small cell lung cancer undergoing complete resection. Cancer Invest 2016;34:378–84. [DOI] [PubMed] [Google Scholar]
- [16].Zhu JY, Liu CC, Wang L, et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer 2017;8:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu G, Liu G, Ma JY, et al. Lymphocyte-to-monocyte ratio in esophageal squamous cell carcinoma prognosis. Clin Chim Acta 2018;486:44–8. [DOI] [PubMed] [Google Scholar]
- [18].Hu RJ, Liu Q, Ma JY, et al. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta 2018;484:1–6. [DOI] [PubMed] [Google Scholar]
- [19].Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis. Clin Chim Acta 2018;481:142–6. [DOI] [PubMed] [Google Scholar]
- [20].Wu Q, Hu T, Zheng E, et al. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine (Baltimore) 2017;96:e7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [22].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [24].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [25].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [27].Altan M, Haberal HB, Akdogan B, et al. A critical prognostic analysis of neutrophil-lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma. Int J Clin Oncol 2017. [DOI] [PubMed] [Google Scholar]
- [28].Chang Y, Fu Q, Xu L, et al. Prognostic value of preoperative lymphocyte to monocyte ratio in patients with nonmetastatic clear cell renal cell carcinoma. Tumour Biol 2016;37:4613–20. [DOI] [PubMed] [Google Scholar]
- [29].Chen Z, Shao Y, Yao H, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget 2017;8:48291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].D’Andrea D, Moschini M, Gust KM, et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol 2017;115:455–61. [DOI] [PubMed] [Google Scholar]
- [31].Gu L, Ma X, Li H, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep 2016;6:23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hutterer GC, Sobolev N, Ehrlich GC, et al. Pretreatment lymphocyte-monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol 2015;68:351–5. [DOI] [PubMed] [Google Scholar]
- [33].Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 2014;32:1041–8. [DOI] [PubMed] [Google Scholar]
- [34].Lucca I, de Martino M, Hofbauer SL, et al. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol 2015;33:2045–52. [DOI] [PubMed] [Google Scholar]
- [35].Mao SY, Huang TB, Xiong DD, et al. Prognostic value of preoperative systemic inflammatory responses in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Int J Clin Exp Pathol 2017;10:5799–810. [Google Scholar]
- [36].Miyake M, Morizawa Y, Hori S, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology 2017;93:259–69. [DOI] [PubMed] [Google Scholar]
- [37].Song X, Zhang GM, Ma XC, et al. Comparison of preoperative neutrophil-lymphocyte, lymphocyte-monocyte, and platelet-lymphocyte ratios in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Onco Targets Ther 2016;9:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Temraz S, Mukherji D, Farhat ZAA, et al. Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xia WK, Xia W, Yu TH, et al. Prognostic significance of lymphocyte-to-monocyte ratio and CRP in patients with nonmetastatic clear cell renal cell carcinoma: a retrospective multicenter analysis. Onco Targets Ther 2016;9:2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yoshida T, Kinoshita H, Yoshida K, et al. A novel risk stratification model, involving preoperative lymphocyte-monocyte ratio and standard pathological factors, for overall survival in patients with bladder cancer undergoing radical cystectomy. Jpn J Clin Oncol 2015;45:1162–7. [DOI] [PubMed] [Google Scholar]
- [41].Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumor Biol 2015;36:1–7. [DOI] [PubMed] [Google Scholar]
- [42].Zhang XK, Yang P, Zhang ZL, et al. Preoperative low lymphocyte-tomonocyte ratio predicts poor clinical outcomes for patients with urothelial carcinoma of the upper urinary tract. Urol J 2018;15:348–54. [DOI] [PubMed] [Google Scholar]
- [43].Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol 2018;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [44].Rajwa P, Zyczkowski M, Paradysz A, et al. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci 2018;22:3027–37. [DOI] [PubMed] [Google Scholar]
- [45].Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33Suppl 1:S79–84. [DOI] [PubMed] [Google Scholar]
- [46].Paik KY, Lee IK, Lee YS, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat 2014;46:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–8. [DOI] [PubMed] [Google Scholar]
- [50].Laughter AH, Twomey JJ. Suppression of lymphoproliferation by high concentrations of normal human mononuclear leukocytes. J Immunol 1977;119:173–9. [PubMed] [Google Scholar]
- [51].Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2017;119:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roupret M, Babjuk M, Comperat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 2018;73:111–22. [DOI] [PubMed] [Google Scholar]
- [53].Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


